Abstract
Rationale
Dopamine D2-like partial agonists such as aripiprazole have received some attention as potential pharmacotherapies for the treatment of psychostimulant addiction. However, the preclinical evaluations so far have focused on acute effects of aripiprazole.
Objectives
We tested the hypothesis that aripiprazole, both as acute and as chronic treatment, would preferentially decrease cocaine self-administration while sparing behavior maintained by a natural reinforcer, resulting in a shift in the allocation of behavior from cocaine-taking towards the alternative reinforcer.
Methods
Rats were trained to self-administer intravenous cocaine in a concurrent choice procedure, with a palatable food as the competing reinforcer, under a FR 1 FR 5 chain schedule. Aripiprazole was then administered as continuous infusion by osmotic minipumps for five days, during which performance in the choice procedure was assessed daily.
Results
An intermediate dose of aripiprazole decreased cocaine self-administration and shifted the cocaine choice curve to the right as an acute treatment. However, as a chronic treatment aripiprazole failed to decrease cocaine self-administration or cocaine choice, despite a dose-dependent decrease in overall response rates and food-maintained behavior.
Conclusions
Our results confirm and extend earlier findings and indicate that acute administration of aripiprazole can decrease cocaine self-administration. However, based on the present data, chronic treatment with aripiprazole does not show much promise as a potential pharmacotherapy for cocaine addiction. Both acute and chronic treatment data are in agreement with published clinical findings, suggesting that the concurrent choice procedure in rats has predictive validity of efficacy in humans.
Introduction
Cocaine abuse and dependence remain a considerable public health problem, for which there are no widely effective treatment medications (Grabowski et al. 2004; Sofuoglu and Kosten 2005). Much evidence indicates that cocaine’s reinforcing effects are directly related to blockade of the dopamine transporter, thereby increasing dopamine binding to postsynaptic dopamine receptors (Ritz et al. 1987; Bannon 2005; Riddle et al. 2005). More specifically, several studies support a crucial role of the dopamine D2 receptor in mediating the reinforcing effects of cocaine. For instance, D2-like receptor agonists are self-administered in animals trained to self-administer cocaine (Woolverton et al. 1984; Wise et al. 1990; Caine and Koob 1993; Grech et al. 1996; Nader and Mach 1996; Sinnot et al. 1999; Ranaldi et al. 2001; Collins and Woods 2007) and pre-treatment with D2-like agonists cause a leftward shift in cocaine self-administration dose-effect functions (Caine and Koob 1995; Caine et al. 1999, 2000; Barrett et al. 2004). Conversely, pre-treatment with D2-like receptor antagonists can cause a rightward shift in cocaine self-administration dose-effect functions (Bergman et al. 1990; Mello and Negus 1996 for review).
Dopamine D2 receptor partial agonists have received some attention as potential medications for cocaine abuse and dependence, based on the idea that they can act as antagonists in states of high D2 receptor stimulation, such as when cocaine is present, while acting as agonists in states of low dopaminergic tone, such as in a cocaine withdrawal state (see Pulvirenti and Koob 2002 for review). Aripiprazole is a non-selective D2 partial agonist that is already approved as an antipsychotic medication and has a favorable side-effect profile, making it an attractive candidate medication (Kikuchi et al. 1995; Semba et al. 1995; Lawler et al. 1999; Nakai et al. 2003). Indeed, preliminary preclinical and clinical studies using acute aripiprazole administration have shown some promise. In rats and mice, acute aripiprazole was found to decrease methamphetamine and cocaine self-administration, respectively (Wee et al. 2007; Sorensen et al. 2008). In non-dependent human volunteers, acute aripiprazole decreased subject-rated effects of d-amphetamine (Lile et al. 2005; Stoops et al. 2006). However, as cocaine dependence is a chronic condition, any medication would arguably have to be given chronically, at least for a period of time. It is therefore important to evaluate the effects of chronic administration of the candidate treatment. In contrast to the acute treatment data, human studies using chronic administration of aripiprazole have shown mixed results. Two small open-label studies in comorbid psychiatric populations of cocaine-dependent users reported a decline in cocaine craving (Brown et al. 2005; Beresford et al. 2005). However, in double-blind placebo-controlled studies, chronic aripiprazole increased “willingness to take drug again” in cocaine users and number of drug-positive urine samples in amphetamine users, relative to placebo (Haney et al. 2007; Stoops et al. 2007; Tiihonen et al. 2007).
To further test whether acute and chronic aripiprazole treatment can decrease the relative reinforcing strength of intravenous cocaine, we used a concurrent choice schedule of reinforcement. In this procedure, the rat may choose either to self-administer cocaine by pressing a lever, or to press a different lever reinforced by an alternative reinforcer (an appetitive food, the vanilla-flavored protein drink Ensure®). Thus the relative reinforcing effect of cocaine is compared to that of an appetitive food, based on lever selection rather than response rate, making the procedure less sensitive to general performance decrements in operant responding produced by a treatment. This procedure also models directly a hallmark of drug addiction in humans: the allocation of behavior to drug taking at the expense of other behaviors (e.g., nutritional, occupational, or family related activities). We also found that once acquired, the baseline behavior was very stable in rats, which makes it suitable for the assessment of chronic drug treatment effects (i.e., no drift of the baseline behavior or need for intervening “training sessions”).
Methods and materials
Animals
Male Sprague-Dawley rats were purchased at approximately 8 weeks of age (Charles River, Wilmington, MA) and acclimated to the laboratory for a minimum of one week. The rats were maintained in the range of 400 to 500 g with once-daily feedings of approximately 17 g of standard rat chow (Rat Diet 5012; PMI Feeds, Inc., St. Louis, MO). Bacon-flavored biscuits (Bio-Serve, Frenchtown, NJ) were also provided once weekly, primarily for enrichment. Rats were housed individually with free access to water in a temperature- and humidity-controlled facility that was maintained on a 12-h light/dark cycle (lights on at 7:00 am). Behavioral testing was conducted in the light phase. Data from a total of 10 rats are presented. Animal maintenance and research were conducted in accordance with the guidelines provided by the National Institutes of Health Committee on Laboratory Animal Resources, and all protocols were approved by the Institutional Animal Care and Use Committee. The health of the rats was periodically monitored by consulting veterinarians.
Apparatus
All studies were conducted in operant conditioning chambers (21 cm × 29.5 cm × 24.5 cm) placed within sound-attenuating cubicles equipped with a house light and an exhaust fan. Each chamber contained three response levers, with two situated on the front wall of the chamber (3.0 cm above the grid floor and 1.5 cm from the side walls), and a third located at the center of the rear wall (3.0 cm above the grid floor). A shallow steel cup was situated between the two levers and 2.0 cm above the floor, as a reservoir for the consumption of liquid food solutions. A panel with three stimulus lights (green, yellow, red) was located above the right lever on the front wall, and four similar stimulus lights were situated above the left lever (green, yellow, red, yellow).
These lights were flashing to signify the availability of specified cocaine doses (no light = no cocaine available; one, two, three and four lights indicating the increasing doses of cocaine) and food (all three lights on). A single white stimulus light was located above the lever on the opposite wall. For each experimental chamber, two 3.3 rpm infusion pumps (PHM-100) were mounted inside the cubicle, with one delivering liquid food via tygon tubing to the food reservoir, and the other delivering i.v. cocaine via tygon tubing and a single channel fluid swivel (Lomir Biomedical, Malone, NY). The swivel was mounted on a balance arm above the chamber and attached to a customized spring lead with an inner tygon tubing, allowing free movement to the rat within the chamber. All components of the operant conditioning chambers and associated hardware were obtained from MED Associates Inc. (Georgia, VT).
Liquid Food Training
In all rats, lever pressing was initially shaped during daily 2-h training sessions in which liquid food (75 μl of 32% EnsureR protein drink in water) reinforced responding under a fixed ratio (FR) 1 schedule of reinforcement. Illumination of the triple cue light above the right lever signaled the availability of food. The house light was turned on at the beginning of the session and stayed on throughout the session. Responding on the left lever had no scheduled consequences, and the rear lever was retracted during those initial training sessions. A few rats failed to initiate lever pressing within five daily sessions, in which case one or (rarely) two 8-h overnight training sessions were added to facilitate acquisition of lever pressing. A maximum of 100 reinforcers were available per session. When at least 50 reinforcers were earned within one 2-h session, the response requirement was gradually increased to FR 5. When rats again earned at least 50 reinforcers in a single session, a chain schedule was introduced in which one response on the rear lever initiated a FR 5 schedule on the right lever. The session started with the delivery of a single non-contingent (“priming”) reinforcer, after which the rear lever was extended and its associated cue light was illuminated. One response on the rear lever had three scheduled consequences: retraction of the rear lever, turning off its associated cue light, and simultaneously turning on the array of cue lights over the right lever. Completion of the FR 5 response requirement on the right lever then turned off the cue light array and initiated the delivery of a food reinforcer (responses during the 4.2-s food delivery time were recorded as timeout responses but had no scheduled consequences). Responding was reinforced under this schedule until rats earned at least 50 food reinforcers per 2-h session, for five consecutive daily sessions. After these criteria were met, rats were implanted with i.v. catheters.
Surgery
Rats were anesthetized with an isoflurane/oxygen vapor mixture and implanted with chronic indwelling i.v. catheters as described in detail elsewhere (Thomsen and Caine, 2005; 2007). Briefly, catheters consisted of a 13-cm length of Silastic tubing fitted to a 22-gauge guide cannula that was bent at a right angle and encased in dental cement anchored with a 28 mm diameter circular nylon mesh. The tubing was passed subcutaneously from the subcapular location of the catheter base to the right external jugular vein, into which it was anchored with sutures. A single dose of the analgesic ketoprofen (5 mg/kg s.c.) as well as the antibiotic amikacin (10 mg/kg s.c.) were administered immediately prior to the surgery. Animals were allowed to recover for 7 days before training was resumed. During this period, each day a prophylactic dose of the antibiotic amikacin (4 mg) was administered s.c., and also the catheters were flushed daily with 0.1 ml of sterile 0.09% saline containing heparin (3 USP U) and cefazoline (6.7 mg). Thereafter, catheters were flushed daily with sterile saline containing heparin only. Patency of the catheters was verified daily by the fact that blood could be withdrawn through the catheter. If this was not the case, catheter patency was tested periodically by administering a solution containing 1.5 mg/0.1 ml ketamine and 0.075 mg/0.1 ml midazolam through the catheter (0.1 ml). Animals with patent catheters exhibited prominent signs of sedation within 3 s of the infusion. One rat failed to demonstrate catheter patency after completion of the aripiprazole testing period, and data from that rat were excluded.
Cocaine self-administration training
After recovery from surgery, cocaine was made available under a chain schedule identical to the food training chain schedule described above, with the following modifications: responding on the left lever was reinforced with 1.0 mg/kg/infusion cocaine (3.2 mg/ml, dose adjusted to bodyweight by varying the infusion volume/pump time, e.g. 125 μl delivered over 7.14 sec for a 400g rat); the light panel over the left lever was flashing to signal drug availability (all four lights; 1 sec on, 1 sec off). Responding on the right lever had no scheduled consequences, and the panel over the right lever stayed off at all times. A maximum of 30 reinforcers were available per session. When at least 15 reinforcers were earned in a 2-h session, the response requirement was gradually increased to FR 5, until at least 15 cocaine reinforcers were again earned per session. Once cocaine self-administration was established (typically within a week), a concurrent choice schedule of i.v. cocaine and liquid food reinforcement was introduced.
Choice training
The choice session was comprised of five 20-min components separated by 2-min intervals. Responding on the rear and then right lever was reinforced under a FR 1 FR 5 chain schedule with 75 μl 32% Ensure solution in all components (with illumination of the triple-light panel signaling availability of food). Responding on the rear and then left lever was reinforced under a FR 1 FR 5 chain schedule with increasing doses of i.v. cocaine: component 1, no infusion (no cue lights); component 2, 0.056 mg/kg/infusion (green light flashing); component 3, 0.18 mg/kg/infusion (green and yellow lights flashing); component 4, 0.56 mg/kg/infusion (green, yellow and red lights flashing); component 5, 1.0 mg/kg/infusion (all four cue lights flashing). The appropriate cocaine doses, adjusted to each rat’s bodyweight, were delivered by varying the infusion duration across components. Each component started with the delivery of one non-contingent food reinforcer and one non-contingent cocaine reinforcer at the dose available, followed by the extension of the rear lever and illumination of its associated cue light. One response on the rear lever retracted this lever, turned off the associated light, and activated the front levers and initiated the appropriate cue lights over the front levers. The house light was on during each component. During the inter-component intervals, the house light and all cue lights were off and the rear lever retracted, and responding on the front levers had no scheduled consequences. In each component, a maximum of 15 reinforcers were available, which could be all cocaine, all food, or a combination of both (e.g., if a rat earned 5 drug reinforcers, it could earn at most 10 food reinforcers, and vice-versa). Rats were maintained under this schedule of reinforcement until baseline criteria were met and three days of stable behavior were observed, showing: A typical inverted-U shaped cocaine self-administration curve with a peak of at least 10 reinforcers earned; at least 10 food reinforcers earned in the first (no cocaine) component; and percent cocaine choice switching from <5% in the first component to >95% in the last component. Cocaine choice was typically 0% in the first component, 0–50% in the second component, 80–100% in the third component and 100% in the last two components (see baseline results).
Testing procedure
Once baseline behavior was established, the rats were anesthetized briefly with isoflurane vapor and one (0.32 and 0.56 mg/kg/hr) or two (1.0 mg/kg/hr) osmotic minipumps were implanted subcutaneously (see Drugs below). The minipumps allowed for constant delivery of aripiprazole over eight days, but were removed after the last day of testing (typically five days). The five day duration was chosen as the first tested rats showed no change in behavior after 4 or 5 days of treatment, and based on the half-life of aripiprazole in rats (≈2 hrs; Shimokawa et al. 2005). Note that this is much shorter than the half-life measured in humans (≈60 hrs; McGavin and Goa 2002), in whom 1–2 weeks treatment are needed to reach steady state. The minipumps were implanted approximately 2 h prior to the first test session, allowing for full recovery from the anesthesia. The rats were then allowed to self-administer cocaine and liquid food in the choice procedure as described above for at least 5 consecutive days. Data from day 1 were used as acute treatment data, and data from day 5 were used as chronic treatment data.
Drugs
Cocaine HCl was supplied by NIDA/NIH, aripiprazole by Lundbeck A/S (Denmark). Cocaine was dissolved in 0.9% saline, aripiprazole was dissolved by gentle heating in isotonic 20% (w/v) hydroxypropyl-β-cyclodextrin (Roquette, Lestrem, France) acidified with methylsulfonic acid in order to ensure complete dissolution of the compound, then titrated back to pH≈4 with NaOH. This formulation was demonstrated to be stable for more than 2 weeks at 37°C (data not shown). Aripiprazole was administered by constant infusion, 10 μl/hr, using one (for 0.32 and 0.56 mg/kg/hr) or two (for 1.0 mg/kg/hr) osmotic minipumps (Alzet® osmotic pumps, Cupertino, CA; model 2ML1, 10 μl/hr flow, 2 ml total volume).
Data analysis
There were five dependent variables: cocaine reinforcers earned, food reinforcers earned, percent cocaine choice, total response rate (responses/min, cumulated across all three levers) and front levers rate (responses/min, cumulated across the two choice levers only). For baseline behavior, repeated measures ANOVA was performed with cocaine dose as the factor on baseline behavior of rats that completed the study, and cocaine doses were compared to no infusion by paired-sample t-test where appropriate. For each aripiprazole dose (analyzed separately), treatment data on day 1 (“acute treatment”) and day 5 (“chronic treatment”) were analyzed separately versus the baseline behavior within subjects, using ANOVA with cocaine dose and treatment as repeated-measures variables. Significant main effects were followed by Bonferroni posttest between treatment and baseline. Vehicle treatment was not included in the experimental design and analysis because the 0.32 mg/kg/hr dose had virtually no effect, showing that the minipump implantation or vehicle had no effect per se.
Results
Baseline behavior
Baseline behavior is shown as open circles with dashed lines in all figures, and is shown for all rats that completed any of these conditions in Figure 1. As expected, cocaine dose significantly affected the number of cocaine reinforcers earned (p<0.001), which demonstrated an inverted-U shaped dose-effect curve typical of cocaine self-administration. Also as predicted, cocaine dose affected the number of food reinforcers earned per component during the concurrent choice procedure (p<0.001), which were high during components when no cocaine or a very low dose of cocaine was available, and very low (or zero) during components when higher cocaine doses were available. Consequently, allocation of lever responding to the cocaine-reinforced lever was a function of cocaine dose (p<0.001), and was practically zero when no cocaine was available and nearly 100% when cocaine doses in the range of 0.18 – 1.0 mg/kg/injection were available. The total response rate was also significantly affected by cocaine dose (p<0.001), with a significant decrease in rates at the two highest doses. In contrast, front lever rates remained relatively constant as a function of cocaine dose (p>0.3) and throughout the multiple component session, indicating that the chain schedule was completed expeditiously once initiated, and that decreases in total rates were due to longer intervals between each trial (i.e., each initiation of the chain schedule).
Figure 1.
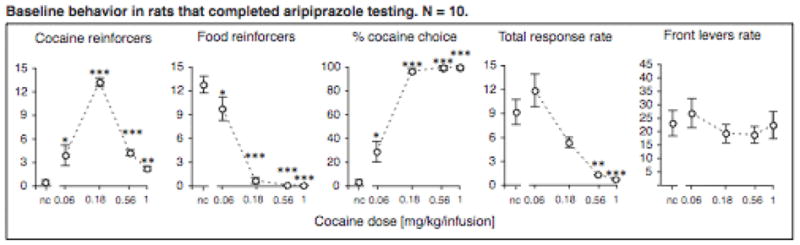
Baseline behavior showing cocaine and food reinforcers earned, percent cocaine choice, total response rates and front levers response rates (ordinates) as a function of cocaine dose (abscissae). Data points are group means, where data from each rat is the mean of three sessions; bars represent one s.e.m. *p<0.05, **p<0.01, ***p<0.001 vs. no cocaine (nc), post-hoc paired-sample t-test.
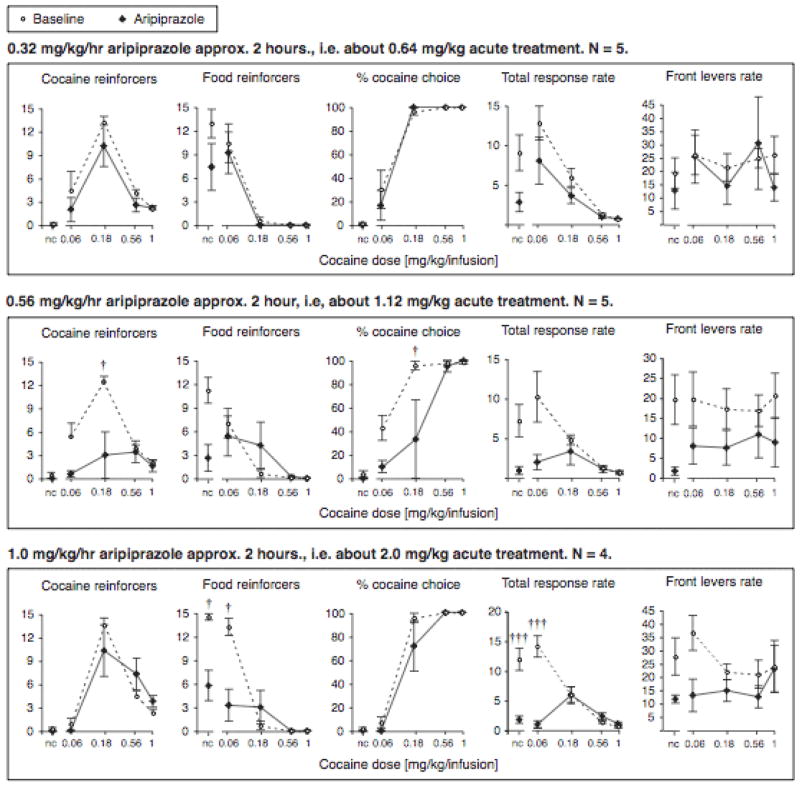
Aripiprazole acute treatment
Similar to the baseline condition, there was a main effect of cocaine dose in all three treatment groups for each of the following measures: cocaine self-administration (all p<0.001), food reinforcers earned (all p<0.001), percent cocaine choice (all p<0.001), and total rate (all p<0.001). Also similar to the baseline condition, cocaine dose did not affect front levers rate significantly in any of the aripiprazole treatment groups.
The lowest aripiprazole dose of 0.32 mg/kg/hr did not have any significant effect on cocaine self-administration, food reinforcers, percent cocaine choice or front levers rate (Fig. 2). It did show a moderate decrease in total rate relative to baseline overall (p<0.05), but no post-hoc tests reached significance.
Figure 2.
Effect of acute aripiprazole treatment on cocaine and food reinforcers earned, percent cocaine choice, total response rates and front levers response rates (ordinates), shown as a function of cocaine dose (abscissae). Data are group means, where treatment points are day 1 data, approximately 2 hours after minipump implantation, and baseline data from corresponding rats are the mean of three sessions for each rat; bars represent one s.e.m. †p<0.05, †††p<0.001 vs. baseline, Bonferroni posttest.
In contrast, the intermediate aripiprazole dose of 0.56 mg/kg/hr significantly reduced cocaine self-administration as acute treatment (main effect p<0.05, interaction with cocaine dose p<0.001; see Fig. 2). Post-hoc comparisons showed that self-administration was suppressed at the peak dose (0.18 mg/kg/infusion; p<0.05), with a similar but non-significant trend at the lowest cocaine dose (p=0.07). Food-maintained responding was not affected as profoundly, with a strong treatment by cocaine dose interaction on day 1 (p<0.001), and while food reinforcers in the first component appeared reduced, this effect did not reach significance post-hoc. Reflecting this relatively selective decrease in cocaine self-administration, the percent cocaine choice was shifted to the right (main effect of treatment p<0.05, interaction with cocaine dose p<0.01). Specifically, percent choice was significantly shifted away from cocaine in the 3rd component (p<0.05), i.e., at the cocaine dose that maintained peak cocaine self-administration, 0.18 mg/kg/infusion, percent cocaine choice was reduced from 96±3.7% cocaine choice under baseline conditions to 33±33% cocaine choice under this aripiprazole treatment A similar trend was seen at the lower cocaine dose of 0.056 mg/kg/infusion. There also was a significant main effect of treatment on total response rate (p<0.05; interaction p<0.01) and front levers response rate (p<0.05, no interaction), but none of these decreases in response rate reached significance post-hoc. Note that only 5 of the 6 rats which completed the treatment period with 0.56 mg/kg/hr aripiprazole were included in the acute treatment data. One rat appeared visibly sedated/inhibited two hours after the minipump implantation and was not tested on the first day out of health concerns. However, data from the remainder of the test period for this rat were comparable to the other rats tested at that aripiprazole dose.
With the highest aripiprazole dose, 1.0 mg/kg/hr, perhaps surprisingly, there was no significant effect of acute treatment on cocaine self-administration, while there was a profound decrease in food reinforcers earned (main effect and interaction with cocaine dose p<0.001; Fig 2). Food-maintained responding was significantly reduced in the first two components, in which behavior was allocated exclusively or primarily to food under baseline conditions (i.e., when no cocaine and a very low cocaine dose were available). Because there was no corresponding increase in responding on the cocaine-reinforced lever in these components, the percent cocaine choice was not significantly affected. The decrease in food consumption was, however, reflected by a decrease in total response rate (main effect and interaction with cocaine dose p<0.001), specifically in the first two components (57% and 65% decrease from baseline, respectively). There was a trend for decreased front levers response rates as well, but this did not reach significance.
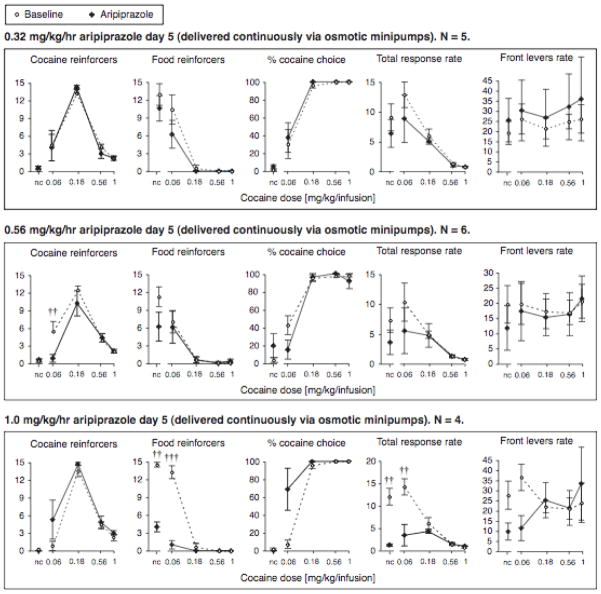
Aripiprazole chronic treatment
There was a main effect of cocaine dose in all three treatment groups for each of the following measures: cocaine self-administration, food reinforcers earned, percent cocaine choice, and total response rate (all p<0.001). Also similar to the baseline condition, cocaine dose did not affect front levers rate significantly in any of the aripiprazole treatment groups.
There were no significant effects of 0.32 mg/kg/hr aripiprazole on days 4/5 (Fig. 3).
Figure 3.
Effect of chronic aripiprazole treatment on cocaine and food reinforcers earned, percent cocaine choice, total response rates and front levers response rates (ordinates), shown as a function of cocaine dose (abscissae). Data are group means, where treatment points are day 5 data, and baseline data from the same rats are the mean of three sessions for each rat; bars represent one s.e.m. ††p<0.01, †††p<0.001 vs. baseline, Bonferroni posttest.
In contrast to the acute treatment, the effects of 0.56 mg/kg/hr aripiprazole dose given as continuous treatment were almost abolished. There remained a main effect on cocaine self-administration (p<0.05; Fig 3), but the effect of aripiprazole on self-administration of the peak cocaine dose had disappeared by day 5. Only self-administration of the lowest cocaine dose remained suppressed (p<0.05). However, it should be noted that this threshold cocaine dose maintained the most variable responding between baseline measures, with the baseline responding in the 0.56 mg/kg/hr group being the highest (see 0.32 and 1.0 mg/kg/hr panels in Fig. 3). Thus the meaningfulness of that decrease in self-administration is difficult to establish. There was no significant effect of chronic aripiprazole treatment on food reinforcers, percent cocaine choice, total or front response rates. Although there remained a significant treatment by cocaine interaction on percent cocaine choice (p < 0.05), no effects reached significance post-hoc and the data did not uniformly suggest a rightward shift of the curve.
Chronic infusion of the highest aripiprazole dose, 1.0 mg/kg/hr, was closer to the acute treatment data: no effect on cocaine self-administration or front levers rate, while the profound suppression of food-maintained behavior and total response rate were maintained (main effect and interaction with cocaine dose p<0.001 for both measures; Fig. 3). Interestingly, there was a significant interaction of treatment with cocaine dose in percent cocaine choice (p<0.001) in the direction of more cocaine choice, but no effect reached significance post-hoc.
Discussion
A first objective of the present investigation was to evaluate the potential for aripiprazole treatment to selectively (or preferentially) reduce cocaine self-administration, while preserving or increasing behavior maintained by an alternative reinforcer. One rationale for this approach was to obtain a measure of cocaine reinforcement that was independent of response rate, to rule out that a decrease in self-administration could be attributed solely to nonspecific effects of aripiprazole on rates of operant behavior (e.g., extrapyramidal motor effects). Another rationale was that an efficient medication in humans should blunt cocaine’s abuse-related effects while sparing healthy motivated behaviors. We therefore used a concurrent choice procedure that was recently established in our laboratory, in which rats can choose between intravenous cocaine self-administration and a palatable food reinforcer during the same session.
Acute treatment with aripiprazole at a moderate dose was able to significantly reduce cocaine self-administration and shift the allocation of behavior towards the alternative choice (food consumption). Although the small sample sizes in the present study limit the power of statistical analyses, our finding corroborates previous acute treatment studies, including our recently observed reduction in cocaine self-administration in mice (Sorensen et al. 2008), and the downward/rightward shift in methamphetamine self-administration reported in rats (Wee et al. 2007). The present data extend those previous findings by showing a significant reduction in cocaine self-administration based on lever selection, supporting the interpretation that acute aripiprazole, at some doses, can decrease the reinforcing effects of cocaine rather than simply producing non-selective rate-suppressing effects. However, preferential decrease in cocaine self-administration was seen only at the intermediate dose, suggesting rather poor selectivity in affecting cocaine over food. The present and previous studies of acute effects of aripiprazole in rodent self-administration procedures are also in agreement with the clinical studies showing decreased subject-rated effects of amphetamine after acute aripiprazole administration (Lile et al. 2005; Stoops et al. 2006).
Despite a preferential decrease in cocaine self-administration at the intermediate dose, a higher dose of aripiprazole also decreased food maintained responding and overall response rates. Interestingly, this dose of aripiprazole produced less effect on cocaine self-administration, suggesting an inverted-U shaped dose-effect curve on the modulation of cocaine self-administration. In contrast, food-maintained responding and overall response rates were uniformly and dose-dependently suppressed with increasing aripiprazole doses. Therefore it appeared that even higher doses of aripiprazole would have produced comparable or less desirable effects on cocaine versus food choice, due to elimination of food-maintained responding.
A second objective of the present study was to evaluate the effect of chronic administration of aripiprazole on cocaine self-administration, as a better predictor of the clinical utility of a candidate medication for cocaine abuse. We found that a 5-day continuous infusion treatment regimen resulted in tolerance to aripiprazole’s ability to block cocaine self-administration under the present conditions. Actually, we observed a slight trend for a leftward shift in cocaine choice after chronic administration of intermediate and high aripiprazole doses. Whereas cocaine self-administration returned to baseline over the treatment period, the dose-dependent suppressing effects of aripiprazole on food-maintained behavior were sustained for at least five days of continuous treatment, suggesting a lack of tolerance to some of aripiprazole’s effects. Accordingly, as with the acute treatment effects, it appeared that even higher doses of chronic aripiprazole would have produced less desirable effects on cocaine versus food choice.
The lack of a decrease in percent cocaine choice after chronic aripiprazole treatment in the present study is also in agreement with recently reported clinical studies in cocaine or amphetamine users. In three independent double-blind, placebo-controlled studies, chronic aripiprazole treatment was found without effect on some subject-rated measures of cocaine, while it increased drug use and the reported desire to take cocaine again, compared to placebo (Haney et al. 2007; Stoops et al. 2007; Tiihonen et al. 2007). Those recent clinical findings are in apparent discordance with earlier findings from open studies in patients with schizophrenia or bipolar/schizoaffective disorders, in which chronic aripiprazole was reported to decrease cocaine craving and/or use (Brown et al. 2005; Beresford et al. 2005). Interestingly in the latter studies, the reported decrease in cocaine craving accompanied an improvement of the comorbid symptoms of schizophrenia or bipolar/schizoaffective disorders, which complicates the interpretation of the results. It cannot be excluded that aripiprazole may have some beneficial effects in some comorbid psychostimulant dependent groups. Limitations of both of those pilot studies included the open-label design and lack of a placebo group, thus the validity of the effect is difficult to assess. Moreover, Beresford and colleagues also reported dropouts (3 subjects of 10 initially entering the study) associated with relapse to cocaine use, suggesting a failure to decrease cocaine craving and use in a substantial proportion of the cohort even after steady-state had been achieved (> 2 weeks). Excluding subjects who relapsed to drug use from the data analysis may well have biased the findings in favor of aripiprazole’s efficacy as a medication for cocaine abuse.
A reversal of the acute effects of candidate medications for cocaine dependence after chronic treatment has been observed before, both in animal studies and in humans. In rats and monkeys, while acute pretreatment with dopamine D1-like or D2-like receptor antagonists decreased the reinforcing and discriminative stimulus effects of cocaine, chronic treatment increased the effects of cocaine (Emmett-Oglesby and Mathis 1988; Kleven and Woolverton 1990; LeDuc and Mittleman 1993; Kosten 1997). The same was observed with the non-selective dopamine receptor antagonist flupenthixol in monkeys (Negus et al. 1996; Negus 2003). In human studies, the dopamine D1-like antagonist ecopipam (SCH 39166) as an acute treatment had shown promising effects on subject-rated effects of cocaine, but the treatment effects were reversed to increased drug-taking, “high”, and “willingness to pay for dose” when ecopipam was administered as a maintenance treatment (Romach et al. 1999; Haney et al. 2001). The reasons for those differences between acute and chronic treatments are not clear, but may be due at least in part to enhanced sensitivity to cocaine. Indeed, chronic treatment with dopamine antagonists has been shown to produce supersensitivity to cocaine and dopamine agonists upon treatment cessation in rats and monkeys (Vaccheri et al. 1987; Barone et al. 1988; Kleven and Woolverton 1990; Howell and Byrd 1992; Braun et al. 1997). The supersensitivity to cocaine may be related to increased density and/or sensitivity of dopamine receptors, including in the nucleus accumbens (Creese and Chen 1985; Hess et al. 1986; Gui-Hua et al. 1992; White et al. 1998). It is possible that aripiprazole acts mainly as a dopamine antagonist and produces similar sensitization of the dopamine system. Interestingly, the delta opioid receptor agonist SNC80 decreased cocaine self-administration more potently than it decreased food-maintained responding as an acute treatment, but the reverse was true after chronic treatment (Do Carmo et al. 2006). The latter finding suggests that reversal of effects can be seen with treatments acting at various classes of receptors, and that cocaine self-administration is an extremely robust behavior, in laboratory animals as in humans (see also Haney et al. 2001 for discussion).
In conclusion, the initial promise of aripiprazole as a medication to control cocaine abuse based on acute studies appeared to be lost after continued treatment. Nevertheless, further assessment of aripiprazole’s potential for treating cocaine abuse is warranted in comorbid psychiatric populations. Finally, our results using a concurrent choice procedure of cocaine self-administration and food-maintained behavior in rats showed very good agreement with the existing clinical findings for both acute and chronic aripiprazole administration. Thus this procedure may be particularly useful in evaluating candidate medications for psychostimulant abuse.
Acknowledgments
This research was supported by NIH grants P01-DA14528, T32-DA07252, and P01-AT002038. Ms. Lauren M. Pepe was supported in part by Boston University’s Undergraduate Research Opportunities Program. All procedures were carried out in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003). We thank Justin Hamilton for expert technical assistance.
References
- Bannon MJ. The dopamine transporter: role in neurotoxicity and human disease. Toxicol Appl Pharmacol. 2005;204:355–360. doi: 10.1016/j.taap.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Barone P, Tucci I, Parashos SA, Chase TN. Supersensitivity to a D-1 dopamine receptor agonist and subsensitivity to a D-2 receptor agonist following chronic D-1 receptor blockade. Eur J Pharmacol. 1988;149:225–32. doi: 10.1016/0014-2999(88)90652-8. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47(Suppl 1):256–273. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Beresford TP, Clapp L, Martin B, Wiberg JL, Alfers J, Beresford HF. Aripiprazole in schizophrenia with cocaine dependence; A pilot study. J Clin Psychopharmacol. 2005;25:363–366. doi: 10.1097/01.jcp.0000169419.38899.5b. [DOI] [PubMed] [Google Scholar]
- Bergman J, Kamien JB, Spealman RD. Antagonism of cocaine self-administration by selective dopamine D(1) and D(2) antagonists. Behav Pharmacol. 1990;1:355–363. doi: 10.1097/00008877-199000140-00009. [DOI] [PubMed] [Google Scholar]
- Braun AR, Laruelle M, Mouradian MM. Interactions between D1 and D2 dopamine receptor family agonists and antagonists: the effects of chronic exposure on behavior and receptor binding in rats and their clinical implications. J Neural Transm. 1997;104:341–62. doi: 10.1007/BF01277656. [DOI] [PubMed] [Google Scholar]
- Brown ES, Jeffres J, Liggin JD, Garza M, Beard L. Switching outpatients with bipolar or schizoaffective disorders and substance abuse from their current antipsychotics to aripiprazole. J Clin Psychiatry. 2005;66:756–760. doi: 10.4088/jcp.v66n0613. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–6. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Pretreatment with the dopamine agonist 7-OH-DPAT shifts the cocaine self-administration dose-effect function to the left under different schedules in the rat. Behav Pharmacol. 1995;6:333–347. [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D(1-like) and D(2-like) agonists in rats that self-administer cocaine. J Pharmacol Exp Ther. 1999;291:353–60. [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK. Effects of dopamine D1-like and D2-like agonists on cocaine self-administration in rhesus monkeys: rapid assessment of cocaine dose-effect functions. Psychopharmacology. 2000;148:41–51. doi: 10.1007/s002130050023. [DOI] [PubMed] [Google Scholar]
- Collins GT, Woods JH. Drug and Reinforcement History as Determinants of the Response-Rate Maintaining Effects of Quinpirole in the Rat. J Pharmacol Exp Ther. 2007;323:599–605. doi: 10.1124/jpet.107.123042. [DOI] [PubMed] [Google Scholar]
- Creese I, Chen A. Selective D-1 dopamine receptor increase following chronic treatment with SCH 23390. Eur J Pharmacol. 1985;109:127–8. doi: 10.1016/0014-2999(85)90549-7. [DOI] [PubMed] [Google Scholar]
- Do Carmo GP, Mello NK, Rice KC, Folk JE, Negus SS. Effects of the selective delta opioid agonist SNC80 on cocaine- and food-maintained responding in rhesus monkeys. Eur J Pharmacol. 2006;547:92–100. doi: 10.1016/j.ejphar.2006.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett-Oglesby M, Mathis D. Chronic administration of SCH 23390 produces sensitization to the discriminative stimulus properties of cocaine. In: Harris LS, editor. Problems of drug dependence; Proc 50th Annual Scientific Meeting, The College on Problems of Drug Dependence; Rockville MD: United States Department of Health and Human Services; 1988. p. 367. [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–64. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Grech DM, Spealman RD, Bergman J. Self-administration of D1 receptor agonists by squirrel monkeys. Psychopharmacology (Berl) 1996;125:97–104. doi: 10.1007/BF02249407. [DOI] [PubMed] [Google Scholar]
- Gui-Hua C, Perry BD, Woolverton WL. Effects of chronic SCH 23390 or acute EEDQ on the discriminative stimulus effects of SKF 38393. Pharmacol Biochem Behav. 1992;41:321–7. doi: 10.1016/0091-3057(92)90105-o. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology (Berl) 2001;155:330–7. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Reed SC, Vosburg SK, Foltin RW. Aripiprazole increases cocaineself-administration in humans. Oral presentation at International Study Group Investigating Drugs as Reinforcers (ISGIDAR); June 2007; Quebec City, Canada. 2007. [Google Scholar]
- Hess EJ, Albers LJ, Le H, Creese I. Effects of chronic SCH23390 treatment on the biochemical and behavioral properties of D1 and D2 dopamine receptors: potentiated behavioral responses to a D2 dopamine agonist after selective D1 dopamine receptor upregulation. J Pharmacol Exp Ther. 1986;238:846–54. [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Enhanced sensitivity to the behavioral effects of cocaine after chronic administration of D2-selective dopamine antagonists in the squirrel monkey. J Pharmacol Exp Ther. 1992;262:907–15. [PubMed] [Google Scholar]
- Kleven MS, Woolverton WL. Effects of continuous infusions of SCH 23390 on cocaine- or food-maintained behavior in rhesus monkeys. Behav Pharmacol. 1990;1:365–373. doi: 10.1097/00008877-199000140-00010. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Tottori K, Uwahodo Y, Hirose T, Miwa T, Oshiro Y, Morita S. 7-(4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butyloxy)-3,4-dihydro-2(1H)-quinolinone (OPC-14597), a new putative antipsychotic drug with both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity. J Pharmacol Exp Ther. 1995;274:329–336. [PubMed] [Google Scholar]
- Kosten TA. Enhanced neurobehavioral effects of cocaine with chronic neuroleptic exposure in rats. Schizophr Bull. 1997;23:203–13. doi: 10.1093/schbul/23.2.203. [DOI] [PubMed] [Google Scholar]
- Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, Gonzalez AM, Sibley DR, Mailman RB. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology. 1999;20:612–27. doi: 10.1016/S0893-133X(98)00099-2. [DOI] [PubMed] [Google Scholar]
- LeDuc PA, Mittleman G. Interactions between chronic haloperidol treatment and cocaine in rats: an animal model of intermittent cocaine use in neuroleptic treated populations. Psychopharmacology (Berl) 1993;110:427–36. doi: 10.1007/BF02244649. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Vansickel AR, Glaser PEA, Lon R, Hays LR, Rush CR. Aripiprazole Attenuates the Discriminative-Stimulus and Subject-Rated Effects of D-Amphetamine in Humans. Neuropsychopharmacology. 2005;30:2103–2114. doi: 10.1038/sj.npp.1300803. [DOI] [PubMed] [Google Scholar]
- McGavin JK, Goa KL. Aripiprazole. CNS Drugs. 2002;16:779–86. doi: 10.2165/00023210-200216110-00008. discussion 787–8. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14(6):375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Nader MA, Mach RH. Self-administration of the dopamine D3 agonist 7-OH-DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology (Berl) 1996;125:13–22. doi: 10.1007/BF02247388. [DOI] [PubMed] [Google Scholar]
- Nakai S, Hirose T, Uwahodo Y, Imaoka T, Okazaki H, Miwa T, Nakai M, Yamada S, Dunn B, Burris KD, Molinoff PB, Tottori K, Altar CA, Kikuchi T. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472:89–97. doi: 10.1016/s0014-2999(03)01857-0. [DOI] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–31. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Lamas X, Mendelson JH. Acute and chronic effects of flupenthixol on the discriminative stimulus and reinforcing effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 1996;278:879–90. [PubMed] [Google Scholar]
- Pulvirenti L, Koob GF. Being partial to psychostimulant addiction therapy. TRENDS in Pharmacological Sciences. 2002;23(4):151–153. doi: 10.1016/s0165-6147(00)01991-x. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Wang Z, Woolverton WL. Reinforcing effects of D2 dopamine receptor agonists and partial agonists in rhesus monkeys. Drug Alcohol Depend. 2001;64:209–17. doi: 10.1016/s0376-8716(01)00124-7. [DOI] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hanson GL. Role of monoamine transporters in mediating psychostimulant effects. APPS Journal. 2005;7:E847–E851. doi: 10.1208/aapsj070481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–23. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Romach MK, Glue P, Kampman K, Kaplan HL, Somer GR, Poole S, Clarke L, Coffin V, Cornish J, O’Brien CP, Sellers EM. Attenuation of the euphoric effects of cocaine by the dopamine D1/D5 antagonist ecopipam (SCH 39166) Arch Gen Psychiatry. 1999;56:1101–6. doi: 10.1001/archpsyc.56.12.1101. [DOI] [PubMed] [Google Scholar]
- Semba J, Watanabe A, Kito S, Toru M. Behavioural and neurochemical effects of OPC-14597, a novel antipsychotic drug, on dopaminergic mechanisms in rat brain. Neuropharmacology. 1995;34:785–791. doi: 10.1016/0028-3908(95)00059-f. [DOI] [PubMed] [Google Scholar]
- Shimokawa Y, Akiyama H, Kashiyama E, Koga T, Miyamoto G. High performance liquid chromatographic methods for the determination of aripiprazole with ultraviolet detection in rat plasma and brain: application to the pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;821:8–14. doi: 10.1016/j.jchromb.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Mach RH, Nader MA. Dopamine D2/D3 receptors modulate cocaine’s reinforcing and discriminative stimulus effects in rhesus monkeys. Drug Alcohol Depend. 1999;54:97–110. doi: 10.1016/s0376-8716(98)00162-8. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Kosten TR. Novel approaches to the treatment of cocaine addiction. CNS Drugs. 2005;19:13–25. doi: 10.2165/00023210-200519010-00002. [DOI] [PubMed] [Google Scholar]
- Sorensen G, Sager TN, Petersen JH, Brennum LT, Thogersen P, Hee-Bengtsen C, Thomsen M, Wörtwein G, Fink-Jensen A, Woldbye DPD. Aripiprazole blocks acute self-administration of cocaine and is not self-administered in mice. (manuscript submitted) [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PEA, Rush CR. A low dose of aripiprazole attenuates the subject-rated effects of d-amphetamine. Drug Alcohol Depend. 2006;84:206–209. doi: 10.1016/j.drugalcdep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Lofwall MR, Rush CR. The Safety, tolerability, and subject-rated effects of acute intranasal cocaine administration during aripiprazole maintenance. American Journal of Drug and Alcohol Abuse. 2007;33(6):769–776. doi: 10.1080/00952990701651556. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Current Protocols in Neuroscience. Unit 920. Wiley and Sons; 2005. Chronic intravenous drug self-administration in rodents. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Intravenous Drug Self-administration in Mice: Practical Considerations. Behav Genet. 2007;37:101–118. doi: 10.1007/s10519-006-9097-0. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuoppasalmi K, Fohr J, Tuomola P, Kuikanmaki O, Vorma H, Sokero P, Haukka J, Meririnne E. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164(1):160–162. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- Vaccheri A, Dall’Olio R, Gandolfi O, Roncada P, Montanaro N. Enhanced stereotyped response to apomorphine after chronic D-1 blockade with SCH 23390. Psychopharmacology (Berl) 1987;91:394–6. doi: 10.1007/BF00518199. [DOI] [PubMed] [Google Scholar]
- Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology. 2007;32:2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Murray A, Bozarth MA. Bromocriptine self-administration and bromocriptine reinstatement of cocaine-trained and heroin-trained lever pressing in rats. Psychopharmacology. 1990;100:355–360. doi: 10.1007/BF02244606. [DOI] [PubMed] [Google Scholar]
- White FJ, Joshi A, Koeltzow TE, Hu XT. Dopamine receptor antagonists fail to prevent induction of cocaine sensitization. Neuropsychopharmacology. 1998;18:26–40. doi: 10.1016/S0893-133X(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Goldberg LI, Ginos J. Intravenous self-administration of dopamine receptor agonists by rhesus monkeys. J Pharmacol Exp Ther. 1984;230:678–683. [PubMed] [Google Scholar]