Abstract
Heparin-like glycans with diverse disaccharide composition and high anticoagulant activity have been described in several families of marine mollusks. The present work focused on the structural characterization of a new heparan sulfate (HS)-like polymer isolated from the mollusk Nodipecten nodosus (Linnaeus, 1758) and on its anticoagulant and antithrombotic properties. Total glycans were extracted from the mollusk and fractionated by ethanol precipitation. The main component (>90%) was identified as HS-like glycosaminoglycan, representing ∼4.6 mg g−1 of dry tissue. The mollusk HS resists degradation with heparinase I but is cleaved by nitrous acid. Analysis of the mollusk glycan by one-dimensional 1H, two-dimensional correlated spectroscopy, and heteronuclear single quantum coherence nuclear magnetic resonance revealed characteristic signals of glucuronic acid and glucosamine residues. Signals corresponding to anomeric protons of nonsulfated, 3- or 2-sulfated glucuronic acid as well as N-sulfated and/or 6-sulfated glucosamine were also observed. The mollusk HS has an anticoagulant activity of 36 IU mg−1, 5-fold lower than porcine heparin (180 IU mg−1), as measured by the activated partial thromboplastin time assay. It also inhibits factor Xa (IC50 = 0.835 μg ml−1) and thrombin (IC50 = 9.3 μg ml−1) in the presence of antithrombin. In vivo assays demonstrated that at the dose of 1 mg kg−1, the mollusk HS inhibited thrombus growth in photochemically injured arteries. No bleeding effect, factor XIIa-mediated kallikrein activity, or toxic effect on fibroblast cells was induced by the invertebrate HS at the antithrombotic dose.
Keywords: Carbohydrate Structure, Drug Action, Extracellular Matrix, Glycosaminoglycan, Heparin
Introduction
Heparin is an effective anticoagulant in the treatment and prevention of venous thrombosis. It is also used to prevent mural thrombosis after myocardial infarction, to treat patients with unstable angina, and, in the absence of aspirin, is the drug of choice to treat acute arterial thrombosis after coronary thrombolysis (1, 2). The anticoagulant activity of heparin is due to the occurrence of various antithrombin binding sequences including the well known pentasaccharide sequence GlcNAc(6S)-GlcA-GlcNS(3S)-IdoA(2S)-GlcNS(6S) (3–5). In the presence of heparin, the rates of inhibition of thrombin, factor IXa, and factor Xa by AT are increased ∼1,000-fold so that inhibition is essentially instantaneous (1, 6).
Currently, commercial heparin preparations are obtained from mammalian sources, either from porcine or bovine intestine or bovine lung (7). However, because of the higher anticoagulant activity of porcine mucosa heparin (8) and the appearance of bovine spongiform encephalopathy, the production of bovine heparin has been almost abolished. Nonanimal sources of heparin for pharmaceutical use are currently not available. However, several authors reported the occurrence of heparin in different classes of invertebrate animals, such as crustaceans (9–11), ascidians (12), and mollusks (13–16). Heparin-like glycans with diverse disaccharide composition and anticoagulant activities varying from <5 up to 363 IU mg−1 (see Table 1) are present in marine bivalve mollusks from six families of the order Veneroida and two families from the order Pectinoida.
TABLE 1.
Characteristics of heparin-like glycans found in marine bivalve mollusk
ND, not determined.
| Class | Order | Family | Genus and species | Average molecular mass | Anticoagulant activity | Major disaccharide units | Reference |
|---|---|---|---|---|---|---|---|
| Da | IU mg−1 | ||||||
| Bivalvia | Veneroida | Arcticidae | Cyprina islandica | ND | 165 | ND | 29 |
| Donacidae | Donax striatus | 20,000 | 180 | [UA-GlcNS6S]/[UA2S-GlcNS6] | 30 | ||
| Galatheoidae | Tagelus gibbus | 24,000 | <5 | [UA-GlcNS6S] | 31 | ||
| Mactridae | Spisula solidissima | ND | 155 | ND | 29 | ||
| Tridacnidae | Tridacna maxima | ND | 75 | ND | 15 | ||
| Veneridae | Anomalocardia brasilianaa | 32,000 | 320 | [UA2SGlcNS6S] /[UAGlcNS3S6S] | 14,30 | ||
| 18,000 | <5 | [UA-GlcNS6S] | 31 | ||||
| Callista chione | 10,950 | 97 | [UA-GlcN2S6S] /[UA-GlcNAc6S] | 32 | |||
| Katelysia opina | 31,000 | 160 | ND | 33 | |||
| Mercenaria mercenaria | 18,000 | 363 | [UA-GlcN3S]b | 34 | |||
| Tapes philippinarum | 13,600 | 358 | [UA2SGlcN2S6S] | 35 | |||
| Tivela mactroides | 25,000 | 220 | [UAGlcNS3S6S]/[UA-GlcNS6S] | 14,30 | |||
| Pectinoida | Pectinidae | Amussium pleuronectus | 7,000 | 95 | ND | 36 | |
| N. nodosus | 27,00 | 36 | [UA-GlcNAc] | This work | |||
| Mytilidae | Perna viridis | ND | 54 | ND | 15 |
a This mollusk contains two compounds: a 32-kDa heparin-like and a 18-kDa HS-like glycan.
b 3-O-Sulfated GlcN-containing disaccharides.
Species of the Pectinidae family of marine bivalve mollusks have great economical importance in countries such as Canada, the United States of America, the United Kingdom, France, Spain, and Japan where they are highly prized and sustained by strong fishery and aquaculture industries (17). The commercial value of these animals results from the presence of a central adductor muscle, called scallop, which is highly appreciated in both Eastern and Western cooking. In Brazil, since 1991, the species Nodipecten nodosus (Linnaeus, 1758) has been successfully cultured throughout the production of larva and post-larva.
The occurrence of anticoagulant heparins and the availability of methods to allow a sustainable production prompt us to study the glycans from the pectinidae N. nodosus. Here, we describe the structure, the anticoagulant and antithrombotic properties, as well as the toxic effects of a unique heparan sulfate (HS)3 isolated from the mollusk. In addition, the localization of the glycan in the tissues of the invertebrate is also described. The mollusk glycan prevents thrombus growth in the carotid artery by a mechanism involving a serpin-mediated inhibition of plasma coagulation proteases. This effect occurs without inducing any significant bleeding, factor XIIa-mediated kallikrein formation, and cellular toxicity. HS was located in the extracellular matrix of the mantle and gills. No glycans were detected in the adductor muscle of the mollusk.
EXPERIMENTAL PROCEDURES
Materials
Heparan sulfate from human aorta was extracted and purified as described previously (18). Chondroitin 4-sulfate from whale cartilage, dermatan sulfate, and heparin from porcine intestinal mucosa (180 IU mg−1), twice-crystallized papain (15 units mg−1 protein), and keratanase from Pseudomonas sp. (EC 3.2.1.103), sulfated dextran 500 and sulfated dextran 8 were purchased from Sigma; chondroitin AC lyase (EC 4.2.2.5) from Arthrobacter aurenses, chondroitin ABC lyase (EC 4.2.2.4) from Proteus vulgaris and heparinase I (EC 4.2.2.7) from Flavobacterium heparinum were from Seikagaku America Inc. (Rockville, MD). Agarose (standard low Mr) was obtained from Bio-Rad; toluidine blue and alcian blue were from Fisher; human antithrombin, human factor Xa, and thrombin were from Hematologic Technologies Inc.; thrombin chromogenic substrate tosyl-Gly-Pro-Arg-p-nitroanilide acetate (Chromozyn TH) and factor Xa chromogenic substrate N-methoxycarbonyl-d-norleucyl-glycyl-l-arginine-4-nitranillide-acetate were from Amersham Biosciences. Human plasmas immunodepleted of heparin cofactor II and/or antithrombin were obtained from Affinity Biologicals (Ancaster, Canada).
Histochemistry
Adult specimens of the bivalve mollusk N. nodosus (Linnaeus, 1758) were collected from Baia da Ilha Grande, Angra dos Reis, Rio de Janeiro, Brazil. For histochemical preparations, N. nodosus viscera was carefully isolated and fixed in 5% formaldehyde, 2.5% glutaraldehyde in 0.1 m sodium cacodylate buffer (pH 7.3) diluted in artificial sea water for 2 h at room temperature. After fixation, the viscera was washed with cacodylate buffer in artificial sea water, dehydrated in graded ethanol, cleared in xylol, and embedded in Para-plast (melting point 55.6 °C). Approximately 7-μm sections were cut longitudinally on a Spencer microtome. The sections were counterstained with periodic acid-Schiff and stained with alcian blue in 0.1 m HCl for 50 min, before or after incubation with chondroitin ABC lyase in 50 mm Tris-HCl buffer (pH 8.0), containing 5 mm EDTA and 15 mm sodium acetate for 2 h at room temperature or with nitrous acid for 2 h at room temperature. The sections were observed under a Zeiss Axioplan light microscope equipped with a CCD color camera (Media Cybernetics, model EvolutionTM MP), and digital images were obtained.
Extraction of the Sulfated Glycans
The bodies of the animals were removed from the shell, and the adductor muscles were separated from the viscera. These tissues were cut in small pieces, immersed in acetone, and kept for 24 h at 4 °C. The dried materials (∼25 g) were separately suspended in 500 ml of 0.1 m sodium acetate buffer (pH 5.5) containing 5 g of papain, 5 mm EDTA, and 5 mm cysteine and incubated at 60 °C overnight. The incubation mixture was then centrifuged (2000 × g for 10 min at room temperature), the supernatant was separated, and the precipitate was incubated with papain. This procedure was repeated three more times, as described above. The clear supernatants from the four extractions were combined and mixed with a solution of cetylpyridinum chloride (final concentration, 0.5%) overnight at room temperature. The precipitate formed was washed with cetylpyridinum chloride (final concentration, 0.05%) and suspended with 2 m NaCl in 95% ethanol (100:15, v/v). The solution was mixed with 2 volumes of 95% ethanol and kept overnight at 4 °C. The precipitate formed (containing the total glycans) was collected by centrifugation (2000 × g for 10 min at room temperature), dried, and dissolved in 50 ml of distilled water.
Fractionation of the Sulfated Glycans
The sulfated glycans extracted from the viscera were fractionated by differential precipitation with ethanol. Briefly, the aqueous solution of the total sulfated glycans was mixed with absolute ethanol to achieve a final concentration of 35.6% and kept at 4 °C overnight. The precipitate formed (glycan P-1) was collected by centrifugation, and the supernatant was mixed with absolute ethanol to achieve a final concentration of 70%. The precipitate formed (glycan P-2) was collected by centrifugation, and the supernatant was mixed with absolute ethanol to achieve a final concentration of 95%. The precipitate was collected by centrifugation and named glycan P-3. The glycans P-1, P-2, and P-3 were dried and suspended in distilled water.
Electrophoretic Analysis
Agarose Gel
The crude or purified glycans from the viscera of N. nodosus (1.5 μg of uronic acid), before or after incubation with specific glycosaminoglycan lyases, or deaminative cleavage with nitrous acid were analyzed by agarose gel electrophoresis, as described previously (19). Briefly, the glycans and a mixture of standard glycosaminoglycans containing chondroitin sulfate, dermatan sulfate, and heparan sulfate (1.5 μg of uronic acid of each) were applied to a 0.5% agarose gel in 0.05 m 1,3-diaminopropane/acetate (pH 9.0) and run for 1 h at 110 mV. After electrophoresis, the glycans were fixed with aqueous 0.1% cetylmethylammonium bromide solution and stained with 0.1% toluidine blue in acetic acid/ethanol/water (0.1:5:5, v/v/v).
Polyacrylamide Gel
The molecular mass of the purified mollusk glycan was estimated by polyacrylamide gel electrophoresis. The samples (∼10 μg) were applied to a 1-mm-thick 6% polyacrylamide slab gel, and after electrophoresis at 100 V for ∼1 h in 0.06 m sodium barbital (pH 8.6), the gel was stained with 0.1% toluidine blue in 1% acetic acid. After staining, the gel was washed overnight in 1% acetic acid. The molecular mass markers used were: dextran 500 Sigma Aldrich (S1) (average molecular weight, 500,000), chondroitin 6-sulfate from shark cartilage (S3) (average molecular weight, 54,000); chondroitin 4-sulfate from whale cartilage (S2) (average molecular weight, 36,000); and dextran 8 (S4) (average molecular weight, 8,000).
Enzymatic Treatments
Chondroitin Lyases
Crude N. nodosus glycans (∼50 μg) were incubated with 0.01 unit of chondroitin AC or ABC lyases in 0.1 ml of 50 mm Tris-HCl buffer (pH 8.0) containing 5 mm EDTA and 15 mm sodium acetate. After incubation at 37 °C for 12 h, the mixtures were analyzed by agarose gel electrophoresis, as described earlier.
Keratanase
Approximately 50 μg (as dry weight) of crude N. nodosus glycan was incubated with 0.005 unit of keratanase in 100 μl in 10 mm Tris-HCl buffer (pH 7.4) overnight at 37 °C. At the end of the incubation period, the mixtures were analyzed by agarose gel electrophoresis, as described earlier.
Heparinase
Approximately 50 μg (as dry weight) of the purified N. nodosus glycan was incubated with 0.005 unit of heparinase I in 100 μl of 100 mm sodium acetate buffer (pH 7.0) containing 10 mm calcium acetate for 17 h at 37 °C. At the end of the incubation period, the mixtures were analyzed by agarose gel electrophoresis, as described earlier.
NMR Experiments and Chemical Structure Design
1H and 13C one- and two-dimensional spectra of the N. nodosus HS were recorded using a Bruker DRX 400 MHz apparatus with a triple resonance probe, and the spectra were processed using the Bruker software TopSpin, as detailed previously (20). Approximately 5 mg of the purified sample were dissolved in 0.5 ml of 99.9% deuterium oxide (Cambridge Isotope Laboratory, Cambridge, MA) inside a 5-mm-diameter NMR tube. The one-dimensional 1H NMR spectrum was recorded with 16 scans. The two-dimensional 1H/1H COSY and 1H/13C HSQC spectra were recorded using state-time proportion phase incrementation for quadrature detection in the indirect dimension. The 1H/13C HSQC spectrum was run with 1024 × 256 points and globally optimized alternating phase rectangular pulses for decoupling. Chemical shifts are displayed relative to external trimethylsilylpropionic acid at 0 ppm for 1H and relative to methanol for 13C at 50 °C. The chemical structure of the bivalve HS was drawn using the Chemoffice 2002 software package (version 7.0) from CambridgeSoft.
Anticoagulant Effect Measured by Activated Partial Thromboplastin Time (aPTT)
Activated partial thromboplastin clotting time assays were carried out as follows: normal human plasma (100 μl) or antithrombin/heparin cofactor II-free plasma was incubated with 10 μl of a solution of mammalian heparin or purified N. nodosus HS (0.001–100 μg) at 37 °C for 1 min. Then 100 μl of aPTT reagent (Celite; Biolab) were added and incubated at 37 °C. After 2 min of incubation, 100 μl of 0.25 m CaCl2 were added to the mixtures, and the clotting time was recorded in a coagulometer (Amelung KC4A).
Inhibition of Thrombin or Factor Xa by Antithrombin or HCII in the Presence of Mammalian Heparin or Mollusk Heparan Sulfate
The inhibition of thrombin or factor Xa by antithrombin or heparin cofactor II were evaluated by the assay of amydolytic activity of thrombin, using chromogenic substrate, as described (12). The incubations were performed in disposable UV semi-microcuvettes. The final concentrations of reactants included 50 nm antithrombin, 68 nm HCII, 15 nm thrombin, 20 nm factor Xa, and 0–100 μg ml−1 mammalian heparin or purified N. nodosus heparan sulfate in 100 μl of 0.02 m Tris/HCl, 0.15 m NaCl, and 1.0 mg ml−1 polyethylene glycol (pH 7.4) (TS/PEG buffer). Thrombin or factor Xa was added last to initiate the reaction. After 60 s of incubation at room temperature, 500 μl of 100 μm chromogenic substrate Chromogenix TH or N-methoxycarbonyl-d-norleucyl-glycyl-l-arginine-4-nitranillide-acetate in TS/PEG buffer was added, and the absorbance at 405 nm was recorded for 100 s. The rate of change of absorbance was proportional to the thrombin or factor Xa activity remaining in the incubation. No inhibition occurred in control experiments in which thrombin or factor Xa was incubated with antithrombin or HCII in the absence of heparin. Nor did inhibition occur when thrombin or factor Xa was incubated with heparin alone over the range of concentrations tested.
Animal Procedures
All of the animal work was carried out in accordance with the Brazilian Animal Protection Law and followed the institutional guidelines for animal care and experimentation (authorization number IBQM 012).
Photochemically Induced Carotid Artery Lesion
The carotid artery thrombosis was induced as described previously (21). Briefly, adult male and female rat (body weight, 200 g) were anesthetized with an intramuscular injection of 100 mg kg−1 of ketamine (Cristália, São Paulo, Brazil) and 16 mg kg−1 of xylazine (Bayer AS, São Paulo, Brazil), supplemented as needed, secured in the supine position, and placed under a dissecting microscope. The right common carotid artery was isolated through a midline cervical incision, and an ultrasonic flow probe (model 0.5 VB; Transonic Systems, Ithaca, NY) was applied. A 1.5-mW, 540-nm laser beam (Melles Griot, Carlsbad, CA) was applied to the artery from a distance of 5 cm. The injury was initiated by injection into the vena cava of rose bengal (90 mg kg−1 of body weight; Fisher) dissolved in phosphate-buffered saline, and flow was monitored until complete and stable (5 min) occlusion occurred. Mammalian heparin or N. nodosus heparan sulfate was dissolved in 0.15 m NaCl and injected intravenously into the vena cava 15 min before the injection of rose bengal, and the occlusion time was determined by a flow meter. The total volume of material injected intravenously did not exceed 10% of the estimated blood volume of the rat. At least five animals were used per group. The time to arterial occlusion was determined in minutes.
Bleeding Effect
For evaluation of the bleeding effect, Wistar rats (both sexes; body weight, ∼200 g) were anesthetized with a combination of xylazine and ketamine, as described above. A cannula was inserted into the right carotid artery for administration of mammalian heparin (0.1 and 1.0 mg kg−1) or N. nodosus heparan sulfate (1.0, 2.0, or 4.0 mg kg−1). The polysaccharide was allowed to circulate for 5 min, and the rat tail was cut 3 mm from the tip. The tail was carefully immersed in 40 ml of distillated water at room temperature. Blood loss was determined 60 min later by measurement of the hemoglobin content in the water solution using a spectrophotometric method, as described (22). The volume of blood was deduced from a standard curve based on absorbance at 540 nm. At least five animals were used per group.
Hexuronic Acid
The hexuronic acid content was estimated by the carbazole reaction (23).
Deaminative Cleavage with Nitrous Acid
Deamination by nitrous acid was performed at pH 1.5. The crude or the purified glycan extracted from N. nodosus (∼20 μg) was incubated with 5 μl of freshly generated HNO2 at room temperature for 1.5 h. The reaction mixtures were then neutralized with 1.0 m Na2CO3. Intact and nitrous acid-degraded glycans were analyzed by agarose gel electrophoresis.
Activation of Factor XII in the Presence of Sulfated Glycans
Activation of factor XII assay was carried out in 96-well plates. Normal human plasma was diluted with 3 volumes in TS/PEG buffer, and the samples (40 μl) were incubated with different concentrations of N. nodosus HS or a chemically oversulfated CS with a structure similar to the oversulfated CS found in heparin preparations (30 μl). After 60 s of incubation at 37 °C, 30 μl of 0.3 mm S-2302 (Chromogenix AB, Mondal, Sweden) was added, and the absorbance at 405 nm recorded for 300 s (Plate reader Thermo-max, America Devices). The S-2302 is a chromogenic substrate for plasma kallikrein, which is activated from its precursor prekallikrein by the action of active factor XII. The method for the determination of activity is based on the difference in absorbance between the p-nitroanilide formed and the original substrate. The rate of p-nitroanilide formation, i.e. the increase in absorbance/s at 405 nm, is proportional to the enzymatic activity. No activation occurred in control experiments in the absence of sulfated glycan.
Citotoxic Effect of the Mollusk HS
BHK 21 cells were seeded in 96-well plates at a density of 1 × 103 cells/well and treated for 48 h with various concentration of mollusk HS (0, 1, 10, 50, 100, and 1000 μg ml−1). The cell viabilities were determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide-based colorimetric assay. The values were described as percentages of the control.
Statistical Analysis
The results are expressed as the means ± standard deviation. The difference between two groups was tested using the t test.
RESULTS
Localization of Glycosaminogycans in N. nodosus
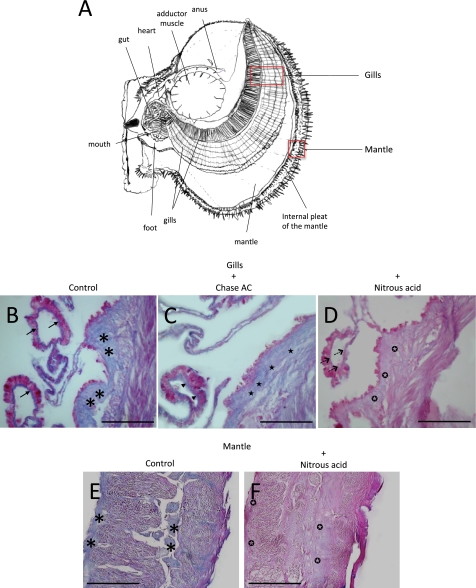
Heparin-like compounds have been described in the internal organs of several species of bivalve mollusks (Table 1). We sought to determine the location of sulfated glycosaminoglycans in N. nodosus by histochemistry with alcian blue, before or after incubation with chondroitinase AC or treatment with nitrous acid, in sections of two organs, the gills and the mantle (Fig. 1A). Two major regions of the gills were highly stained, the basement membrane of the gill epithelium (Fig. 1B, arrows) and the extracellular matrix of the internal part of the gill, which has a fibrillar appearance (Fig. 1B, asterisks). The staining at the basement membrane resisted chondroitinase AC (Fig. 1C, arrowhead) but completely disappeared after treatment with nitrous acid (Fig. 1D, dashed arrows). The extracellular fibrillar material at the interior of the gills was partially degraded with chondroitinase AC, as indicated by the slight reduction in the intensity of the staining (Fig. 1C, stars), but was almost completely degraded by nitrous acid treatment, as indicated by a drastic reduction in the intensity of the staining (Fig. 1D, white stars in black circles). In the mantle, the alcian blue staining was restricted to the extracellular matrix (Fig. 1E, asterisks) and was completely abolished after nitrous acid treatment (Fig. 1F, white stars in black circles). Overall, these results suggest that a heparin-like glycosaminoglycan occurs at the basement membrane of the gills epithelium and dispersedly throughout the extracellular matrix of the gills and mantle, along with chondroitin sulfate (CS).
FIGURE 1.
Histological analysis of sections from N. nodosus stained with alcian blue. A, schematic drawing of N. nodosus showing the internal organs. The sections from the gills (A–D) and mantle (E and F) were stained with alcian blue before (B and E) or after incubation with chondroitinase AC (C) or nitrous acid treatment (D and F). The arrows indicate alcian blue staining of the basement membrane. Arrowheads indicate alcian blue staining of the basement membrane after chondroitinase AC treatment. The dashed arrows indicate alcian blue staining of the basement membrane after nitrous acid treatment. The asterisks indicate alcian blue staining at the extracellular matrix. The black stars indicate alcian blue staining at the extracellular matrix after chondroitinase AC treatment. The white stars in black circles indicate alcian blue staining at the extracellular matrix after nitrous acid treatment. Bars, 200 μm.
Isolation and Characterization of N. nodosus Glycans
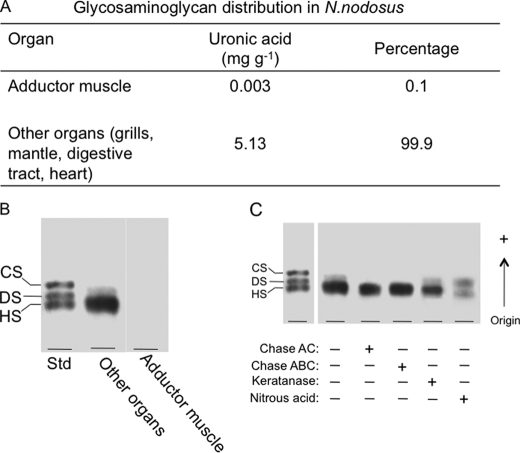
The glycans were extracted separately from the adductor muscle and viscera (gills, digestive tract, heart, and mantle) of N. nodosus with four consecutive digestions with proteolytic enzyme. After cetylpyridinum chloride and ethanol precipitation, the crude glycans were recovered from the supernatant, yielding ∼5.13 mg g−1 of dry organs. No significant amount of glycans was detected in the material extracted from the adductor muscle, as indicated by the content of uronic acid (Fig. 2A) and agarose gel electrophoresis (Fig. 2B). Densitometry of the agarose gel revealed the presence of a major (> 90%, based on densitometry units) metachromatic band migrating as standard HS. This band resists incubation with chondroitinases AC/ABC and keratanase but disappears after treatment with nitrous acid, revealing another tenuous metachromatic band with a slightly lower mobility in the agarose gel (Fig. 2C). This band resists incubation with chase AC/ABC, keratanase, and nitrous acid (not shown). A minor band displaying slower mobility in the agarose gel, migrating between standard dermatan sulfate and CS, was also observed. This material resists keratanase and nitrous acid treatment but completely disappears after incubation with chondroitinase AC or ABC (Fig. 2C). These results indicate that a heparinoid is the preponderant glycan present in the N. nodosus. CS and an unidentified glycan are also present as minor components.
FIGURE 2.
Distribution and initial characterization of glycosaminoglycans in N. nodosus. A, glycosaminoglycans were detected in the organs by the amount (mg g−1) of uronic acid, determined by the carbazole reaction (23). B, agarose gel electrophoresis of the glycans isolated from adductor muscle and viscera (pool of different organs: gills, mantle, digestive tract, and heart). C, agarose gel electrophoresis of the total glycans from the organs before (−) or after (+) incubation with chondroitinase AC (Chase AC), chondroitinase ABC (Chase ABC), keratanase, and nitrous acid. DS, dermatan sulfate.
Fractionation of N. nodosus Glycans
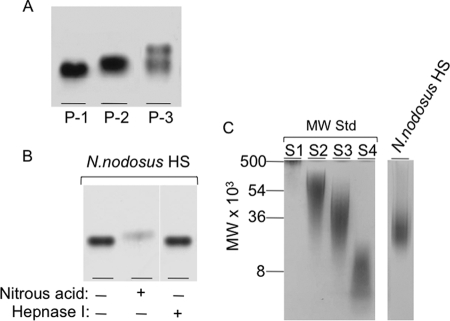
The crude glycans from the viscera were easily fractionated by precipitation with different ethanol concentrations, as described under “Experimental Procedures” (Fig. 3A). The material precipitated with 35.6% ethanol contains a mixture of the low mobility unidentified glycan and mollusk HS (P-1). HS (∼59% of the starting material) free of contaminating glycans was precipitated with 70% ethanol (P-2), and a mixture containing HS and CS was precipitated with 95% ethanol (P-3).
FIGURE 3.
Fractionation and electrophoretic analysis of the N. nodosus glycans. A, Total glycans isolated from the viscera of N. nodosus were precipitated with increasing concentrations of ethanol, 35.6% (P-1), 70% (P-2), and 95% (P-3) and analyzed by agarose gel electrophoresis. B, the purified HS obtained in the 70% precipitate (P-2) was analyzed by agarose gel electrophoresis before (−) or after (+) deaminative cleavage with nitrous acid or incubation with heparanase I. C, the purified mollusk HS was analyzed by polyacrylamide gel electrophoresis along molecular weight standard glycans: dextran 500 (S1) (average molecular weight, 500,000); chondroitin 6-sulfate from shark cartilage (S3) (average molecular weight, 54,000); chondroitin 4-sulfate from whale cartilage (S2) (average molecular weight, 36,000); and dextran 8 (S4) (average molecular weight, 8,000).
The purified mollusk HS (P-2) was analyzed by agarose gel electrophoresis before or after deaminative cleavage with nitrous acid or incubation with heparinase I (Fig. 3B). The deaminiative cleavage with nitrous acid gives rise to a degradation product displaying a higher mobility in the gel, when compared with the intact glycan. Heparinase I, on the other hand, was clearly ineffective.
Polyacrylamide gel electrophoresis in barbital buffer was used to estimate the molecular weight of the mollusk HS (P-2) (Fig. 3C). Based on comparison with the electrophoretic motilities of molecular mass markers, the N. nodosus heparinoid has a molecular mass of ∼27,000 Da.
NMR Structural Characterization of the Heparinoid from N. nodosus
Attempts to determine the main structural features of the N. nodosus heparinoid were successfully accomplished by a combination of multiple NMR experiments. Through this kind of structural analysis, largely employed for structural characterization of glycosaminoglycans, we observed that the mollusk glycan possesses a novel structure with unique features, such as a high degree of N-acetyl-α-d-glucosamine residues, a lack of α-iduronate residues, and the occurrence of 2- and 3-sulfated β-glucosaminyl units. These characteristics are less commonly found among mammalian and mollusk heparinoids (Table 1), described so far.
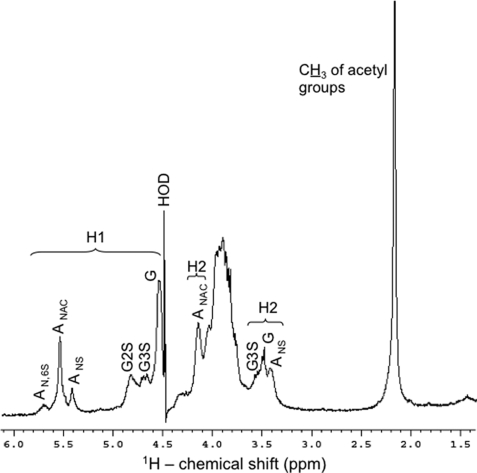
The one-dimensional 1H spectrum of the N. nodosus heparinoid (Fig. 4) shows, besides a reasonable level of sample homogeneity (only NMR signals of HS), equivalent amounts of anomeric protons of α-glucosamine (A) and β-glucuronic acid (G) residues, respectively. This is the major evidence indicating, unequivocally, that the N. nodosus glycan is a HS rather than a heparin-like compound with the preponderance of α-anomeric protons. In addition, we observed considerable amounts of 1H-anomerics (δH 5.56–5.44 ppm; Table 2) attributable to N-acetyl-α-d-glucosaminyl units, which comprise ∼60% of all α-residues of the mollusk glycan (Fig. 4, ANAC). This demonstrates that the mollusk HS contains more N-acetylated (ANAC) domains than regular heparosans and provides additional evidence supporting that the invertebrate glycan is a HS, because heparins usually possess an average of 10–15% of ANAC residues (24). Moreover, the one-dimensional 1H spectrum reveals considerable amounts of 1H-anomeric of glucuronic acid residues with sulfate esters located at the less common 2- and 3-positions (G2S at δH 4.73–4.8 ppm and G3S at δH 4.61–4.72 ppm; Table 2). Based on 1H-anomeric integral values, the percentages of nonsulfated, 2-sulfated, and 3-sulfated glucuronic acid residues in the mollusk HS backbone are 25.4, 14.2, and 10.4%, respectively (Fig. 4).
FIGURE 4.
1H NMR spectrum of the N. nodosus HS. Approximately 5 mg of the purified sample were dissolved in 0.5 ml of D2O, and the one-dimensional NMR spectrum was recorded at 50 °C. The residual water signal was suppressed by presaturation. Chemical shifts are relative to external trimethylsilylpropionic acid at 0 ppm. The H1 signals correspond to the both α- and β-anomeric protons at δH 5.8–5.3 and δH 4.9–4.5 ppm, respectively. The H2 signals denote protons at the 2-position in the sugar rings. AN,6S, N,6-di-sulfated-d-glucosaminyl; ANAC, N-acetyl-d-glucosaminyl; ANS, N-sulfated-d-glucosaminyl; G2S, 2-sulfated-glucuronate; G3S, 3-sulfated glucuronate; G, glucuronate. The percentages (relative integral values of all α-glucosamine residues) of the AN,6s, ANAC, and ANS signals are 16:61:23, respectively. The percentages (also based on relative integral values of 1H-anomeric) of the different types of glucuronates are 50.7:28.42:20.81, respectively for G, G2S, and G3S.
TABLE 2.
1H and 13C chemical shifts of glucuronic acid and glucosamine units from N. nodosus HS
Chemical shifts are relative to external trimethylsilylpropionic acid to 0 ppm for 1H, and methanol for 13C at 50 °C. G, glucuronic acid; A, glucosamine; NA, not assigned.
| Nucleus | G | G2S | G3S | ANAc | ANS |
|---|---|---|---|---|---|
| H1 | 4.61-4.45 | 4.8-4.73 | 4.72-4.61 | 5.56-5.44 | 5.43-5.35 |
| C1 | 105.1-102.0 | 105.0-100.9 | 103.2-101.0 | 98.2-95.3 | 98.1-95.9 |
| H2 | 3.57-3.38 | 4.15-4.02 | 3.8-3.67 | 4.14-3.95 | 3.42-3.3 |
| C2 | 74-8-70.2 | 75.3-72.0 | 73.0-70.5 | 54.8-51.5 | 59.0-56.2 |
| H3 | 3.99-3.72 | NA | 4.73-4.58 | 3.87-3.78 | 3.99-3.89 |
| C3 | 77.3-72.9 | NA | 85-80.4 | 69.8-67.0 | 69.0-67.2 |
| H4 | 3.99-3.72 | 3.99-3.72 | NA | NA | 3.9-3.86 |
| C4 | 77.3-72.9 | 77.3-72.9 | NA | NA | 82.8-81 |
| H5 | 3.99-3.72 | 3.99-3.72 | NA | 4.0-3.88 | 69.9-61.2 |
| C5 | 77.3-72.9 | 77.3-72.9 | NA | 71.8-66.9 | 4.19-4.03 |
| 6S-H6 | 4.31-4.22 | 4.57-4.45 | |||
| 6S-C6 | 66.9-64.5 | 65.9-65.1 | |||
| 6OH-H6 | 4.02-3.68 | ||||
| 6OH-C6 | 62.1-58.9 |
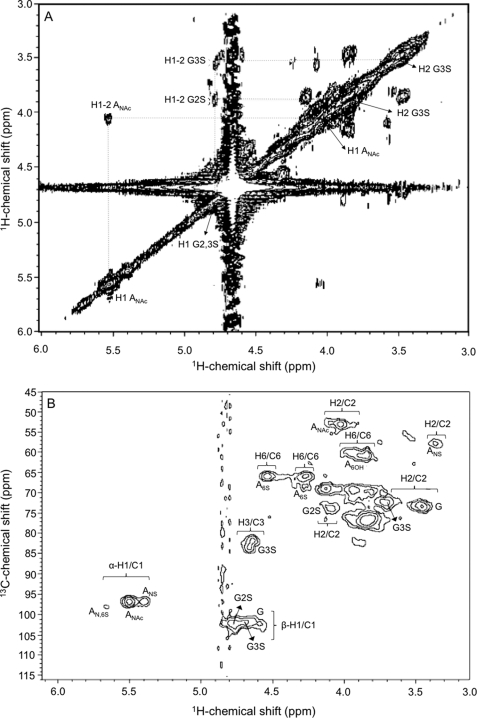
The 1H NMR assignment of the invertebrate HS (Fig. 4 and Table 2) was feasible because of the establishment of 1H-1H connectivities for vicinal protons in the COSY spectrum (Fig. 5A) where the most intense auto- and cross-peaks are labeled. These 1H chemical shifts together with standard 1H chemical shifts of β-glucuronic acids and α-glucosamines from the heparan sulfates described previously (24) facilitated unequivocal assignment for the majority of the cross-peaks in the 1H/13C HSQC spectrum (Fig. 5B) and subsequently fill out Table 2 with the respective 13C chemical shifts of the novel HS from N. nodosus. These 1H/13C NMR signals show suitable agreement with the chemical shifts of other heparan sulfates from the literature with these same structural characteristics (24).
FIGURE 5.
1H/1H COSY (A) and 1H/13C HSQC (B) NMR spectra of the N. nodosus HS. Approximately 5 mg of the purified sample were dissolved in 0.5 ml of D2O, and the two-dimensional NMR spectra were recorded at 50 °C. The residual water signal was suppressed by presaturation. Chemical shifts are relative to external trimethylsilylpropionic acid at 0 ppm for 1H and relative to methanol for 0 ppm of 13C. The H1 signals correspond to the both α- and β-anomeric protons at δH 5.8–5.3 and δH 4.9–4.5 ppm, respectively. The numbers at the right side of H (A) or H/C (B) indicate the positions of the nuclei in the sugar rings. AN,6S, N,6-di-sulfated-d-glucosaminyl; ANAC, N-acetyl-d-glucosaminyl; ANS, N-sulfated-d-glucosaminyl; G2S, 2-sulfated-glucuronate; G3S, 3-sulfated glucuronate; G, glucuronate.
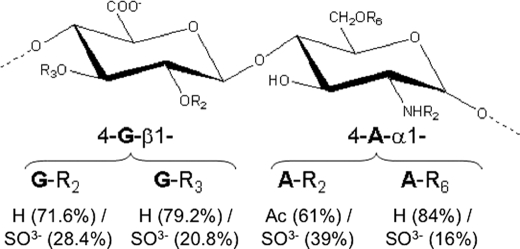
Taking into account all of the NMR data (Figs. 4 and 5 and Table 2), as well as the respective relative integral values of the 1H-anomerics, a chemical structure for the major repeating disaccharide unit of the N. nodosus HS can therefore be suggested (Fig. 6). Certainly, a more extensive structural analysis can be carried out to determine whether there are some specific clusters (such as NA, NA/NS, and NS domains, and/or G-, G2S-, or G3S-enriched regions) throughout the chain of the mollusk HS.
FIGURE 6.
Suggested structure of the major disaccharide unit of the N. nodosus HS. The percentages of the radicals are based in integral values of the NMR signals (Fig. 3 and 4). The residues denoted with G and A are glucoronate and d-glucosamine, respectively. Rn represents the radicals, where their respective chemical groups are down listed, and where the respective subscript numbers correspond to the positions in the sugar ring.
Anticoagulant Properties of N. nodosus HS
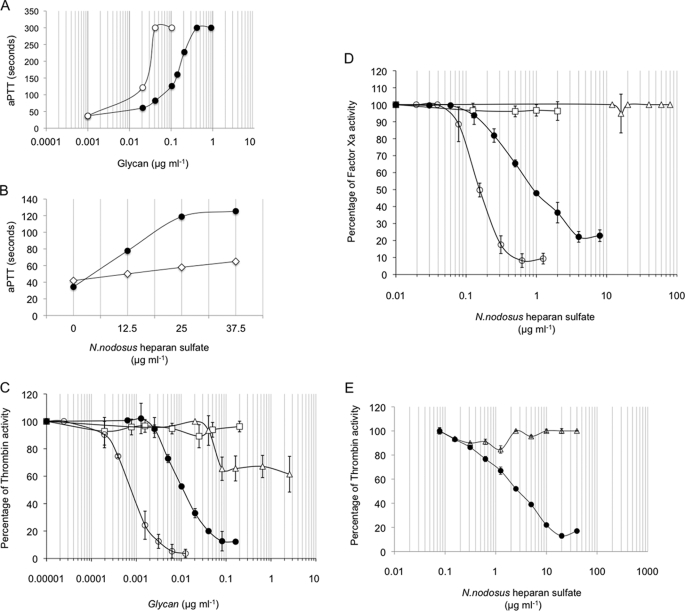
The anticoagulant properties of the N. nodosus HS was evaluated by the aPTT assay using human plasma and by measuring the inhibition of thrombin or factor Xa by AT in the presence of increasing concentrations of the mollusk glycan (Fig. 7). Using a parallel standard curve based on the aPTT activity of a heparin containing 180 IU mg−1, the anticoagulant activity of the mollusk HS was estimated in ∼36 IU mg−1 (Fig. 7A). When antithrombin/heparin cofactor II-free plasma replaced normal plasma in the aPTT assay, a drastic reduction in the anticoagulant activity of the mollusk HS was observed, indicating a serpin-mediated anticoagulant mechanism (Fig. 7B).
FIGURE 7.
Anticoagulant properties of N. nodosus HS. Measurement of the aPTT was carried according to the manufacture's specifications (Biolab-Merieux AS, Rio de Janeiro, Brazil). Normal human (A) was incubated with mammalian heparin (○) or purified N. nodosus HS (●) (0.001–100 μg ml−1) at 37 °C for 1 min. Then 100 μl of APTT reagent (Celite; Biolab) were added and incubated at 37 °C. After 2 min of incubation, 100 μl of 0.25 m CaCl2 were added to the mixtures, and the clotting time was recorded in a coagulometer (Amelung KC4A). B, normal (●) or antithrombin/heparin cofactor II-free plasma (◇) was incubated with purified N. nodosus HS (0–37.5 μg ml−1), and the clotting time recorded in a coagulometer (Amelung KC4A), as described above. C–E, inhibition of thrombin (C) or factor Xa (D) activity by AT or HCII (E) in the presence of intact (○) or nitrous acid-treated (□) mammalian or intact (●) or nitrous acid-treated (▵) N. nodosus HS. AT (50 nm) or HCII (68 nm) was incubated with thrombin (15 nm) or factor Xa (20 nm) in the presence of various concentrations of glycans. After 60 s, the remaining thrombin or factor Xa activity was determined with a chromogenic substrate (ΔA405/min). The results are expressed as percentages of thrombin activity.
Assays with purified serpins (antithrombin and heparin cofactor II) and proteases (thrombin and Factor Xa) showed that the N. nodosus HS inhibits thrombin (Fig. 7C) and factor Xa (Fig. 7D) in the presence of antithrombin, with 12.5- and 6-fold less potency than porcine heparin, respectively (Table 3). Nitrous acid treatment completely abolished thrombin inhibition by porcine heparin in the presence of antithrombin but only partially abolished thrombin inhibition by the mollusk HS (Fig. 7C). Nitrous acid treatment completely abolishes the inhibitory activity of antithrombin by porcine heparin and N. nodosus HS (Fig. 7D), when thrombin is replaced by Factor Xa.
TABLE 3.
Anticoagulant activity of mammalian and mollusk heparinoids
ND, not determined.
| Heparin/heparan sulfate | Anticoagulant activity by aPTT |
Antithrombin activity |
IC50 protease inhibition |
||||
|---|---|---|---|---|---|---|---|
| Plasma |
Anti-IIa | Anti-Xa | Antithrombin |
HCII (IIa) | |||
| Normal | Serpin-free | IIa | Xa | ||||
| IU mg−1 | IU mg−1 | μg ml−1 | |||||
| Mammalian | |||||||
| Porcine intestinal mucosa heparina | 180 | ND | 180 | 180 | 0.001d | 0.18d | 0.001e |
| Bovine lung heparinb | 139 | ND | 136 | 135 | ND | ND | ND |
| Bovine pancreas heparan sulfatec | 1 | ND | ND | 4 | ND | ND | ND |
| Mollusk | |||||||
| N. nodosus | 38.3 | 7.6 | 16 | 36 | 0.012 | 0.9 | 4 |
The mollusk HS also inhibits thrombin in the presence of heparin cofactor II, with a concentration >1000-fold higher than that of porcine heparin required to achieve the same inhibitory activity. Nitrous acid completely abolishes the HCII activity of the mollusk HS (Fig. 7E and Table 3).
Antithrombotic Activity of N. nodosus HS
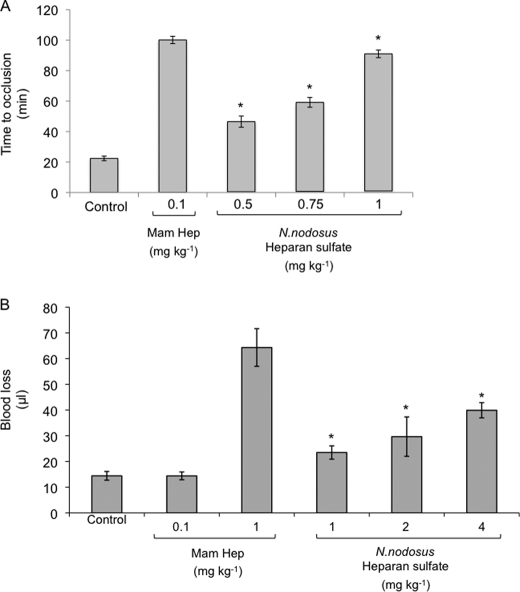
The effects of mammalian heparin and N. nodosus HS on arterial thrombosis in vivo were investigated in the carotid artery after a photochemical lesion, as described under “Experimental Procedures.” Mammalian heparin or N. nodosus HS was injected intravenously into the vena cava 15 min before the injection of rose bengal, and the occlusion time was determined by a flow meter. The interval between endothelial injury and total thrombotic occlusion of the carotid artery in animals not treated with any of the glycans was ∼22 min. Administration of the mollusk HS increased the occlusion time in a dose-dependent manner, up to ∼5 times the base-line value at a dose of 1.0 mg kg−1 (Fig. 8A). Doses lower than 0.5 mg kg−1 did not change the occlusion time. Mammalian heparin at a dose of 0.1 mg kg−1 prolonged the occlusion time to the same value obtained with 1.0 mg kg−1 of the mollusk HS.
FIGURE 8.
In vivo antithrombotic and bleeding effect of N. nodosus HS. A, antithrombotic effect. Mammalian or N. nodosus HS was administered intravenously at the doses indicated. Then 15 min later, vascular injury was initiated by injection of rose bengal, and the time to thrombotic occlusion was determined. The error bars represent the means ± S.D. Five animals were used per dose. *, p ≤ 0.002 versus control (no glycan). B, bleeding effect. Mammalian or N. nodosus HS were infused into rats at the doses indicated and allowed to circulate for 5 min. The rat tail was cut 3 mm from the tip and immersed in 40 ml of distilled water at room temperature. Blood loss was determined 60 min later by measuring the hemoglobin in the water. The error bars represent the means ± S.D. Five animals were used per dose. *, p ≤ 0.002 versus control (no glycan).
Hemorrhagic Effect of N. nodosus HS
The hemorrhagic effect of mammalian heparin or N. nodosus HS was assessed based on blood loss from a cut rat tail after intravascular administration. At the dose of 1 mg kg−1, which induced the maximum antithrombotic effect, mollusk HS increased blood loss only 1.7-fold (Fig. 8B). The same dose of mammalian heparin resulted in a blood loss ∼5-fold higher. No bleeding was observed at the dose of 0.1 mg kg−1 of mammalian heparin. Administration of mollusk HS at doses up to four times that required for higher antithrombotic effect produced a maximum blood loss of 2.7 times the base-line value.
Factor XIa-mediated Kallikrein Activity
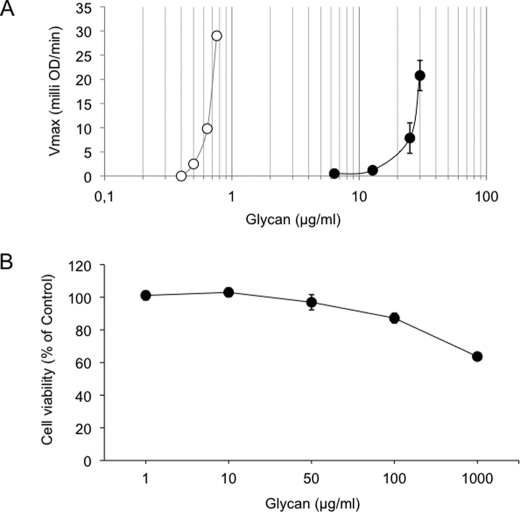
To examine the ability of N. nodosus HS to activate the amidolytic activity of kallikrein in human plasma, different concentrations of the mollusk HS or a chemically oversulfated CS (as positive control) were incubated in human plasma, and kallikrein activity was determined as described above. As shown in Fig. 9A, potent activation of kallikrein occurred with oversulfated chondroitin sulfate at 0.6–0.8 μg ml−1 but not with N. nodosus HS. Activation of kallikrein by the mollusk glycan was observed only at 11.5–12 μg ml−1.
FIGURE 9.
Effect of glycans on factor XII activation and cell viability. A, factor XII activation. Normal human plasma was incubated with increasing concentrations of oversulfated chondroitin (○) or purified N. nodosus HS (●). After 60 s of incubation at 37 °C, 0.3 mm of chromogenic substrate for plasma kallikrein was added. The increase in absorbance at 405 nm was expressed by milli optical density min-1 (means ± S.E., n = 3). B, cell viability. The cells were treated with the indicated concentration of purified N. nodosus HS for 24 h. The percentage of viable cells was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The data representative of three independent experiments are expressed as the means ± S.E.
Citotoxic Effect
To examine the effect of HS extracted from the N. nodosus on BHK 21 cell viability, the cells were incubated with various concentration of mollusk HS for 48 h. As shown in Fig. 9B, glycan treatment began to significantly decreased BHK 21 cell viability at 100 μg ml−1. At 1000 μg ml−1, cell viability was reduced in ∼35%. However, no significant change in cell viability was observed at the concentration, which causes the higher antithrombotic effect (1 μg ml−1).
DISCUSSION
Unfractionate heparin is the main anticoagulant drug used for cardiovascular surgery and for the prevention of arterial or venous thrombosis (2). The worldwide consumption of heparin has been augmented to 100 tons/year, partially because of an increasing use of low molecular weight heparin (25). However, the source of pharmaceutical heparins is very limited, because it is primarily obtained from porcine intestine (7). It was estimated that in 2008 ∼20 million people suffered from thromboembolic diseases, and 200 million pigs were needed to meet this demand (25). Therefore, there is an urgent necessity to find alternative sources of heparin.
Heparin-like polymers with significant anticoagulant activity have been extensively described in mollusks (Table 1). Fourteen species from eight families of bivalve mollusks contain heparins with different disaccharide compositions, molecular weights, and anticoagulant activities ranging from <5 to 365 IU mg−1. Among them, mollusk species from the Pectinidae family have been successfully cultivated in different parts of the world because of the high value of its meat. In 2007, ∼3 tons of the pectinidae N. nodosus was produced by laboratory culture of larvae and post-larvae in Brazil. Thus, this species provides an interesting subject for a prospective study of the antithrombotic properties of the mollusk glycans.
In the present work, a HS was identified in the viscera of N. nodosus. Histochemistry using alcian blue showed that the mollusk HS was localized at the extracellular matrix of the gills and mantle. In the gills it was also present at the epithelial basement membrane. CS was also detected in these organs, but its occurrence is restricted to the extracellular matrix. Perlecan, Agrin, and Bamacan are vertebrate extracellularly secreted proteoglycans found in basement membranes. Among them, Agrin is the only exclusively glycanated with HS chains. Perlecan proteoglycan with the addition of HS also contains CS chains, whereas Bamacan is a CS proteoglycan (28). Therefore, considering that CS was not detected in the basement membrane and by analogy with vertebrates, N. nodosus HS would most likely be linked to an Agrin- or Perlecan-like proteoglycan. However, presently this is only a hypothesis, which will be investigated in future work.
A very simple procedure using sequential ethanol precipitation was employed to successfully purify the mollusk HS in a high yield. One gram of the mollusk viscera (without the adductor muscle) yielded 5.13 mg of crude glycans from which ∼3.03 mg is the heparinoid. This recovery (0.9%) is higher than that obtained in the isolation of heparin from the ascidian Styela plicata (0.26%) (26) and much higher than that from pig intestinal mucosa (∼0.022%) (27). CS and an unidentified sulfated glycan are minor components that can be easily removed from the major HS component by a straightforward precipitation method with ethanol. HS is obtained in the 70% ethanol fraction free of contaminating glycans. The adductor muscle, which is the part of the mollusk that is most appreciated for consumption, is free of glycans. The HS is restricted to organs commonly discarded during preparation for commercialization, which increases the potential value of the mollusk.
The average molecular mass of the mollusk HS, estimated by polyacrylamide gel, is ∼27,000 kDa, which is higher than that of mammalian heparin. Considering the average molecular weight of heparin/HS disaccharides to be 486, the average length of the mollusk HS is ∼55 disaccharides long.
The mollusk HS contains characteristics found both in HS and heparin. Thus, the polymer resisted the action of heparinase I, which cleaves the glycosidic bound between hexosamine and 2-O-sulfated iduronic acid, commonly found in heparin; the NMR analysis, provided a clear 1H spectrum characteristic of HS, suggesting that ∼60% of the mollusk HS contains nonsulfated, N-acetylated disaccharides. However, the glycan was partially degraded by nitrous acid at low pH (Fig. 3B), which cleaves glycosidic bound of N-sulfated glucosamine. The NMR analysis showed that sulfation occurs in smaller percentage of the disaccharide units, either in the glucuronic acid or in the glucosamine; ∼39% of the glucosamine residues are N-sulfated and are therefore susceptible to deaminative cleavage with nitrous acid. This indicates the occurrence of heparin-like region in the mollusk HS, which might account for the anticoagulant activity of the polymer, observed in experiments using plasma and purified coagulation proteases and inhibitors.
The mollusk HS shows mostly mono-sulfated GluA (G2S or G3S) and insignificant amounts of 2,3-di-O-sulfated GluA. Even thought 2- and 3-sulfated GluA residues are rare in nature, the combination of COSY and 13C HSQC spectra support their presence in the mollusk glycan. The first supporting evidence is the presence of very well resolved H2/C2 and H3/C3 cross-peaks in the heteronuclear spectrum (Fig. 5B). These peaks have proton and carbon chemical shifts of δH/δC 4.15–4.02/75.3–72.0 and 4.73–4.58/85–80.4, respectively. These HSQC peaks are quite distinct, well separated, and cannot be misassigned as other resonances from HS units. These particular peaks are also not easily found in the spectrum of regular HS described so far (10), meaning they are from a particular structure. The second point consists of the assigned peaks in the COSY spectrum. Even though it contains an intense water peak, this homonuclear spectrum shows the right 1H-1H connectivities between the anomeric protons of G2S and G3S with their respective 1H2s, where 1H2 of G3S units are more downfield-shifted (0.55 ppm) than 1H2 of G2S units, as assigned in the HSQC (Fig. 5A). Therefore, these two resonances are consistent with those from 2- and 3-sulfated GluA.
The anticoagulant activity of the N. nodosus HS is mainly mediated by antithrombin and heparin cofactor II, because the absence of these inhibitors drastically reduces anticoagulant activity (Fig. 6B). However, some activity remains even in the absence of the two inhibitors (Table 3), suggesting that a minor additional serpin-independent anticoagulant mechanism might be present.
The mollusk HS efficiently inhibited thrombus formation in a model of arterial thrombosis, which involves endothelial damage by a photochemical reaction. In this model, thrombosis is initiated by platelet adherence to the subendothelial space, and both activation of platelets and coagulation contribute to thrombus formation (1). Therefore, the model mimics the pathophysiology of most heart attacks and many strokes that are triggered by thrombosis secondary to disrupted atherosclerotic plaques. Doses of mammalian heparin as low as 0.1 mg kg−1 drastically inhibit arterial thrombus formation in this model. Mollusk HS requires a dose 10-fold higher to produce the same effect. However, a significant antithrombotic effect is observed with only 0.5 mg kg−1 of the HS. At this dose no bleeding effect was observed, and at the full antithrombotic dose (1 mg kg−1), the bleeding increased only slightly.
The HS described in the present study contains small but significant amounts of sulfated groups that do not exist in mammalian glycosaminoglycans. This fact raises concerns about the toxic effect of the mollusk glycan, especially in view of recent facts involving the contamination of heparin preparations with oversulfated chondroitin sulfate (37). It has been shown that the acute toxic effects of contaminated heparin were the result of factor XII-dependent kallikrein production and complement pathway activation by the oversulfated chondroitin sulfate (37). As shown in Fig. 9A, N. nodosus HS started to activate factor XII-mediated kallikrein production in the concentration range of 20–30 μg ml−1, which is 20–30-fold higher than the equivalent dose required for complete inhibition of arterial thrombosis in vivo (1.0 μg ml−1) (Fig. 8A). In addition, the toxic effects of the mollusk HS in cells is only observed at 100–1000 μg ml−1.
The critical question related to therapeutics from natural sources, in addition to toxic matters, is the technical and economic possibility of obtaining very large quantities of the compounds in a constant and ecologically correct manner. Overall, the mollusk HS is isolated at reasonable yields, by procedures similar to those already employed in the preparation of pharmaceutical heparin. Several species of mollusks, including those containing high quantities of heparin analogs, have been successfully cultivated in different parts of the world. The culture employs developed aquaculture technologies capable to produce ton quantities of starting material (38–41). For example, in 1999, ∼73,000 tons of scallops (41) were produced. Therefore, the critical conditions required to use marine invertebrates as a source of natural therapeutic compounds have already been established. What is necessary now is a cooperative effort from scientists of related areas to specifically adapt current methodologies.
Overall, the present work contributes to increasing the knowledge of potential sources of new heparin analogs. The study of the effects of new glycosaminoglycans on thrombosis models that mimic different pathophysiological conditions may contribute to the discovery of new antithrombotic agents that are safe and effective in specific thrombotic conditions.
Acknowledgment
We thank Carlos Alberto Marques de Carvalho for helping with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
The work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico, the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro, and the Mizutani Foundation for Glycoscience (to M. S. G. P.).
- HS
- heparan sulfate
- COSY
- correlated spectroscopy
- HSQC
- heteronuclear single quantum coherence
- aPTT
- activated partial thromboplastin time
- CS
- chondroitin sulfate.
REFERENCES
- 1.Hirsh J., Anand S. S., Halperin J. L., Fuster V. (2001) Circulation 103, 2994–3018 [DOI] [PubMed] [Google Scholar]
- 2.Mackman N. (2008) Nature 451, 914–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindahl U., Bäckström G., Thunberg L., Leder I. G. (1980) Proc. Natl. Acad. Sci. U.S.A. 77, 6551–6555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casu B., Oreste P., Torri G., Zoppetti G., Choay J., Lormeau J. C., Petitou M., Sinäy P. (1981) Biochem. J. 197, 599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choay J., Lormeau J. C., Petitou M., Sinaÿ P., Fareed J. (1981) Ann. N.Y. Acad. Sci. 370, 644–649 [DOI] [PubMed] [Google Scholar]
- 6.Jordan R. E., Oosta G. M., Gardner W. T., Rosenberg R. D. (1980) J. Biol. Chem. 255, 10073–10080 [PubMed] [Google Scholar]
- 7.Liu H., Zhang Z., Linhardt R. J. (2009) Nat. Prod. Rep. 26, 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulloy B., Gray E., Barrowcliffe T. W. (2000) Thromb. Haemost. 84, 1052–1056 [PubMed] [Google Scholar]
- 9.Dietrich C. P., Paiva J. F., Castro R. A., Chavante S. F., Jeske W., Fareed J., Gorin P. A., Mendes A., Nader H. B. (1999) Biochim. Biophys. Acta. 1428, 273–283 [DOI] [PubMed] [Google Scholar]
- 10.Chavante S. F., Santos E. A., Oliveira F. W., Guerrini M., Torri G., Casu B., Dietrich C. P., Nader H. B. (2000) Int. J. Biol. Macromol. 27, 49–57 [DOI] [PubMed] [Google Scholar]
- 11.Demir M., Iqbal O., Dietrich C. P., Hoppensteadt D. A., Ahmad S., Daud A. N., Fareed J. (2001) Clin. Appl. Thromb. Hemost. 7, 44–52 [DOI] [PubMed] [Google Scholar]
- 12.Cavalcante M. C., Allodi S., Valente A. P., Straus A. H., Takahashi H. K., Mourão P. A., Pavão M. S. (2000) J. Biol. Chem. 275, 36189–36196 [DOI] [PubMed] [Google Scholar]
- 13.Dietrich C. P., de Paiva J. F., Moraes C. T., Takahashi H. K., Porcionatto M. A., Nader H. B. (1985) Biochim. Biophys. Acta. 843, 1–7 [DOI] [PubMed] [Google Scholar]
- 14.Pejler G., Danielsson A., Björk I., Lindahl U., Nader H. B., Dietrich C. P. (1987) J. Biol. Chem. 262, 11413–11421 [PubMed] [Google Scholar]
- 15.Arumugam M., Shanmugam A. (2004) Indian J. Exp. Biol. 42, 529–532 [PubMed] [Google Scholar]
- 16.Hovingh P., Linker A. (1982) J. Biol. Chem. 257, 9840–9844 [PubMed] [Google Scholar]
- 17.Maeda-Martinez A. N., Abarca A., Avendaño M., Barracco M. A., Blanco I., Cáceres-Martinez I., Cantillanez M., Freites L., Ibarra A. M., Lodeiros C., Merino G., Mendo J., Navarte M., Vargas-Albores E., Pena J., Roman C., Stolz W., Uriarte J., von Brand E. (2001) in Los Moluscos Pectínidos de Iberoamerica: Ciencia y Acuicultura (Maeda-Martinez A. N. ed) 1st Ed., pp. 469–476, Editorial Limusa, Mexico City, Mexico [Google Scholar]
- 18.Cardoso L. E., Mourão P. A. (1994) Arterioscler. Thromb. 14, 115–124 [DOI] [PubMed] [Google Scholar]
- 19.Pavão M. S., Aiello K. R., Werneck C. C., Silva L. C., Valente A. P., Mulloy B., Colwell N. S., Tollefsen D. M., Mourão P. A. (1998) J. Biol. Chem. 273, 27848–27857 [DOI] [PubMed] [Google Scholar]
- 20.Pomin V. H., Valente A. P., Pereira M. S., Mourão P. A. (2005) Glycobiology 15, 1376–1385 [DOI] [PubMed] [Google Scholar]
- 21.Vicente C. P., He L., Pavão M. S., Tollefsen D. M. (2004) Blood 104, 3965–3970 [DOI] [PubMed] [Google Scholar]
- 22.Herbert J. M., Hérault J. P., Bernat A., van Amsterdam R. G., Vogel G. M., Lormeau J. C., Petitou M., Meuleman D. G. (1996) Circ. Res. 79, 590–600 [DOI] [PubMed] [Google Scholar]
- 23.Bitter T., Muir H. M. (1962) Anal. Biochem. 4, 330–334 [DOI] [PubMed] [Google Scholar]
- 24.Guerrini M., Naggi A., Guglieri S., Santarsiero R., Torri G. (2005) Anal. Biochem. 337, 35–47 [DOI] [PubMed] [Google Scholar]
- 25.Melo E. I., Pereira M. S., Cunha R. S., Sá M. P., Mourão P. A. (2008) Rev. Bras. Cir. Cardiovasc. 23, 169–174 [DOI] [PubMed] [Google Scholar]
- 26.Santos J. C., Mesquita J. M., Belmiro C. L., da Silveira C. B., Viskov C., Mourier P. A., Pavão M. S. (2007) Thromb. Res. 121, 213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linhardt R. J., Ampofo S. A., Fareed J., Hoppensteadt D., Mulliken J. B., Folkman J. (1992) Biochemistry 31, 12441–12445 [DOI] [PubMed] [Google Scholar]
- 28.Iozzo R. V. (1998) Annu. Rev. Biochem. 67, 609–652 [DOI] [PubMed] [Google Scholar]
- 29.Burson S. L., Jr., Fahrenbach M. J., Frommhagen L. H., Riccardi B. A., Brown R. A., Brockman J. A., Lewry H. V., Stokstad E. L. (1956) J. Am. Chem. Soc. 78, 5874–5878 [Google Scholar]
- 30.Dietrich C. P., Nader H. B., de Paiva J. F., Santos E. A., Holme K. R., Perlin A. S. (1989) Int. J. Biol. Macromol. 11, 361–366 [DOI] [PubMed] [Google Scholar]
- 31.Nader H. B., Ferreira T. M., Paiva J. F., Medeiros M. G., Jerônimo S. M., Paiva V. M., Dietrich C. P. (1984) J. Biol. Chem. 259, 1431–1435 [PubMed] [Google Scholar]
- 32.Luppi E., Cesaretti M., Volpi N. (2005) Biomacromolecules 6, 1672–1678 [DOI] [PubMed] [Google Scholar]
- 33.Vijayabaskar P., Balasubramanian T., Somasundaram S. T. (2008) Methods Find. Exp. Clin. Pharmacol. 30, 175–180 [DOI] [PubMed] [Google Scholar]
- 34.Jordan R. E., Marcum J. A. (1986) Arch. Biochem. Biophys. 248, 690–695 [DOI] [PubMed] [Google Scholar]
- 35.Cesaretti M., Luppi E., Maccari F., Volpi N. (2004) Glycobiology 14, 1275–1284 [DOI] [PubMed] [Google Scholar]
- 36.Saravanan R., Shanmugam A. (2010) Appl. Biochem. Biotechnol. 160, 791–799 [DOI] [PubMed] [Google Scholar]
- 37.Kishimoto T. K., Viswanathan K., Ganguly T., Elankumaran S., Smith S., Pelzer K., Lansing J. C., Sriranganathan N., Zhao G., Galcheva-Gargova Z., Al-Hakim A., Bailey G. S., Fraser B., Roy S., Rogers-Cotrone T., Buhse L., Whary M., Fox J., Nasr M., Dal Pan G. J., Shriver Z., Langer R. S., Venkataraman G., Austen K. F., Woodcock J., Sasisekharan R. (2008) N. Engl. J. Med. 358, 2457–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chantal C. (2004) in Advances in Sea Cucumber Aquaculture and Management (Lovatelli A., Conand C., Purcell S., Uthicke S., Hamel J. F., Mercier A. eds) pp. 439, FAO, Rome, Italy [Google Scholar]
- 39.Helm M. M., Bourne N. (2004) in The Hatchery Culture of Bivalves: A Practical Manual (Lovatelli A. ed) pp. 201, FAO, Rome, Italy [Google Scholar]
- 40.Bourne N. F. (2000) Aquaculture Int. 8, 113–122 [Google Scholar]
- 41.Lem A. (2005) in FAO Fisheries and Aquaculture Department, FAO, Rome, Italy [Google Scholar]