Abstract
Background
Late complications of the Fontan operation represent a significant management challenge. In general, failing Fontan patients have two modes of presentation: those with impaired ventricular function (IVF group) and those with preserved ventricular function but with failing Fontan physiology (protein-losing enteropathy-PLE, and plastic bronchitis-PB) (PVF group). In this study we evaluated whether failing Fontan patients referred for heart transplantation had a different outcome based on the mode of presentation.
Methods
We conducted a retrospective chart review of all Fontan patients evaluated for heart transplantation at a single institution from 1994–2008. Demographic, hemodynamic, and laboratory data were collected. Patients were stratified into IVF group or PVF group based on echocardiographic criteria. Descriptive statistics and Kaplan-Meier analysis were used for hypothesis testing.
Results
Thirty four Fontan patients were evaluated for heart transplantation. Based on echo description of systolic function, 18 were categorized as IVF and 16 patients as PVF. The IVF group had significantly lower cardiac index, SvO2, and significantly higher SVR compared to the PVF group (p<0.05). PLE or PB was present in 13/16 in the PVF group and 0/18 in the IVF group. Of the 34 patients, 20 received transplantation with similar rates amongst the IVF and PVF groups. Within one year from evaluation there were 2/18 deaths in the IVF group and 7/16 deaths in the PVF group (p=0.052)
Conclusions
Failing Fontans with PVF have decreased overall survival independent of whether they were transplanted. This trend indicates a need to improve the management and/or timing for transplantation amongst this population.
Keywords: Heart Transplantation, Congenital heart disease, CHD Fontan
Introduction
In 1968, Fontan and Baudet introduced total right heart bypass via an atriopulmonary connection in a patient with tricuspid atresia—the first Fontan (1,2). Since that time palliation of functional single ventricles has undergone many revisions in order to improve survival and decrease long term morbidity (3,4). Although survival has improved markedly since the first palliative operations these patients are still at increased risk of late morbidity and mortality (5). Late complications of Fontan palliation include progressive ventricular dysfunction, atrioventricular valve regurgitation, progressive cyanosis, thromboembolism, protein-losing enteropathy (PLE), plastic bronchitis, atrial tachyarrythmias, and Fontan pathway obstruction (6–9). If medical, surgical and catheter based interventions are unable to improve symptoms, cardiac transplantation may provide the only therapeutic treatment for many of these patients (5,9–11).
The factors contributing to “heart failure” or failing Fontan physiology in patients with Fontan surgery remain poorly defined. Identifying which factors may contribute to mortality or increased risk of failure requiring medical or surgical intervention is difficult given that Fontan patients are a heterogeneous group that differ in underlying anatomic malformation, type of Fontan operation, age at Fontan operation, and eras in which operations were performed. Understanding of these factors will aid in the management decisions for this group of complex patients.
Amongst the failing Fontan patients, systolic ventricular dysfunction is a major indication for heart transplantation (12). However another group of patients exist who have relatively preserved systolic ventricular function but suffer from complications of failed Fontan physiology such refractory ascites, pleural effusions, PLE, and plastic bronchitis (8,12,13). Because these conditions persist in the setting of relatively normal cardiac output the advent of a new heart via transplantation may not have an immediate impact on the course of the disease. Furthermore, the long term consequences of malnutrition, immunosuppression, and pulmonary complications associated with both PLE and plastic bronchitis may increase the risks of heart transplantation in these patients. Because of these findings we hypothesized that patients with failed Fontan physiology and preserved ventricular function are at increased risk for death both before and after cardiac transplantation.
Patients and Methods
The study was conducted in accordance with institutional Human Subjects Committee guidelines and was approved by the Institutional Review Board. The need for individual consent was waived. The authors had full access to the data and take full responsibility for their integrity. All authors have read and agree to the manuscript as written.
Patients were identified by a database searching the Children’s Hospital cardiovascular program database for patients who had undergone Fontan surgery at any institution but were evaluated for heart transplantation at Children’s Hospital Boston between January 1,1994 and January 1, 2008. The criterion for referring the patient for heart transplant evaluation was physician dependant. All patients had been discharged from the hospital and were more than 60 days from the time of last Fontan operation and had at least 6 months of follow-up from time of evaluation. Patients who had a previous Fontan takedown to a superior caval-pulmonary anastomosis (bidirectional Glenn) were excluded.
A detailed retrospective review was conducted of all medical records. Demographic data including inpatient, clinic and operative notes were collected. Laboratory, functional and hemodynamic data including cardiac catheterization and echocardiographic data were analyzed. Patient survival and transplant status were also recorded. As many patients had subsequent functional and hemodynamic testing after transplantation evaluation, only data available at the time of transplant evaluation was collected for analysis. The type of Fontan was classified as right atrium to pulmonary artery anastomosis, a lateral tunnel, or extracardiac conduit.
Atrioventricular valve anatomy and systemic ventricular morphology were categorized according to previously defined nomenclature on the basis of preoperative studies and surgical findings. Cardiac function was evaluated by two-dimensional echocardiography and cardiac catheterization. Ventricular dysfunction was defined qualitatively as none, mild, moderate, or severe by a clinician not involved in the care of the patient. Atrioventricular valve regurgitation was graded by echocardiography as mild, moderate, or severe based on the width of the Doppler color flow jet and the extent of the regurgitation into the atrium.
The patient cohort was divided into 2 groups based on echocardiographic findings of ventricular systolic function. Patients who were diagnosed with moderate to severe ventricular dysfunction on 2-dimensional echocardiography were assigned to the impaired ventricular function group (IVF). Patients with mild or no ventricular dysfunction by 2-dimensional echocardiography were assigned to the preserved ventricular function group (PVF). The diagnosis of protein-losing enteropathy (PLE) required the following criteria: hypoalbuminemia (≤3.0 mg/dL) for ≥ 3 months in the absence of liver or renal disease and accompanying ascites, pleural effusions, edema, diarrhea, or abdominal pain for ≥ 3 months (7,14). Plastic bronchitis (PB) was diagnosed by recurrent formation of branching bronchial casts of the tracheobronchial tree (8,15).
Statistical Analysis
Un-paired Student’s t-test was used to compare the following continuous variables: age at evaluation, time since Fontan operation, pre-transplantation hemodynamics including cardiac index, systemic oxygen saturation, Fontan pathway pressures, pulmonary vascular resistance, and systemic vascular resistance, and pre-transplantation laboratory values. Continuous variables were expressed as mean ± standard deviation unless otherwise stated. Categorical variables including gender, anatomical classification, LV versus RV ventricle dominance, ventricular dysfunction, AV valve regurgitation, protein-losing enteropathy, plastic bronchitis, arrhythmias, and use of pacemaker, were summarized as proportion ± standard deviation and compared using Pearson’s chi-square test and where appropriate Fisher’s exact test. The Bonferroni correction was used to account for multiple comparisons. In order to estimate proportional survival after evaluation for transplantation, a Kaplan-Meier analysis was performed and patients with preserved ventricular function were compared to individuals with impaired ventricular function. Furthermore, survival was compared between transplanted and non-transplanted subjects to estimate the overall outcome in both groups including deaths occurring after transplantation. Statistical significance was inferred at a 2-sided value of P < .05. The statistical analyses were computed by use of STATA (Stata Corporation, College Station, TX, Version 10.1 for PC).
Results
Group Characteristics
A total of 34 patients met criteria for inclusion into the study. Patients were divided into 2 groups based on ventricular function by echocardiography: those with preserved ventricular function complicated by failed Fontan physiology (PVF n=16), and those with impaired ventricular function (IVF n=18). A summary of the baseline characteristics of the two groups is shown in table 1. There were no differences in mean age at heart transplantation evaluation (IVF: 12.8 ± 5.9 vs PVF: 10.9 ± 6.0 yrs) or time since Fontan operation (IVF: 8.8 ± 5.2 vs PVF: 6.1 ± 6.2 yrs). The types of Fontan surgery were similar in both groups as well as the percentage of those with systemic RV (IVF: 66.7% vs PVF: 75.0%). There was no difference between the two groups in arrhythmia complications requiring pacemaker implantation. The preserved ventricular function group was complicated by PLE only in 8 patients, plastic bronchitis only in 5 patients, one patient with both PLE and plastic bronchitis, and 3 patients with preserved ventricular function complicated by ascites, pulmonary edema, and/or pleural effusions but did not meet our criteria for the diagnosis of PLE or plastic bronchitis. There were no cases of PLE or plastic bronchitis in the group of failing Fontans with impaired ventricular function.
Table 1.
Comparison of Impaired Ventricular Function versus Preserved Ventricular Function with heart failure
| Characteristic | Impaired Ventricular Function (n=18) | Preserved Ventricular Function (n=16) | p* |
|---|---|---|---|
| Age at Evaluation, yrs mean ± SD (range) | 12.8 ± 5.9 (2.3–19.9) | 10.9 ± 6.0 (4.6–26.1) | * |
| Time since Fontan, yrs mean ± SD (range) | 8.8 ± 5.2 (0.2–16.9) | 6.1 ± 6.2 (0.2–24.8) | * |
| Male sex, n (%) | 12 (66.7%) | 9 (56.3%) | * |
| Congenital heart defect n, (%) | * | ||
| TA | 4 (22.2%) | 2 (12.5%) | |
| DORV | 5 (27.8%) | 7 (43.75%) | |
| HLHS | 6 (33.4%) | 5 (31.3%) | |
| DILV | - | 2 (12.5%) | |
| Other | 3 (16.7%) | - | |
| Fontan Surgery, n (%) | * | ||
| Lateral Tunnel | 15 (83.3%) | 11 (68.8%) | |
| Atriopulmonary | 2 (11.1%) | 3 (18.8%) | |
| Extracardiac conduit | 1 (5.6%) | 2 (12.5%) | |
| Systemic Ventricle | |||
| Right, n (%) | 12 (66.7%) | 12 (75.0%) | * |
| AV valve regurgitation, n (%) | |||
| Moderate to Severe | 10 (55.6%) | 1 (6.3%) | .006 |
| Pacemaker, n (%) | 5 (27.8%) | 6 (37.5%) | * |
| Plastic Bronchitis, n (%) | 0 | 5 (31.3%) | .016 |
| PLE, n (%) | 0 | 9 (56.3%) | <.001 |
AV valve, atrioventricular valve; DILV, double inlet left ventricle; DORV, double outlet right ventricle; HLHS, hypoplastic left heart syndrome; PLE, protein-losing enteropathy; TA, tricuspid atresia;
Table 2 shows the comparison of hemodynamic and laboratory data. The IVF group had significantly lower cardiac index (IVF: 2.3 ± 0.4 vs PVF: 3.1 ± 0.9, p<0.001) and mixed venous saturation (IVF: 56.5 ± 8.5 vs PVF: 63.6 ± 8.0, p=.02), and higher systemic vascular resistance (IVF: 27.1 ± 12.2 vs PVF: 18.7 ± 8.5). There was no difference in Fontan baffle pressures, caval pressures, or pulmonary capillary wedge pressures. The impaired ventricular function group had moderate to severe AV valve regurgitation in 55.6% patients as compared to 6.3% (p=0.006) in the preserved ventricular function group. Once referred for transplant, no patients had attempts at AV valve repair. Laboratory data were only significant for an increased hemoglobin in the IVF group (IVF: 16.0 ± 1.6 vs PVF: 14.4 ± 2.0 mg/dL, p=.027) and significantly decreased albumin in the PVF group (IVF: 3.6 ± 0.5 vs PVF: 2.4 ± 0.8 mg/dL, p=0.002).
Table 2.
Comparison of Hemodynamic and Laboratory Data
| Characteristic | Impaired Ventricular Function (n=18) | Preserved Ventricular Function (n=16) | p* |
|---|---|---|---|
| Cardiac Index (L/min/m2) | 2.25 ± 0.38 | 3.12 ± 0.86 | < 0.001 |
| SaO2 (%) | 86.5 ± 6.7 | 85.6 ± 6.4 | * |
| MvO2 (%) | 56.5 ± 8.5 | 63.6 ± 8.0 | .020 |
| Fontan Pathway, mm Hg | 17.4 ± 5.4 | 17.4 ± 6.4 | * |
| PCWP, mm Hg | 13.8 ± 5.1 | 14.3 ± 6.5 | * |
| SVR, Wood units | 27.1 ± 12.2 | 18.7 ± 8.5 | .032 |
| PVR, Wood units | 2.3 ± 0.9 | 2.9 ± 1.0 | * |
| Hemoglobin, mg/dL | 16.0 ± 1.6 | 14.4 ± 2 | .027 |
| Creatinine, mg/dL | 0.78 ± 0.24 | 0.74 ± 0.70 | * |
| Albumin, mg/dL | 3.6 ± 0.5 | 2.4 ± 0.8 | .002 |
MvO2, mixed venous saturation; PCWP, pulmonary capillary wedge pressure; SaO2, saturation arterial oxygen; SVR, systemic vascular resistance; PVR, pulmonary vascular resistance.
Survival
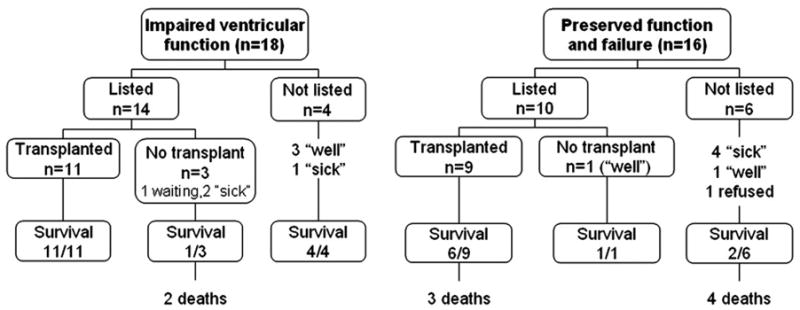
Of the 34 patients identified in this study, 24 were listed for transplantation. See figure 1. The rates of listing and transplantation were similar in both groups (table 3). There were no differences in waitlist time between the two groups, (178 days vs 192 days, p=0.87). Of the 4 patients not listed for transplantation in the IVF group, 3 were considered too well for listing based institutional criteria, and 1 patient was ineligible based on multiple medical risk factors. Those considered ineligible had a combination of multiple risk factors such as renal dysfunction, high pulmonary vascular resistance, lung disease, and/or liver cirrhosis. Of the 14 patients listed for transplantation in the IVF group, 2 died prior to transplantation, 11 were transplanted, and one patient was currently listed awaiting transplantation.
Figure 1.
Listing for Transplantation
Table 3.
Comparison of Mortality
| Characteristic | Impaired Ventricular Function (n=18) | Preserved Ventricular Function (n=16) | p* |
|---|---|---|---|
| Transplanted | 11 (61.1%) | 9 (56.3%) | * |
| Deaths, n (%) | 2 (11.1%) | 7 (43.8) | .052 |
| Transplanted | 0/11 | 3/9 (33.3%) | .074 |
| Non-transplanted | 2/7 (28.6%) | 4/8 (50%) | * |
There were 10 out 16 patients listed for transplant in the PVF group and nine of those patients were transplanted with 3 deaths (33%) early post transplant. The tenth patient was delisted as his conditioned improved. There were 3 deaths in those patients transplanted. There were 7 PVF patients not listed for transplantation: 4 were considered ineligible for transplant based on standard institutional criteria as listed above (ie: “too sick”), 2 were eligible but considered “too well,” and one patient refused. All four patients who were “too high risk” for transplantation died.
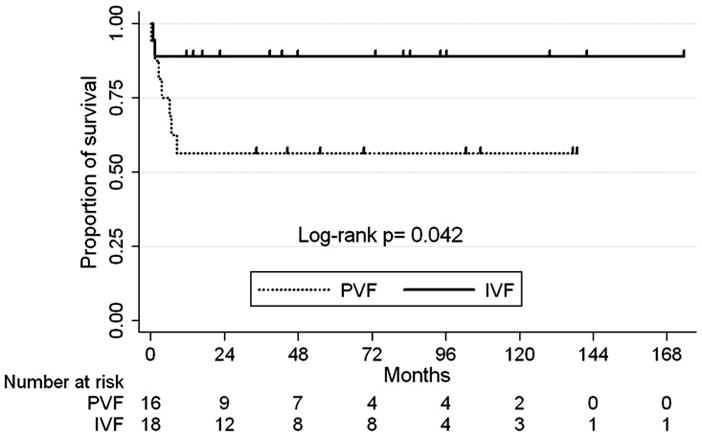
Overall, there were 7 deaths (43.8%) in the preserved ventricular function group compared to 2 deaths (12.5%) in the systolic ventricular dysfunction group (p=0.052) during a mean follow up period of 55.2 ± 50 months. See table 3. Overall survival determined by Kaplan-Meier analysis (figure 2) demonstrated patients with preserved ventricular function with failing Fontan physiology had significantly worse mid-term survival compared to patients with impaired ventricular function. All deaths occurred within one year from time of transplant evaluation demonstrating a 1 year actuarial survival of 88.9% in the IVF group and 56.2% in the PVF group (p=0.042). There were no significant differences in the causes of death between the two groups listed in table 4. In the preserved ventricular function group with failed Fontan physiology, 3 of the 7 deaths occurred in transplanted patients. Two deaths were due to acute graft failure without evidence of cellular rejection and 1 due to multi-organ failure. Two of the patients were diagnosed with plastic bronchitis and 1 with PLE. The remaining deaths in the PVF group occurred in those not listed for transplantation with a median time from evaluation to death of 142.5 days (range, 6–252 days). Cardiac failure was reported as the cause of death in 2 patients, multi-organ failure in 1, and the cause of death was unknown in 1 patient. Cardiac failure and multi-organ failure also accounted for the 2 deaths in the IVF group.
Figure 2.
Actuarial Survival from Time of Evaluation for Heart Transplantation.
IVF, impaired ventricular function group; PVF, preserved ventricular function group.
Patients were censored at time of last follow-up date. Mean follow-up time 55.2 ± 50 months.
Table 4.
Cause of Death
| Impaired Ventricular Function (n=2) | Preserved Ventricular Function (n=7) | |
|---|---|---|
| Cardiac failure | 1 (50%) | 2 (29%) |
| Multiorgan failure | 1 (50%) | 2 (29%) |
| Acute graft failure | 2 (29%) | |
| Unknown | 1 (14) |
Effects of Transplantation
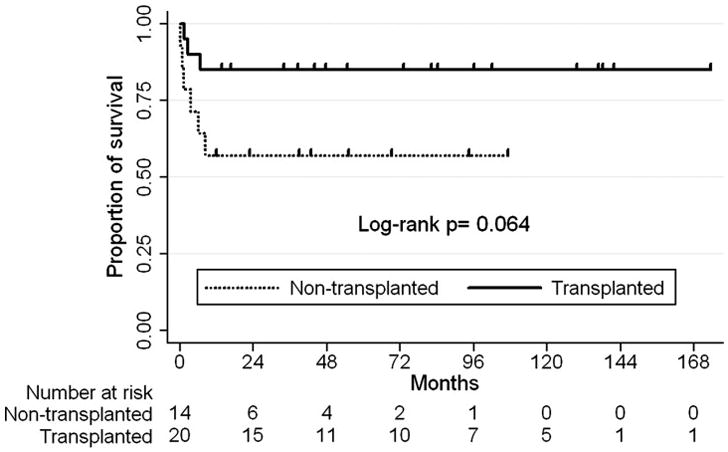
In order to examine the effects of transplantation on survival in all single ventricle palliation patients referred for transplantations we analyzed the overall survival of those who were transplanted versus those who were not transplanted. Of the 34 patients evaluated, 24 were listed for transplantation and 20 were transplanted. There were 3 deaths in the 20 patients transplanted (15 %) and 6 deaths in the 14 patients that were not transplanted (42.9%) (p=0.064). See figure 3. Survival analysis showed a strong trend towards improved survival in those patients who received transplantation.
Figure 3.
Actuarial Survival for Transplantation
Patients were censored at time of last follow-up date.
Mean follow-up time 55.2 ± 50 months.
Comment
Management of late failure of Fontan palliation is a significant challenge with transplantation often being the only available option. Additionally, there are currently no standard mechanical ventricular assist strategies for this complex group of patients. Of the failed Fontan patients referred to our center for transplant evaluation, we found that categorizing them on the basis of ventricular systolic function, as determined by echocardiography, correlated strongly with the presence or absence of secondary sequelae of failed Fontan physiology such as PLE, plastic bronchitis and refractory ascites and edema. Furthermore, there was a significant decrease in survival in the group who presented with preserved ventricular function but with signs and symptoms of failed Fontan physiology such as ascites, edema, and pleural effusions. The possible cause for this concerning finding remains speculative as a wide range of variables including type of systemic ventricle, pacemaker dependency, atrial tachyarrythmias, age at Fontan, and time since follow-up on univariate analysis did not show statistically significant risk.
Because PLE and plastic bronchitis were only found in the preserved ventricular function group it is tempting to impugn these two conditions as likely contributors. PLE is a devastating complication of Fontan physiology with a 50% mortality within 5 years after diagnosis (13,16). There exist multiple treatment modalities which likely reflect the paucity of definitive management principles based on a clear understanding of its cause (17). Bernstein et al, reported that 100% of patients who survived the initial post transplant period had improvement of their PLE symptoms making a compelling argument for use of transplantation as a final treatment modality for refractory PLE (9). Others as well have documented an improvement in PLE after transplantation (18). However, a recent review of long term survival in patients with Fontan surgery found PLE to be an increased risk factor for mortality(7). We found 33% of patients with PLE died--2 before transplantation and 1 after.
In our study, 5 patients evaluated for heart transplantation were diagnosed with plastic bronchitis with 3 deaths overall, 2 after transplantation, during the follow up period. Plastic bronchitis is becoming an increasingly recognized complication of congenital heart disease and particularly Fontan patients. Little is known about the cause of this difficult condition and there exist multiple therapies without a clear consensus on best management (15,19,20). Furthermore there is little data examining its role in Fontan failure or contribution to post transplant mortality. We are limited by the small sample size, however the results are concerning and point to the need for further study into the natural history of plastic bronchitis including possible resolution after heart transplantation.
Three patients with preserved ventricular function lacked the diagnosis of plastic bronchitis or PLE but had significant complications of pleural effusions, edema, and refractory ascites. These did not meet the stringent criteria for diagnosis of PLE in this study but could represent a spectrum of disease with PLE as the extreme manifestation (13). The validity of grouping these patients together is yet to be proven although PLE and plastic bronchitis may share a similar etiology (12,21,22).
Impaired ventricular function is a well recognized indication for transplantation (12,18). In our study 47% of patients were classified as having impaired ventricular function by echocardiography and confirmed by catheterization, consistent with other reported rates of ventricular dysfunction (12). Prior studies have suggested that progressive ventricular dysfunction is more common with the RV (23,24). In our study the morphological right ventricle was the systemic ventricle in approximate two thirds of all patients with equal distribution in both the preserved and impaired ventricular function groups. By log-rank test a systemic RV was not significantly associated with decreased survival.
All mortality within our study occurred within 1 year from time of referral for transplantation indicating that this is a very sick patient population at time of evaluation. Survivors from the early mortality period had equivalent mid-term survival indicating the increased risk of dying for those with preserved function and failed Fontan physiology is in the early time period. Earlier referral of these patients may limit the negative consequences of the non-cardiac complications of failed Fontan physiology such as PLE and plastic bronchitis which could contribute to the increased mortality.
There are several limitations to our study. First, the small sample size in each subgroup may limit detection of statistically significant differences. In addition, the study is limited by its retrospective nature. In particular, at our large institution, the criteria for referral for cardiac transplantation evaluation is not standardized. This may contribute to the differences in outcome between the two groups, as one group may have been referred later. In addition, certain eligible patients may have been excluded due to practitioner bias. Possible considerations include those who are felt to be too sick or high risk for transplantation or those whose family are not interested in transplantation as a therapeutic option and therefore referral was not made. More standardized criteria for evaluation are needed in late Fontan patients before we can definitively answer those who benefit most or are at greatest risk during heart transplantation. Lastly, the study is limited by the use of echocardiographic estimates of systolic function in single ventricle patients and the difficulty in estimating diastolic function’s contribution to the pathology.
As increasing numbers of patients are palliated with Fontan operations, the number of children, adolescents, and young adults requiring late rescue therapy with heart transplantation will increase (11). The sparse availability of hearts for transplantation and the associated morbidities with immunosuppression make it imperative that it is utilized appropriately. Therefore much benefit would be derived from identifying those patients with failing Fontan physiology who might benefit from other modes of therapy and optimizing the timing of transplantation in order to exact the greatest benefit from such a scarce resource. Further study of diastolic dysfunction following the Fontan procedure may further clarify high risk patients. In addition, developing ventricular support devices that may bridge patients more successfully are needed. It may be possible to better prepare the Fontan pulmonary circulation over months with pulsitile flow in order to better select patients for improved transplant outcomes.
In conclusion, we have found that patients referred for transplant evaluation with failing Fontan circulation have two different modes of presentation and can be categorized on the basis of their ventricular systolic function. This method of categorization identifies a higher risk group which also present with sequelae of failed Fontan physiology including PLE, plastic bronchitis, refractory ascites and edema. This high risk group has a greater than threefold risk of death within one year compared to the group that presents with poor ventricular function. Earlier referral for transplantation or alternative medical or surgical interventions should be considered for the high risk group.
Acknowledgments
Dr. Griffiths was supported by the Harvard-Longwood Research Training in Vascular Surgery: T32 HL 007734 to FW Logerfo.
Acronyms and Abbreviations
- AV valve
Atrioventricular valve
- DILV
Double inlet left ventricle
- DORV
Double outlet right ventricle
- HLHS
Hypoplastic left heart syndrome
- IVF
Impaired ventricular function
- MvO2
Mixed venous saturation
- PB
Plastic Bronchitis
- PCWP
Pulmonary capillary wedge pressure
- PLE
Protein losing enteropathy
- PVF
Preserved ventricular function
- PVR
Pulmonary vascular resistance
- SaO2
Saturation arterial oxygen
- SVR
Systemic vascular resistance
- TA
Tricuspid atresia
Footnotes
Meeting Presentation: Society of Thoracic Surgeons 45th Annual Meeting, San Francisco, California, January 26–28, 2009.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26(3):240–248. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontan F, Deville C, Quaegebeur J, Ottenkamp J, Sourdille N, Choussat A, Brom GA. Repair of tricuspid atresia in 100 patients. The Journal of thoracic and cardiovascular surgery. 1983;85(5):647–660. [PubMed] [Google Scholar]
- 3.de Leval MR, Kilner P, Gewillig M, Bull C. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. The Journal of thoracic and cardiovascular surgery. 1988;96(5):682–695. [PubMed] [Google Scholar]
- 4.Marcelletti C, Corno A, Giannico S, Marino B. Inferior vena cava-pulmonary artery extracardiac conduit. A new form of right heart bypass. The Journal of thoracic and cardiovascular surgery. 1990;100(2):228–232. [PubMed] [Google Scholar]
- 5.Alphonso N, Baghai M, Sundar P, Tulloh R, Austin C, Anderson D. Intermediate-term outcome following the fontan operation: a survival, functional and risk-factor analysis. Eur J Cardiothorac Surg. 2005;28(4):529–535. doi: 10.1016/j.ejcts.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 6.Huddleston CB. The failing Fontan: options for surgical therapy. Pediatric cardiology. 2007;28(6):472–476. doi: 10.1007/s00246-007-9008-z. [DOI] [PubMed] [Google Scholar]
- 7.Khairy P, Fernandes SM, Mayer JE, Jr, Triedman JK, Walsh EP, Lock JE, Landzberg MJ. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117(1):85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 8.Brogan TV, Finn LS, Pyskaty DJ, Jr, Redding GJ, Ricker D, Inglis A, Gibson RL. Plastic bronchitis in children: a case series and review of the medical literature. Pediatric pulmonology. 2002;34(6):482–487. doi: 10.1002/ppul.10179. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein D, Naftel D, Chin C, Addonizio LJ, Gamberg P, Blume ED, Hsu D, Canter CE, Kirklin JK, Morrow WR. Outcome of listing for cardiac transplantation for failed Fontan: a multi-institutional study. Circulation. 2006;114(4):273–280. doi: 10.1161/CIRCULATIONAHA.105.548016. [DOI] [PubMed] [Google Scholar]
- 10.Carey JA, Hamilton JR, Hilton CJ, Dark JH, Forty J, Parry G, Hasan A. Orthotopic cardiac transplantation for the failing Fontan circulation. Eur J Cardiothorac Surg. 1998;14(1):7–13. doi: 10.1016/s1010-7940(98)00130-4. discussion 13–14. [DOI] [PubMed] [Google Scholar]
- 11.Michielon G, Parisi F, Di Carlo D, Squitieri C, Carotti A, Buratta M, Di Donato RM. Orthotopic heart transplantation for failing single ventricle physiology. Eur J Cardiothorac Surg. 2003;24(4):502–510. doi: 10.1016/s1010-7940(03)00342-7. discussion 510. [DOI] [PubMed] [Google Scholar]
- 12.Jayakumar KA, Addonizio LJ, Kichuk-Chrisant MR, Galantowicz ME, Lamour JM, Quaegebeur JM, Hsu DT. Cardiac transplantation after the Fontan or Glenn procedure. J Am Coll Cardiol. 2004;44(10):2065–2072. doi: 10.1016/j.jacc.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 13.Mertens L, Hagler DJ, Sauer U, Somerville J, Gewillig M. Protein-losing enteropathy after the Fontan operation: an international multicenter study. PLE study group. The Journal of thoracic and cardiovascular surgery. 1998;115(5):1063–1073. doi: 10.1016/s0022-5223(98)70406-4. [DOI] [PubMed] [Google Scholar]
- 14.Powell AJ, Gauvreau K, Jenkins KJ, Blume ED, Mayer JE, Lock JE. Perioperative risk factors for development of protein-losing enteropathy following a Fontan procedure. The American journal of cardiology. 2001;88(10):1206–1209. doi: 10.1016/s0002-9149(01)02066-5. [DOI] [PubMed] [Google Scholar]
- 15.Costello JM, Steinhorn D, McColley S, Gerber ME, Kumar SP. Treatment of plastic bronchitis in a Fontan patient with tissue plasminogen activator: a case report and review of the literature. Pediatrics. 2002;109(4):e67. doi: 10.1542/peds.109.4.e67. [DOI] [PubMed] [Google Scholar]
- 16.Silvilairat S, Cabalka AK, Cetta F, Grogan M, Hagler DJ, O’Leary PW. Protein-losing enteropathy after the Fontan operation: associations and predictors of clinical outcome. Congenital heart disease. 2008;3(4):262–268. doi: 10.1111/j.1747-0803.2008.00200.x. [DOI] [PubMed] [Google Scholar]
- 17.Gersony WM. Fontan operation after 3 decades: what we have learned. Circulation. 2008;117(1):13–15. doi: 10.1161/CIRCULATIONAHA.107.748566. [DOI] [PubMed] [Google Scholar]
- 18.Gamba A, Merlo M, Fiocchi R, Terzi A, Mammana C, Sebastiani R, Ferrazzi P. Heart transplantation in patients with previous Fontan operations. The Journal of thoracic and cardiovascular surgery. 2004;127(2):555–562. doi: 10.1016/j.jtcvs.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Quasney MW, Orman K, Thompson J, Ring JC, Salim M, Schoumacher RA, Watson D, Novick W, Deitcher SR, Joyner R. Plastic bronchitis occurring late after the Fontan procedure: treatment with aerosolized urokinase. Critical care medicine. 2000;28(6):2107–2111. doi: 10.1097/00003246-200006000-00074. [DOI] [PubMed] [Google Scholar]
- 20.Do TB, Chu JM, Berdjis F, Anas NG. Fontan Patient with Plastic Bronchitis Treated Successfully Using Aerosolized Tissue Plasminogen Activator: A Case Report and Review of the Literature. Pediatric cardiology. 2008 doi: 10.1007/s00246-008-9312-2. [DOI] [PubMed] [Google Scholar]
- 21.Shah SS, Drinkwater DC, Christian KG. Plastic bronchitis: is thoracic duct ligation a real surgical option? The Annals of thoracic surgery. 2006;81(6):2281–2283. doi: 10.1016/j.athoracsur.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Stiller B, Riedel F, Paul K, van Landeghem FK. Plastic bronchitis in children with Fontan palliation: analogue to protein losing enteropathy? Pediatric cardiology. 2002;23(1):90–94. doi: 10.1007/s00246-001-0024-0. [DOI] [PubMed] [Google Scholar]
- 23.Julsrud PR, Weigel TJ, Van Son JA, Edwards WD, Mair DD, Driscoll DJ, Danielson GK, Puga FJ, Offord KP. Influence of ventricular morphology on outcome after the Fontan procedure. The American journal of cardiology. 2000;86(3):319–323. doi: 10.1016/s0002-9149(00)00922-x. [DOI] [PubMed] [Google Scholar]
- 24.Gentles TL, Mayer JE, Jr, Gauvreau K, Newburger JW, Lock JE, Kupferschmid JP, Burnett J, Jonas RA, Castaneda AR, Wernovsky G. Fontan operation in five hundred consecutive patients: factors influencing early and late outcome. The Journal of thoracic and cardiovascular surgery. 1997;114(3):376–391. doi: 10.1016/s0022-5223(97)70183-1. [DOI] [PubMed] [Google Scholar]


