Abstract
In γ-aminobutyric acid type A (GABAA) receptors, the structural elements that couple ligand binding to channel opening remain poorly defined. Here, site-directed mutagenesis was used to determine if Loop 9 on the non-GABA binding site interface of the β2-subunit may be involved in GABAA receptor activation. Specifically, residues Gly170-Gln185 of the β2-subunit were mutated to alanine, co-expressed with wild-type α1- and γ2S-subunits in human embryonic kidney (HEK) 293 cells and assayed for their activation by GABA, the intravenous anesthetic propofol and the endogenous neurosteroid pregnanolone using whole cell macroscopic recordings. Three mutants, G170A, V175A, and G177A, produced 2.5-, 6.7-, and 5.6-fold increases in GABA EC50 whereas one mutant, Q185A, produced a 5.2-fold decrease in GABA EC50. None of the mutations affected the ability of propofol or pregnanolone to potentiate a submaximal GABA response, but the Q185A mutant exhibited 8.3- and 3.5-fold increases in the percent direct activation by propofol and pregnanolone, respectively. Mutant Q185A receptors also had an increased leak current that was sensitive to picrotoxin, indicating an increased gating efficiency. Further Q185E, Q185L, and Q185W substitutions revealed a strong correlation between the hydropathy of the amino acid at this position and the GABA EC50. Taken together, these results indicate that β2 Loop 9 is involved in receptor activation by GABA, propofol, and pregnanolone and that β2(Q185) participates in hydrophilic interactions that are important for stabilizing the closed state of the GABAA receptor.
Keywords: Channels/Chloride, Protein/Allosteric, Protein/Ligand Binding, Receptors/Structure-Function, GABA Receptors
Introduction
γ-Aminobutyric acid type A (GABAA)2 receptors (GABAARs) are responsible for the majority of fast inhibitory synaptic transmission in the central nervous system and are, consequently, critical to the proper functioning of a number of neural networks, including those involved in sleep and pain sensation (1, 2). Many exogenous and endogenous allosteric modulators, such as general anesthetics and neurosteroids, enhance the activation of GABAARs by GABA and can also directly activate these receptors at higher concentrations (3, 4). Indeed, many of the behavioral effects associated with these compounds, including sedation and analgesia, have been linked to altered GABAAR function (4, 5).
A key component to understanding GABAAR function and modulation is identification of the underlying structural correlates. GABAARs are members of the Cys-loop ligand-gated family of receptors, which includes nicotinic acetylcholine (nACh), 5-hydroxytryptamine (5-HT)3, and glycine receptors, all of which share similar structural characteristics (6). In particular, receptors in this family are formed by the pentameric assembly of subunits, with the most common GABAAR stoichiometry being (α1)2(β2)2(γ2)1 (7). Despite the diversity of pharmacological properties associated with specific subunit incorporation, all subunits have similar structural components, with extracellular, transmembrane, and intracellular domains (7). The neurotransmitter binding sites are formed by the extracellular domains, located at the β/α interfaces for GABAARs. The binding sites of positive allosteric modulators have been located in both extracellular and transmembrane subunit interfaces (8–14). Even though the binding sites for a number of agonists have been localized, the structural elements that couple agonist binding to channel opening are not clearly defined.
One region of the Cys-loop receptors that has been implicated as an important structural element in channel activation is Loop 9 (alternatively, Loop F or the β8-β9 loop) within the extracellular domain. Structural models predict that Loop 9 is close to the transmembrane region, ideally positioned to couple ligand binding to channel opening (15), and chimera studies indicate that this loop is required for formation of functional receptors (16, 17). In α1β2 GABAARs, Loop 9 of the α1-subunit undergoes structural rearrangements upon activation by pentobarbital (18). Similarly, mutations within γ2 Loop 9 of α1β2γ2L GABAARs alter benzodiazepine (BZD) efficacy, affecting the ability of diazepam, flurazepam, and zolpidem to potentiate submaximal GABA responses, without affecting BZD binding (19, 20).
Because Loop 9 of the α- and γ-subunits is important for transducing ligand binding to channel opening, we used Ala-scanning mutagenesis to determine if Loop 9 of the β2-subunit plays a role in gating α1β2γ2S GABAARs. Even though Loop 9 of the GABAAR β2-subunit is located at a non-GABA binding interface, our results suggest that several residues within this region are important for conferring agonist sensitivity. Furthermore, mutations at one residue, β2(Q185), gave rise to a PTX-blockable leak current and enhanced activation by GABA, propofol, and pregnanolone, suggesting that this residue is important for stabilizing the closed state of the receptor.
EXPERIMENTAL PROCEDURES
Mutagenesis and Expression of GABAAR Subunit cDNA
Human α1, rat β2, and human γ2S GABAAR subunit cDNAs in the pCIS2 expression vector were a gift from Neil L. Harrison, Ph.D., Professor, Department of Anesthesiology, Columbia University, New York, NY. Site-directed mutagenesis was performed to introduce point mutations in the β2-subunit cDNA using the QuikChange kit (Stratagene, La Jolla, CA) and confirmed by DNA sequencing of the complete insert.
Wild-type or mutant cDNAs were transiently expressed in HEK 293 cells using a calcium phosphate transfection technique (21). Cells were transfected with 2.5 μg each of GABAAR α1-, γ2S-, β2-, or mutant β2-subunits along with 2.5 μg of an adenosine-associated virus green fluorescent protein (GFP) cDNA to enable identification of transfected cells. HEK 293 cells were maintained in culture (37 °C and 5% CO2) on poly-d-lysine-treated glass coverslips in a solution containing Eagle's minimum essential medium supplemented with 5% fetal bovine serum (Hyclone, Logan, UT), l-glutamine (0.292 mg/ml), penicillin G sodium (100 units/ml), and streptomycin sulfate (100 mg/ml). After 24 h of exposure to precipitate, HEK 293 cells were washed with culture medium. Electrophysiological studies were performed 24–72 h after washing.
Electrophysiological Procedures and Analysis
Whole cell patch-clamp experiments were performed on fluorescing HEK 293 cells under constant perfusion with an external bathing solution (160 mm NaCl, 3 mm KCl, 1 mm MgCl2, 1.5 mm CaCl2, 10 mm HEPES, 6 mm d-glucose, adjusted to pH 7.4 with HCl). Patch pipettes were filled with an intracellular solution (1 mm MgCl2, 5 mm CsCl, 1 mm CaCl2, 1.1 mm EGTA, 147 mm N-methyl-d-glucamine, 5 mm K2ATP, 5 mm HEPES, adjusted to pH 7.2 with HCl) and had resistances of 1–4 MΩ in the external bathing solution. Whole cell currents were recorded at a holding potential of −60 mV using a Multi-Clamp 700B amplifier. Stock solutions of GABA were prepared in water, picrotoxin was prepared in external bathing solution, and propofol (2,6-di-isopropylphenol) and pregnanolone (3α-hydroxy-5β-pregnan-20-one) were prepared in dimethyl sulfoxide. All drugs were obtained from Sigma, and all final dilutions were prepared fresh daily in the external bathing solution. Both propofol and pregnanolone were diluted such that the final concentration of dimethyl sulfoxide was ≤ 0.04% (v/v), which does not affect GABAAR currents (22). All compounds were applied to the cells using a motor-driven solution exchange device (Rapid Solution Changer RSC-160; Molecular Kinetics), with which complete solution exchange was achieved in ∼100–200 ms. Currents were low-pass filtered (100 Hz) and digitized with a 1322A interface (Molecular Devices) using pClamp 9.2.
To measure GABA sensitivity, GABA (0.1 μm - 10 mm) was applied via the motor-driven solution exchanger for 2 s with a 10-s washout between successive applications. Longer washout periods were tested to ensure that accumulated desensitization did not affect the results. The peak current response to each GABA concentration was recorded. Concentration-response curves were generated for each recombinant receptor. Data were fit by non-linear regression analysis (Matlab, Mathworks, Natick, MA) to the Hill equation: I = Imax/(1+(EC50/[A]))nH where I is the peak current amplitude for a concentration of agonist ([A]), Imax is the maximum current amplitude, EC50 is the agonist concentration required for half-maximal receptor activation, and nH is the Hill coefficient.
The ability of propofol (0.5 or 2 μm) and pregnanolone (100 or 500 nm) to directly activate the receptors and to potentiate an EC20 GABA response was also measured. Specifically, potentiation was measured as the percent increase in the current response to co-application of the modulator and an EC20 of GABA relative to a control EC20 response. Modulators were pre-applied for 3–10 s before co-application, and an EC100 GABA response was also measured for each experiment to ensure that the analysis only included potentiation of EC15-EC30 GABA responses. The percent potentiation was calculated as (IMod+EC20GABA − IMod − IEC20GABA)/IEC20GABA × 100, where IMod+EC20GABA is the peak current after pre-application and co-application of the modulator with an EC20 of GABA, IMod is the peak current response from the 3–10 s pre-application of modulator alone and IEC20GABA is the peak current elicited by an EC20 of GABA. Direct activation is defined as IMod/Imax × 100, where Imax corresponds to the EC100 GABA response for each experiment. The propofol concentration (2 μm) used in most of the experiments corresponds to the clinically relevant anesthetic EC50 (22), and the pregnanolone concentration (500 nm) corresponds to the GABAAR EC50 for direct activation (data not shown). Lower modulator concentrations were also tested on select receptors to ensure that the potentiation results were not confounded by a ceiling effect.
Finally, picrotoxin (PTX) responsiveness was measured by applying 2 mm PTX for 3 s after recording baseline/leak activity for 3 s. The response to an EC100 of GABA was also recorded for each experiment. Subsequently, the percent of leak current reduction by PTX (% PTX) was calculated by (IPTX − Ileak)/(IPTX − Imax) × 100, where Ileak and IPTX are the average and maximal currents in the 3 s prior to and during PTX application, respectively. For all experiments, statistical significance was assessed using a one-way analysis of variance (ANOVA) followed by a Dunnett's post-hoc test (SPSS, SPSS Inc., Chicago, IL).
RESULTS
Expression and Functional Characterization of Ala Mutations
Alanine mutations were introduced at 15 individual positions (Fig. 1) in the GABAAR β2-subunit (Gly170, Asp171, Asp172, Asn173, Val175, Thr176, Gly177, Val178, Thr179, Lys180, Ile181, Glu182, Leu183, Pro184, and Gln185), co-expressed with GFP, wild-type α1- and γ2S-subunits in HEK 293 cells and assayed for functionality using whole cell voltage-clamp measurements. Expression of all mutant subunits resulted in fluorescing cells with GABA-activated chloride current. Full GABA concentration-response relationships were used to compare receptor activity (Fig. 2). Three mutations significantly increased the GABA EC50; mutants G170A, V175A, and G177A resulted in 2.5-, 6.7-, and 5.6-fold EC50 increases relative to wild-type (EC50 = 47.6 ± 5.0 μm) (Table 1). Although not statistically significant, the mutant Q185A produced a 5.2-fold reduction in GABA EC50 compared with wild-type. None of the mutations significantly altered the Hill coefficient for GABA activation.
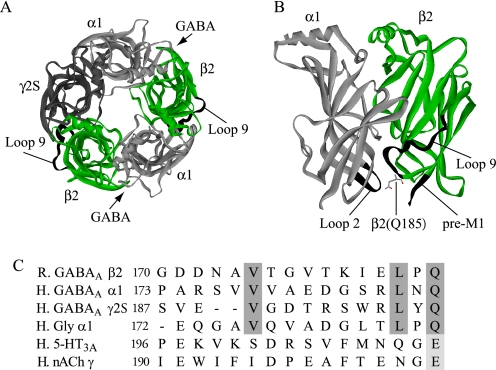
FIGURE 1.
Location of Gly170–Gln185 (Loop 9) of the GABAAR β2-subunit. A, homology model (44) of the extracellular α1β2γ2S GABAAR subunit domains viewed from the extracellular side. Arrows indicate the subunit interfaces at which GABA binding occurs. Loop 9 is shown in black on the green β2-subunits. B, alternate view showing adjacent GABAAR α1- and β2-subunits and highlighting the location of Loop 9 on the β2-subunit. The pre-M1 region of the β2-subunit and Loop 2 of the α1-subunit are also labeled to indicate their proximity to the β2(Q185) residue. C, alignment of Loop 9 sequences from several Cys-loop receptor subunits: rat GABAA β2 (accession number: NP_037089), human GABAA α1 (accession number: CAA32874), human GABAA g2S (accession number: CAA33437), human glycine (Gly) α1 (accession number: NP_000162), human 5-hydroxytryptamine (5-HT)3A (accession number: AAH04453), and human nicotinic acetylcholine (nACh) γ (accession number: NP_005190). Conserved residues within this region are highlighted. Dashes indicate gaps in the amino acid sequence alignment, and numbering is from Cromer et al. (45).
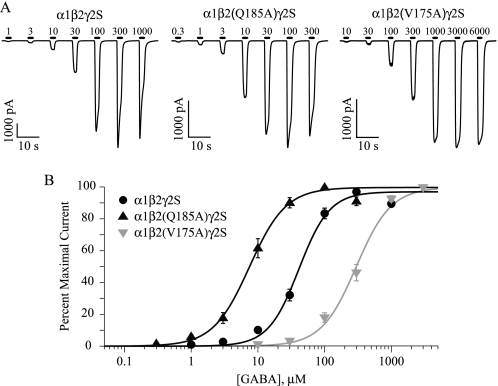
FIGURE 2.
GABA-activated currents and concentration-response relationships for wild-type α1β2γ2S, mutant α1β2(Q185A)γ2S, and mutant α1β2(V175A)γ2S receptors. A, average time course of wild-type and mutant receptor currents in response to successive 2-s applications of GABA. Black bars indicate the timing of GABA application, and the numbers indicate the μm GABA concentration. B, concentration-response relationships for the data shown in A. Raw data were normalized to the maximum GABA-elicited current for each experiment and are shown as the mean ± S.E. of at least eight experiments. EC50 values and Hill coefficients were determined by a fit of these data to the Hill equation (see “Experimental Procedures”), and Table 1 contains values for all Ala mutations.
TABLE 1.
Effect of β2 Ala substitutions on the EC50 values and Hill coefficients (nH) for GABA activation
Data represent the mean ± S.E. for n cells and were determined from fits of the Hill equation to normalized current responses. The EC50ratio is given as the mutant (MUT) divided by the WT value.
| Receptor | EC50 | nH | n | EC50 ratio |
|---|---|---|---|---|
| μm | MUT/WT | |||
| α1β2γ2S | 47.6 ± 5.0 | 2.02 ± 0.09 | 20 | – |
| α1β2 (G170A) γ2S | 121.1 ± 14.9a | 1.73 ± 0.14 | 12 | 2.54 |
| α1β2 (D171A) γ2S | 60.2 ± 8.8 | 1.78 ± 0.11 | 12 | 1.26 |
| α1β2 (D172A) γ2S | 29.9 ± 4.1 | 1.96 ± 0.14 | 9 | 0.63 |
| α1β2 (N173A) γ2S | 30.6 ± 1.7 | 2.02 ± 0.11 | 14 | 0.64 |
| α1β2 (V175A) γ2S | 317.3 ± 33.8a | 1.94 ± 0.12 | 12 | 6.67 |
| α1β2 (T176A) γ2S | 33.3 ± 2.9 | 2.48 ± 0.14 | 13 | 0.70 |
| α1β2 (G177A) γ2S | 264.8 ± 22.9a | 1.64 ± 0.04 | 12 | 5.56 |
| α1β2 (V178A) γ2S | 56.5 ± 9.8 | 2.07 ± 0.08 | 11 | 1.19 |
| α1β2 (T179A) γ2S | 55.7 ± 8.4 | 1.84 ± 0.07 | 17 | 1.17 |
| α1β2 (K180A) γ2S | 44.6 ± 5.2 | 2.20 ± 0.13 | 11 | 0.94 |
| α1β2 (I181A) γ2S | 34.3 ± 3.1 | 2.01 ± 0.11 | 15 | 0.72 |
| α1β2 (E182A) γ2S | 40.6 ± 4.2 | 1.98 ± 0.06 | 12 | 0.85 |
| α1β2 (L183A) γ2S | 18.3 ± 2.0 | 2.18 ± 0.16 | 14 | 0.38 |
| α1β2 (P184A) γ2S | 28.5 ± 3.1 | 2.17 ± 0.12 | 16 | 0.60 |
| α1β2 (Q185A) γ2S | 8.9 ± 1.5 | 1.91 ± 0.10 | 14 | 0.19 |
a Significantly different from wild-type (ANOVA with Dunnett's post test: p < 0.01).
Effect of Ala Mutations on Direct Activation and Potentiation of GABA Responses by Propofol and Pregnanolone
To determine the importance of the β2 Loop 9 region in allosteric modulation of the GABAAR, the percent activation and potentiation of an EC20 GABA-activated current by 2 μm propofol and 500 nm pregnanolone was measured for all 15 Ala mutations. All mutant receptors retained sensitivity to both propofol and pregnanolone, exhibiting some degree of direct activation and potentiation of an EC20 GABA response (Fig. 3). One mutant, Q185A, showed significant 8.3- and 3.5-fold enhancement of propofol and pregnanolone direct activation compared with wild-type (propofol = 2.1 ± 0.5%; pregnanolone = 8.3 ± 1.1%) (Fig. 4 and Table 2). There were no significant differences in the percent potentiation.
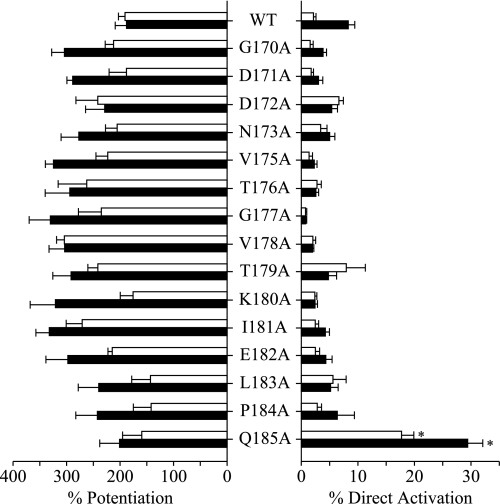
FIGURE 3.
Percent potentiation and direct activation of α1β2γ2S WT and β2 mutant receptors in response to 2 μm propofol (white bars) or 500 nm pregnanolone (black bars). Potentiation was measured as the percent enhancement of an EC20 GABA response. Direct activation was measured as a percent of the maximum GABA-elicited current. Results represent the mean ± S.E. for six to seven experiments. *, significantly different from wild-type (ANOVA with Dunnett's post test: p < 0.01).
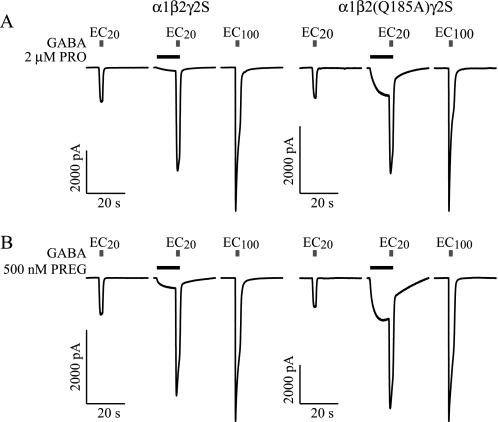
FIGURE 4.
Direct activation and potentiation of GABA-activated Cl− currents in wild-type α1β2γ2S and mutant α1β2(Q185A)γ2S receptors by A, 2 μm PRO, and B, 500 nm PREG. Bars above the current traces indicate the duration of agonist application, with the gray and black bars corresponding to GABA and modulator application, respectively. GABA EC20 and EC100 were calculated from the concentration-response data summarized in Table 1. Percent PRO and PREG direct activation and potentiation for all Ala mutants is summarized in Fig. 3.
TABLE 2.
Effect of amino acid substitution at α1β2(Q185)γ2S on the activation and modulation of receptor current
GABA EC50 values and Hill coefficients (nH) were determined from fits of the Hill equation to normalized current responses. The apparent PTX and leak (Ileak) currents as well as the PRO and PREG direct activation (DA) measurements are reported as percentages of the maximum elicited GABA current (Imax) for each experiment. Data represent the mean ± S.E. for (n) cells.
| β 2(Q185) | β 2(Q185A) | β 2(Q185L) | β 2(Q185W) | β 2(Q185E) | |
|---|---|---|---|---|---|
| EC50 (μm) | 47.6 ± 5.0 (20) | 8.9 ± 1.5 (14) | 4.2 ± 0.6 (10) | 20.4 ± 2.7 (16) | 18.9 ± 2.6 (13) |
| nH | 2.02 ± 0.09 | 1.91 ± 0.08 | 1.86 ± 0.09 | 1.92 ± 0.06 | 1.98 ± 0.10 |
| % PTX | 0.06 ± 0.02 (9) | 0.57 ± 0.17 (10) | 0.91 ± 0.29a (9) | 0.13 ± 0.04 (8) | 0.19 ± 0.07 (9) |
| % Ileak | 1.35 ± 0.38 | 2.52 ± 0.53 | 2.26 ± 0.46 | 1.11 ± 0.20 | 0.97 ± 0.20 |
| Imax (nA) | 6.52 ± 1.84 | 5.16 ± 1.32 | 14.97 ± 1.71 | 7.55 ± 1.08 | 9.03 ± 1.34 |
| % PRO DA | 2.14 ± 0.46 (6) | 17.7 ± 2.2a (6) | 12.9 ± 3.1a (5) | 5.13 ± 0.70 (6) | 5.15 ± 0.60 (6) |
| % PREG DA | 8.30 ± 1.15 (6) | 29.4 ± 2.7a (6) | 39.9 ± 5.7a (5) | 10.4 ± 0.65 (6) | 13.5 ± 1.39 (6) |
a Significantly different from wild-type (ANOVA with Dunnett's post test: p < 0.01).
Because the potentiated currents of the Q185A mutant were close to the maximum GABA-elicited current (Fig. 4), the wild-type and Q185A measurements were repeated with lower modulator concentrations (0.5 μm propofol and 100 nm pregnanolone) to ensure that the potentiation was not truncated. As with the higher concentrations, direct activation was significantly increased in the Q185A mutant (propofol = 3.9 ± 0.9%; n = 5; pregnanolone = 12.8 ± 1.4%; n = 6) compared with wild-type (propofol = 0.4 ± 0.1%, n = 5; pregnanolone = 1.0 ± 0.2%, n = 5), but there were no significant differences in potentiation (Q185A: propofol = 69 ± 19%, n = 5; pregnanolone = 204 ± 58%, n = 6; wild-type: propofol = 105 ± 14%, n = 5; pregnanolone = 224 ± 37%, n = 5).
Spontaneous Openings in Mutant β2(Q185A) Receptors
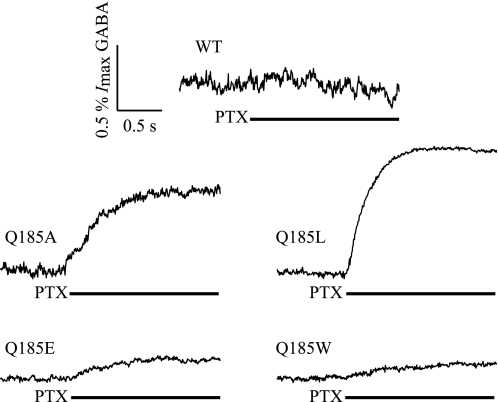
In addition to increased sensitivity to direct activation by propofol and pregnanolone, mutant Q185A receptors had a 1.7-fold greater leak current (Ileak = −116 ± 37 nA, n = 10) compared with wild-type receptors (Ileak = −67 ± 13 nA, n = 11). The GABAAR blocker PTX (2 mm) reduced the leak current of Q185A-containing receptors by 0.57 ± 0.17% of the maximum GABA-activated current (Table 2 and Fig. 5). Comparatively, there was no discernable reduction in the leak current of wild-type receptors upon PTX application. Together, this suggests that the Q185A mutation increases the number of spontaneous, unliganded open GABAARs.
FIGURE 5.
Average traces showing reduction of leak current in WT α1β2γ2S as well as mutant α1β2(Q185A)γ2S, α1β2(Q185L)γ2S, α1β2(Q185E)γ2S, and α1β2(Q185W)γ2S receptors by application of 2 mm PTX (black bar indicates timing of application). The scale bar applies to all five receptors, and Table 2 summarizes the average percent of leak reduction by PTX (% PTX).
Characterization of Additional Amino Acid Substitutions at β2(Q185)
To gain insight into the role of residue β2(Q185) during normal receptor function, we introduced three additional mutations at this site: Q185E, Q185L, and Q185W. These substitutions were chosen to investigate the importance of side chain size and hydrogen bonds on receptor activity. Similar to the Ala mutation, Q185L-containing receptors exhibited statistically significant 6.0- and 4.8-fold increases in direct activation by propofol and pregnanolone, respectively, compared with wild type (Table 2). A PTX-blockable leak current was evident in all three mutants, but the percent of leak current reduction was only statistically significant in Q185L-containing receptors, where PTX reduced the leak current by 0.91 ± 0.29% of the maximum GABA response (Fig. 5 and Table 2). GABA concentration-response curves for all three mutants yielded no statistically significant differences compared with wild type; however, the Q185L mutant was similar to Q185A, with a 11.3-fold reduction in GABA EC50 (Table 2).
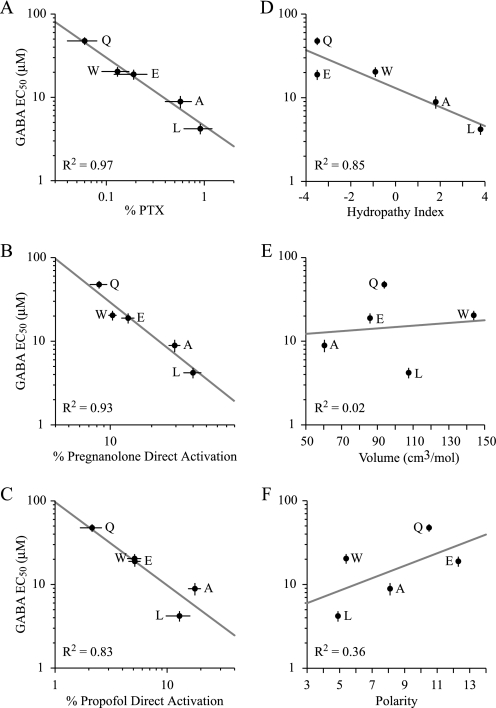
Although the GABA EC50 values of the β2(Q185) mutants were not significantly different from wild type, they were correlated to several experimental metrics (Fig. 6, A–C). In particular, the GABA EC50 was negatively correlated with the apparent PTX current, pregnanolone direct activation and propofol direct activation. In addition, the GABA EC50 was highly correlated with the hydropathy (23) of the amino acid substituted at β2(Q185) but not with the volume (24) or polarity (25) (Fig. 6, D–F).
FIGURE 6.
The GABA EC50 for wild-type and α1β2(Q185)γ2S mutant receptors is correlated to the percent of leak reduction by PTX, the percent direct activation by propofol and pregnanolone, as well as the hydropathy of the amino acid at the β2(Q185) position. Correlations between GABA EC50 (μm) and A, the percent of leak current reduction by 2 mm PTX; B–C, the percent direct activation by 500 nm pregnanolone and 2 μm propofol; and D–F, the hydropathy index, volume, and polarity of amino acids substituted at the β2(Q185) position. Letters adjacent to points indicate the amino acid substituted at β2(Q185), and the R2 value indicates the goodness of fit for each relationship.
DISCUSSION
Previous studies indicate that Loop 9 within the extracellular domains of both the α and γ GABAAR subunits is involved in transducing ligand binding to channel opening (18–20). To determine if Loop 9 of the β2-subunit may play a similar role, we performed a site-directed mutagenesis study, assaying individual mutants for their responsiveness to GABA, propofol, pregnanolone, and picrotoxin. Overall, our results indicate that the β2Gly170-Gln185 region is important for gating the receptor in response to neurotransmitter binding and that Gln185 is important for stabilizing the closed state of the receptor.
To investigate the functionality of β2 Loop 9 in GABAAR activation, we first performed an Ala scan of this region. By substituting with Ala, we assumed that the specific side-chain interactions were eliminated but that the backbone conformation of the protein was unaffected (26). Our results indicate that β2 Loop 9 is important for GABA activation. Specifically, Ala substitution at three residues in the GABAAR β2 subunit, G170A, V175A, and G177A, significantly increased the GABA EC50. Altered apparent GABA affinity can be caused by changes in binding affinity, channel gating, or a combination of both (27). However, because Loop 9 of the GABAAR β2-subunit is located at the non-GABA binding site interface, it is unlikely that the observed EC50 effects are due to rearrangement of the GABA binding site. Instead, the altered apparent GABA affinity is likely due to an increased efficacy of channel gating.
The significant shift in GABA EC50 observed for several β2 Loop 9 mutants indicates that structural components spatially removed from the ligand binding site can affect receptor activation. Previous experiments provide further evidence that spatial coupling across long distances is possible in GABAARs. For instance, cysteine accessibility studies have shown that GABA binding to α1β2γ2S receptors can induce structural rearrangements in the extracellular α/γ interface and that benzodiazepine binding at the extracellular α/γ interface can induce structural movements at the extracellular α/β interface (28). Fluorescent labeling studies have also shown that GABA binding induces movement of Loop E on the non-GABA binding interface of the β2-subunit in α1β2 receptors (29). Our results suggest that GABA binding may also induce movement of Loop 9 on the analogous interface in α1β2γ2S receptors.
Because several β2 Loop 9 mutations altered GABA sensitivity, we compared the ability of two allosteric modulators, propofol and pregnanolone, to activate all Ala mutants. In doing so, we sought to determine if the role of β2 Loop 9 in GABAAR activation is specific to GABA-initiated openings. Only one Ala mutation, β2(Q185A), significantly affected the actions of these two compounds, dramatically increasing their ability to directly activate the receptor. Because Loop 9 is spatially distinct from the proposed binding sites of these two agonists in the α- and β-subunit transmembrane domains (11–14), the increase in direct activation is likely caused by changes in gating efficiency. Coupled with the modest decrease in GABA EC50 introduced by the Q185A mutation, this result indicates that β2(Q185) may play a similar role in transducing activation by GABA, propofol, and pregnanolone. A previous study indicates that activation by GABA and general anesthetics induces similar structural movements of the transmembrane domain (TM) 2 region (30), but a comparable technique showed distinct N terminus and TM2 movements upon activation by the barbiturate pentobarbital and GABA (31). Along with further structural studies, it would be interesting to use rate equilibrium linear free energy analysis to compare the predicted location of β2(Q185) along the closed to open pathway when different agonists are used to initiate receptor activation (32).
Although receptors with the β2(Q185A) mutation exhibited enhanced direct activation by both propofol and pregnanolone, this mutation did not affect the ability of either modulator to potentiate submaximal GABA responses. There are other examples where the abilities of propofol and neurosteroids to potentiate and directly activate GABAARs have been uncoupled. For example, α4β1γ2 GABAARs show propofol potentiation but not direct activation (33). A mutation in TM2, α2β1(S265I), markedly reduced propofol direct activation but did not significantly affect GABA potentiation (34) whereas a mutation in TM3, α1β2(M286W)γ2S, was associated with the opposite effect (11). Similarly, TM1 substitutions α1(T236I)β2γ2S and α1(C233W)β2γ2S as well as TM3 substitution α1β2(Y284F)γ2S reduced direct activation of GABAARs by neurosteroids without affecting submaximal GABA potentiation (13). In addition to these functional assays, several cysteine accessibility studies indicate that the TM2 segment of GABAARs undergoes distinct structural rearrangements during direct activation and potentiation by propofol (30, 35). Even though previous work has focused on the transmembrane domains, our results suggest that direct activation and potentiation by propofol and pregnanolone may also elicit distinct N terminus movements. Studies that specifically measure protein movement are, however, necessary to verify this finding.
In addition to enhancing the ability of several modulators to directly activate the receptor, the β2(Q185A) mutation significantly increased the leak and apparent PTX currents, suggesting an increase in the resting open probability of the mutant receptor. Mutations at several structural locations within GABAARs give rise to spontaneously active receptors. A majority of these residues are located within TM2 and, as with the β2(Q185A) mutation, are associated with an increased leak current along with a decreased GABA EC50 (36–38). However, a mutation in β-sheet 7 of the extracellular domain, α1β2(E155C), also increased the probability of spontaneously open receptors (39). Taken together with this study, it appears that the extracellular domain of the β-subunit is an important region in conferring gating efficiency and, more specifically, for stabilizing the closed state of the GABAA receptor.
To further characterize the physiochemical amino acid properties that are important at the β2(Q185) position for GABAAR activation, Q185L, Q185W, and Q185E mutants were assayed for their responsiveness to GABA, propofol, pregnanolone, and picrotoxin. In all mutants, a decrease in GABA EC50 was correlated with an increase in spontaneous receptor activity and with an increase in direct activation by propofol and pregnanolone. The most dramatic effects were observed with the Leu and Ala mutations, which eliminated all side-chain hydrogen bonds. Comparatively, the Glu and Trp substitutions, which introduced more modest electrophysiological changes, reduced the wild-type hydrogen donor capability by one. The importance of side-chain interactions at β2(Q185) is further suggested by the apparent tolerance of backbone distortions at this position, which were likely introduced by the Trp substitution. Moreover, the GABA EC50 values of the β2(Q185) mutants were correlated with the hydropathy of the substituted amino acid but not with molecular volume or polarity. Specifically, a more hydrophobic amino acid was associated with a decrease in the GABA EC50. The predicted spatial proximity of β2 Loop 9 to the pre-M1 region of the same subunit and to Loop 2 of the adjacent subunit (α1 or γ2S), as shown in Fig. 1B, makes these regions likely candidates for intra- or intersubunit hydrogen bond pairings. There is precedence for molecular interactions between Loop 9 and the pre-M1 region in Cys-loop receptors. In 5-HT3 receptors, it has been suggested that a charge interaction between the Glu215 in Loop 9 with Arg246 in the pre-M1 region is involved in the gating process (40). Similarly, our results suggest that increased hydropathy at β2(Q185) eliminates or weakens molecular interactions that are important for stabilizing the closed state of the GABAA receptor.
The receptor dysfunction associated with mutating homologous β2(Q185) positions within the Cys-loop family underscores the importance of this residue. In the neuronal nicotinic acetylcholine receptors, the α7(E172) position is important for modulating the effects of calcium on current amplitudes and agonist affinity, where an α7(E172Q) mutation eliminates these effects (41, 42). In GABACR homomers, a ρ1(Q226C) substitution decreases GABA efficacy (43). Furthermore, the α1(Q189C)β2 mutation prevents expression of functional GABAA receptors (18). Thus, this study and others indicate that Loop 9, and particularly the Q185 position, is important for transducing activation and modulation of Cys-loop receptors.
Acknowledgment
We thank Kate O'Toole for critical reading of the manuscript.
This work was supported, in whole or in part, by NIGMS, National Institutes of Health Grants 073959 (to A. J.) and F32 084621 (to C. A. W.).
- GABAA
- γ-aminobutyric acid type A
- GABAAR
- type A GABA receptor
- nACh
- nicotinic acetylcholine
- 5-HT
- 5-hydroxytryptamine
- HEK
- human embryonic kidney
- GFP
- green fluorescent protein
- TM1–4
- transmembrane domain 1–4
- WT
- wild-type α1β2γ2s GABAAR
- PTX
- picrotoxin
- PRO
- propofol
- PREG
- pregnanolone
- ANOVA
- analysis of variance.
REFERENCES
- 1.Franks N. P. (2008) Nat. Rev. Neurosci. 9, 370–386 [DOI] [PubMed] [Google Scholar]
- 2.Price T. J., Cervero F., Gold M. S., Hammond D. L., Prescott S. A. (2009) Brain Res. Rev. 60, 149–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callachan H., Cottrell G. A., Hather N. Y., Lambert J. J., Nooney J. M., Peters J. A. (1987) Proc. R. Soc. Lond., Ser. B: Biol. Sci. 231, 359–369 [DOI] [PubMed] [Google Scholar]
- 4.Hemmings H. C., Jr., Akabas M. H., Goldstein P. A., Trudell J. R., Orser B. A., Harrison N. L. (2005) Trends Pharmacol. Sci. 26, 503–510 [DOI] [PubMed] [Google Scholar]
- 5.Mitchell E. A., Herd M. B., Gunn B. G., Lambert J. J., Belelli D. (2008) Neurochem. Int. 52, 588–595 [DOI] [PubMed] [Google Scholar]
- 6.Ortells M. O., Lunt G. G. (1995) Trends Neurosci. 18, 121–127 [DOI] [PubMed] [Google Scholar]
- 7.Olsen R. W., Sieghart W. (2009) Neuropharmacology 56, 141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigel E., Buhr A. (1997) Trends Pharmacol. Sci. 18, 425–429 [DOI] [PubMed] [Google Scholar]
- 9.Li G. D., Chiara D. C., Sawyer G. W., Husain S. S., Olsen R. W., Cohen J. B. (2006) J. Neurosci. 26, 11599–11605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart D., Desai R., Cheng Q., Liu A., Forman S. A. (2008) Mol. Pharmacol. 74, 1687–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krasowski M. D., Nishikawa K., Nikolaeva N., Lin A., Harrison N. L. (2001) Neuropharmacology 41, 952–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bali M., Jansen M., Akabas M. H. (2009) J. Neurosci. 29, 3083–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosie A. M., Wilkins M. E., da Silva H. M., Smart T. G. (2006) Nature 444, 486–489 [DOI] [PubMed] [Google Scholar]
- 14.Li G. D., Chiara D. C., Cohen J. B., Olsen R. W. (2009) J. Biol. Chem. 284, 11771–11775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zouridakis M., Zisimopoulou P., Poulas K., Tzartos S. J. (2009) IUBMB Life 61, 407–423 [DOI] [PubMed] [Google Scholar]
- 16.Bouzat C., Gumilar F., Spitzmaul G., Wang H. L., Rayes D., Hansen S. B., Taylor P., Sine S. M. (2004) Nature 430, 896–900 [DOI] [PubMed] [Google Scholar]
- 17.Paulo J. A., Hawrot E. (2009) Protein Expr. Purif. 67, 76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newell J. G., Czajkowski C. (2003) J. Biol. Chem. 278, 13166–13172 [DOI] [PubMed] [Google Scholar]
- 19.Padgett C. L., Lummis S. C. (2008) J. Biol. Chem. 283, 2702–2708 [DOI] [PubMed] [Google Scholar]
- 20.Hanson S. M., Czajkowski C. (2008) J. Neurosci. 28, 3490–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C., Okayama H. (1987) Mol. Cell Biol. 7, 2745–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krasowski M. D., Jenkins A., Flood P., Kung A. Y., Hopfinger A. J., Harrison N. L. (2001) J. Pharmacol. Exp. Ther. 297, 338–351 [PubMed] [Google Scholar]
- 23.Kyte J., Doolittle R. F. (1982) J. Mol. Biol. 157, 105–132 [DOI] [PubMed] [Google Scholar]
- 24.Zamyatnin A. A. (1984) Annu. Rev. Biophys. Bioeng. 13, 145–165 [DOI] [PubMed] [Google Scholar]
- 25.Grantham R. (1974) Science 185, 862–864 [DOI] [PubMed] [Google Scholar]
- 26.Cunningham B. C., Wells J. A. (1989) Science 244, 1081–1085 [DOI] [PubMed] [Google Scholar]
- 27.Colquhoun D. (1998) Br. J. Pharmacol. 125, 924–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharkey L. M., Czajkowski C. (2008) Mol. Pharmacol. 74, 203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muroi Y., Czajkowski C., Jackson M. B. (2006) Biochemistry 45, 7013–7022 [DOI] [PubMed] [Google Scholar]
- 30.Rosen A., Bali M., Horenstein J., Akabas M. H. (2007) Biophys. J. 92, 3130–3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muroi Y., Theusch C. M., Czajkowski C., Jackson M. B. (2009) Biophys. J. 96, 499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grosman C., Zhou M., Auerbach A. (2000) Nature 403, 773–776 [DOI] [PubMed] [Google Scholar]
- 33.Wafford K. A., Thompson S. A., Thomas D., Sikela J., Wilcox A. S., Whiting P. J. (1996) Mol. Pharmacol. 50, 670–678 [PubMed] [Google Scholar]
- 34.Krasowski M. D., Koltchine V. V., Rick C. E., Ye Q., Finn S. E., Harrison N. L. (1998) Mol. Pharmacol. 53, 530–538 [DOI] [PubMed] [Google Scholar]
- 35.Williams D. B., Akabas M. H. (2002) J. Neurosci. 22, 7417–7424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang Y., Weiss D. S. (1998) Mol. Pharmacol. 53, 511–523 [DOI] [PubMed] [Google Scholar]
- 37.Scheller M., Forman S. A. (2002) J. Neurosci. 22, 8411–8421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Findlay G. S., Ueno S., Harrison N. L., Harris R. A. (2001) Neurosci. Lett. 305, 77–80 [DOI] [PubMed] [Google Scholar]
- 39.Newell J. G., McDevitt R. A., Czajkowski C. (2004) J. Neurosci. 24, 11226–11235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price K. L., Millen K. S., Lummis S. C. (2007) J. Biol. Chem. 282, 25623–25630 [DOI] [PubMed] [Google Scholar]
- 41.Eddins D., Sproul A. D., Lyford L. K., McLaughlin J. T., Rosenberg R. L. (2002) Am. J. Physiol. 283, C1454–C1460 [DOI] [PubMed] [Google Scholar]
- 42.Galzi J. L., Bertrand S., Corringer P. J., Changeux J. P., Bertrand D. (1996) EMBO J. 15, 5824–5832 [PMC free article] [PubMed] [Google Scholar]
- 43.Sedelnikova A., Smith C. D., Zakharkin S. O., Davis D., Weiss D. S., Chang Y. (2005) J. Biol. Chem. 280, 1535–1542 [DOI] [PubMed] [Google Scholar]
- 44.O'Mara M., Cromer B., Parker M., Chung S. H. (2005) Biophys. J. 88, 3286–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cromer B. A., Morton C. J., Parker M. W. (2002) Trends Biochem. Sci. 27, 280–287 [DOI] [PubMed] [Google Scholar]