Abstract
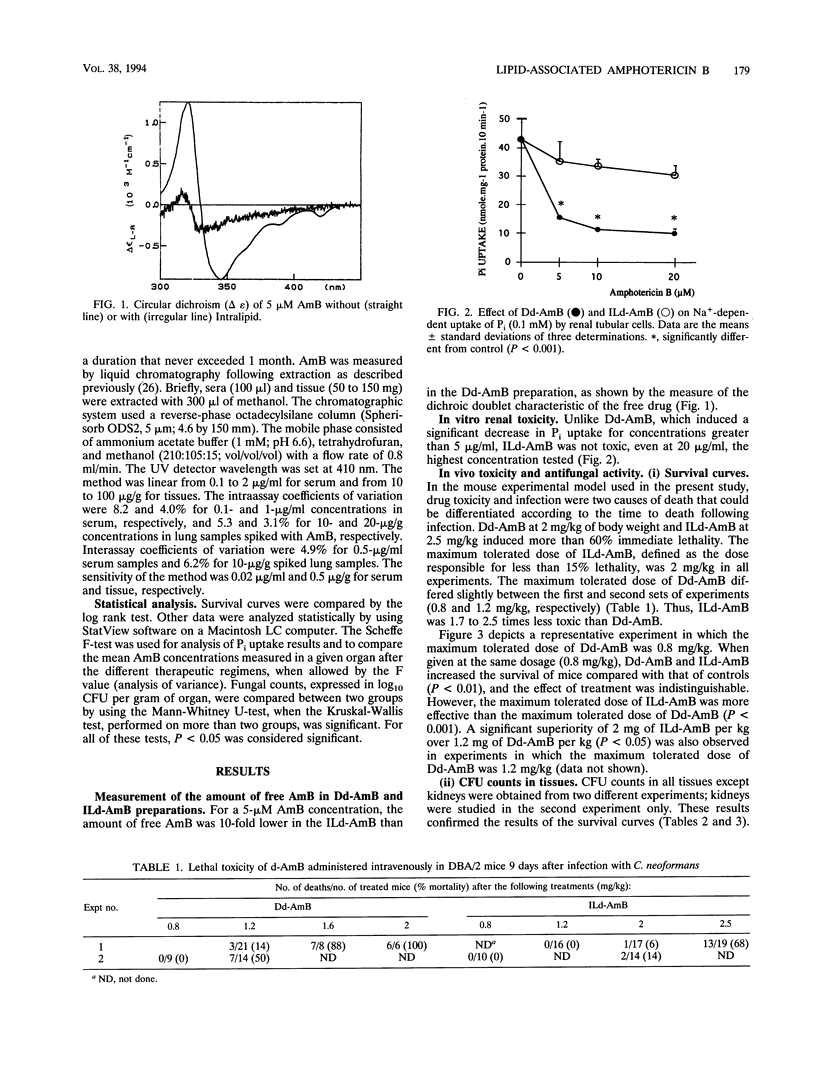
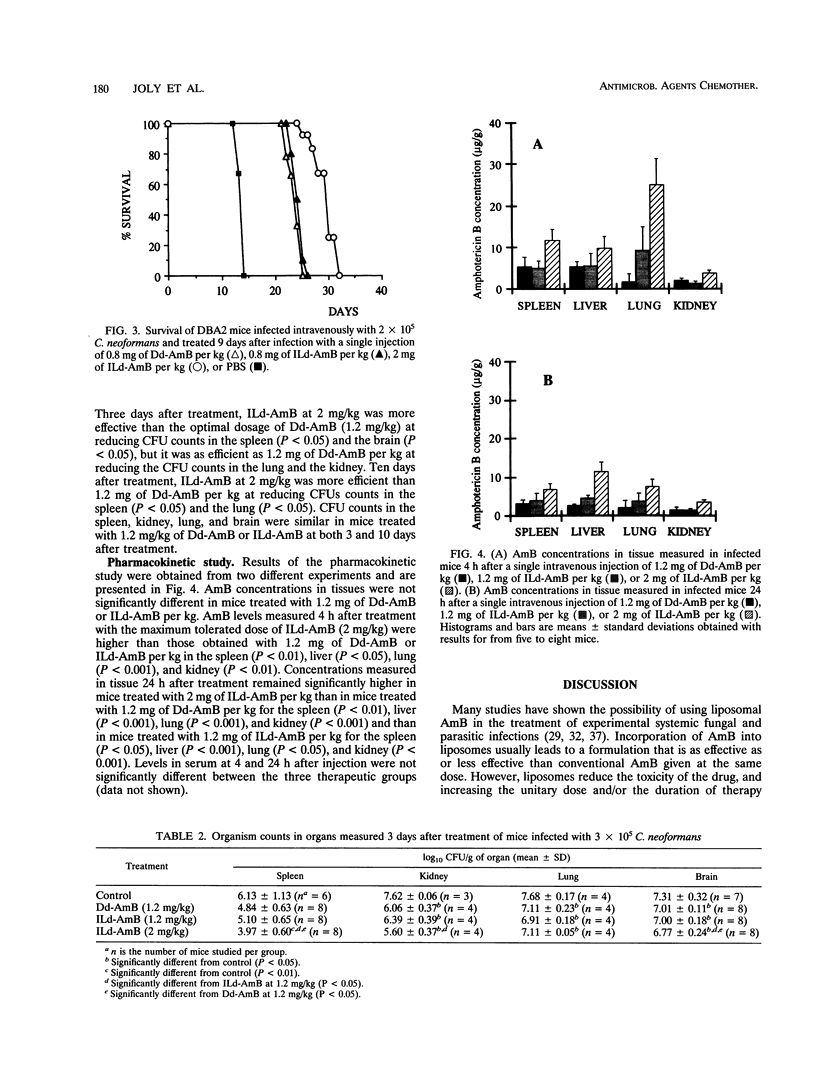
We compared the experimental toxicities and activities of deoxycholate amphotericin B (d-AmB) dissolved in glucose (Dd-AmB) or mixed with 20% Intralipid (ILd-AmB). In vitro, ILd-AmB against renal tubular cells in primary culture. In vivo, the toxicities and activities of Dd-AmB and ILd-AmB were studied in DBA2 mice with cryptococcosis. The maximum tolerated dose of intravenously administered d-AmB, i.e., the dose that induced less than 15% mortality because of toxicity, was 1.7 to 2.5 times higher when it was administered as ILd-AmB than when it was administered as Dd-AmB. Both treatments given intravenously at the same dose were equivalent for improving the survival of mice and reducing CFU counts in infected tissue, but at maximum tolerated doses, ILd-AmB (2 mg/kg of body weight) was more effective than Dd-AmB (0.8 to 1.2 mg/kg). AmB concentrations in spleen, liver, lung, and kidney were measured by high-pressure liquid chromatography 4 and 24 h after a single injection of 1.2 mg of Dd-AmB per kg, 1.2 mg of ILd-AmB per kg, or 2 mg of ILd-AmB per kg. In a given organ, AmB levels were similar after administration of 1.2 mg of Dd-AmB or ILd-AmB per kg but were significantly higher after administration of 2 mg of ILd-AmB per kg. The lower level of toxicity of ILd-AmB might be explained by circular dichroism experiments, showing that ILd-AmB contained 10-fold less soluble oligomeric AmB, which is believed to be the toxic form of the drug, than Dd-AmB. We conclude that ILd-AmB is as efficient as Dd-AmB and is better tolerated than Dd-AmB in mice with experimental cryptococcosis. By allowing higher doses of AmB to be infused, Intralipid enhances AmB concentrations in infected sites, and thus the therapeutic activity of the drug.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. T., HILL G. J., 2nd, SZWED C. F., KNIGHT V. AMPHOTERICIN B RENAL TOXICITY IN THE DOG. J Pharmacol Exp Ther. 1964 Jan;143:47–56. [PubMed] [Google Scholar]
- Barwicz J., Christian S., Gruda I. Effects of the aggregation state of amphotericin B on its toxicity to mice. Antimicrob Agents Chemother. 1992 Oct;36(10):2310–2315. doi: 10.1128/aac.36.10.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolard J., Legrand P., Heitz F., Cybulska B. One-sided action of amphotericin B on cholesterol-containing membranes is determined by its self-association in the medium. Biochemistry. 1991 Jun 11;30(23):5707–5715. doi: 10.1021/bi00237a011. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Bolard J., Kobayashi G. S., Levy R. A., Ostlund R. E., Jr, Schlessinger D., Medoff G. Interaction of plasma proteins and lipoproteins with amphotericin B. J Infect Dis. 1984 Jun;149(6):986–997. doi: 10.1093/infdis/149.6.986. [DOI] [PubMed] [Google Scholar]
- Burgess J. L., Birchall R. Nephrotoxicity of amphotericin B, with emphasis on changes in tubular function. Am J Med. 1972 Jul;53(1):77–84. [PubMed] [Google Scholar]
- Caillot D., Casasnovas O., Solary E., Chavanet P., Bonnotte B., Reny G., Entezam F., Lopez J., Bonnin A., Guy H. Efficacy and tolerance of an amphotericin B lipid (Intralipid) emulsion in the treatment of candidaemia in neutropenic patients. J Antimicrob Chemother. 1993 Jan;31(1):161–169. doi: 10.1093/jac/31.1.161. [DOI] [PubMed] [Google Scholar]
- Fisher M. A., Talbot G. H., Maislin G., McKeon B. P., Tynan K. P., Strom B. L. Risk factors for Amphotericin B-associated nephrotoxicity. Am J Med. 1989 Nov;87(5):547–552. doi: 10.1016/s0002-9343(89)80612-6. [DOI] [PubMed] [Google Scholar]
- Gondal J. A., Swartz R. P., Rahman A. Therapeutic evaluation of free and liposome-encapsulated amphotericin B in the treatment of systemic candidiasis in mice. Antimicrob Agents Chemother. 1989 Sep;33(9):1544–1548. doi: 10.1128/aac.33.9.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouge T. H., Andriole V. T. An experimental model of amphotericin B nephrotoxicity with renal tubular acidosis. J Lab Clin Med. 1971 Nov;78(5):713–724. [PubMed] [Google Scholar]
- Holleran W. M., Wilbur J. R., DeGregorio M. W. Empiric amphotericin B therapy in patients with acute leukemia. Rev Infect Dis. 1985 Sep-Oct;7(5):619–624. doi: 10.1093/clinids/7.5.619. [DOI] [PubMed] [Google Scholar]
- Holmberg K., Meyer R. D. Fungal infections in patients with AIDS and AIDS-related complex. Scand J Infect Dis. 1986;18(3):179–192. doi: 10.3109/00365548609032326. [DOI] [PubMed] [Google Scholar]
- Janoff A. S., Boni L. T., Popescu M. C., Minchey S. R., Cullis P. R., Madden T. D., Taraschi T., Gruner S. M., Shyamsunder E., Tate M. W. Unusual lipid structures selectively reduce the toxicity of amphotericin B. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6122–6126. doi: 10.1073/pnas.85.16.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly V., Bolard J., Saint-Julien L., Carbon C., Yeni P. Influence of phospholipid/amphotericin B ratio and phospholipid type on in vitro renal cell toxicities and fungicidal activities of lipid-associated amphotericin B formulations. Antimicrob Agents Chemother. 1992 Feb;36(2):262–266. doi: 10.1128/aac.36.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly V., Saint-Julien L., Carbon C., Yeni P. Interactions of free and liposomal amphotericin B with renal proximal tubular cells in primary culture. J Pharmacol Exp Ther. 1990 Oct;255(1):17–22. [PubMed] [Google Scholar]
- Jullien S., Brajtburg J., Bolard J. Affinity of amphotericin B for phosphatidylcholine vesicles as a determinant of the in vitro cellular toxicity of liposomal preparations. Biochim Biophys Acta. 1990 Jan 15;1021(1):39–45. doi: 10.1016/0005-2736(90)90381-w. [DOI] [PubMed] [Google Scholar]
- Jullien S., Vertut-Croquin A., Brajtburg J., Bolard J. Circular dichroism for the determination of amphotericin B binding to liposomes. Anal Biochem. 1988 Jul;172(1):197–202. doi: 10.1016/0003-2697(88)90432-0. [DOI] [PubMed] [Google Scholar]
- Kan V. L., Bennett J. E., Amantea M. A., Smolskis M. C., McManus E., Grasela D. M., Sherman J. W. Comparative safety, tolerance, and pharmacokinetics of amphotericin B lipid complex and amphotericin B desoxycholate in healthy male volunteers. J Infect Dis. 1991 Aug;164(2):418–421. doi: 10.1093/infdis/164.2.418. [DOI] [PubMed] [Google Scholar]
- Kirsh R., Goldstein R., Tarloff J., Parris D., Hook J., Hanna N., Bugelski P., Poste G. An emulsion formulation of amphotericin B improves the therapeutic index when treating systemic murine candidiasis. J Infect Dis. 1988 Nov;158(5):1065–1070. doi: 10.1093/infdis/158.5.1065. [DOI] [PubMed] [Google Scholar]
- Larsen R. A., Leal M. A., Chan L. S. Fluconazole compared with amphotericin B plus flucytosine for cryptococcal meningitis in AIDS. A randomized trial. Ann Intern Med. 1990 Aug 1;113(3):183–187. doi: 10.7326/0003-4819-113-3-183. [DOI] [PubMed] [Google Scholar]
- Legrand P., Romero E. A., Cohen B. E., Bolard J. Effects of aggregation and solvent on the toxicity of amphotericin B to human erythrocytes. Antimicrob Agents Chemother. 1992 Nov;36(11):2518–2522. doi: 10.1128/aac.36.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longuet P., Joly V., Amirault P., Seta N., Carbon C., Yeni P. Limited protection by small unilamellar liposomes against the renal tubular toxicity induced by repeated amphotericin B infusions in rats. Antimicrob Agents Chemother. 1991 Jul;35(7):1303–1308. doi: 10.1128/aac.35.7.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Berestein G., Bodey G. P., Frankel L. S., Mehta K. Treatment of hepatosplenic candidiasis with liposomal-amphotericin B. J Clin Oncol. 1987 Feb;5(2):310–317. doi: 10.1200/JCO.1987.5.2.310. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G., Fainstein V., Hopfer R., Mehta K., Sullivan M. P., Keating M., Rosenblum M. G., Mehta R., Luna M., Hersh E. M. Liposomal amphotericin B for the treatment of systemic fungal infections in patients with cancer: a preliminary study. J Infect Dis. 1985 Apr;151(4):704–710. doi: 10.1093/infdis/151.4.704. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G., Mehta R., Hopfer R. L., Mills K., Kasi L., Mehta K., Fainstein V., Luna M., Hersh E. M., Juliano R. Treatment and prophylaxis of disseminated infection due to Candida albicans in mice with liposome-encapsulated amphotericin B. J Infect Dis. 1983 May;147(5):939–945. doi: 10.1093/infdis/147.5.939. [DOI] [PubMed] [Google Scholar]
- Medoff G., Brajtburg J., Kobayashi G. S., Bolard J. Antifungal agents useful in therapy of systemic fungal infections. Annu Rev Pharmacol Toxicol. 1983;23:303–330. doi: 10.1146/annurev.pa.23.040183.001511. [DOI] [PubMed] [Google Scholar]
- New R. R., Chance M. L., Heath S. Antileishmanial activity of amphotericin and other antifungal agents entrapped in liposomes. J Antimicrob Chemother. 1981 Nov;8(5):371–381. doi: 10.1093/jac/8.5.371. [DOI] [PubMed] [Google Scholar]
- Proffitt R. T., Satorius A., Chiang S. M., Sullivan L., Adler-Moore J. P. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J Antimicrob Chemother. 1991 Oct;28 (Suppl B):49–61. doi: 10.1093/jac/28.suppl_b.49. [DOI] [PubMed] [Google Scholar]
- Sawaya B. P., Weihprecht H., Campbell W. R., Lorenz J. N., Webb R. C., Briggs J. P., Schnermann J. Direct vasoconstriction as a possible cause for amphotericin B-induced nephrotoxicity in rats. J Clin Invest. 1991 Jun;87(6):2097–2107. doi: 10.1172/JCI115240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculier J. P., Coune A., Meunier F., Brassinne C., Laduron C., Hollaert C., Collette N., Heymans C., Klastersky J. Pilot study of amphotericin B entrapped in sonicated liposomes in cancer patients with fungal infections. Eur J Cancer Clin Oncol. 1988 Mar;24(3):527–538. doi: 10.1016/s0277-5379(98)90033-5. [DOI] [PubMed] [Google Scholar]
- Stamm A. M., Diasio R. B., Dismukes W. E., Shadomy S., Cloud G. A., Bowles C. A., Karam G. H., Espinel-Ingroff A. Toxicity of amphotericin B plus flucytosine in 194 patients with cryptococcal meningitis. Am J Med. 1987 Aug;83(2):236–242. doi: 10.1016/0002-9343(87)90691-7. [DOI] [PubMed] [Google Scholar]
- Tremblay C., Barza M., Fiore C., Szoka F. Efficacy of liposome-intercalated amphotericin B in the treatment of systemic candidiasis in mice. Antimicrob Agents Chemother. 1984 Aug;26(2):170–173. doi: 10.1128/aac.26.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasan K. M., Brazeau G. A., Keyhani A., Hayman A. C., Lopez-Berestein G. Roles of liposome composition and temperature in distribution of amphotericin B in serum lipoproteins. Antimicrob Agents Chemother. 1993 Feb;37(2):246–250. doi: 10.1128/aac.37.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasan K. M., Vadiei K., Lopez-Berestein G., Luke D. R. Pharmacokinetics, tissue distribution, and toxicity of free and liposomal amphotericin B in diabetic rats. J Infect Dis. 1990 Mar;161(3):562–566. doi: 10.1093/infdis/161.3.562. [DOI] [PubMed] [Google Scholar]



