Abstract
Objectives
The cerebellar vermis is increasingly implicated in bipolar disorder (BD). In this study, we investigated vermis morphology in BD using a quantitative volumetric analysis.
Methods
Volumes for total vermis and vermis subregions, V1 (lobules I–V), V2 (lobules VI–VII), and V3 (lobules VIII–X), were calculated using high resolution structural magnetic resonance imaging obtained from 44 individuals with BD (25 females and 19 males) and 43 healthy comparison (HC) subjects (26 females and 17 males). Total vermis volumes were compared between the BD and HC groups. Potential effects of vermis subregions and clinical features were explored.
Results
Total vermis volumes were significantly larger in the BD group than in the HC group (P=0.02). There was a significant group by sex interaction (P=0.02). Total vermis volumes were significantly larger in males with BD than HC males (P=0.004); vermis volumes did not differ significantly between females with and without BD (P=0.95). Subregion analyses showed a trend-level interaction between diagnosis and subregion (P=0.07), in which subregion V1 volumes were significantly larger in BD participants (P=0.001), with differences primarily driven by males (P=0.001).
Conclusions
Our findings demonstrate increases in cerebellar vermis volumes in males with BD. These findings support the presence of structural alterations in the cerebellar vermis in BD and furthermore the influence of sex on such changes.
Keywords: Bipolar disorder, structural MRI, cerebellum, cerebellar vermis, sex difference
INTRODUCTION
A role for the cerebellar vermis in bipolar disorder (BD) was first suggested by observations of affective changes in humans with vermis lesions, specifically manic-like symptoms (1–4). Its role in BD is further supported by preclinical literature as animal studies have shown modulation of emotional behavior through electrical stimulation and lesions in the vermis (5–7). Moreover, the vermis is interconnected with brain regions strongly implicated in BD, including the hypothalamus, amygdala, hippocampus, anterior cingulate and ventral prefrontal cortices (8–12).
Prior magnetic resonance imaging (MRI) studies have demonstrated variable findings of vermis structural abnormalities in BD (13–16). These studies have examined total vermis as well as vermis subregions, V1 (lobules I–V), V2 (lobules VI–VII), and V3 (lobules VIII–X) (14). Brambilla et al. suggested that abnormalities in total vermis volumes may be associated with familial cases of BD (13). An initial study by DelBello et al. reported smaller V3 in BD and was followed by a study of a larger sample by Mills et al. reporting smaller V2 and V3 in BD (14,15). Both these studies found that vermis abnormalities may be related to the number of prior affective episodes. Monkul et al. reported a similar trend in the effect of episode number on vermis subregion (V2) in adolescents and young adults with BD (16). In addition, they suggested that this trend may be more prominent in young males with BD. With the exception of Mills et al (15) who utilized volumetric measurements, the above studies investigated vermis subregions by measuring mid-sagittal cross-sectional areas.
In this study, our primary aim was to compare total vermis size between individuals with BD and healthy individuals using a quantitative volumetric approach. The potential effects of vermis subregions and clinical features on vermis abnormalities were explored.
METHODS
Participants
Participants with BD were recruited from the Yale School of Medicine Medical Center (New Haven, CT), the Veterans Affair Connecticut Healthcare System (West Haven, CT) and the greater New Haven community through clinical referrals and advertisements. Healthy comparison participants were recruited from the surrounding community through word-of-mouth and advertisements. Individuals were excluded if they 1) had a significant medical condition (with the exception of 4 BD females with treated hypothyroidism), 2) had a significant neurological condition, or 3) experienced loss of consciousness for 5 minutes or more.
The presence or absence of Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV) (17) diagnoses were determined by the Structured Clinical Interview for DSM-IV Disorders Version 2.0 (SCID) (18) for 44 individuals with BD (25 females and 19 males, ages 18 to 55 years) and 43 healthy comparison (HC) individuals (26 females and 17 males, ages 18 to 53 years). HC participants had neither personal history of DSM-IV Axis I disorders nor a history of a mood, psychotic, anxiety, or substance use disorder in their first-degree relatives. Written informed consent was obtained from all participants in accordance with the human investigation committees of the Yale School of Medicine and the Department of Veterans Affairs following a complete description of the research protocol.
Of the BD participants, 28 (64%) met criteria for rapid cycling, 21 (48%) had at least one first-degree relative with a primary mood disorder, and 13 (30%) had a history of psychotic features during mood episodes. At the time of scanning, 10 (23%) met DSM-IV criteria for a depressive episode, 13 (30%) met DSM-IV criteria for a manic, hypomanic or mixed episode, and 21 (48%) were euthymic at time of scanning. Co-morbidities included post-traumatic stress disorder (4, 9%), panic disorder (3, 7%), and specific phobia (1, 2%). Ten BD participants (23%) were unmedicated. The remaining participants were prescribed psychotropic medications that included lithium (14, 32%), anticonvulsants (22, 50%), atypical antipsychotics (17, 39%), antidepressants (13, 30%), benzodiazepines (7, 16%), and levothyroxine (4, 9%).
MRI Acquisition and Processing
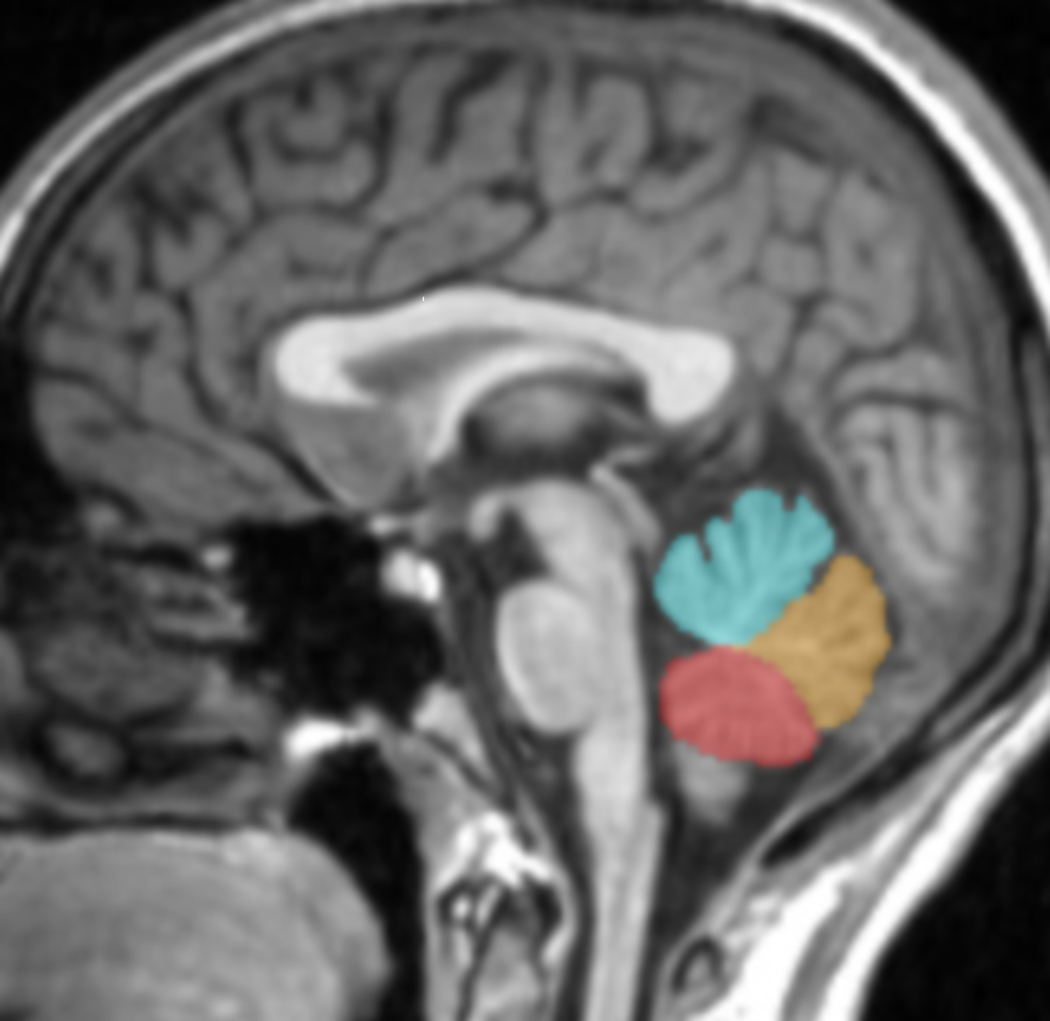
MRI scans were obtained on a 3T Trio MR Scanner (Siemens, Erlangen, Germany) using a three-dimensional Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) T1-weighted sequence (TR=1500ms, TE=2.83ms, FOV=256 × 256 mm2, matrix=256 × 256, slice thickness=1.0mm without gap, 160 slices, NEX=2). Images were adjusted for head tilt and rotation that occurred during scanning using both cerebral and cerebellar landmarks (19). The vermis was delineated in the sagittal plane on each slice that contained it using BioImage Suite software (www.bioimagesuite.org) by operators blinded to participant characteristics. The presence of the vermis was defined by 1) the presence of the prepyramidal fissure, 2) the retention of vermis shape, and 3) the presence of thin, branching white matter (characteristic of the vermis) (19,20). The vermis was divided into 3 subregions, V1 (lobules I–V), V2 (lobules VI–VII), and V3 (lobules VIII–X). The boundaries of V1 were determined superiorly by the superior medullary velum and inferiorly by the primary fissure. V2 was demarcated superiorly by the primary fissure and inferiorly by the prepyramidal fissure, and V3 was defined superiorly by the prepyramidal fissure and inferiorly by the vallecula with careful delineation from the cerebellar tonsils (14,15,19) (Figure 1). The cerebellar tonsils were identified by 1) ovoid shape, 2) an axis oriented parallel to the posterior surface of the medulla, and 3) gray and white matter appearing in a regular hatch-mark pattern at a 45–90 degree angle to the posterior surface of the medulla (19). Automated skull stripping and gray matter, white matter, and cerebrospinal fluid (CSF) segmentation were performed using the Statistical Parametric Mapping 99 (SPM99) (www.fil.ion.ucl.ac.uk) tissue classification algorithm (21). Total vermis, V1, V2, V3, and total brain volumes were calculated using all gray and white matter voxels within the volumes. Inter-rater reliabilities for total vermis, V1, V2, and V3 volumes, presented as intra-class coefficients, were 0.92, 0.99, 0.99 and 0.99 respectively.
Figure 1.
Delineation of the Vermis Subregions
The T1-weighted image displays the delineation of vermis subregions (V1 in blue, V2 in yellow and V3 in red) in the sagittal plane.
Statistical Analysis
All statistical analyses were performed using the Statistical Analysis Software (SAS) version 9.1 (SAS Institute, Cary, NC). Vermis volumes were tested for normality using Kolmogorov-Smirnov test statistics. All p-values presented are two-tailed.
Total vermis volumes were analyzed using Analysis of Covariance (ANCOVA) with diagnostic group (BD, HC) and sex as between-subject factors and with age and total brain volume (TBV) as covariates. All multi-way interactions were tested. Least squares (LS) means were calculated from the model and plotted to interpret significant effects.
Exploratory analyses were performed to examine the effects of vermis subregion volumes using a linear mixed model with diagnostic group and sex as between-subject factors, vermis subregions as within-subject factors, and age and TBV as covariates. All multi-way interactions were tested. Exploratory analyses were also performed to determine the effects of each clinical variable within the BD group on total vermis volumes. These analyses were carried out separately for each clinical variable with the clinical variable and sex as between-subject factors and with age and TBV as covariates. The clinical effects analyzed included presence of rapid cycling, presence of a first-degree relative with a primary mood disorder, mood state at the time of scan, presence of lifetime psychosis, and medication status at scanning.
RESULTS
The HC and BD groups did not differ statistically in sex distribution (Χ2(1)=0.12, P = 0.73), but did differ in age (t85=2.9, P=0.005), such that participants with BD (mean age 33.9 ± SD 10.8 years) were older than HC participants (mean age 27.7 ± SD 9.1 years). Mean ages for female and male participants were 31.4 ± SD 10.5 years and mean age 30.1 ± SD 10.4 years respectively. Female and male BD participants did not differ significantly in age, presence of rapid cycling, family history of a primary mood disorder, mood state, presence of lifetime psychosis, or medication status at scanning (P’s >0.15).
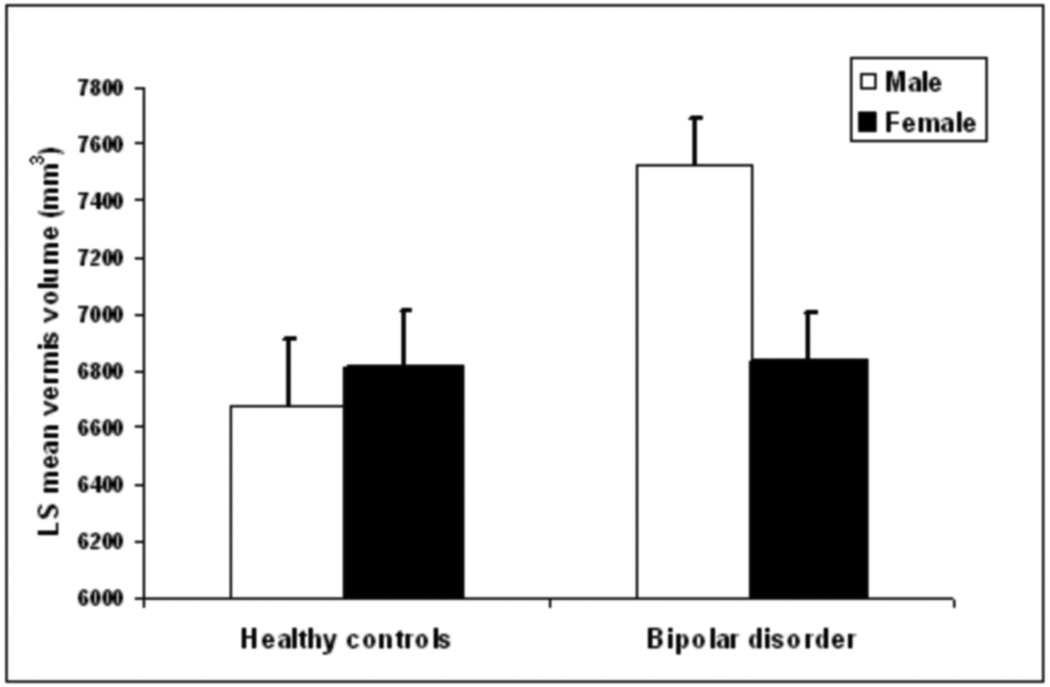
Vermis volumes were approximately normal in distribution. There was a significant main effect of diagnostic group [F(1,81)=5.49, P=0.02] where BD participants had significantly larger total vermis volumes compared to HC participants. The diagnosis by sex interaction was also significant [F(1,81)=5.49, P=0.02]. BD males had significantly larger total vermis volumes than HC males [F(1,81)=8.81, P=0.004], while vermis volumes did not differ significantly between the female groups (P=0.94) (Figure 2). Exploratory analyses did not demonstrate any significant main effects of clinical variables or of their interactions with sex in influencing total vermis volume (P’s>0.15).
Figure 2.
Vermis Volumes in Females and Males with and without Bipolar Disorder Least square means and standard errors of total vermis volumes for the BD and HC groups. Means are adjusted for age and TBV. The HC group consisted of 26 females and 17 males, and the BD group consisted of 25 females and 19 males.
Exploratory analysis of the vermis subregions revealed significant main effects of diagnosis [F(1,81)=5.46, P=0.02] and subregion [F(2,166)=422.52, P<0.0001]. There was a trend toward a diagnostic group by subregion interaction [F(2,166)=2.64, P=0.07]. This trend appeared to be driven by larger V1 volumes in the BD group when compared to the HC group [F(1,166)=10.58, P=0.001], particularly among males [F(1,166)=10.79, P=0.001], while groups did not differ significantly in volumes in V2 or V3 (P’s > 0.20).
DISCUSSION
In this study, total vermis volumes were significantly larger in the BD group when compared to the HC group. Moreover, it appeared that these increases were specific to males; total vermis volumes were significantly larger in males with BD than in HC males, whereas vermis volumes did not differ significantly between females with and without BD. In addition, vermis subregion analyses suggested that the volume increases are most prominent in V1 with males primarily driving the differences in this subregion. Although the significant age difference between the BD and HC group poses a potential limitation, we are not aware of any evidence suggesting increased cerebellar volumes with age. Rather, evidence from post-mortem and imaging studies suggest that cerebellar volumes, including vermis volumes, decrease with age (22–25). Thus, the older age of BD participants likely limited our abilities to detect further differences between the BD and HC groups.
Increases in the vermis volume have been observed in other neuropsychiatric disorders, specifically autism and schizophrenia (20,26,27). This is of interest as there is evidence of shared genetic vulnerabilities among BD, autism and schizophrenia (28–31). These findings suggest commonalities in the pathophysiology of these disorders. For example, as evidence of white matter abnormalities in BD and schizophrenia are emerging, such commonalities could involve alterations in vermis white matter connectivity (27,32,33). However, studies of vermis morphology in autism and schizophrenia have had disparate findings (34–40). Thus, such implications of commonalities in the vermis are speculative until further investigation.
Previous neuroimaging studies have suggested decreases in the vermis in BD when compared to healthy individuals (14,15,41). However other studies in BD have reported findings of no differences in the vermis (13,16). In this study, vermis increases were found. The disparate findings among studies could reflect methodological differences, such as in measuring vermis area versus volume (13,14,42), determining the lateral boundaries of the vermis (43), or correcting for head tilt during scanning (19). They could also reflect differences in the demographic and clinical features of the study samples. The increases reported herein appeared specific to males suggesting that the distribution of males and females in the study samples, and sufficient power to detect sexually dimorphic features, might influence findings. Other factors that might also influence findings include age, as well as aspects of clinical course such as episode history and medication exposure.
Prior MRI studies have not reported direct effects of sex on vermis volumes in BD, as was found in this study (13–16). However sexually dimorphic influences of genetic factors and early exposures on cerebellar development have been shown in rodent studies (44–50). These include more severe effects of mutations studied as models for neuropsychiatric disorders (e.g. mice heterozygous for the reeler mutation and mutation of the RORα gene of the staggerer mouse) and of prenatal and early environmental exposures to substances of abuse (e.g. cocaine) and neurotoxic agents (e.g. polychlorinated biphenyls) on the cerebellum of males (45–47,49). The findings reported herein raise interesting questions regarding how sex-related factors, such as hormonal levels, may interact with the development of the vermis in BD and whether vermis abnormalities may contribute to clinical features of BD that tend to be more characteristic of males with the disorder. These features include a greater tendency in males with BD to experience manic than depressive episodes, as compared to females with BD (51). A role for the vermis in mania is suggested by the reports of manic-like behaviors subsequent to vermis lesions (2,4). In our sample, the large proportion of BD participants with rapid-cycling limited our ability to quantify manic episodes as these participants often had difficulty approximating the number of manic or depressive episodes in retrospective recall. The association between vermis abnormalities and number of manic episodes would be of interest to pursue in future studies. It is also possible that males are more likely to have other clinical characteristics that might produce the vermis differences. For example, males may differ in the likelihood of experiencing psychotic symptoms or being exposed to certain medications. In this study, males with BD did not differ from females with BD in any of the clinical features examined, nor were there any significant interactions between vermis volumes, sex and clinical features; however, our power to detect these associations was limited.
Previous studies of the vermis in BD have reported abnormalities in V2 and V3 in association with episode number rather than alterations in V1 (14,15). The reason for the differences between our study and previous studies is unclear. It may relate to methodological differences such as in delineation of the vermis subregions. Alternatively, it could relate to differences in the features of the participant samples. Our diagnosis by subregion interaction was at trend-level, so conclusions regarding the effects specific to subregion are tentative. However, the findings in V1 in BD are of interest as V1 has known connections with regions in which abnormalities have been previously demonstrated in BD, including the hypothalamus, hippocampus and orbitofrontal cortex (8). Further investigations are needed to determine how the vermis subregions are affected in BD.
In summary, the findings of this study support the presence of structural alterations in the cerebellar vermis in BD, and furthermore they demonstrate the influence of sex on these changes. The findings also underscore the need for future investigations to elucidate the role of the vermis in BD pathophysiology and the potential influences of sex on this role.
Acknowledgments
We wish to thank Ms. Kathleen Colonese who was devoted to helping those suffering from psychiatric illnesses and to advancing the field of bipolar disorder research. The authors thank Cheryl Lacadie, B.S., Karen Martin, R.T.R.M.R., Terry Hickey, R.T.R.M.R. and Hedy Sarofin, R.T.R.M.R. for their technical expertise, Allison McDonough, B.S. and Lindsay Warren for assistance with the study, and the research subjects for their participation.
Funding Acknowledgments: This study was supported by grants from the National Institute of Mental Health R01MH69747 (HPB), R01MH070902 (HPB), R25MH071240 (FYW), T32MH14276 (JHK, LGC), the Department of Veterans Affairs Research Enhancement Award Program (REAP) (HPB, LGC) programs, the National Alliance for Research in Schizophrenia and Depression (Great Neck, NY) (HPB, JHK, FW), The Attias Family Foundation (HPB), Marcia Simon Kaplan (JHK), The Ethel F. Donaghue Women’s Investigator Program at Yale (New Haven, CT) (HPB), and the Klingenstein Foundation (JHK).
Footnotes
These data were presented in part at the Sixty-third Annual Scientific Meeting and Convention of the Society of Biological Psychiatry, May 1–3, 2008, Washington, D.C.
Financial Disclosures: FYW, FW, LGC, JHK, LS, EE, BPP, RTC, and XP report no conflicts of interest. HPB has received consultant fees from Pfizer, Inc and has received honoraria from Abbott Laboratories and Eli Lilly.
REFERENCES
- 1.Lauterbach EC. Bipolar disorders, dystonia, and compulsion after dysfunction of the cerebellum, dentatorubrothalamic tract, and substantia nigra. Biol Psychiatry. 1996;40:726–730. doi: 10.1016/0006-3223(96)82516-9. [DOI] [PubMed] [Google Scholar]
- 2.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 3.Tavano A, Grasso R, Gagliardi C, Triulzi F, Bresolin N, Fabbro F, et al. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007;130:2646–2660. doi: 10.1093/brain/awm201. [DOI] [PubMed] [Google Scholar]
- 4.Yadalam KG, Jain AK, Simpson GM. Mania in two sisters with similar cerebellar disturbance. Am J Psychiatry. 1985;142:1067–1069. doi: 10.1176/ajp.142.9.1067. [DOI] [PubMed] [Google Scholar]
- 5.Schmahmann JD. The role of the cerebellum in affect and psychosis. J Neurolinguistics. 2000;13:189–214. [Google Scholar]
- 6.Supple WF, Jr, Cranney J, Leaton RN. Effects of lesions of the cerebellar vermis on VMH lesion-induced hyperdefensiveness, spontaneous mouse killing, and freezing in rats. Physiol Behav. 1988;42:145–153. doi: 10.1016/0031-9384(88)90290-9. [DOI] [PubMed] [Google Scholar]
- 7.Supple WF, Jr, Leaton RN, Fanselow MS. Effects of cerebellar vermal lesions on species-specific fear responses, neophobia, and taste-aversion learning in rats. Physiol Behav. 1987;39:579–586. doi: 10.1016/0031-9384(87)90156-9. [DOI] [PubMed] [Google Scholar]
- 8.Anand BK, Malhotra CL, Singh B, Dua S. Cerebellar projections to limbic system. J Neurophysiol. 1959;22:451–457. doi: 10.1152/jn.1959.22.4.451. [DOI] [PubMed] [Google Scholar]
- 9.Blumberg HP, Charney DS, Krystal JH. Frontotemporal neural systems in bipolar disorder. Semin Clin Neuropsychiatry. 2002;7:243–254. doi: 10.1053/scnp.2002.35220. [DOI] [PubMed] [Google Scholar]
- 10.Heath RG, Dempesy CW, Fontana CJ, Myers WA. Cerebellar stimulation: effects on septal region, hippocampus, and amygdala of cats and rats. Biol Psychiatry. 1978;13:501–529. [PubMed] [Google Scholar]
- 11.Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- 12.Snider RS, Maiti A. Cerebellar contributions to the Papez circuit. J Neurosci Res. 1976;2:133–146. doi: 10.1002/jnr.490020204. [DOI] [PubMed] [Google Scholar]
- 13.Brambilla P, Harenski K, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, et al. MRI study of posterior fossa structures and brain ventricles in bipolar patients. J Psychiatr Res. 2001;35:313–322. doi: 10.1016/s0022-3956(01)00036-x. [DOI] [PubMed] [Google Scholar]
- 14.DelBello MP, Strakowski SM, Zimmerman ME, Hawkins JM, Sax KW. MRI analysis of the cerebellum in bipolar disorder: a pilot study. Neuropsychopharmacology. 1999;21:63–68. doi: 10.1016/S0893-133X(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 15.Mills NP, Delbello MP, Adler CM, Strakowski SM. MRI analysis of cerebellar vermal abnormalities in bipolar disorder. Am J Psychiatry. 2005;162:1530–1532. doi: 10.1176/appi.ajp.162.8.1530. [DOI] [PubMed] [Google Scholar]
- 16.Monkul ES, Hatch JP, Sassi RB, Axelson D, Brambilla P, Nicoletti MA, et al. MRI study of the cerebellum in young bipolar patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:613–619. doi: 10.1016/j.pnpbp.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Text Revision. 4th edition. Washington, D.C.: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 18.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 19.Courchesne E, Press GA, Murakami J, Berthoty D, Grafe M, Wiley CA, et al. The cerebellum in sagittal plane--anatomic-MR correlation: 1. The vermis. AJR Am J Roentgenol. 1989;153:829–835. doi: 10.2214/ajr.153.4.829. [DOI] [PubMed] [Google Scholar]
- 20.Levitt JJ, McCarley RW, Nestor PG, Petrescu C, Donnino R, Hirayasu Y, et al. Quantitative volumetric MRI study of the cerebellum and vermis in schizophrenia: clinical and cognitive correlates. Am J Psychiatry. 1999;156:1105–1107. doi: 10.1176/ajp.156.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 22.Ellis RS. Norms for some structural changes in the human cerebellum from birth to old age. J Comp Neurol. 1920;32:1–33. [Google Scholar]
- 23.Raz N, Dupuis JH, Briggs SD, McGavran C, Acker JD. Differential effects of age and sex on the cerebellar hemispheres and the vermis: a prospective MR study. AJNR Am J Neuroradiol. 1998;19:65–71. [PMC free article] [PubMed] [Google Scholar]
- 24.Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD. Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. AJNR Am J Neuroradiol. 2001;22:1161–1167. [PMC free article] [PubMed] [Google Scholar]
- 25.Torvik A, Torp S, Lindboe CF. Atrophy of the cerebellar vermis in ageing: a morphometric and histologic study. J Neurol Sci. 1986;76:283–294. doi: 10.1016/0022-510x(86)90176-0. [DOI] [PubMed] [Google Scholar]
- 26.Courchesne E, Saitoh O, Yeung-Courchesne R, Press GA, Lincoln AJ, Haas RH, et al. Abnormality of cerebellar vermian lobules VI and VII in patients with infantile autism: identification of hypoplastic and hyperplastic subgroups with MR imaging. AJR Am J Roentgenol. 1994;162:123–130. doi: 10.2214/ajr.162.1.8273650. [DOI] [PubMed] [Google Scholar]
- 27.Lee KH, Farrow TF, Parks RW, Newton LD, Mir NU, Egleston PN, et al. Increased cerebellar vermis white-matter volume in men with schizophrenia. J Psychiatr Res. 2007;41:645–651. doi: 10.1016/j.jpsychires.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Berrettini W. Bipolar disorder and schizophrenia: not so distant relatives? World Psychiatry. 2003;2:68–72. [PMC free article] [PubMed] [Google Scholar]
- 29.Hennah W, Thomson P, McQuillin A, Bass N, Loukola A, Anjorin A, et al. DISC1 association, heterogeneity and interplay in schizophrenia and bipolar disorder. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.22. [DOI] [PubMed] [Google Scholar]
- 30.Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rzhetsky A, Wajngurt D, Park N, Zheng T. Probing genetic overlap among complex human phenotypes. Proc Natl Acad Sci U S A. 2007;104:11694–11699. doi: 10.1073/pnas.0704820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter CJ. eIF2B and oligodendrocyte survival: where nature and nurture meet in bipolar disorder and schizophrenia? Schizophr Bull. 2007;33:1343–1353. doi: 10.1093/schbul/sbm007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sussmann JE, Lymer GK, McKirdy J, Moorhead TW, Maniega SM, Job D, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord. 2009;11:11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 34.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Hardan AY, Minshew NJ, Harenski K, Keshavan MS. Posterior fossa magnetic resonance imaging in autism. J Am Acad Child Adolesc Psychiatry. 2001;40:666–672. doi: 10.1097/00004583-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Ichimiya T, Okubo Y, Suhara T, Sudo Y. Reduced volume of the cerebellar vermis in neuroleptic-naive schizophrenia. Biol Psychiatry. 2001;49:20–27. doi: 10.1016/s0006-3223(00)01081-7. [DOI] [PubMed] [Google Scholar]
- 37.Joyal CC, Pennanen C, Tiihonen E, Laakso MP, Tiihonen J, Aronen HJ. MRI volumetry of the vermis and the cerebellar hemispheres in men with schizophrenia. Psychiatry Res. 2004;131:115–124. doi: 10.1016/j.pscychresns.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Nopoulos PC, Ceilley JW, Gailis EA, Andreasen NC. An MRI study of cerebellar vermis morphology in patients with schizophrenia: evidence in support of the cognitive dysmetria concept. Biol Psychiatry. 1999;46:703–711. doi: 10.1016/s0006-3223(99)00093-1. [DOI] [PubMed] [Google Scholar]
- 39.Okugawa G, Sedvall GC, Agartz I. Smaller cerebellar vermis but not hemisphere volumes in patients with chronic schizophrenia. Am J Psychiatry. 2003;160:1614–1617. doi: 10.1176/appi.ajp.160.9.1614. [DOI] [PubMed] [Google Scholar]
- 40.Stanfield AC, McIntosh AM, Spencer MD, Philip R, Gaur S, Lawrie SM. Towards a neuroanatomy of autism: a systematic review and meta-analysis of structural magnetic resonance imaging studies. Eur Psychiatry. 2008;23:289–299. doi: 10.1016/j.eurpsy.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Lippmann S, Manshadi M, Baldwin H, Drasin G, Rice J, Alrajeh S. Cerebellar vermis dimensions on computerized tomographic scans of schizophrenic and bipolar patients. Am J Psychiatry. 1982;139:667–668. doi: 10.1176/ajp.139.5.667. [DOI] [PubMed] [Google Scholar]
- 42.Aylward EH, Reiss A. Area and volume measurement of posterior fossa structures in MRI. J Psychiatr Res. 1991;25:159–168. doi: 10.1016/0022-3956(91)90020-b. [DOI] [PubMed] [Google Scholar]
- 43.Schmahmann JDDJ, Toga AW, Petrides M, Evans AC. MRI atlas of the human cerebellum. San Diego, CA: Academic Press; 2000. [Google Scholar]
- 44.Dean SL, McCarthy MM. Steroids, sex and the cerebellar cortex: implications for human disease. Cerebellum. 2007:1–10. doi: 10.1007/s12311-008-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doulazmi M, Frederic F, Lemaigre-Dubreuil Y, Hadj-Sahraoui N, Delhaye-Bouchaud N, Mariani J. Cerebellar Purkinje cell loss during life span of the heterozygous staggerer mouse (Rora(+)/Rora(sg)) is gender-related. J Comp Neurol. 1999;411:267–273. [PubMed] [Google Scholar]
- 46.Hadj-Sahraoui N, Frederic F, Delhaye-Bouchaud N, Mariani J. Gender effect on Purkinje cell loss in the cerebellum of the heterozygous reeler mouse. J Neurogenet. 1996;11:45–58. doi: 10.3109/01677069609107062. [DOI] [PubMed] [Google Scholar]
- 47.Nguon K, Ladd B, Baxter MG, Sajdel-Sulkowska EM. Sexual dimorphism in cerebellar structure, function, and response to environmental perturbations. Prog Brain Res. 2005;148:341–351. doi: 10.1016/S0079-6123(04)48027-3. [DOI] [PubMed] [Google Scholar]
- 48.Ramirez O, Jimenez E. Sexual dimorphism in rat cerebrum and cerebellum: different patterns of catalytically active creatine kinase isoenzymes during postnatal development and aging. Int J Dev Neurosci. 2002;20:627–639. doi: 10.1016/s0736-5748(02)00102-8. [DOI] [PubMed] [Google Scholar]
- 49.Vathy I, Katay L, Mini KN. Sexually dimorphic effects of prenatal cocaine on adult sexual behavior and brain catecholamines in rats. Brain Res Dev Brain Res. 1993;73:115–122. doi: 10.1016/0165-3806(93)90053-d. [DOI] [PubMed] [Google Scholar]
- 50.Vathy I, Rimanoczy A, Eaton RC, Katay L. Sex dimorphic alterations in postnatal brain catecholamines after gestational morphine. Brain Res Bull. 1995;36:185–193. doi: 10.1016/0361-9230(94)00192-4. [DOI] [PubMed] [Google Scholar]
- 51.Arnold LM. Gender differences in bipolar disorder. Psychiatr Clin North Am. 2003;26:595–620. doi: 10.1016/s0193-953x(03)00036-4. [DOI] [PubMed] [Google Scholar]