Abstract
Volume-sensitive outwardly rectifying (VSOR) Cl− channels are critical for the regulatory volume decrease (RVD) response triggered upon cell swelling. Recent evidence indicates that H2O2 plays an essential role in the activation of these channels and that H2O2 per se activates the channels under isotonic isovolumic conditions. However, a significant difference in the time course for current onset between H2O2-induced and hypotonicity-mediated VSOR Cl− activation is observed. In several cell types, cell swelling induced by hypotonic challenges triggers the release of ATP to the extracellular medium, which in turn, activates purinergic receptors and modulates cell volume regulation. In this study, we have addressed the effect of purinergic receptor activation on H2O2-induced and hypotonicity-mediated VSOR Cl− current activation. Here we show that rat hepatoma cells (HTC) exposed to a 33% hypotonic solution responded by rapidly activating VSOR Cl− current and releasing ATP to the extracellular medium. In contrast, cells exposed to 200 μm H2O2 VSOR Cl− current onset was significantly slower, and ATP release was not detected. In cells exposed to either 11% hypotonicity or 200 μm H2O2, exogenous addition of ATP in the presence of extracellular Ca2+ resulted in a decrease in the half-time for VSOR Cl− current onset. Conversely, in cells that overexpress a dominant-negative mutant of the ionotropic receptor P2X4 challenged with a 33% hypotonic solution, the half-time for VSOR Cl− current onset was significantly slowed down. Our results indicate that, at high hypotonic imbalances, swelling-induced ATP release activates the purinergic receptor P2X4, which in turn modulates the time course of VSOR Cl− current onset in a extracellular Ca2+-dependent manner.
Keywords: Channels/Chloride, ATP, Calcium, Purinergic Receptor, Reactive Oxygen Species (ROS), Cell Volume, Hydrogen Peroxide, Volume-sensitive Chloride Channels
Introduction
Volume-sensitive outwardly rectifying (VSOR)2 Cl− channels are ubiquitously expressed in most mammalian cells types playing a central role in maintaining normal cell volume. However, increasing evidence supports the notion that these channels participate in several other physiological processes, such as salt transport and metabolic stress, apoptosis, proliferation, migration, and cell cycle regulation (1–5). Furthermore, it has also been suggested that a failure in the activation of these channels upon cell swelling is involved in necrotic cell death (6). VSOR Cl− currents have been well studied and characterized; however, intracellular signal transduction pathways responsible for VSOR Cl− channel activation in these diverse physiological circumstances remain less understood and appear to be rather contradictory (7). The mechanisms of activation and regulation of VSOR Cl− channels comprise, depending on the cell type studied, a large variety of factors including ionic strength, macromolecular crowding, intracellular Ca2+, G proteins, arachidonic acid, and metabolites, protein kinase C, ATP release, ROS production, cytoskeleton proteins, tyrosine kinase-mediated phosphorylation of several proteins, and inactivation of phosphatases (2, 8–14).
Nonetheless, the precise roles of these swelling-induced cellular responses on the activation of VSOR Cl− channels are not completely identified. In addition, it is evident that they depend on the cell type studied (8). Participation of intracellular Ca2+ in VSOR Cl− channel activation exemplifies this issue. In bovine pulmonary artery cells, hypotonicity-activated Cl− currents were found to be already maximally activated at 50–100 nm [Ca2+]i (15), and in Ehrlich ascites tumor cells 25 nm [Ca2+]i was reported to be sufficient (16). In contrast, in a rat hepatoma cell line (17) (HTC), it was demonstrated that hypotonicity-induced cell swelling elicits an increase in [Ca2+]i, which proved necessary for volume recovery (18).
On the other hand, extracellular Ca2+ (Cao2+) has been shown to be fundamental in mouse kidney proximal tubular cells (19), whereas in astrocytes the current development was found to be independent of Cao2+ (20). Conversely, in non-pigmented ciliary epithelial cells Cao2+ removal slowed down the current onset as well as decreased the maximal current amplitude, suggesting a role for Cao2+ (21). Finally, in HTC cells, despite the fact that the regulatory volume decrease was impaired in the absence of external Ca2+, maximal VSOR Cl− current measured at steady-state remained unaffected by external Ca2+ removal (18).
In the same cells, it has been observed that after exposure to a hypotonic solution, these cells respond with a transient increase in NAD(P)H oxidase-dependent H2O2 production (22), [Ca2+]i (18) and release of ATP (23). The [Ca2+]i increase in these cells induced by extracellular exposure to hypotonicity was demonstrated to be mediated by PLCγ1 phosphorylation (10). More recently, we established in the same cell system that exogenous application of H2O2 induced PLCγ1 phosphorylation in isotonic conditions. Also, we demonstrated that the activation of this phospholipase was found to be essential for H2O2-dependent VSOR Cl− channel activation (24).
In this study, we explored the influence of Cao2+ and extracellular ATP on VSOR Cl− channel activation. Here we show that the ionotropic purinergic receptor P2X4 is activated in HTC cells as part of the mechanism responsible for VSOR Cl− channel activation. Our results demonstrate that cells exposed to 33% hypotonic solution (200 mosmol/liter), in contrast to cells exposed to 200 μm H2O2, release ATP to the medium, which in turn activates the purinergic receptor P2X4, promoting Ca2+ entry. This additional increase in [Ca2+]i was found to have a profound impact on the half-time constant for VSOR Cl− current onset without affecting the activation and the steady-state amplitude of the current.
EXPERIMENTAL PROCEDURES
Cell Culture
HTC cells passages 6–15 were grown at 37 °C in a 5% CO2-95% air atmosphere in DMEM (GIBCO) supplemented with 10% fetal bovine serum (GIBCO), 2 mm l-glutamine, and 50 units of ml−1 penicillin-streptomycin (Sigma). All experiments were performed at room temperature.
Transfection
Subconfluent (∼80%) cultures of HTC cells were co-transfected with 1 μg of P2X4 K313A (non-functional) plasmid (25, 26) and 0.2 μg of pEGFP plasmid (Clontech) using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Electrophysiological experiments were performed 48 h post-transfection on GFP-positive cells.
Electrophysiological Measurements
For electrophysiological experiments, cells were grown on 12-mm coverslips and directly mounted on the experimental chamber (RC-25, Warner Instruments) installed on the stage of an inverted microscope (Nikon Diaphot, Nikon America). Solution changes were made by a gravity-fed perfusion system, and the solution level in the chamber was kept constant by a peristaltic pump. In the case of Cl− current recordings, the bath solution contained (mm): 5 NaCl, 95 NMDGCl, 2 CaCl2, 1 MgCl2, 100 sorbitol, and 10 Hepes, pH 7.4, adjusted with Tris. Osmolality was adjusted with sorbitol to 300 ± 5 mosmol (Kg H2O)−1 using an osmometer (Advanced Instruments). The pipette solution contained (mm): 5 NaCl, 133 CsCl, 1 MgCl2, and 10 Hepes, pH 7.2 adjusted with Tris and an osmolality of 295 ± 5 mosmol (Kg H2O)−1. 33% hypotonic solutions (200 ± 5 mosmol (Kg H2O)−1) were made by omitting sorbitol. For ATP-induced currents, the bath solution contained (mm): NaCl 140, KCl 5, CaCl2 2, MgCl2 1, HEPES 10, and glucose 10 (pH 7.4) and the pipette solution NaCl 5, KCl 140, MgCl2 1, and HEPES 10 (pH 7.2). Patch-clamp pipettes were made from thin borosilicate (hard) glass capillary tubing with an outer diameter of 1.5 mm (Harvard Apparatus), using a BB-CH puller (Mecanex). Whole-cell currents were recorded with an EPC-7 (List Medical, Germany) amplifier. Command voltage protocols and whole-cell currents acquisition were controlled by pClamp 8 (Molecular Devices Corp.) via a laboratory interface (Digidata 1200, Molecular Devices Corp.). The bath was grounded via an agar-KCl bridge. The nystatin perforated-patch configuration was used as described (27, 28). Nystatin (Sigma) stock solution was freshly made in DMSO at 50 mg/ml. Aliquots of stock solution were added to the pipette solution to obtain a final concentration of 165 mg ml−1. Square pulses of 5 μV were used to monitor the electrical access to the cell. Usually, a stable access resistance (Ra) of 8 ± 3 mΩ (mean ± S.E.) was achieved after 15 min. The membrane cell capacitance ranged from 20 to 30 pF. The acquisition rate was 1 kHz and the experiments were performed at room temperature. Current analysis was performed as described elsewhere (29).
Calcium Measurements
[Ca2+]i was measured by dual-wavelength emission ratiometric laser scanning confocal microscope, using the Ca2+-sensitive fluorescent dyes fluo-3 and fura-red (18). HTC cells were loaded with fluo-3 and fura-red for 20–45 min at room temperature in the presence of 5 μm fluo-3 acetoxymethyl ester (fluo-3-AM) and 15 μm fura-red-AM, dissolved in pluronic acid/DMSO (Invitrogen) in the bath solution described above. Cells were thoroughly washed, and experiments were started after 30 min. Each coverslip was placed in a perfusion chamber mounted on an Axiovert 200 inverted fluorescence microscope equipped with a LSM 5 Pascal laser scanning confocal system (Carl Zeiss). Dyes were excited with the 488-nm line of an argon-krypton laser and emission was detected simultaneously at 515–530 nm (fluo-3) and long pass ≥ 670 nm (fura-red) with a 40×/1.4 NA oil immersion objective. Changes in [Ca2+]i were measured in a field-of-view consisting of 10–30 cells. Fluo-3 and fura-red fluorescence emission intensity were acquired every 30 s. Changes in [Ca2+]i were inferred from changes in the relative fluorescence ratio, calculated by dividing R at each time point by R0, the fluorescence ratio measured as the average fluorescence ratio 1–2 min before stimulation.
Bulk ATP Measurements
Bulk ATP measurements for experiments with 200 μm H2O2 and 33% hypotonic solution were performed as previously described (30). Briefly, HTC cells were grown on 6-well plates (106 cells/well). Before stimulation, cells were washed and equilibrated in isotonic medium for 15 min. Four min after stimulation, the medium was carefully removed and kept on ice. Samples were derivatized in the presence of 1 m chloroacetaldehyde and 25 mm Na2HPO4, pH 4 (200 μl final volume), and incubated for 40 min at 72 °C. Samples were transferred to ice, alkalinized with 50 μl of 0.5 m NH4HCO3, and analyzed by HPLC within 24 h. Identification and quantification of ethenylated species were performed with an automated Merck HPLC apparatus equipped with a fluorescence detector. A 100-μl sample aliquot was injected into Sep-Pak C-18 reverse phase cartridges (Waters). The mobile phase (2 ml min−1, 30% methanol) contained 100 mm KH2PO4, 5 mm tetrabutylammonium, 4% acetonitrile, pH 3. Typical elution times (in min) of etheno-standards were as follows: -ADO, 1.8; -ADP, 3.6; and -ATP, 9.3. The detection limit of this technique is 3 pmol (31).
Cell Volume Measurement
Changes in cell water volume of individual cells were assessed by measuring variations in the concentration of an intracellularly trapped fluorescent dye as previously described (29, 32). Briefly, HTC cells grown were plated on round coverslips, loaded with 2 μm calcein-AM (Molecular Probes) for 5 min and then superfused with an isosmotic solution for 10 min before subjecting the cells to hypotonicity. Changes in fluorescence were monitored using an Olympus DSU confocal microscope. Excitation light was 495 nm, and emitted light was measured at 521 nm. Images were obtained at 15-s intervals, and the fluorescence of a 10-μm2 area in the center of a cell was measured. The data are presented as Vt/V0 values, where V0 is the cell water volume in iso-osmotic solution at time 0, and Vt is the cell water volume at time t. Vt/V0 was calculated from the fluorescence intensity ratio F0/Ft as previously described (32).
Reagents
All reagents were of analytical grade and were purchased from Sigma and Merck. EGTA, suramin, ivermectin, and ATP (sodium salt) were purchased from Sigma. Pluronic acid, fura red, and fluo-3 were purchased from Invitrogen (Carlsbad, CA).
Statistics
All results are presented as means ± S.E. from 4–10 independent experiments. Statistical analysis of the data was performed using Statgraphics Plus 5.0 (Statistical Graphics Corp., USA). The unpaired Student's t test was performed. Statistical differences in Ca2+ measurements experiments were assessed by two-way analysis of variance (ANOVA) for multiple comparisons, followed by the Bonferroni post-hoc test or nonparametric ANOVA (Kruskal-Wallis). Significance was considered at p < 0.05.
RESULTS
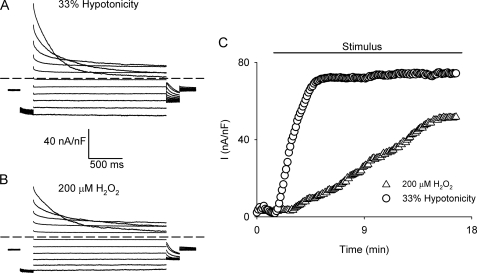
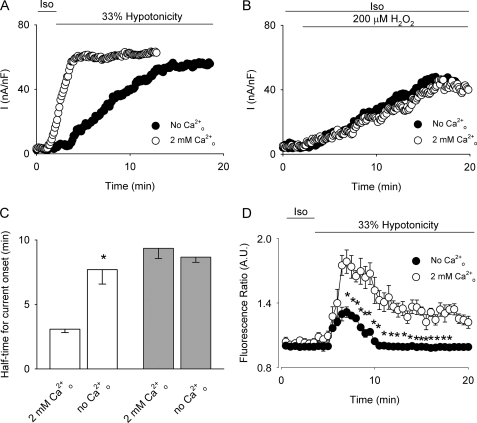
To clarify the involvement of Ca2+ in VSOR Cl− activity, HTC cells were exposed to 33% hypotonicity (Fig. 1A) or 200 μm H2O2 (Fig. 1B). As anticipated, cells responded by activating a Cl− current that displayed the characteristics of the VSOR Cl− current. The hypotonicity-triggered as well as the H2O2-induced current was indistinguishable at steady state, and as expected, the current magnitude was smaller in the H2O2-induced current (22). However, a significant difference in the half-time for current onset was observed (Fig. 1C). Hypotonicity (33%) in the presence of 2 mm extracellular Ca2+ triggered the activation of VSOR Cl− currents within the first 5 min. However, when Cao2+ was omitted, the half-time for current onset was significantly increased without affecting the maximal current (Fig. 2, A and C). In contrast, the half-time for current onset as well as the current magnitude of the current induced by 200 μm H2O2 was found to be independent of Cao2+ (Fig. 2, B and C).
FIGURE 1.
Hypotonicity- and H2O2-evoked VSOR Cl− currents. Representative current traces of nystatin-perforated whole-cell currents activated by a voltage step protocol (2000 ms) from a holding potential of −30 mV ranging from −100 to 100 mV in 20 mV steps. The test pulse was preceded by a pulse to −100 mV (200 ms) and followed by a pulse to −60 mV (200 ms), for cells exposed to 33% hypotonic solution (A) or 200 μm H2O2 (B). C, representative experiment showing the time course of VSOR Cl− current development evoked by exposure to 33% hypotonicity (○) or 200 μm H2O2 in isotonicity (▵) in the presence of 2 mm external Ca2+. Currents were measured at 80 mV every 7 s and normalized to cell capacitance.
FIGURE 2.
Effect of external Ca2+ removal on VSOR Cl− currents and Cao2+ changes. Representative experiments of the time course of VSOR Cl− current development evoked by exposure to 33% hypotonicity (A) or by 200 μm H2O2 (B) in a extracellular solution containing 2 mm Ca2+ (○) or in the absence of external Ca2+ (no Ca2+ added plus 5 mm EGTA, ●). The experimental protocol is the same as for experiments depicted in Fig. 1C. C, summary of the data for half-time for current onset obtained from n = 5–8 independent experiments, for hypotonically (empty bars) or H2O2 (light gray bars)-stimulated cells in 2 mm external Ca2+ solution or in the absence of external Ca2+. D, time course of Cai2+ signal (averaged fluorescence ratio ± S.E.). Cells were exposed to 33% hypotonicity during the time depicted by the bar in the presence (○, control) or in the absence of external Ca2+ (●). n = 4 independent experiments. *, p < 0.05 compared with control conditions (2 mm Cao2+).
To assess the influence of Cao2+ on Cai2+, we monitored the relative changes in Cai2+ in cells stimulated with 33% hypotonicity. As displayed in Fig. 2D, cells exposed to a hypotonic stimulus showed a biphasic increase in Cai2+, which was significantly reduced upon extracellular Ca2+ removal (no Ca2+ added plus 5 mm EGTA), suggesting that Ca2+ entry is responsible, at least partially, for the observed increase in Cai2+. This result is at variance with the observation that H2O2-induced Cai2+ increase is independent of Ca2+ influx (24). Therefore, we hypothesized that Ca2+ entry could be playing a role in modulating the half-time for current onset.
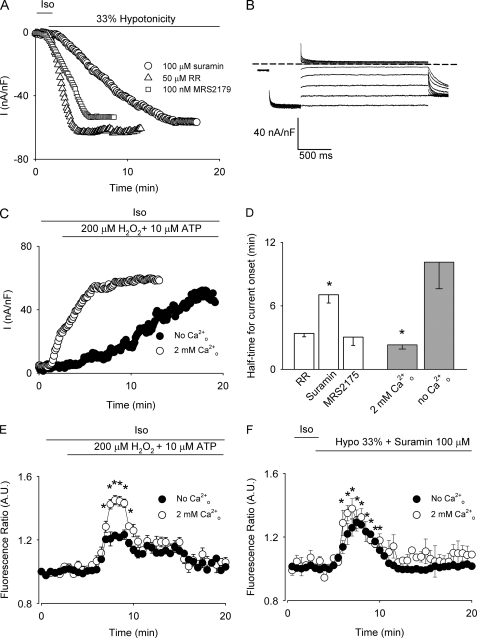
Based on the observation that hypotonically swollen HTC cells (33) and other cell types (2, 34) release ATP to the medium, we examined next the effect of suramin (100 μm), a nonspecific purinergic receptor inhibitor. As shown in Fig. 3, A and D, suramin significantly increased the half-time for current onset. Because suramin exerts a strong voltage-dependent blockade of VSOR Cl− channels producing a complete inhibition of the outward currents (Fig. 3B) (35), the currents were measured at −80 mV. The participation of TRPV4 (36) was ruled out because the nonspecific TRPV4 inhibitor ruthenium red (50 μm) did not affect the half-time for current onset (Fig. 3, A and D). If the observed increase in the half-time for current onset upon suramin exposure is due to the inhibition of purinergic receptors and not to nonspecific effects on VSOR Cl− channels, it can be anticipated that application of ATP together with H2O2 in the presence of external Ca2+ should decrease the half-time for current onset. As depicted in Fig. 3C, in cells stimulated with 200 μm H2O2 and 10 μm ATP, the steady-state current was achieved in a time frame comparable to that observed after a hypotonic challenge (<5 min, Fig. 1C). Moreover, the half-time for current onset became Cao2+-dependent, as shown in Fig. 3, C and D. Similarly, the increase in Cai2+ was also dependent on Cao2+ upon exposure to H2O2 and ATP (Fig. 3E). Conversely, in cells challenged with 33% hypotonicity in the presence of suramin, the increase in Cai2+ was independent of Cao2+ (Fig. 3F), suggesting that Ca2+ entry following the activation of purinergic receptors accounts for the difference in the half-time for current onset observed in hypotonicity and H2O2-challenged HTC cells.
FIGURE 3.
Effect of purinergic receptor activity on VSOR Cl− currents. A, representative experiment of the time course of VSOR Cl− currents in cells exposed to 33% hypotonicity and 50 μm ruthenium red (▵), 100 μm suramin (○), or 100 nm MRS2175 (□). Currents were recorded at −80 mV every 7 s, and normalized to cell capacitance. B, representative steady-state nystatin-perforated whole-cell current traces obtained using the same protocol as in Fig. 1, A and B of a cell exposed to 33% hypotonicity and 100 μm suramin. C, representative time course of VSOR Cl− currents in cells exposed to 200 μm H2O2 and 10 μm ATP in the presence (○) or absence (●) of external Ca2+. The experimental protocol is as in Fig. 1C. D, summary of the data for half-time for current onset obtained from n = 5–6 independent experiments for 33% hypotonic solution (empty bars) or 200 μm H2O2 plus 10 μm ATP (light gray bars)-stimulated cells. E, time course of Cai2+ signal (averaged fluorescence ratio ± S.E.). Cells were exposed to 200 μm H2O2 and 10 μm ATP during the time depicted by the bar in the presence (○, control) or absence of external Ca2+ (●). n = 4 independent experiments. F, time course of Cai2+ signal (averaged fluorescence ratio ± S.E.). Cells were exposed to 33% hypotonicity and 100 μm suramin during the time depicted by the bar in the presence (○, control) or absence of external Ca2+ (●). n = 5 independent experiments. *, p < 0.05 compared with control conditions.
As the metabotropic purinergic receptor P2Y1 has been involved in cell volume regulation in the same cell line (37) and because suramin inhibits this receptor, we explored the effect of MRS2179, a specific P2Y1 inhibitor, on VSOR Cl− channel activation. As depicted in Fig. 3, A and D no change was observed either in the maximal current attained or in the half-time for current onset.
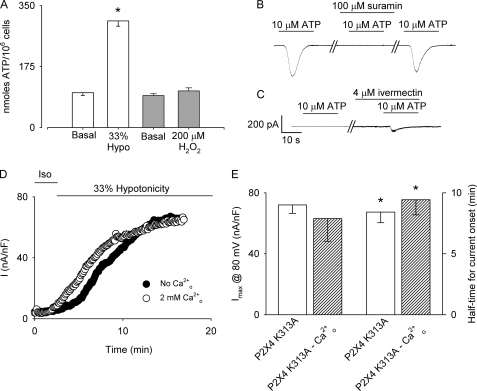
Prompted by these results and taking into account that substantial evidence indicate that ATP is released from mechanically and hypotonically stressed cells triggering autocrine/paracrine activation of purinergic receptors (23, 38, 39), we compared ATP release from HTC cells exposed to hypotonicity and H2O2. To that end, bulk extracellular ATP concentration was measured after 5 min of exposing HTC cells to either H2O2 or hypotonicity. As illustrated in Fig. 4A, a significant increase in bulk ATP was detected in cells exposed to 33% hypotonicity when compared with non-stimulated cells. In contrast, in cells stimulated with 200 μm H2O2, no ATP released could be detected in the medium.
FIGURE 4.
Role of ATP release and P2X4 activation on VSOR Cl− currents. A, bulk ATP released from HTC cells measured after 5 min of exposure to 33% hypotonicity (empty bars) or 200 μm H2O2 (light gray bars). n = 3 independent experiments. *, p < 0.05 respect to basal release. B, representative current record of a cell kept at a holding potential of −80 mV exposed to 10 μm ATP. The same cell exposed to a second puff of ATP in the presence of 100 μm suramin. After suramin wash-out, partial recovery of the current is observed. ATP was delivered for the time indicated by the bar using a puffing pipette located nearby the cell. For these experiments, a 15% hypertonic solution was uses to avoid any contribution from volume-sensitive Cl− currents. C, representative current record of a cell kept at a holding potential of −80 mV exposed to 10 μm ATP in a cell overexpressing a dominant-negative mutant for P2X4 (P2X4 K313A). The same cell exposed to a second puff of ATP after 15 min of preincubation with 4 μm ivermectin. n = 3–4 independent experiments. D, representative experiments showing the time course VSOR Cl− currents evoked by 33% hypotonicity in cells overexpressing P2X4 K313A in the absence (●) or in the presence (○) of 2 mm external Ca2+. Currents were measured at 80 mV every 7 s and normalized by cell capacitance. E, summary of the data presented in D for normalized maximal currents at 80 mV (left axis) and half-time for current onset (right axis) obtained from n = 6 independent experiments. *, p < 0.05 compared with control conditions.
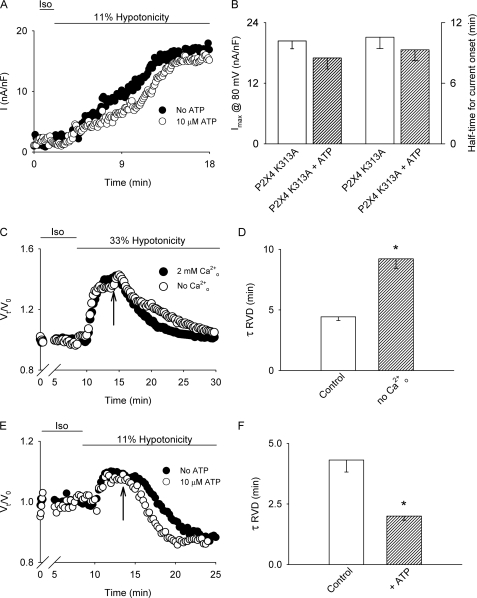
To establish which purinergic receptor is responsible for the ATP-dependent Ca2+ entry in hypotonically swollen HTC cells, we measured ATP-induced currents. As depicted in Fig. 4B, 10 μm ATP triggers a fast, suramin-blockable inward current with a slow deactivation kinetic (τ = 10 ± 2.6 s, single exponential fitted to the data; n = 10). Furthermore, this current was potentiated after preincubation with 4 μm ivermectin (not shown). Based on the deactivation kinetic and the potentiating effect of ivermectin, we explored the role of the ionotropic purinergic receptor P2X4, known to be expressed in liver and in HTC cells (40). To that end, HTC cells were transfected with a non-functional dominant-negative mutant in which the lysine 313 is exchanged by alanine (P2X4 K313A) (41). As shown in Fig. 4C, in HTC cells overexpressing this mutant no current could be measured after application of 10 μm ATP, and only a small current was detected upon preincubation with ivermectin. This result indicates that in HTC cells the P2X4 purinergic receptor is responsible for the inward current induced by ATP.
To determine the role of P2X4 on the extracellular Ca2+ dependence of the half-time for VSOR Cl− current onset, P2X4 K313A-transfected cells were subjected to 33% hypotonicity. As shown in Fig. 4D, overexpression of P2X4 K313A was sufficient to increase the half-time for current onset without affecting the maximal current. In addition, in these cells the half-time for current onset became Cao2+ independent (Fig. 4, D and E).
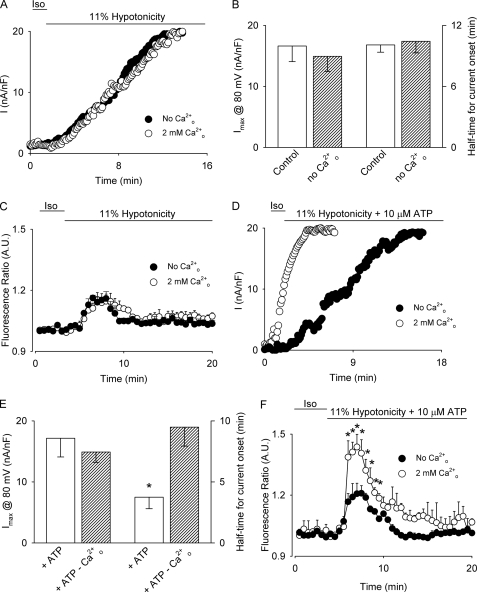
Because the amount of ATP released from swollen cells has been found to be dependent on the magnitude of cell swelling (33, 42), we explored the role of P2X4 in VSOR Cl− channel activity at a smaller hypotonic challenge. As depicted in Fig. 5, A and B, in cells exposed to 11% hypotonicity the half-time for current onset was Cao2+ independent resembling the time course observed in H2O2-stimulated cells. As expected, no difference in the Cai2+ changes was observed when Cao2+ was omitted (Fig. 5C), suggesting that no Ca2+ entry pathway is activated at 11% hypotonicity.
FIGURE 5.
Cao2+ and ATP modulation of 11% hypotonicity-induced VSOR Cl− currents. A, representative experiment showing the time course of the development of VSOR Cl− currents evoked by 11% hypotonicity in the absence (●) or in the presence (○) of 2 mm external Ca2+. Currents were measured at 80 mV every 7 s and normalized by cell capacitance. B, summary of the data for maximal normalized currents at 80 mV (left axis) and half-time for current onset (right axis) obtained from n = 4–6 independent experiments described in A. C, time course of Cai2+ signal (averaged fluorescence ratio ± S.E.). Cells were exposed to 11% hypotonicity in the absence (●) or in the presence (○) of 2 mm external Ca2+ during the time depicted by the bar. n = 4–5 independent experiments. D, representative experiments showing the time course of VSOR Cl− currents evoked by 11% hypotonicity and 10 μm ATP in the absence (●) or in the presence (○) of 2 mm external Ca2+. Currents were measured at 80 mV every 7 s and normalized by cell capacitance. E, summary of the data for normalized maximal currents at 80 mV (left axis) and half-time for current onset (right axis) obtained from n = 4–6 independent experiments described in D. F, time course of Cai2+ signals (averaged fluorescence ratio ± S.E.). Cells were exposed to 11% hypotonicity in the presence of 10 μm ATP (2 mm external Ca2+, ○; 0 mm external Ca2+, ●) during the time depicted by the bar. n = 4–5 independent experiments. *, p < 0.05 compared with control conditions (no ATP added, 2 mm Cao2+).
To confirm the relevance of purinergic receptor activation on VSOR Cl− channel activity, cells were stimulated with 11% hypotonicity and 10 μm ATP. In this condition, the half-time for current onset was dependent on Cao2+ becoming significantly faster in the presence of Cao2+ without variation in the maximal current attained (Fig. 5, D and E). As shown in Fig. 5F, Cai2+ significantly increased with ATP, similarly as obtained in cells stimulated with 200 mm H2O2 and ATP.
To establish that the purinergic receptor responsible for the acceleration of the current onset upon low hypotonicity and ATP exposure can be also attributable to P2X4 activation, we performed experiments in which HTC cells overexpressing the dominant-negative mutant of this receptor were stimulated with 11% hypotonicity in the presence and absence of 10 μm ATP. As depicted in Fig. 6, A and B overexpression of P2X4 K313A prevented the expected decrease of the half-time for current onset upon stimulation with 11% hypotonicity and ATP, without affecting the current magnitude at steady state.
FIGURE 6.
Effect of P2X4 dominant-negative mutant overexpression on 11% hypotonicity-induced VSOR Cl− currents and Cao2+ and ATP on RVD. A, representative experiments showing the time course of VSOR Cl− currents evoked by 11% hypotonicity in cells overexpressing the P2X4 K313A dominant-negative construct in the presence of 2 mm external Ca2+ with (●) or without (○) 10 μm ATP. Currents were measured at 80 mV every 7 s and normalized by cell capacitance. B, summary of the data presented in A for normalized maximal currents at 80 mV (left axis) and half-time for current onset (right axis) obtained from n = 5–6 independent experiments. C, cell volume was monitored in single HTC cells loaded with calcein. Representative experiment in which cell swelling was induced by exposure to 33% hypotonicity during the time depicted by the bar is shown. At the time indicated by the arrow, the solution was switched to a NMDG-Cl (replacing all NaCl) containing 10 μm gramicidin solution with (●) or without Cao2+ (○). D, summary of the rate constants obtained by adjusting the fall in cell volume in each case to a single decreasing exponential from experiments depicted in C. Results shown are averages of 15–20 cells measured in single coverslips, n = 3–4 independent experiments. E, cell volume was monitored in single HTC cells loaded with calcein. Representative experiment in which cell swelling was induced by exposure to 11% hypotonicity during the time depicted by the bar is shown. At the time indicated by the arrow, the solution was switched to a NMDG-Cl (replacing all NaCl) containing 10 μm gramicidin solution with (○) or without 10 mm ATP (●) in the presence of 2 mm Cao2+. F, summary of the rate constants obtained by adjusting the fall in cell volume in each case to a single decreasing exponential from experiments depicted in E. Results shown are averages of 15–20 cells measured in single coverslips, n = 3–4 independent experiments. *, p < 0.05 compared with control conditions.
Next we addressed the impact of Ca2+ entry on the regulatory volume decrease (RVD) process. For this purpose, cells were loaded with the fluorescent probe calcein for 5 min (2 μm) and exposed to 33% hypotonicity in the presence or absence of Cao2+ or 11% hypotonicity in the presence or absence of ATP. To force the RVD response to be dependent only on the Cl− conductance (GCl), cells were exposed, after swelling, to a NMDG-gramicidin hypotonic solution (43). As depicted in Fig. 6, C and D, in cells challenged with 33% hypotonicity and 2 mm Cao2+, the RVD response was faster (τ = 4.4 ± 0.3 min, single exponential fitted to the data) when compared with cells exposed to 33% hypotonicity in the absence of Cao2+ (τ = 9.2 ± 0.8 min, single exponential fitted to the data). On the other hand, cells subjected to 11% hypotonicity (Fig. 6, E and F) have a faster RVD response in the presence of ATP (τ = 2.0 ± 0.2 min, single exponential fitted to the data) when compared with cells exposed to 11% hypotonicity without ATP added to the medium (τ = 4.3 ± 0.5 min, single exponential fitted to the data).
DISCUSSION
VSOR Cl− channels participate in maintaining normal cell volume as well as in a number of other physiological processes (13). Here we expand our knowledge on how hypotonicity activates VSOR Cl− channels in HTC cells demonstrating that, depending on the magnitude of the hypotonic challenge, the ionotropic purinergic receptors P2X4 are activated and thus, the ensuing Ca2+ entry modulates the time course of VSOR Cl− currents and RVD response.
Concerning VSOR Cl− channel modulation, Ca2+-mobilizing G protein-coupled receptor (GPCR) activation has been studied in many cell types. For example, in an intestinal cell line (44) and in a neuroblastoma cell line (45), activation of the thrombin receptor induces at least a 2-fold increase in I− efflux. Furthermore, fibroblasts (46) show not only an increase in VSOR Cl− current but also a decrease in the half-time for current onset after thrombin receptor activation. Other GPCR widely studied in this context are the metabotropic purinergic receptors (P2Y family). For instance, some groups suggest that purinergic receptor activation is mandatory for VSOR Cl− activation (23, 38), nevertheless, others authors propose that purinergic receptors only modulate VSOR Cl− channel activation (47). Similarly, in intestinal epithelial and HTC cells, VSOR Cl− activation has been shown to be independent of ATP release and purinergic receptor activation (37, 48).
In this work, we demonstrate, for the first time, that in HTC cells in addition to the activation of P2Y1 receptors, ATP released from hypotonically stimulated cells activates the ionotropic purinergic receptor P2X4. In contrast with previous results, the data presented here demonstrate a modulatory role of ionotropic purinergic receptor on VSOR Cl− current activation, where P2X4 opening after ATP release allows Ca2+ entry, which is responsible for modulating the half-time for VSOR Cl− current onset.
In cells exposed to H2O2, VSOR Cl− current activation (this work) as well as Cai2+ mobilization is independent of Cao2+ (24) suggesting that Ca2+ permeability pathways are not necessary for VSOR Cl− activation. However, upon exposure to extracellular ATP H2O2-induced VSOR Cl− currents display a faster current onset. Likewise, in cells stimulated with 33% hypotonicity, Cao2+ removal as well as overexpression of a non-functional dominant-negative mutant of P2X4, only slows down VSOR Cl− channel activation without affecting the maximal current achieved, demonstrating that Ca2+ entry through P2X4 is a key modulator of the current.
Although cells are exposed rarely to high (i.e. 33%) extracellular hypotonicity, they are frequently exposed to lower magnitude anisotonic conditions. Here we show that a relatively low magnitude of extracellular hypotonicity (11%) the time course of VSOR Cl− current is slower compared with 33% hypotonicity and in addition, independent of extracellular Ca2+. Also, it is demonstrated that Cai2+ mobilization is independent of Cao2+, a fact that emphasizes the absence of a role for Ca2+ entry pathways under these conditions.
The mechanisms for cell swelling-induced ATP release are still under debate (49). The main ones proposed comprise exocytosis (42) and ATP4− translocation via VSOR Cl− channels at high positive voltages (50). However, as no ATP release was detected in cells stimulated with 200 μm H2O2 (a concentration twice the EC50 for VSOR Cl− channel activation (22)), our results suggest that ATP is not released through VSOR Cl− channels.
In view of the data presented here and previous findings in HTC cells, a model for VSOR Cl− channel activation and modulation is suggested. In this model, we propose that cells subjected to a high osmotic imbalance generate H2O2 upon NAD(P)H oxidase activation (22) promoting Src-mediated PLCγ1 phosphorylation (10, 24) leading to IP3-sensitive intracellular Ca2+ mobilization (18, 51) and subsequent VSOR Cl− channel activation. On the other hand, cell swelling triggers the release of ATP (23, 33), which in turn acts on P2X4 receptors increasing the Ca2+ permeability and thus, modulating the time course of VSOR Cl− currents (this work). The decrease in the half-time of current onset would then allow a faster volume recovery. Conversely, in cells exposed to a relatively small osmotic imbalance, ATP release is not triggered, despite full activation of the current.
Acknowledgments
We thank Juan Pablo Huidobro-Toro from the Centro de Regulación Celular y Patología J.V. Luco, Departamento de Fisiología, Unidad de Regulación Neurohumoral, Facultad de Ciencias Biológicas Pontificia Universidad Católica de Chile for the use of the ATP measurement facility and his invaluable help in performing these experiments, and to Stanko S. Stojilkovic for kindly providing the P2X4 K313L dominant-negative mutant.
This work was supported by FONDAP-Fondecyt 15010006, Chile.
- VSOR
- volume-sensitive outwardly rectifying
- RVD
- regulatory volume decrease
- HTC
- rat hepatoma cells
- ROS
- reactive oxygen species
- PLC
- phospholipase C.
REFERENCES
- 1.Friis M. B., Friborg C. R., Schneider L., Nielsen M. B., Lambert I. H., Christensen S. T., Hoffmann E. K. (2005) J. Physiol. 567, 427–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann E. K. (2000) Cell Physiol. Biochem. 10, 273–288 [DOI] [PubMed] [Google Scholar]
- 3.Shen M. R., Droogmans G., Eggermont J., Voets T., Ellory J. C., Nilius B. (2000) J. Physiol. 529, 385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimizu T., Numata T., Okada Y. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 6770–6773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szabò I., Lepple-Wienhues A., Kaba K. N., Zoratti M., Gulbins E., Lang F. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 6169–6174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mori S., Morishima S., Takasaki M., Okada Y. (2002) Brain Res. 957, 1–11 [DOI] [PubMed] [Google Scholar]
- 7.Nilius B., Droogmans G. (2003) Acta Physiol. Scand. 177, 119–147 [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann E. K., Lambert I. H., Pedersen S. F. (2009) Physiol. Rev. 89, 193–277 [DOI] [PubMed] [Google Scholar]
- 9.McCarty N. A., O'Neil R. G. (1992) Physiol. Rev. 72, 1037–1061 [DOI] [PubMed] [Google Scholar]
- 10.Moore A. L., Roe M. W., Melnick R. F., Lidofsky S. D. (2002) J. Biol. Chem. 277, 34030–34035 [DOI] [PubMed] [Google Scholar]
- 11.Nilius B., Voets T., Prenen J., Barth H., Aktories K., Kaibuchi K., Droogmans G., Eggermont J. (1999) J. Physiol. 516, 67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okada Y. (1997) Am. J. Physiol. Cell Physiol. 273, C755–C789 [DOI] [PubMed] [Google Scholar]
- 13.Stutzin A., Hoffmann E. K. (2006) Acta Physiol. 187, 27–42 [DOI] [PubMed] [Google Scholar]
- 14.Voets T., Droogmans G., Raskin G., Eggermont J., Nilius B. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 5298–5303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szücs G., Heinke S., Droogmans G., Nilius B. (1996) Pflugers Arch.-Eur. J. Physiol. 431, 467–469 [DOI] [PubMed] [Google Scholar]
- 16.Pedersen S. F., Prenen J., Droogmans G., Hoffmann E. K., Nilius B. (1998) J. Membr. Biol. 163, 97–110 [DOI] [PubMed] [Google Scholar]
- 17.Thompson E. B., Tomkins G. M., Curran J. F. (1966) Proc. Natl. Acad. Sci. U.S.A. 56, 296–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roe M. W., Moore A. L., Lidofsky S. D. (2001) J. Biol. Chem. 276, 30871–30877 [DOI] [PubMed] [Google Scholar]
- 19.Barrière H., Rubera I., Belfodil R., Tauc M., Tonnerieux N., Poujeol C., Barhanin J., Poujeol P. (2003) J. Membr. Biol. 193, 153–170 [DOI] [PubMed] [Google Scholar]
- 20.Crépel V., Panenka W., Kelly M. E., MacVicar B. A. (1998) J. Neurosci. 18, 1196–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi C., Ryan J. S., French A. S., Coca-Prados M., Kelly M. E. (1999) J. Physiol. 521, 57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varela D., Simon F., Riveros A., Jørgensen F., Stutzin A. (2004) J. Biol. Chem. 279, 13301–13304 [DOI] [PubMed] [Google Scholar]
- 23.Wang Y., Roman R., Lidofsky S. D., Fitz J. G. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 12020–12025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varela D., Simon F., Olivero P., Armisén R., Leiva-Salcedo E., Jørgensen F., Sala F., Stutzin A. (2007) Cell Physiol. Biochem. 20, 773–780 [DOI] [PubMed] [Google Scholar]
- 25.Yan Z., Liang Z., Obsil T., Stojilkovic S. S. (2006) J. Biol. Chem. 281, 32649–32659 [DOI] [PubMed] [Google Scholar]
- 26.Yan Z., Liang Z., Tomic M., Obsil T., Stojilkovic S. S. (2005) Mol. Pharmacol. 67, 1078–1088 [DOI] [PubMed] [Google Scholar]
- 27.Horn R., Marty A. (1988) J. Gen. Physiol. 92, 145–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varela D., Niemeyer M. I., Cid L. P., Sepúlveda F. V. (2002) J. Physiol. 544, 363–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stutzin A., Eguiguren A. L., Cid L. P., Sepulveda F. V. (1997) Am. J. Physiol. Cell Physiol. 42, C999–C1007 [DOI] [PubMed] [Google Scholar]
- 30.Lazarowski E. R., Tarran R., Grubb B. R., van Heusden C. A., Okada S., Boucher R. C. (2004) J. Biol. Chem. 279, 36855–36864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwata K., Haruki S., Kimura T. (1995) J. Chromatogr. B Biomed. Appl. 667, 339–343 [DOI] [PubMed] [Google Scholar]
- 32.Alvarez-Leefmans F. J., Altamirano J., Crowe W. E. (1995) Methods Neuroscience 27, 361–391 [DOI] [PubMed] [Google Scholar]
- 33.Feranchak A. P., Roman R. M., Schwiebert E. M., Fitz J. G. (1998) J. Biol. Chem. 273, 14906–14911 [DOI] [PubMed] [Google Scholar]
- 34.Okada Y., Maeno E., Shimizu T., Dezaki K., Wang J., Morishima S. (2001) J. Physiol. 532, 3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poletto Chaves L. A., Varanda W. A. (2008) Pflugers Arch. 457, 493–504 [DOI] [PubMed] [Google Scholar]
- 36.Fernández-Fernández J. M., Nobles M., Currid A., Vázquez E., Valverde M. A. (2002) Am. J. Physiol. Cell Physiol. 283, C1705–C1714 [DOI] [PubMed] [Google Scholar]
- 37.Junankar P. R., Karjalainen A., Kirk K. (2002) J. Biol. Chem. 277, 40324–40334 [DOI] [PubMed] [Google Scholar]
- 38.Darby M., Kuzmiski J. B., Panenka W., Feighan D., MacVicar B. A. (2003) J. Neurophysiol. 89, 1870–1877 [DOI] [PubMed] [Google Scholar]
- 39.Roman R. M., Feranchak A. P., Salter K. D., Wang Y., Fitz J. G. (1999) Am. J. Physiol. Gastrointest. Liver Physiol. 276, G1391–G1400 [DOI] [PubMed] [Google Scholar]
- 40.Emmett D. S., Feranchak A., Kilic G., Puljak L., Miller B., Dolovcak S., McWilliams R., Doctor R. B., Fitz J. G. (2008) Hepatology 47, 698–705 [DOI] [PubMed] [Google Scholar]
- 41.Zemkova H., Yan Z., Liang Z., Jelinkova I., Tomic M., Stojilkovic S. S. (2007) J. Neurochem. 102, 1139–1150 [DOI] [PubMed] [Google Scholar]
- 42.Gatof D., Kilic G., Fitz J. G. (2004) Am. J. Physiol. Gastrointest. Liver Physiol. 286, G538–G546 [DOI] [PubMed] [Google Scholar]
- 43.Stutzin A., Torres R., Oporto M., Pacheco P., Eguiguren A. L., Cid L. P., Sepúlveda F. V. (1999) Am. J. Physiol. 277, C392–C402 [DOI] [PubMed] [Google Scholar]
- 44.Tilly B. C., Edixhoven M. J., van den Berghe N., Bot A. G., de Jonge H. R. (1994) Am. J. Physiol. 267, C1271–C1278 [DOI] [PubMed] [Google Scholar]
- 45.Cheema T. A., Pettigrew V. A., Fisher S. K. (2007) J. Pharmacol. Exp. Ther. 320, 1068–1077 [DOI] [PubMed] [Google Scholar]
- 46.Vázquez-Juárez E., Ramos-Mandujano G., Lezama R. A., Cruz-Rangel S., Islas L. D., Pasantes-Morales H. (2008) Pflugers Arch. 455, 859–872 [DOI] [PubMed] [Google Scholar]
- 47.Mongin A. A., Kimelberg H. K. (2005) Am. J. Physiol. Cell Physiol. 288, C204–C213 [DOI] [PubMed] [Google Scholar]
- 48.Hazama A., Shimizu T., Ando-Akatsuka Y., Hayashi S., Tanaka S., Maeno E., Okada Y. (1999) J. Gen. Physiol. 114, 525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fitz J. G. (2007) Trans. Am. Clin. Climatol. Assoc. 118, 199–208 [PMC free article] [PubMed] [Google Scholar]
- 50.Hisadome K., Koyama T., Kimura C., Droogmans G., Ito Y., Oike M. (2002) J. Gen. Physiol. 119, 511–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varela D., Simon F., Riveros A., Jørgensen F., Stutzin A. (2004) Adv. Exp. Med. Biol. 559, 141–145 [PubMed] [Google Scholar]