Abstract
The calcium-activated chloride channel anoctamin1 (ANO1; TMEM16A) is fundamental for the function of epithelial organs. Mice lacking ANO1 expression exhibit transport defects and a pathology similar to cystic fibrosis. They also show a general defect of epithelial electrolyte transport. Here we analyzed expression of all ten members (ANO1–ANO10) in a broad range of murine tissues and detected predominant expression of ANO1, 6, 7, 8, 9, 10 in epithelial tissues, while ANO2, 3, 4, 5 are common in neuronal and muscle tissues. When expressed in Fisher Rat Thyroid (FTR) cells, all ANO proteins localized to the plasma membrane but only ANO1, 2, 6, and 7 produced Ca2+-activated Cl− conductance, as analyzed by ATP-induced iodide quenching of YFP fluorescence. In contrast ANO9 and ANO10 suppressed baseline Cl− conductance and coexpression of ANO9 with ANO1 inhibited ANO1 activity. Patch clamping of ANO-expressing FRT cells indicated that apart from ANO1 also ANO6 and 10 produced chloride currents, albeit with very different Ca2+ sensitivity and activation time. We conclude that each tissue expresses a set of anoctamins that form cell- and tissue-specific Ca2+-dependent Cl− channels.
Keywords: Calcium/Channels, Cell, Channels/Chloride, Channel/Other, Anion transport, Ca2+-activated Cl− currents, Epithelial, TMEM16A, Anoctamin
Introduction
Endogenous Ca2+-activated Cl− channels have been characterized in detail in a large number of tissues, where they show variable properties (1–6). Three independent groups recently identified the gene encoding Ca2+-activated Cl− channels (TMEM16A, anoctamin1, ANO1)3 or a major component of it (7–9). Expression of ANO1 in Xenopus oocytes or mammalian cells induces a Ca2+-activated Cl− channel resembling the properties found for endogenous Ca2+-activated Cl− channels. ANO1 is widely expressed in epithelial tissues where it seems to play an important role. ANO1 knock-out mice die briefly after birth probably due to pronounced tracheomalacia, with incomplete cartilage rings causing instable airways (10). Moreover, the airway epithelium of these mice shows a largely reduced Ca2+-dependent Cl− conductance (11, 12). Ca2+-activated Cl− secretion is of particular importance in mouse, because murine airways express only small amounts of CFTR and therefore secretion relies on Ca2+-activated Cl− conductance (13–15). As a result of impaired Ca2+-dependent Cl− secretion, ANO1−/− tracheas show a reduced mucociliary clearance and exhibited significant, neonatal, luminal mucus accumulation (11, 12). A detailed functional analysis of other epithelial tissues of ANO1 knock-out mice indicated impaired electrophysiological properties in epithelial tissues that show prominent expression of ANO1 such as salivary and pancreatic glands, hepatocytes, and large intestinal epithelium (12). Thus the function of multiple organs is impaired by the ANO1 knock-out, which may all contribute to the high lethality of these animals.
In the airways and probably in other epithelial organs of ANO1 KO animals, Ca2+-dependent Cl− secretion is not completely absent, suggesting that other members of the anoctamin family contribute to Ca2+-activated Cl− conductance in these tissues (11, 12). However apart from ANO1 and ANO2, it is currently not clear whether all anoctamins produce Ca2+-activated Cl− currents (7, 16, 17). It may well be that different cell types express a pattern of anoctamins that supplies a specific cell type with Ca2+-activated Cl− conductance of a particular property. Anoctamin proteins appear to have quite homologous structures apart from ANO8, which has a largely extended p-loop (15). In the present study we therefore analyzed expression of all ten members (ANO1- ANO10) in a broad range of murine tissues. We found predominant expression of ANO 1, 2, 5, 6, 7, 8, 9, 10 in epithelial tissues, which were examined more closely by overexpression in FRT cells. Fluorescence quenching of halide-sensitive yellow fluorescence protein and patch clamping suggested that some but not all investigated anoctamins are able to produce Ca2+-dependent Cl− currents, albeit with variable regulation and functional properties.
MATERIALS AND METHODS
Real-time RT-PCR of Mouse Tissue
From 3 male (7 month) C57BL/6 mice, total RNA was isolated from different tissues using the RNeasy Mini- or Micro kit from Qiagen (Hilden, Germany). RNA was reverse transcribed for 1 h at 37 °C using random primer and M-MLV Reverse Transcriptase (Promega). Real time reverse transcriptase-polymerase chain reaction (RT-PCR) was performed in a plate reader Light Cycler 480 (Roche Applied Science) and by using a Sybrgreen I PCR Kit (Roche Applied Science). Each reaction contained 5 μl of Sybrgreen mastermix, 1 pm of each primer (supplemental Table S1) and 1 μl of cDNA. After 5 min at 94 °C for activation of Taq polymerase, cDNA was amplified by 15 s at 94 °C, 10 s at 62 °C, and 10 s at 72 °C, for 50 cycles. Pooled cDNA from all organs served as standard. To compare different runs, a calibrator was used. The amplification was followed by a melting curve analysis to control the PCR products. As negative controls, water instead of cDNA was run with every PCR experiment. To verify accuracy of the amplification, PCR products were further analyzed on ethidium bromide-stained 2% agarose gels. Data were analyzed with Light Cycler 480 software (Roche Applied Science). The target expressions were normalized using β-actin expression as reference. A mean value of target expression from three mice for each tissue was calculated.
cDNA for ANO and EYFP-I152L
ANO1, ANO6, and ANO9 cDNA was amplified from total RNA of 16HBE-14o cells (bronchial epithelium; kindly provided by Prof. D. Gruenert, CPMRI, San Francisco, CA) by RT-PCR using the primer for ANO1: sense 5′-AAAAGCGGCCGCGGCCACGATGAGGGTC-3′, antisense 5′-AAATCTAGAAACAGGACGCCCCCGTGGTA-3′, for ANO6: sense 5′-TTAGCGGCCGCCATGAAAAAGATGAGCAGGAATG-3′, antisense 5′-TTTTTCTAGATTTTCTGATTTTGGCCGTAAATTG-3′ and for ANO9: sense 5′-AAAGATATCATGCAGGGCGAAGAGAGC-3′, antisense 5′-AAATCTAGATTCACGTCTGTGCTCCTGGC-3′. The cDNAs were subcloned into pcDNA3.1 V5-His (Invitrogen, Karlsruhe, Germany). 16HBE-14o cells express an ANO1 isoform containing the exons a, b, and c, according to Caputo et al. (9). The His tag is located at the intracellular C-terminal end of the different anoctamins. Mouse ANO2 was purchased from ImaGenes GmbH (Berlin, Germany, Clone: IRAVp968H1167D). Human Ano5 cDNA was cloned by RT-PCR from total RNA of A549 cells (carcinomic human alveolar basal epithelial cells, kindly provided by Prof. M. D. Amaral, Department of Chemistry and Biochemistry, Faculty of Sciences, University of Lisboa, Portugal) using the primers 5′-AAAAGGATCCGGCGAAGATGGGCGACCC-3′ and 5′-TTTTCTCGAGTTGAGTGTTGATTTAGCCAGCTG-3′ and subcloned into pcDNA3.1V5His. Human ANO7L and human ANO7S cDNA were kindly provided by I. Pastan (NCI, National Institutes of Health) and subcloned into pcDNA3.1 V5-His. Human ANO8 (GenBankTM Accession No: BC037307) was purchased from imaGenes (Clone: IMAGp998FF0411661Q, Berlin, Germany) and subcloned without intron into pcDNA3.1 V5-His). Human ANO10 was purchased from OriGene (Rockville, MD, catalogue no SC113757) and subcloned into pcDNA3.1V5His. The EYFP-I152L plasmid was kindly provided by Dr. A. Verkman (UCSF, San Francisco). All cDNAs were verified by sequencing.
Cell Culture, RT-PCR, and Transfection
Fisher rat thyroid (FRT) cells cultured in Invitrogen's DMEM/F-12+GlutaMAXTM-I medium, supplemented with 5% fetal calf serum and 1% penicillin (100,000 units/liter)/streptomycin (100 mg/liter). Total RNA was isolated, and RT-PCR was performed to identify endogenous expression of ANO proteins using primers against rat ANO1, 3, 4, 6, 7 (supplemental Table S2) and mouse primer for ANO2, 5, 8, 9, and 10. Plasmids were transfected into FRT cells using standard methods (Lipofectamine, Invitrogen, Karlsruhe, Germany). All experiments were performed 48 h after the transfection.
Immunoblotting
Cells were lysed in radioimmune precipitation assay buffer (1% Triton X-100, 0.01% SDS, 150 mm NaCl, 20 mm Tris-HCl, pH 7.5, 0.08% deoxycholic acid, 5 units/ml benzonase + protease inhibitor) and separated on an 8% polyacrylamide gel. Proteins were transferred to Immobilon-P polyvinylidene difluoride (PVDF) membranes (Millipore) for 90 min on ice at 200 mA. Membranes were blocked with 5% bovine serum albumin in PBS-Tween 20 and probed overnight at 4 °C with a rabbit polyclonal primary anti-His antibody (Cell Signaling, 2365) diluted 1:1000. A secondary horseradish peroxidase-conjugated anti-rabbit was used at 1:5000 dilution (Acaris, R136HRP), and blots were visualized using chemiluminescence (Pierce).
Immunocytochemistry
Transfected FRT cells were grown on glass coverslips and fixed for 10 min with 4% (w/v) paraformaldehyde at room temperature. Cells were incubated for 5 min with 0.1% SDS in PBS. After washing, cells were permeabilized and blocked with 2% (w/v, PBS) bovine serum albumin and 0.04% (v/v, PBS) Triton X-100 and incubated for 1 h with primary antibody mouse anti-His tag (1:500, Qiagen, Hilden, Germany) at 37 °C. Binding of the primary antibody was visualized by incubation with a secondary donkey anti-mouse antibodies conjugated with AlexaFluor® 488 (1:1.000, Molecular Probes, Invitrogen). Nuclei were stained with Hoe33342 (0.1 μg/ml PBS, Aplichem, Darmstadt, Germany). β-Catenin was visualized using an Alexa 568-labeled secondary antibody. F-actin was labeled with phalloidin-conjugated AlexaFluor® 647 (1:40, Molecular Probes, Invitrogen). Cells were mounted on glass slides with fluorescent mounting medium (DAKO Cytomation, Hamburg, Germany) and examined with an ApoTome Axiovert 200 m fluorescence microscope (Zeiss, Göttingen, Germany).
Iodide Quenching
Quenching of the intracellular fluorescence generated by the iodide-sensitive enhanced yellow fluorescent protein (EYFP-I152L) was used to measure anion conductance. YFP-I152L fluorescence was excited at 490 nm using a high speed polychromatic illumination system for microscopic fluorescence measurement (Visitron Systems, Puchheim, Germany) and the emitted light at 535 ± 25 nm was detected with a Coolsnap HQ CCD camera (Roper Scientific). Quenching of YFP-I152L fluorescence by I− influx was induced by replacing 20 mm extracellular Cl− by I−. Images were analyzed with Metafluor software (Universal Imaging). Cells were grown on coverslips and mounted in a thermostatically controlled imaging chamber maintained at 37 °C. Cells were continuously perfused at 4–5 ml/min with Ringer solution and exposed to I− concentration of 20 mm by replacing same amount of NaCl with equimolar NaI. Background fluorescence was subtracted, and autofluorescence was negligible. Changes in fluorescence induced by I− are expressed as initial rates of maximal fluorescence decrease (ΔRF/Δt). For quantitative analysis, cells with low or excessively high fluorescence were discarded. We observed that the slope for fluorescence quenching for any given condition was not dependent on the absolute YFP fluorescence.
Patch Clamping
FTR cells were grown on coverslips, which were mounted in a perfused bath on the stage of an inverted microscope (Axiovert 100, Zeiss) and kept at 37 °C. The bath was perfused continuously with Ringer solution at about 10 ml/min. Patch-clamp experiments were performed in the fast whole-cell configuration. Patch pipettes had an input resistance of 4–6 MΩ, when filled with ringer solution or an intracellular-like solution containing (mm) KCl 30, potassium gluconate 95, NaH2PO4 1.2, Na2HPO4 4.8, EGTA 1, calcium gluconate 0.758, MgCl2 1.034, d-glucose 5, ATP 3. pH was 7.2, the Ca2+ activity was 0.1 μm. The access conductance was measured continuously and was 70–140 nS. Currents (voltage clamp) and voltages (current clamp) were recorded using a patch-clamp amplifier (EPC 7, List Medical Electronics, Darmstadt, Germany), the LIH1600 interface and PULSE software (HEKA, Lambrecht, Germany) as well as Chart software (AD-Instruments, Spechbach, Germany). Data were stored continuously on a computer hard disc and were analyzed using PULSE software. In regular intervals, membrane voltages (Vc) were clamped in steps of 10 mV from −50 to +50 mV. Membrane conductance Gm was calculated from the measured current (I) and Vc values according to Ohm's law.
Materials and Statistical Analysis
All compounds used were of high grade of purity and were from Sigma (Taufkirchen, Germany) or Merck (Darmstadt, Germany). All cell culture reagents were from Invitrogen. Student's t test (for paired or unpaired samples as appropriate), and analysis of variance (ANOVA) was used for statistical analysis. p values<0.05 were accepted as significant.
RESULTS
Expression of Anoctamins in Murine Tissues
The relative expression levels of mRNA encoding the 10 anoctamins was measured in a broad range of murine tissues, using real time RT-PCR (Fig. 1). Expression of the different ANO mRNAs can be subdivided into three groups. ANO2, 3, and 4 are predominantly expressed in neuronal tissues. For ANO2 the highest mRNA expression was found in the eye. In contrast ANO3 and ANO4 mRNA were equally expressed in spinal cord, brain stem, cerebellum, and eye. As a second group ANO6, ANO8, and ANO10 are equally expressed in all tissues. A third group is formed by ANO proteins that are expressed in particular tissues: Highest levels of ANO5 expression was found in skeletal muscle and thyroid gland. The thyroid gland shows high levels of expression for a number of different anoctamins, such as ANO1, ANO5, ANO8, ANO9, and ANO10. ANO7 is found predominantly in the stomach. ANO1 mRNA is expressed in all electrolyte transporting tissues like trachea, pancreas, colon, salivary gland, and prostate tissue. Epithelial tissues therefore express ANO1, ANO5, ANO6, ANO7, ANO8, ANO9, and ANO10 (“epithelial anoctamins”), which were examined more closely in subsequent functional studies.
FIGURE 1.
Expression of ANO proteins in murine tissues. Real-time RT-PCR was performed from total RNA of different mouse tissues. Expression of mRNA for ANO1–10 was normalized using expression of β-actin as a reference (relative expression). A mean value for expression was calculated from three mice and at least one PCR run per tissue.
Endogenous Expression of Anoctamins in FRT Cells
To screen the different anoctamins for Cl− channel function we decided to use FTR cells as an expression system. Filter cultures of FRT cells form high-resistance epithelial monolayers and have very low endogenous ion conductance. Also cells grown on glass coverslips have a low membrane conductance and lack of endogenous Ca2+-activated Cl− channels (9). Also in the present YFP-based fluorescence assays, endogenous Ca2+-activated Cl− conductance was not detectable in mock-transfected cells, after stimulation with ATP or ionomycin (Figs. 4 and 6). Moreover, CFTR currents have not been detected in FRT cells (18) and thus stimulation with IBMX and forskolin was without any effect (Fig. 4). However, we recently found that various TMEM16 proteins are expressed in basically every cell line (15) and therefore performed RT-PCR to detect expression of endogenous anoctamins in FRT cells. Whereas FRT cells do not express ANO1, ANO2, ANO3, ANO4, ANO5, ANO7, and ANO9, they clearly show expression of ANO6, ANO8, and ANO10 (Fig. 2A). Because all cell lines express anoctamins, there is always the probability that ion currents produced by overexpression of anoctamins are contaminated to some degree by endogenous anoctamins (15).
FIGURE 4.
Purinergic activation of ANO1 in FRT cells. Original traces of mock- (A) and ANO1- (B) transfected FRT cells. Activation of Ca2+-activated chloride channels was measured by I− influx measurements using the I−-sensitive protein YFP-I152L. Initial slopes of YFP fluorescence quenching by I− influx (red lines) correlates to the size of the chloride conductance. ANO1 expression increased baseline I− uptake, which was further accelerated by purinergic stimulation (A = 100 μm ATP). Elevation of cAMP by IBMX (100 μm) and forskolin (2 μm, IBMX/Fors) had no effects on I− influx.
FIGURE 6.
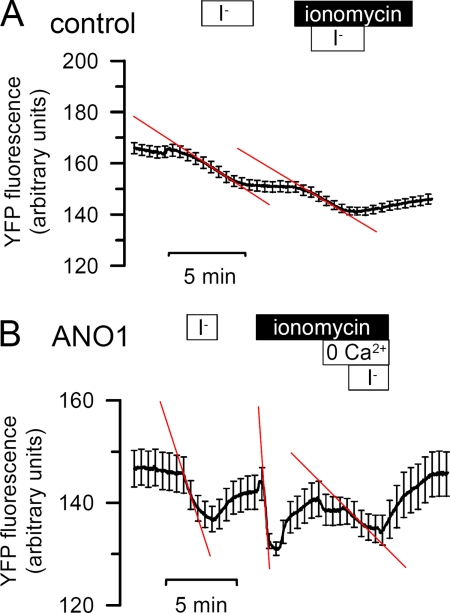
Activation of ANO1 by ionomycin is Ca2+ dependent. Original traces of mock (A) and ANO1 (B)-transfected FRT cells. Treatment of FRT cells with ionomycin (1 μm)-activated I− uptake, which was clearly reduced by removal of Ca2+ from the extracellular bath solution (0 Ca2+).
FIGURE 2.
Expression of ANO proteins in FRT cells. A, total RNA was isolated from Fisher rat thyroid (FRT) cells and RT-PCR was performed to identify endogenous expression of ANO proteins. FRT cells expressed mRNA for ANO6, ANO8, and ANO10, whereas no detectable signal was found for ANO1, ANO2, ANO3, ANO4, ANO5, ANO7, and ANO9. ± indicates presence or absence of reverse transcriptase. B, overexpression of His-tagged human anoctamins in FRT cells as detected by Western blotting using a His antibody. Actin indicates equal loading.
Membrane Localization of His-tagged Anoctamins
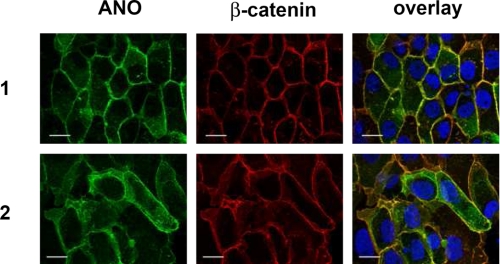
Different epithelial anoctamins and ANO2 were His-tagged and overexpressed in FRT cells. His-tagged anoctamins were readily expressed in FRT cells as demonstrated by Western blotting, as shown for ANO1, -2, and -6 (Fig. 2B). Expression of His-tagged anoctamins (ANO1, ANO2, ANO5, ANO6, ANO7L (large isoform), ANO7S (short isoform), ANO9, and ANO10) was verified by immunocytochemistry using a primary anti-His antibody. Co-staining of β-catenin suggested plasma membrane expression for most ANO proteins (yellow color in the overlays) (Fig. 3 and supplemental Fig. S1). Membrane expression was most obvious for ANO1 and ANO2. It was much reduced for ANO10 and was intracellular for ANO7, ANO8, ANO9, and ANO10. Notably, the short isoform of ANO7 (ANO7S) was detected in the nucleus (19). Similar data were obtained when anoctamins were co-stained with F-actin (supplemental Fig. S2). No immunofluorescence was detected in mock-transfected control cells.
FIGURE 3.
ANO1 and ANO2 are associated with the plasma membrane of FRT cells. His-tagged ANO1 and ANO2 were overexpressed in FRT cells and were detected with anti-His antibody. β-Catenin was visualized using an Alexa 568-labeled phalloidin. Plasma membrane staining of ANO1 and ANO2 is shown by colocalization with β-catenin (yellow color in the overlay picture). No immunofluorescence was detected in mock-transfected control cells.
Functional Screening of ANO Proteins in FRT Cells
The activity of ANO proteins overexpressed in FRT cells was assessed by I− quenching of coexpressed halide-sensitive YFP (20). To that end FRT cells were cotransfected with anoctamin and the yellow fluorescence protein YFP-I152L. The initial slope of fluorescence quenching by I− correlates directly with the Cl− channel activity (20) (Fig. 4). In cells transfected with empty vector only (control), the initial I− uptake rate was not increased by purinergic stimulation with ATP (100 μm) (Fig. 4A). We also examined if a cAMP-activated whole-cell conductance is present in control cells, by stimulating with IBMX (100 μm) and forskolin (2 μm), which, however, did not enhance YFP-quenching (Figs. 4A and 5). In contrast expression of ANO1 caused an increase in initial (basal) I− uptake rate and provided an ATP-induced increase in I− uptake (Figs. 4B and 5).
FIGURE 5.
ATP- but not cAMP-activated ANO family members in FRT cells. Summarized effects of purinergic and cAMP stimulation on I− uptake in ANO-overexpressing FRT cells. Basal I− uptake (basal) was increased by ANO1 expression in FRT cells. ANO1, ANO2, ANO6, and ANO7 (L and S) were activated by purinergic stimulation (ATP, 100 μm), therefore cAMP elevation by IBMX (100 μm) and forskolin (2 μm) had no further effects on basal activity of Ca2+-activated Cl− channels. Values are mean ± S.E. (#, paired t test to basal activity, p < 0.05; §, ANOVA to control, p < 0.05; n = number of cells).
Compared with ANO1, expression of the other epithelial anoctamins (ANO5, ANO6, ANO7, ANO8, ANO9, ANO10) and ANO2 induced very little Ca2+ (ATP)-activated Cl− conductance (Fig. 5). Ca2+-activated Cl− conductance was only detected for ANO2, ANO6, and both the long and the short form of ANO7 and none of the expressed anoctamins induced a cAMP-(IBMX/forskolin) dependent anion conductance. Rather surprisingly, ANO9 and ANO10 reduced both basal and ATP-induced anion conductance. Notably, coexpression of ANO9 inhibited both basal and ATP-induced anion conductance produced by ANO1 (Fig. 8). These results suggest that not all anoctamins produce Ca2+-activated Cl− channels and that some members of the anoctamin family have rather a dominant negative effect on Ca2+-activated Cl− conductance.
FIGURE 8.
Activity of ANO1 is inhibited by coexpression of ANO9. Basal (basal) and ATP (100 μm) activated I− uptake in ANO1-expressing FRT cells was reduced in the presence of coexpressed ANO9 and was slightly reduced after coexpression of ANO10. Treatment with IBMX (100 μm) and forskolin (2 μm) (I/F) had no effects on I− uptake. Note that also basal I− uptake is inhibited by ANO9. Values are mean ± S.E. (#, paired t test to basal activity, p < 0.05; §, ANOVA to control, p < 0.05; n, number of cells).
To exclude the possibility that not all anoctamins examined here are activated by purinergic receptors, we decided to increase intracellular Ca2+ directly using the Ca2+ionophore ionomycin (1 μm). Control cells did not respond to ionomycin and showed only little quenching by I− (Fig. 6A). Of all anoctamins examined, only ANO1 induced a higher basal anion conductance and an ionomycin-activated Cl− conductance, whereas ANO9 and ANO10 reduced both basal and ionomycin-induced anion conductance (Figs. 6 and 7). The ANO1-induced Cl− conductance was strongly inhibited by removal of Ca2+ from the extracellular bath solution, indicating that the ANO1 current is strictly depending on Ca2+.
FIGURE 7.
Ionomycin-activated ANO1 in FRT cells. Summary of the effects of ionomycin (1 μm) on I− uptake in FRT cells overexpressing anoctamins. Basal I− uptake (basal) in FRT cells was increased by expression of ANO1. I− uptake was activated by ionomycin (1 μm) in cells expressing ANO1, but not in cells expressing other anoctamin proteins. Values are mean ± S.E. (#, paired t test to basal activity, p < 0.05; §, ANOVA to control, p < 0.05; n, number of cells).
ANO9 and ANO10 Inhibit ANO1
FRT cells have a small but detectable basal Cl− conductance that could be produced by any of the endogenously expressed anoctamins (ANO6, 8, 10) (Fig. 2). We noticed that expression of ANO9 and ANO10 inhibited this basal anion conductance (Figs. 5 and 7). We would therefore suggest that the endogenous anion conductance is produced by ANO6 but is antagonized by endogenous ANO10, probably due to hetero-oligomerization of both anoctamins or by reduced membrane trafficking of ANO6 in the presence of ANO10. To further verify the inhibitory effects of ANO9 and ANO10 on other anoctamins, we expressed ANO1, which induced a large Cl− conductance, in the absence or presence of ANO9 or ANO10. In fact, both basal and ATP-induced ANO1 conductance was largely inhibited by coexpression of ANO9, while coexpression of ANO10 showed only little effects, possible because endogenous ANO10 levels are already high (Fig. 8). However, this result clearly indicates that different anoctamins interfere with each other.
Anoctamins Show Different Activation Profile and Ca2+ Sensitivity
We further examined Ca2+ (ATP)-dependent activation of anoctamins in patch-clamp experiments. To our surprise, we found that FRT cells show endogenous ATP-activated whole-cell Cl− currents (Fig. 9A, left trace). Unlike the typical fast-activating Cl− current that is generated by ANO1 (Fig. 9A, right trace), this endogenous Cl− current is slowly activating, and of somewhat smaller amplitude and has not been detected in the YFP-quenching experiments. The endogenous Ca2+-activated whole-cell current in mock-transfected cells, which is probably because of expression of endogenous anoctamins (ANO 6, 8, 10) showed a larger current for Cl− than I−, whereas the whole-cell current that was produced by overexpressed ANO1, was increased when bath Cl− was replaced by I− (Fig. 9B). ANO1 and ANO10 overexpressed in FRT cells showed a permeability sequence of I− > Br− > Cl−, while their conductivity was similar for I− and Cl− (Table 1).
FIGURE 9.
Anoctamins form Ca2+-dependent Cl− currents with different properties. A, original continuous recording of the whole-cell current measured in mock-transfected FRT cells expressing endogenous anoctamins only (left trace) or overexpressing ANO1 (right trace). Stimulation with ATP (100 μm)-induced slow activation of endogenous Cl− currents in mock-transfected cells, but fast activation of whole-cell Cl− currents in ANO1-expressing cells. B, summary of the whole-cell conductances activated by ATP in mock-transfected and ANO1-overexpressing cells. Whereas both endogenous and ANO1-related currents were inhibited by replacement of extracellular Cl− with gluconate, only ANO1 currents demonstrated a higher conductance in the presence of iodide. C, continuous recording of the whole-cell current after establishing whole-cell configuration (arrow and line). Patch pipettes were filled with a solution containing 1 μm Ca2+. Whereas whole-cell currents were quickly activated in ANO1-expressing cells, ANO10-expressing or mock-transfected cells only slowly activated a whole-cell current. Niflumic acid (F; 100 μm) potently inhibited ANO1 currents but had little effects of ANO10 and endogenous currents. Values are mean ± S.E. (*, significant effect of iodide and gluconate, paired-test); n, number of cells.
TABLE 1.
Halide permeability (P) and conductivity (G) ratio of ANO1 and ANO10
Values are means ± S.E. (n = number of cells).
| PI/PCl | PBr/PCl | GI/GCl | GBr/GCl | |
|---|---|---|---|---|
| AMO1 (10) | 1.50 ± 0.09 | 1.21 ± 0.04 | 1.13 ± 0.03 | 1.09 ± 0.03 |
| AMO10 (6) | 1.45 ± 0.19 | 1.20 ± 0.07 | 1.04 ± 0.16 | 1.00 ± 0.03 |
Anoctamins currents were also activated directly by an increase in intracellular Ca2+ through perfusion of the Ca2+ containing (1 μm) pipette-filling solution into the cytosol, upon establishing a whole-cell configuration. Whole-cell currents were activated in mock-transfected cells and cells overexpressing ANO1, ANO2, (ANO6), and ANO10 (Fig. 10B). Activation was rapid in ANO1 expressing cells, but was slow for control (mock) or ANO2,6,10-expressing cells (Figs. 9C and 10B). Notably, activation of ANO1 was suppressed by Ca2+-free pipette solution (dashed red line), while slow activation of endogenous currents was not inhibited (black dashed line in Fig. 10B). Moreover, slow current activation was not inhibited by 5 μm of the CAMKII inhibitor KN62 (data not shown). Notably, ANO1 currents were well inhibited (about 60%) by 100 μm niflumic acid (F; 100 μm), while ANO10 currents, or endogenous currents (mock) were only slightly inhibited (about 20%) (Figs. 9C and 10D). Niflumic acid had no effects on ANO6-expressing cells.
FIGURE 10.
ANO1 produces large and rapidly activating Ca2+-dependent Cl− currents. A, Ca2+ sensitivity of different anoctamins and endogenous currents (mock). ANO1 required 10 μm of cytosolic Ca2+ for full activation but was inhibited at higher Ca2+ concentrations. B, summary of the time course for activation of the different anoctamins and endogenous currents (mock). 80% of the ANO1 current is activated after 20 s, while 80% activation of the other anoctamins requires 6 min. C, I/V curves of whole-cell currents in ANO1-expressing FRT cells, measured 30 s after establishing a whole-cell configuration. Activation of ANO1 currents is reduced in the presence of siRNA for ANO10 (si-16K). D, concentration-dependent inhibition of whole-cell currents by NFA in ANO1 and ANO 10-expressing cells. Values are mean ± S.E. (*, significant effect of iodide and gluconate, paired-test); n, number of cells.
Moreover we examined the Ca2+ sensitivity for the different anoctamins by loading the patch pipette with different Ca2+ concentrations. We found that ANO1 and ANO6 require somewhat higher cytosolic Ca2+ concentrations (EC50% = 0.7 μm) than endogenous Ca2+-activated Cl− currents or ANO10 (EC50% = 0.2 μm) (Fig. 10A). At about 10 μm [Ca2+]i, ANO1 was maximally activated, which corresponds well to earlier data (7), and was then inhibited by further increase of cytosolic Ca2+. Cl− currents generated by endogenous anoctamins or ANO10 were inhibited already at 10 μm Ca2+, indicating enhanced inhibitory effect of Ca2+ on these anoctamins. Notably, although ANO9 and ANO10 reduced maximal currents produced by ANO1 (Fig. 8), initial activation of the current (30 s after establishing a whole-cell recording) was reduced by siRNA suppression of endogenous ANO10 (Fig. 10C). Taken together, these results indicate that the different members of the anoctamin family produce Ca2+-activated Cl− currents of remarkably different regulatory properties, which may be essential for their function in different types of epithelial cells (1).
DISCUSSION
Anoctamins Form Ca2+-activated Cl− Channels
ANO1 has been identified recently as a protein that produces Ca2+-activated Cl− currents with properties, archetypical for endogenous CaCCs (7–9). Evidence has been provided that ANO1 itself operates as a Cl− channel, however it is currently not clear whether additional channel subunits are required to build a complete CaCC (7, 8). The channel is clearly regulated by cytosolic Ca2+, however, no obvious Ca2+ binding site has been identified within ANO1, which may suggest additional channel subunits (discussed in Ref. 15). Alternatively the channel is not directly regulated by Ca2+ but rather through calmodulin (CAM) and CAM-dependent kinase. Both direct activation by Ca2+ and CAMKII-dependent regulation has been described extensively (discussed in Refs. 1, 15).
Although ANO1 belongs to a larger family of 10 homologous proteins, it was unclear whether all anoctamins produce Ca2+-activated Cl− channels. The present data, obtained in fluorescence-based assay and by patch clamping suggest that at least some of the epithelial anoctamins produce Ca2+-activated Cl− channels, or major components of it. While some anoctamins (ANO1) produce strong currents, others generate small CaCCs that are only detected by patch clamping. A remarkable finding was that the endogenous ATP-activated Cl− current and also overexpressed ANO10 is only very slowly activated over a time frame of about 10 min (and may therefore escape detection in the fluorescence-based assay). This is in sharp contrast to ANO1, which is activated within seconds of exposure to Ca2+ (Fig. 9). This differences could be sue to a missing accessory subunit that is required (but missing in FRT cells) to speed up activation of ANO10. Similar has been found for KCNQ-K+ channels that have KCNE proteins as β-subunits (21, 22). Alternatively the mechanism of activation could be variable for the different anoctamins (Ca2+, CAM, or CAMKII). These and other aspects such as differential Ca2+ sensitivity need to be clarified in subsequent studies.
Endogenous Expression of Anoctamins
The present report shows that FRT cells express ANO6, 8, and 10 endogenously. Several anoctamins are also expressed in each mouse (Fig. 1) and human (15) tissue examined so far. This correlates well with the fact that CaCC is detected in almost every type of tissue (1, 23). We also observed that CaCC, even if it is not detected in situ in the native cell, will appear during dedifferentiation, proliferation, and development of cancer (15, 24–26). Moreover during inflammation CaCC is up-regulated; a fact that was utilized for cloning of TMEM16A (ANO1) (9, 27). Because several anoctamins are always present in a given cells, it is difficult to examine overexpressed anoctamins without any background. When screening of a number of cell lines we identified typically 2–5 anoctamin paralogs (15). Assuming that all anoctamins induce CaCC activity, it is surprising to find that not all of the cell lines examined express Ca2+-activated Cl− currents (15, 28). Thus not every anoctamin paralog produces CaCC, as shown in this study. Alternatively, additional proteins could be required, or plasma membrane localization varies between cell lines. It is also entirely possible that some epithelial anoctamins require polarization of the cells for proper membrane expression.
Endogenous CaCC May Be Composed of a Set of Anoctamins
The present data show that Ca2+-activated Cl− currents produced by different anoctamins are variable in terms of size, Ca2+ sensitivity, halide permeability, sensitivity toward inhibitors, and time required for activation, The data show that several anoctamins are expressed in epithelial cells and may interfere with each other (Fig. 8). This may provide the characteristic features of Ca2+-activated Cl− conductance in a particular cell type and may explain why Ca2+-activated Cl− currents are so heterogeneous in different cell types (1, 23).
Supplementary Material
This work was supported by Deutsche Forschungsgemeinschaft DFG KU 756/8-1, DFG SFB699 A6 and A7, and TargetScreen (EU-FP6- 2005-LH-037365).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2 and Figs. S1 and S2.
- ANO
- anoctamin
- PBS
- phosphate-buffered saline
- YFP
- yellow fluorescent protein
- FRT
- Fisher rat thyroid.
REFERENCES
- 1.Hartzell C., Putzier I., Arreola J. (2005) Annu. Rev. Physiol 67, 719–758 [DOI] [PubMed] [Google Scholar]
- 2.Leblanc N., Ledoux J., Saleh S., Sanguinetti A., Angermann J., O'Driscoll K., Britton F., Perrino B. A., Greenwood I. A. (2005) Can. J. Physiol. Pharmacol. 83, 541–556 [DOI] [PubMed] [Google Scholar]
- 3.Frings S., Reuter D., Kleene S. J. (2000) Prog. Neurobiol. 60, 247–289 [DOI] [PubMed] [Google Scholar]
- 4.Eggermont J. (2004) Proc. Am. Thorac. Soc. 1, 22–27 [DOI] [PubMed] [Google Scholar]
- 5.Kidd J. F., Thorn P. (2000) Annu. Rev. Physiol. 62, 493–513 [DOI] [PubMed] [Google Scholar]
- 6.Melvin J. E., Yule D., Shuttleworth T., Begenisich T. (2005) Annu. Rev. Physiol. 67, 445–469 [DOI] [PubMed] [Google Scholar]
- 7.Yang Y. D., Cho H., Koo J. Y., Tak M. H., Cho Y., Shim W. S., Park S. P., Lee J., Lee B., Kim B. M., Raouf R., Shin Y. K., Oh U. (2008) Nature 455, 1210–1215 [DOI] [PubMed] [Google Scholar]
- 8.Schroeder B. C., Cheng T., Jan Y. N., Jan L. Y. (2008) Cell 134, 1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caputo A., Caci E., Ferrera L., Pedemonte N., Barsanti C., Sondo E., Pfeffer U., Ravazzolo R., Zegarra-Moran O., Galietta L. J. (2008) Science 322, 590–594 [DOI] [PubMed] [Google Scholar]
- 10.Rock J. R., Futtner C. R., Harfe B. D. (2008) Dev. Biol. 321, 141–149 [DOI] [PubMed] [Google Scholar]
- 11.Rock J. R., O'Neal W. K., Gabriel S. E., Randell S. H., Harfe B. D., Boucher R. C., Grubb B. R. (2009) J. Biol. Chem. 284, 14875–14880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ousingsawat J., Martins J. R., Schreiber R., Rock J. R., Harfe B. D., Kunzelmann K. (2009) J. Biol. Chem., 284, 28698–28703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rochelle L. G., Li D. C., Ye H., Lee E., Talbot C. R., Boucher R. C. (2000) Am. J. Physiol. Lung Cell. Mol. Physiol. 279, L14–L24 [DOI] [PubMed] [Google Scholar]
- 14.Kreda S. M., Mall M., Mengos A., Rochelle L., Yankaskas J., Riordan J. R., Boucher R. C. (2005) Mol. Biol. Cell 16, 2154–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunzelmann K., Kongsuphol P., AlDehni F., Tian Y., Ousingsawat J., Warth R., Schreiber R. (2009) Cell Calcium 46, 233–241 [DOI] [PubMed] [Google Scholar]
- 16.Stöhr H., Heisig J. B., Benz P. M., Schöberl S., Milenkovic V. M., Strauss O., Aartsen W. M., Wijnholds J., Weber B. H., Schulz H. L. (2009) J. Neurosci. 29, 6809–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pifferi S., Dibattista M., Menini A. (2009) Pflügers Arch. 458, 1023–1038 [DOI] [PubMed] [Google Scholar]
- 18.Galietta L. J., Springsteel M. F., Eda M., Niedzinski E. J., By K., Haddadin M. J., Kurth M. J., Nantz M. H., Verkman A. S. (2001) J. Biol. Chem. 276, 19723–19728 [DOI] [PubMed] [Google Scholar]
- 19.Galindo B. E., Vacquier V. D. (2005) Int. J. Mol. Med. 16, 919–924 [PubMed] [Google Scholar]
- 20.Pedemonte N., Lukacs G. L., Du K., Caci E., Zegarra-Moran O., Galietta L. J., Verkman A. S. (2005) J. Clin. Invest. 115, 2564–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder B. C., Waldegger S., Fehr S., Bleich M., Warth R., Greger R., Jentsch T. J. (2000) Nature 403, 196–199 [DOI] [PubMed] [Google Scholar]
- 22.Heitzmann D., Warth R. (2008) Physiol. Rev. 88, 1119–1182 [DOI] [PubMed] [Google Scholar]
- 23.Nilius B., Droogmans G. (2003) Acta Physiol. Scand. 177, 119–147 [DOI] [PubMed] [Google Scholar]
- 24.Aldehni F., Spitzner M., Martins J. R., Barro-Soria R., Schreiber R., Kunzelmann K. (2009) J. Am. Soc. Nephrol. 20, 1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spitzner M., Martins J. R., Soria R. B., Ousingsawat J., Scheidt K., Schreiber R., Kunzelmann K. (2008) J. Biol. Chem. 283, 7421–7428 [DOI] [PubMed] [Google Scholar]
- 26.Kunzelmann K., Milenkovic V. M., Spitzner M., Soria R. B., Schreiber R. (2007) Pflügers Arch. 454, 879–889 [DOI] [PubMed] [Google Scholar]
- 27.Galietta L. J., Pagesy P., Folli C., Caci E., Romio L., Costes B., Nicolis E., Cabrini G., Goossens M., Ravazzolo R., Zegarra-Moran O. (2002) J. Immunol. 168, 839–845 [DOI] [PubMed] [Google Scholar]
- 28.Soria R. B., Spitzner M., Schreiber R., Kunzelmann K. (2009) J. Biol. Chem. 284, 29405–29412 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.