Abstract
Carboxypeptidase E (CPE) is involved in maturation of neuropeptides and sorting of brain-derived neurotrophic factor (BDNF) to the regulated pathway for activity-dependent secretion from CNS neurons. CPE knock-out (CPE-KO) mice have many neurological deficits including learning and memory. Here, we analyzed the dendritic arborization and spine morphology of CPE-KO mice to determine a possible correlation of defects in such structures with the neurological deficits observed in these animals. Analysis of pyramidal neurons in layer V of cerebral cortex and in hippocampal CA1 region in 14 week old CPE-KO mice showed more dendritic complexity compared to wild type (WT) mice. There were more dendritic intersections and more branch points in CPE-KO versus WT neurons. Comparison of pyramidal cortical neurons in 6 week versus 14 week old WT mice showed a decrease in dendritic arborization, reflecting the occurrence of normal dendritic pruning. However, this did not occur in CPE-KO neurons. Furthermore, analysis of spine morphology demonstrated a significant increase in the number of D-type spines regarded as non-functional in the cortical neurons of CPE-KO animals. Our findings suggest that CPE is an important novel player in mediating appropriate dendritic patterning and spine formation in CNS neurons.
Keywords: Carboxypeptidase E, dendritic pruning, hippocampus, BDNF
Introduction
Formation of an appropriately connected neural network is fundamental to proper functioning of the central nervous system (CNS). Establishment of such a network requires precisely regulated growth and branching of dendritic arbors, formation of dendritic spines, activity-dependent synaptogenesis, and simultaneous pruning of overgrowth of dendritic material. The dendritic architecture and formation of specific types of spines are also crucial for neurotransmission and accurate functioning of the CNS as an integral unit (Schaefer et al. 2003). Synaptic connections and spine morphology are plastic, and changes with learning and memory. Thus far, the molecular mechanisms governing these phenomena remain poorly understood.
The development of dendritic arbors is controlled at different levels which encompass various types of extracellular signals and transcription factors. Extracellular signals include diffusible molecules [eg. Slit, reelin and Semaphorin3A (Tolwani et al. 2002), (Morita et al. 2006), (Jossin and Goffinet 2007)]; cell contacts mediated by Notch, cadherins, and Dscam (Hughes et al. 2007; Redmond et al. 2000); and neuronal activity, which cause calcium influx and neurotransmitter release (Haas et al. 2006; Sin et al. 2002) (Wayman et al. 2006; Wu et al. 2007). These extracellular signals have different signal transduction pathways that involves members of small G-proteins, protein kinases and protein phosphatases (Chen et al. 2005; Fink et al. 2003; Fu et al. 2007; Kumar et al. 2005; Rosso et al. 2005; Tolias et al. 2005). Transcriptional factors such as Hamlet, Cut, Abrupt, and Spineless in Drosophila (Parrish et al. 2007), and Neurogenin 2 and Dlx homeobox in mammals (Cobos et al. 2007; Cobos et al. 2005; Hand et al. 2005) also play a role in controlling dendritic arborization. Additionally, neurotrophins and their receptors have been implicated in mediating the fine-tuning and plasticity of the mature neuronal network (Johnson et al. 2007; Lou et al. 2005; Prithviraj et al. 2008; Singh et al. 2008; Wirth et al. 2003).
Advances in imaging techniques have permitted detail studies of dendritic spines, the small protrusions on the surface of dendrites, which are necessary for synapse formation and function. It has been shown that dendritic spine numbers and structure change with synaptic activity and developmental stage (Bourne and Harris 2008; De Roo et al. 2008; Matsuzaki 2007). For example, the size of the head and length of neck of the spine reflect its function. Spines with a bigger head and a longer neck will generally reflect more activity and show greater plasticity, as opposed to those which are small and stubby. Spines are crucial in activation of neurotransmitter receptors, the generation of long-term potentiation (LTP) and long-term depression (LTD), and many other biological functions necessary for trans-synaptic signaling.
Here, we discover a new player, carboxypeptidase E (CPE) that appears to be important for maintaining proper dendritic arborization and spine morphology in CNS neurons. CPE is a multifunctional glycoprotein expressed in endocrine cells and neurons. Soluble CPE is a proneuropeptide processing enzyme (Fricker et al. 1986) that cleaves C-terminal basic amino acid residue extensions from neuropeptide intermediates to produce active peptides. The membrane form of CPE functions acts as a sorting receptor at the trans-Golgi network, directing proneuropeptides and proform brain-derived neurotrophic factor (proBDNF) to the regulated secretory pathway vesicles for activity-dependent secretion (Cool et al. 1997; Lou et al. 2005). The cytoplasmic tail of a transmembrane form of CPE has been shown to anchor BDNF-containing vesicles to the microtubules for transport in neurites for secretion (Park et al. 2008). The importance of CPE in the nervous system was further demonstrated in CPE-KO mice, which exhibited diminished reactivity to stimuli and toe-pinch reflexes, poor muscle strength, as well as loss of neurotransmission in the retina (Cawley NX et al. 2004; Zhu X 2005). There are morphological and physiological abnormalities in the CNS of CPE-KO mice including a degenerated CA3 region in the adult hippocampus, LTP impairment in CA1 neurons and deficits in memory and learning (Woronowicz et al. 2008).
In this study we examined the dendritic arborization and spine morphology of CNS neurons in the CPE-KO mouse. Sholl analysis of pyramidal neurons in cerebral cortex layer V and hippocampal CA1 region revealed that the dendritic arborizations in the CPE-KO mice were more complex than their WT littermates. Also more immature dendritic spines were found in CPE-KO versus WT mice. Thus CPE plays a role in regulating and maintaining these neuronal structures which are essential for brain function.
Materials and Methods
Animals and Golgi staining of brains
Two 6 and 14 week old CPE-KO mice and 2 WT littermates were anesthetized and then perfused with fix solution (containing: 4% paraformaldehyde, 4% sucrose and 1.4% sodium cacodylate). Animals were then euthanized by cervical dislocation, followed by decapitation and the brains were dissected. All experimental procedures for this study were approved by the National Institute of Child Health and Human Development Animal Care and Use Committee and followed the NIH Guide for the Care and Use of Laboratory Animals.
From each brain, a coronal block of tissue approximately 2.5 mm thick encompassing the parietal cortex and the underlying hippocampus was dissected from each hemisphere and stained using a variant of the Rapid Golgi impregnation method (Valverde 2002). Briefly, the blocks were initially immersed in a solution of osmium tetroxide and potassium dichromate for 5–6 days, rinsed and blotted and then placed in a 0.75% solution of freshly prepared silver nitrate for 39–42 hours. The blocks were then dehydrated through ascending solutions of alcohols, placed in ether-alcohol, and then infiltrated with solutions of nitrocellulose of increasing viscosity. The tissue blocks were then placed in a 30% solution of nitrocellulose, which was hardened by exposure to chloroform vapors. The hardened blocks of nitrocellulose (containing the mouse brains) were affixed to fiber blocks for sectioning on an AO sliding microtome (American Optical, Buffalo, NY). Tissue sections were cut in the coronal plane at 120 µm thickness. Sections were cleared in alpha-terpineol thoroughly rinsed in xylene, placed on coded slides, cover-slipped under Permount (Fisher Scientific, Pittsburgh, PA) and permitted to thoroughly dry.
Quantification of dendritic branching, spine density and spine type
From the coded slides, cerebral cortex layer V pyramidal and CA1 hippocampal neurons were randomly selected for analysis of dendritic branching and spines. Dendritic branching and spine analysis was carried out on the basilar tree of the layer V cerebral cortex and CA1 hippocampal neurons.
Dendritic Branching
For the dendritic branching analysis, cerebral cortex layer V pyramidal and CA1 hippocampal pyramidal neurons were selected which met the following criteria: (1) neurons had to be fully impregnated, (2) somas had to be located in the middle third of the thickness of the tissue section, (3) the basilar trees could not be obscured by capillaries, other dendritic or neuritic processes, or non-descript precipitate. Using a Zeiss brightfield research microscope with a drawing tube (Carl Zeiss, Oberkochen, Germany), camera lucida drawings were made of the basilar tree of the selected neurons. To quantify the amount of dendritic material of the basilar tree and its distribution, a Sholl analysis was carried out. The Sholl analysis quantifies the number of intersections (or “hits”) of the dendrites with each of the circles (or “shells”). This generates a profile of the amount of dendritic intersections at increasing distances from the soma. The distance between each shell was equivalent to 10 µm. The branching data in each profile was statistically compared between the wild type controls and the knock outs and evaluated using the Wilcoxon rank sign test. An adjusted (conservative) alpha level is assigned to denote statistical significance between groups. Branching data from the Sholl analysis can also be expressed as a cumulative value (e.g., “total hits”) which can be used to assess the estimated total dendritic length.
Dendritic Spine Analysis
Dendritic spines are small excrescences which represent the loci for the majority of synapses in the brain. In general, each spine can be thought of as being equivalent to a single excitatory synapse. Two regions of the basilar tree were selected since they would provide a broader picture of the overall spine changes occurring on this part of the tree. On the basilar tree of pyramidal neurons in cerebral cortex and hippocampal CA1 regions, spines were counted along two regions: on terminal tip segments and on internal branch segments. The basilar tree of cerebral cortical pyramids receives the majority of synaptic input to the neuron. Spines were counted directly on the Zeiss microscope using a long-working distance oil immersion objective lens at a 63× magnification and a 2.0 Optivar (intermediate) magnification. Only flanking spines were counted, e.g., those extending laterally from the dendritic segments. This is because spines that are directed essentially up toward - or away from - the observer are likely to be partially or wholly obscured by the shadow of the impregnated dendritic branch. Thus, the data do not reflect the actual total number of spines and would be an underestimate of the true number. However, for the purposes of the study, both spines from WT controls and from CPE-KO mice would be equally underreported and so can still be statistically compared. We used three categories of spines: 'M-type' (mushroom), characterized by a large, well-defined spine head and a thick spine neck; 'N-type' (nubby), characterized by a poorly defined spine head and a thickened spine neck; and 'D-type' (dimple), characterized by a poorly defined spine head and the absence of a definitive spine neck. Similar spine configuration categories have been used previously (Neigh et al. 2004). Spines per neuron (n = 18 neurons per group) were counted in WT and CPE-KO mice and the value for CPE KO mice were expressed as a % wild-type (made equal to 100%). For means of comparison, statistics on the graphs are calculated using the unpaired student’s t test.
Results
Dendritic branching of cortical pyramidal neurons in wild-type and CPE-KO mice
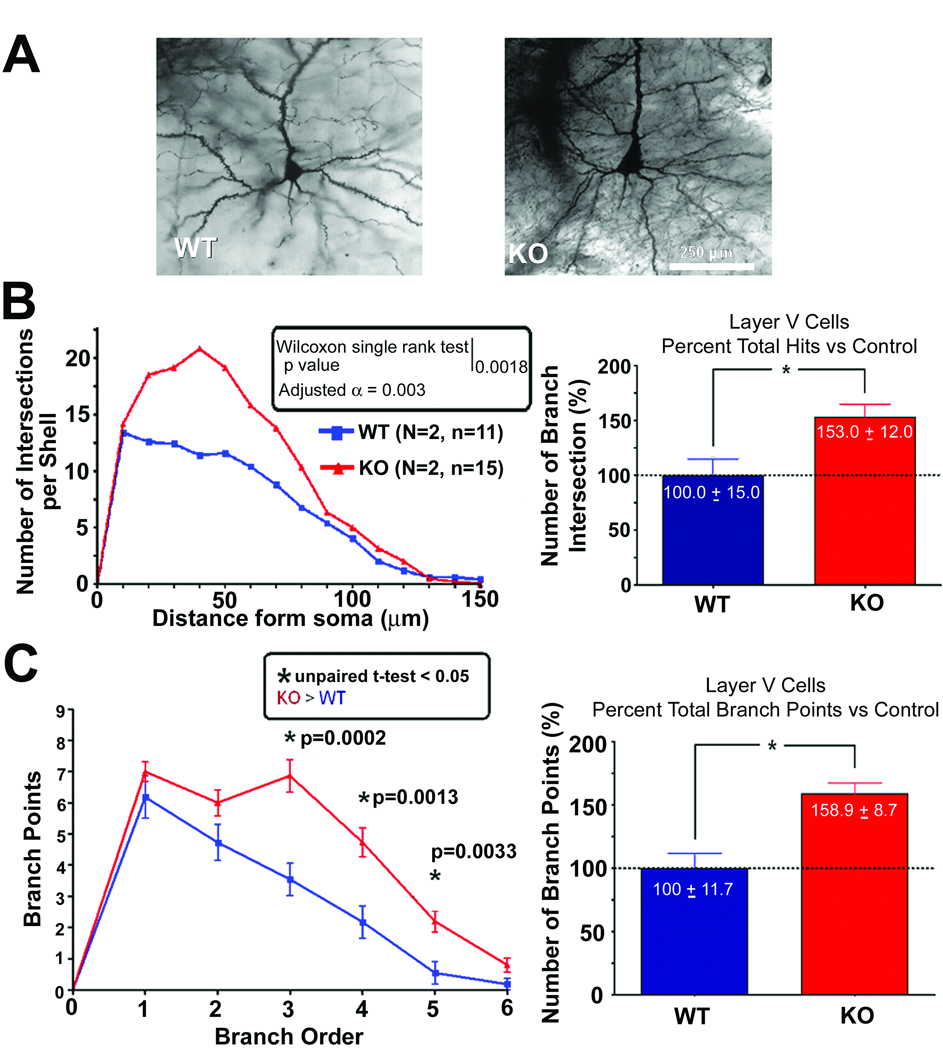
The dendritic complexity of the pyramidal layer V cortical neurons from 14 week old CPE-KO and WT mice were analyzed (Fig.1). Fig 1A shows representative photomicrographs of neurons from WT and CPE-KO mice. Sholl analysis showed a significant difference in the number of intersections at various distances from the cell soma (Fig1B). The dendritic branching of neurons from CPE-KO mice displayed a more complex dendritic pattern than that observed in WT animals. Quantitative analysis of WT and CPE-KO neurons showed that there was significantly more (53%) dendritic intersections in Sholl analysis in CPE-KO mice compared to the WT control (Fig.1B, bar graph). Moreover, there were more dendritic intersections proximal to the soma, at a distance of ~20–60 µm in CPE-KO mice compared to WT mice (Fig.1B, line graph). The complexity of the dendritic arbor was assessed using the branch point analysis (Fig1C). The branch point is the place where a dendritic segment bifurcates into two higher order branches. Therefore, the more branch points there are the more complex the dendritic tree is. As shown in Fig.1C there were more 3rd, 4th and 5th order branch points in CPE-KO neurons compared to WT neurons, indicating that the dendritic tree of CPE-KO neurons is more complex than WT neurons. The statistical analysis showed that there was a 59% increase in total dendritic branch points in the basilar tree of the CPE-KO versus WT mice.
Fig. 1.
Dendritic branching of cortex layer V pyramidal neurons of WT and CPE-KO mice at 14 weeks of age. (A) Representative Golgi-impregnated WT and (KO) neurons at age 14 weeks. (B) (Left panel) The number of dendritic intersections at different distances from the soma of neurons from CPE-KO and WT littermates as analyzed by Sholl analysis. There were a significantly greater number of dendritic intersections at 20–60 µm from the soma in CPE-KO versus WT animals. (Right panel) Bar graphs showing the percentage of the number of branch intersections. There was 53% more dendritic branching in the KO versus WT neurons. (C) Left panel: Graph showing branch point analysis assessing the complexity of the dendritic arbor of neurons from CPE-KO mice and WT mice. There was more complexity in CPE-KO neurons versus WT neurons as evidenced by the significant difference in the 3rd, 4th, and 5th branch order segments (unpaired T-test, p < 0.05). Right panel: Bar graphs showing the total number of branch points (regardless of branch order). There was a 59% higher number of branch points in KO versus WT animals.
Dendritic branching of cortex layer V pyramidal neurons differ in 6 versus 14 week old CPE-KO and WT mice
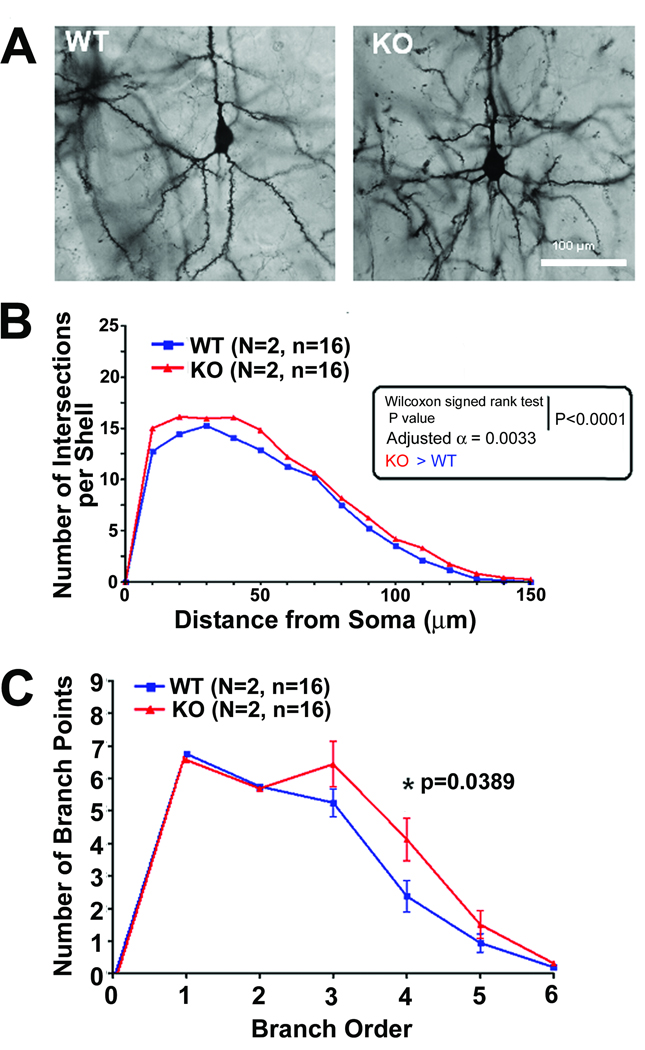
We further broadened our studies by comparing animals of different ages. Sholl analysis was carried out on the basilar tree of 6 week old CPE-KO and age-matched WT mice (Fig. 2). Fig 2A shows representative photomicrographs of neurons from WT and CPE-KO mice. The Sholl analysis (Fig 2B) showed a significant difference in the dendritic basilar tree between the two groups (Wilcoxon signed rank test p < 0.0001). While the dendritic arbor of the CPE-KO mice was significantly larger than the WT controls, the difference was smaller than in the 14 week mice. The CPE-KO mice showed 14% more dendritic intersections per shell in the Sholl analysis of the basilar tree compared to WT animals. This finding is in agreement with the results for the same analysis of the 14 week old mice. The branch point analysis illustrated that, even at 6 weeks of age, the CPE-KO mice tended to have more complex arbors throughout the extent of the tree, but the significant difference was only for the 4th order branch points (p = 0.0389) (Fig. 2C).
Fig. 2.
Dendritic branching of cortex layer V pyramidal neurons of WT and CPE-KO mice at 6 weeks of age. (A) Representative Golgi-impregnated WT and (KO) neurons at age 6weeks. (B) The number of dendritic intersections at different distances from the soma of neurons from CPE-KO and WT littermates as analyzed by Sholl analysis. There was a small but significant difference in the number of dendritic intersections at 20–60 µm from the soma between the 2 groups (Wilcoxon signed rank test, p < 0.0001; Adjusted α = 0.0033). (C) Graph showing branch point analysis of dendritic arbors from WT and CPE-KO mice. There were more complex arbors at 3–6 branch point orders in CPE-KO mice compared to WT mice, but only the 4th order showed statistical significance (p = 0.0389).
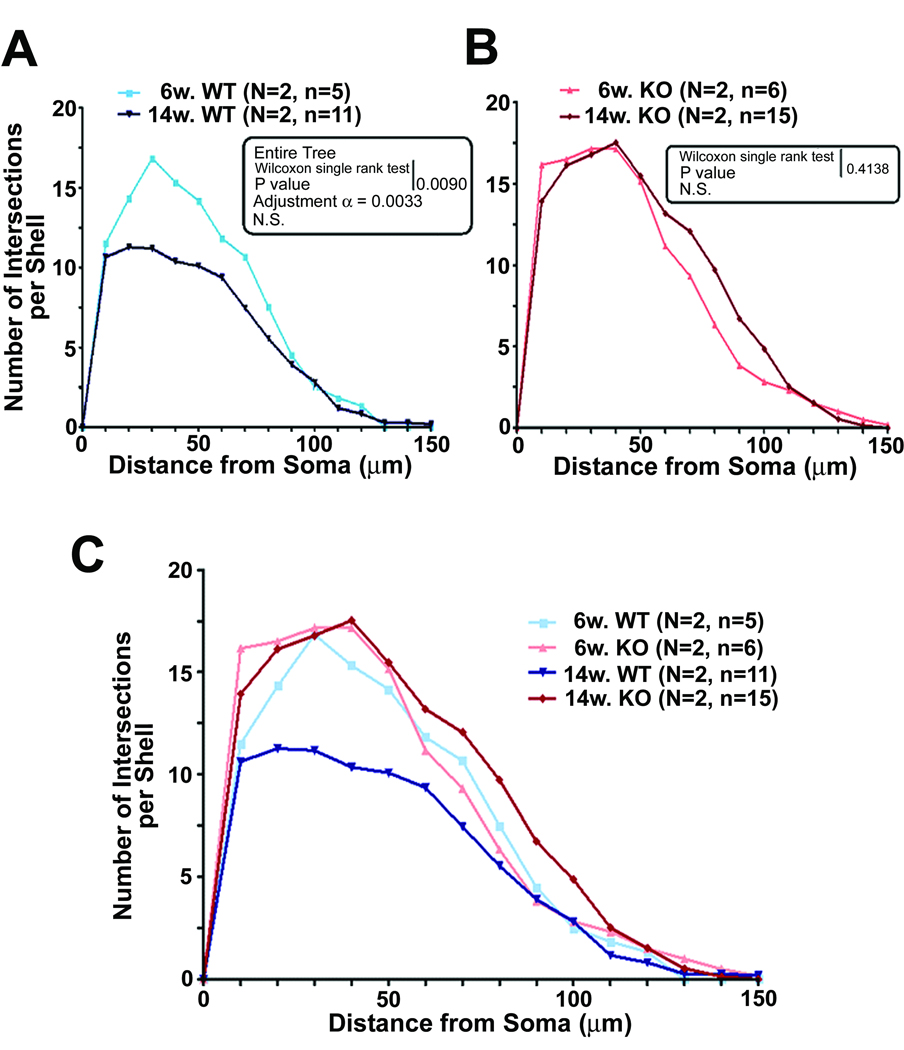
Comparison of results from 6 and 14 week old animals (Fig. 3) showed that there is an age difference in the pattern of dendritic arborization in the WT and CPE-KO mice. In the WT controls, there was a decrease of dendritic intersections per shell in the basilar tree of the neurons between 6 and 14 weeks (Fig. 3A,C). It is apparent that during this period, a major loss of dendritic intersections per shell in the inner two-thirds of the basilar tree occurred in WT but not in CPE-KO mice. This observation suggests that the physiological pruning process was halted by lack of CPE during that period. Conversely, there was an increase in the amount of the dendritic intersections per shell in CPE-KO mice between 6 to 14 weeks of age (Fig. 3B). However, Wilcoxon analysis of the entire tree was not significant (p = 0.4138), although there appears to be more dendritic intersections per shell in the middle third of the arbor at 14 weeks versus 6 weeks, a pattern different from that seen in the WT mice at the same age.
Fig. 3.
Comparison of dendritic arborization using Sholl analysis in 6 and 14 week old cortical neurons. The amount of dendritic branching on neurons at age 6 and 14 weeks was compared for WT (panel A) and CPE-KO mice (panel B). Note the significantly lesser amount of dendritic intersections per shell (Wilcoxon signed rank test p value = 0.009) in 14 week old compared to 6 week old WT mice, (panel A), whereas, the number of dendritic intersections per shell was the same at both ages in CPE-KO mice (panel B) (Wilcoxon signed rank test, p = 0.4138). (C) Graph showing a summary of dendritic arborization from both ages and genotypes. Note that WT animals at 14 weeks of age showed a significant reduction of the dendritic arborizations compared to 6 week WT, 6 week CPE-KO and 14 week CPE-KO mice.
Dendritic branching of hippocampal neurons is more complex in CPE-KO mice
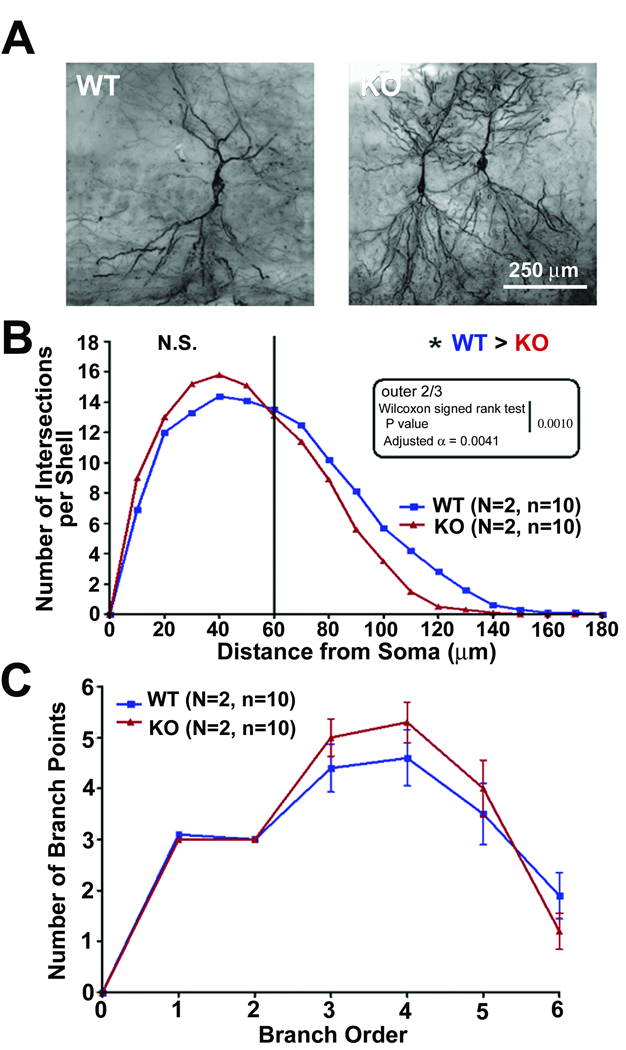
We also examined the dendritic branching in CA1 neurons in the hippocampus of 14 week old WT and CPE-KO mice that were stained with Golgi impregnation (Fig. 4A). The Sholl analysis of the basilar tree of the CA1 pyramidal neurons (Fig. 4B) showed that there was a trend towards increased dendritic intersections per shell proximal to the soma, at a distance of 30–50 µm, in CPE-KO mice compared to WT mice, although it did not reach statistical significance. However, in the more distal part of the arbor (further than 60 µm from the soma), the amount of dendritic intersections per shell in the WT mice was greater than in the KO mice. There was no difference in total hits, which suggests that the estimated total dendritic length is the same in the both groups. This is due to the differences between proximal and distal dendritic regions. Thus, the data show that there was remodeling of the CA1 basilar dendritic arbor in the CPE-KO mice such that there were less dendritic intersections per shell in the distal portion of the dendritic tree compared to the WT controls. The branch point analysis, which reflects dendritic branching complexity, showed that there was a trend for more dendritic complexity in the CPE-KO mice (Fig. 4C).
Fig. 4.
Dendritic branching of hippocampal CA1 neurons of WT and CPE-KO mice at 14 weeks of age. (A) Representative Golgi-impregnated WT and CPE-KO (KO) neurons at age 14 weeks. (B) The number of dendritic intersections at different distances from the soma of neurons from CPE-KO and WT littermates was analyzed by Sholl analysis. There was a tendency for a greater number of dendritic intersections at the proximal 1/3 part of the dendritic tree (20–60 µm from soma) in CPE-KO versus WT animals, although not statistically significant, However, there were statistically fewer numbers of dendritic intersections per shell in the outer 2/3 part of the dendritic tree in CPE-KO mice compared to WT mice (p = 0.001). (C) Graph showing branch point analysis of the neurons from the CA1 region of the hippocampus assessing the complexity of the dendritic arbor of neurons from CPE-KO mice and WT mice. There was no statistical difference although there was a tendency towards more complexity in the dendritic arbor of CPE-KO versus WT hippocampal CA1 neurons.
Differences in the spine morphology of CPE-KO and WT mice
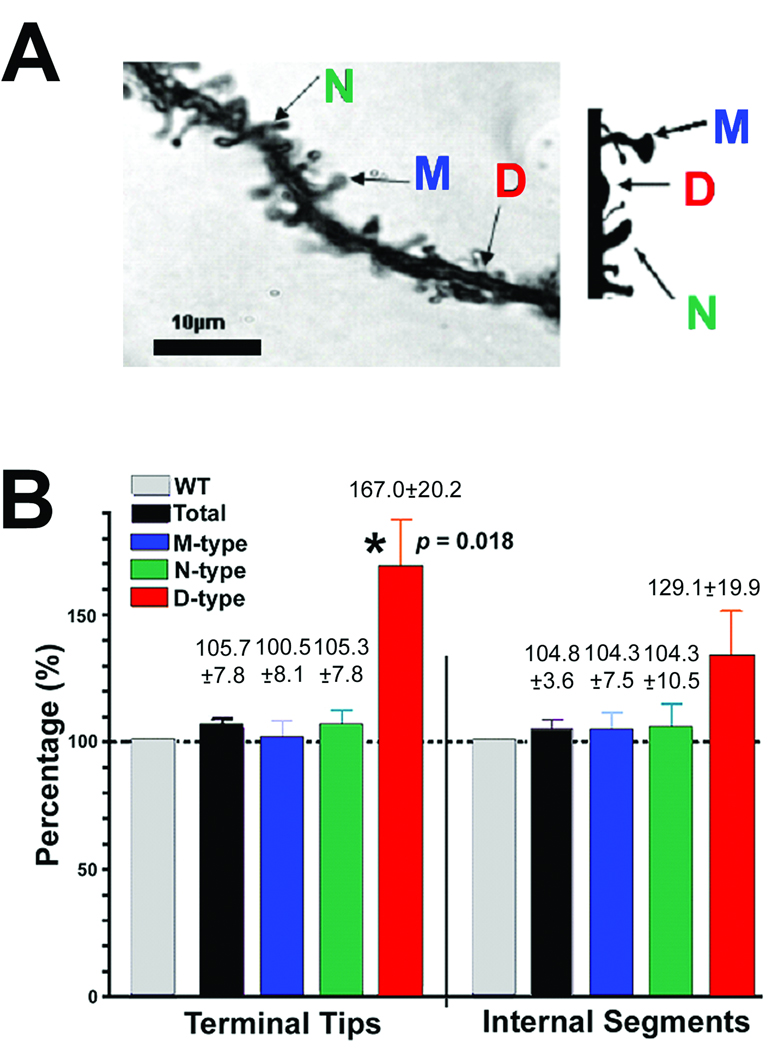
Dendritic spines were analyzed on the basilar tree of cortical layer V neurons of 14 week old WT and CPE-KO mice. Fig. 5A shows examples of M, N and D spines that were analyzed. The total number of dendritic spines on the basilar tree of cortical layer V pyramidal neurons showed no significant difference in CPE-KO versus WT neurons (Fig. 5B). Analysis of spine morphology revealed that within the internal segments and the terminal tips, there was no difference in the % of M and N spines in the CPE-KO versus the WT animals. Only the number of D-type spines, (also referred to as stubby spines by others, (Bourne and Harris 2008), which are characterized as not fully functional were significantly higher at the terminal tips in CPE-KO versus WT mice. Analysis of the total number of dendritic spines on the basilar tree of CA1 hippocampal neurons showed no significant difference in CPE-KO versus WT neurons. Additionally, analysis of spine morphology revealed that the number of D-type of spines tended to be higher in CPE-KO mice compared to WT animals (data not shown).
Fig. 5.
Analysis of dendritic spines on the basilar tree of cerebral cortical neurons of 14week old WT and CPE-KO mice. (A) Left: Photomicrograph showing representative examples of the three types of dendritic spines on pyramidal cerebral cortex neurons. 'M-type' (mushroom), characterized by a large, well-defined spine head and a thick spine neck; 'N-type' (nubby), characterized by a poorly defined spine head and a thickened spine neck; and 'D-type' (dimple), characterized by a poorly defined spine head and the absence of a definitive spine neck. Right: A diagrammatic representation of the different types of spines. (B) Analysis of the total number of dendritic spines on the basilar tree of cortical neurons showed no significant difference in CPE-KO versus WT neurons (n = 18 per group), the data is expressed as a percent change in relation to the wild type control value (which is assigned 100%). Analysis of spine morphology revealed that only the number of D-type spines, which are regarded as not fully functional were significantly higher in CPE-KO versus WT mice (for terminal tips, p = 0.018).
Discussion
Dendrite development is required for establishment of appropriate neuronal connectivity. The pattern of dendritic growth and branching critically determines neuronal functions (Jan and Jan 2003). Many environmental and molecular cues, such as neuronal activity and neurotrophins, regulate dendritic morphogenesis (Scott and Luo 2001; Wong and Ghosh 2002). Moreover, molecules such as Rho family small GTPases and their activators which are key regulators of actin cytoskeleton are also important in dendritic growth and morphology (Negishi and Katoh 2002; Ueda et al. 2008). In this study, we show that CPE plays a role in modeling the pattern of dendritic branching. Our analysis showed that in 14 week old CPE-KO mice which lack CPE, the arborization in pyramidal layer V cerebral cortex and hippocampal CA1 neurons were more complex than in WT animals (Fig 1, Fig 4). There were more dendrites just proximal to the soma and more branch points. Additionally, there were age-dependent changes in arborization in cerebral cortical neurons that differed between WT and CPE-KO animals. Between 6 and 14 weeks of age, WT mice showed a significant decrease in dendritic intersections per shell. This decrease between 6 weeks and 14 weeks in the WT mice is generally interpreted as being due to dendritic pruning, resulting in fewer dendritic arbors. (Groc et al. 2002; Pokorny and Trojan 1983; Pokorny and Trojan 1986; Silva-Gomez et al. 2003). In contrast, there were similar amounts of dendritic intersections per shell in the cortical neurons of CPE-KO mice at age 14 weeks and at 6 weeks. Thus our findings suggest that the lack of CPE results in an aberrant pattern of dendritic growth, as well as inhibition of dendritic pruning in cerebral cortical and hippocampal neurons. Besides differences in dendritic arborization, CPE-KO mice also showed an increase in D-type dendritic spines in cortical layer V neurons, although the total spine density was not significantly different than in WT mice. D-type spines are characterized by a lack of a distinctive spine head or neck and regarded as not fully competent (Diamond et al. 2006; Liu et al. 2008; Malone et al. 2008). In general, presence of D-type spines is an indication of spine degeneration; however, they could also represent early spine formation. Given the greater dendritic growth and branching in the CPE-KO mice, the early spine formation hypothesis is certainly a possibility. Nevertheless, since the D-type spines are not fully functional, synaptogenesis and consequently neuronal connectivity would be affected in the CPE-KO mice. Thus CPE is involved not only in efficient dendritic pruning, but also in appropriate spine formation and synaptogenesis. These dendritic architectural defects in cortical and CA1 neurons might contribute to the neurological deficits observed in the CPE-KO mouse, such as poor memory consolidation and learning (Cawley NX et al. 2004; Woronowicz et al. 2008).
It is well established that pruning which occurs in the distal part of the axon and in dendritic branches by means of retraction, degeneration and dendritic shedding is an essential part of the maintenance of the neuronal network (Singh et al. 2008). Although some studies on the molecular basis of axonal pruning have been reported, the molecular mechanisms underlying the regulation of dendritic pruning is less understood. Studies on Drosophila have shown a complex process of growing, pruning back and regrowth of an axonal network of a special group of peripheral sensory neurons during metamorphosis that is mediated by the ubiquitin-proteasome pathway. In mammals, axonal pruning has been described at the neuromuscular junction, climbing fibers in cerebellum, sympathetic eye-projecting neurons, and the hippocampal mossy fiber system (Johnson et al. 2007; Liu et al. 2005; Singh et al. 2008). The involvement of a number of molecules, such as AMPA glutamate receptors (Prithviraj et al. 2008), semaphorin and plexin ligand-receptor pairs (Waimey and Cheng 2006), as well as neurotrophins, particularly BDNF and proBDNF (Hu et al. 2005; Jansen et al. 2007; Lush et al. 2005) have been implicated in axonal pruning. The BDNF/proBDNF receptors: TrkB and p75NTR, have also been shown to be involved in axonal pruning of CNS neurons including climbing-fibers in cerebellum (Jaworski and Sheng 2006; Johnson et al. 2007; Kohn et al. 1999; Lee et al. 1994; Singh and Miller 2005; Singh et al. 2008). We have previously shown that the membrane-bound form of CPE is required as a sorting receptor to target proBDNF into the regulated secretory pathway vesicles for processing and activity–dependent secretion in cerebral cortical neurons (Cool et al. 1997; Lou et al. 2005). As a result, cerebral cortical neurons from CPE-KO mice showed poor processing of proBDNF to mature BDNF (Lou et al., 2005).One possibility is the decreased dendritic pruning in CPE-KO mice could be due to insufficient mature BDNF in these animals.
In conclusion, this study provides evidence that CPE plays a novel role in regulating proper dendritic patterning, especially dendritic pruning and spine formation which are necessary for appropriate synaptogenesis and the establishment of neuronal network. Further study would be required to identify the molecular mechanisms underlying CPE’s action in regulating dendritic pruning and spine morphogenesis in CNS neurons.
Acknowledgements
We thank Dr. Milton Brightman (NINCDS/NIH, Bethesda, Maryland) for his advice on the interpretation of our data and Dr. Ron Mervis (Neurostructural Research Labs, Inc, Center of Excellence in Aging and Brain Repair, University of South Florida) for help with the statistical analysis of the dendritic branching and spine morphology.
This research was supported by NIH grant #RO1NS049470 (Z-G.X.) and the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, USA.
Reference
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annual review of neuroscience. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley NX, Zhou J, Hill JM, Abebe D, Romboz S, Yanik T, Rodriguiz RM, Wetsel WC, YP L. The carboxypeptidase E knockout mouse exhibits endocrinological and behavioral deficits. Endocrinology. 2004;145(12):5807–5819. doi: 10.1210/en.2004-0847. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang PY, Ghosh A. Regulation of cortical dendrite development by Rap1 signaling. Mol Cell Neurosci. 2005;28(2):215–228. doi: 10.1016/j.mcn.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Cobos I, Borello U, Rubenstein JL. Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron. 2007;54(6):873–888. doi: 10.1016/j.neuron.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nature neuroscience. 2005;8(8):1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Cool DR, Normant E, Shen F, Chen HC, Pannell L, Zhang Y, Loh YP. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell. 1997;88(1):73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- De Roo M, Klauser P, Garcia PM, Poglia L, Muller D. Spine dynamics and synapse remodeling during LTP and memory processes. Progress in brain research. 2008;169:199–207. doi: 10.1016/S0079-6123(07)00011-8. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Woodson JC, Conrad CD, Bachstetter AD, Mervis RF. Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus. 2006;16(7):571–576. doi: 10.1002/hipo.20188. [DOI] [PubMed] [Google Scholar]
- Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39(2):283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- Fricker LD, Evans CJ, Esch FS, Herbert E. Cloning and sequence analysis of cDNA for bovine carboxypeptidase E. Nature. 1986;323(6087):461–464. doi: 10.1038/323461a0. [DOI] [PubMed] [Google Scholar]
- Fu Z, Lee SH, Simonetta A, Hansen J, Sheng M, Pak DT. Differential roles of Rap1 and Rap2 small GTPases in neurite retraction and synapse elimination in hippocampal spiny neurons. J Neurochem. 2007;100(1):118–131. doi: 10.1111/j.1471-4159.2006.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Petanjek Z, Gustafsson B, Ben-Ari Y, Hanse E, Khazipov R. In vivo blockade of neural activity alters dendritic development of neonatal CA1 pyramidal cells. The European journal of neuroscience. 2002;16(10):1931–1938. doi: 10.1046/j.1460-9568.2002.02264.x. [DOI] [PubMed] [Google Scholar]
- Haas K, Li J, Cline HT. AMPA receptors regulate experience-dependent dendritic arbor growth in vivo. Proc Natl Acad Sci U S A. 2006;103(32):12127–12131. doi: 10.1073/pnas.0602670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand R, Bortone D, Mattar P, Nguyen L, Heng JI, Guerrier S, Boutt E, Peters E, Barnes AP, Parras C, Schuurmans C, Guillemot F, Polleux F. Phosphorylation of Neurogenin2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron. 2005;48(1):45–62. doi: 10.1016/j.neuron.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Hu B, Nikolakopoulou AM, Cohen-Cory S. BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development. 2005;132(19):4285–4298. doi: 10.1242/dev.02017. [DOI] [PubMed] [Google Scholar]
- Hughes ME, Bortnick R, Tsubouchi A, Baumer P, Kondo M, Uemura T, Schmucker D. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron. 2007;54(3):417–427. doi: 10.1016/j.neuron.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY. The control of dendrite development. Neuron. 2003;40(2):229–242. doi: 10.1016/s0896-6273(03)00631-7. [DOI] [PubMed] [Google Scholar]
- Jansen P, Giehl K, Nyengaard JR, Teng K, Lioubinski O, Sjoegaard SS, Breiderhoff T, Gotthardt M, Lin F, Eilers A, Petersen CM, Lewin GR, Hempstead BL, Willnow TE, Nykjaer A. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nature neuroscience. 2007;10(11):1449–1457. doi: 10.1038/nn2000. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34(3):205–219. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- Johnson EM, Craig ET, Yeh HH. TrkB is necessary for pruning at the climbing fibre-Purkinje cell synapse in the developing murine cerebellum. J Physiol. 2007;582(Pt 2):629–646. doi: 10.1113/jphysiol.2007.133561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y, Goffinet AM. Reelin signals through phosphatidylinositol 3-kinase and Akt to control cortical development and through mTor to regulate dendritic growth. Mol Cell Biol. 2007;27(20):7113–7124. doi: 10.1128/MCB.00928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn J, Aloyz RS, Toma JG, Haak-Frendscho M, Miller FD. Functionally antagonistic interactions between the TrkA and p75 neurotrophin receptors regulate sympathetic neuron growth and target innervation. J Neurosci. 1999;19(13):5393–5408. doi: 10.1523/JNEUROSCI.19-13-05393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci. 2005;25(49):11288–11299. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KF, Bachman K, Landis S, Jaenisch R. Dependence on p75 for innervation of some sympathetic targets. Science. 1994;263(5152):1447–1449. doi: 10.1126/science.8128229. [DOI] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nature neuroscience. 2008;11(3):334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Liu XB, Low LK, Jones EG, Cheng HJ. Stereotyped axon pruning via plexin signaling is associated with synaptic complex elimination in the hippocampus. J Neurosci. 2005;25(40):9124–9134. doi: 10.1523/JNEUROSCI.2648-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase e. Neuron. 2005;45(2):245–255. doi: 10.1016/j.neuron.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Lush ME, Ma L, Parada LF. TrkB signaling regulates the developmental maturation of the somatosensory cortex. Int J Dev Neurosci. 2005;23(6):523–536. doi: 10.1016/j.ijdevneu.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Malone JI, Hanna S, Saporta S, Mervis RF, Park CR, Chong L, Diamond DM. Hyperglycemia not hypoglycemia alters neuronal dendrites and impairs spatial memory. Pediatric diabetes. 2008;9(6):531–539. doi: 10.1111/j.1399-5448.2008.00431.x. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M. Factors critical for the plasticity of dendritic spines and memory storage. Neuroscience research. 2007;57(1):1–9. doi: 10.1016/j.neures.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Morita A, Yamashita N, Sasaki Y, Uchida Y, Nakajima O, Nakamura F, Yagi T, Taniguchi M, Usui H, Katoh-Semba R, Takei K, Goshima Y. Regulation of dendritic branching and spine maturation by semaphorin3A-Fyn signaling. J Neurosci. 2006;26(11):2971–2980. doi: 10.1523/JNEUROSCI.5453-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi M, Katoh H. Regulation of neurite formation by Rho family GTPases. Seikagaku. 2002;74(5):395–398. [PubMed] [Google Scholar]
- Neigh GN, Glasper ER, Kofler J, Traystman RJ, Mervis RF, Bachstetter A, DeVries AC. Cardiac arrest with cardiopulmonary resuscitation reduces dendritic spine density in CA1 pyramidal cells and selectively alters acquisition of spatial memory. Eur J Neurosci. 2004;20(7):1865–1872. doi: 10.1111/j.1460-9568.2004.03649.x. [DOI] [PubMed] [Google Scholar]
- Park JJ, Cawley NX, Loh YP. A bi-directional carboxypeptidase E-driven transport mechanism controls BDNF vesicle homeostasis in hippocampal neurons. Mol Cell Neurosci. 2008;39(1):63–73. doi: 10.1016/j.mcn.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish JZ, Emoto K, Kim MD, Jan YN. Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annual review of neuroscience. 2007;30:399–423. doi: 10.1146/annurev.neuro.29.051605.112907. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Trojan S. Chronic changes in the receptive field of the pyramidal cells of the rat hippocampus after intermittent postnatal hypoxia. Physiologia Bohemoslovaca. 1983;32(5):393–402. [PubMed] [Google Scholar]
- Pokorny J, Trojan S. The development of hippocampal structure and how it is influenced by hypoxia. Acta Universitatis Carolinae Medica. 1986;113:1–79. [PubMed] [Google Scholar]
- Prithviraj R, Kelly KM, Espinoza-Lewis R, Hexom T, Clark AB, Inglis FM. Differential regulation of dendrite complexity by AMPA receptor subunits GluR1 and GluR2 in motor neurons. Dev Neurobiol. 2008;68(2):247–264. doi: 10.1002/dneu.20590. [DOI] [PubMed] [Google Scholar]
- Redmond L, Oh SR, Hicks C, Weinmaster G, Ghosh A. Nuclear Notch1 signaling and the regulation of dendritic development. Nature neuroscience. 2000;3(1):30–40. doi: 10.1038/71104. [DOI] [PubMed] [Google Scholar]
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nature neuroscience. 2005;8(1):34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- Schaefer AT, Larkum ME, Sakmann B, Roth A. Coincidence detection in pyramidal neurons is tuned by their dendritic branching pattern. J Neurophysiol. 2003;89(6):3143–3154. doi: 10.1152/jn.00046.2003. [DOI] [PubMed] [Google Scholar]
- Scott EK, Luo L. How do dendrites take their shape? Nature neuroscience. 2001;4(4):359–365. doi: 10.1038/86006. [DOI] [PubMed] [Google Scholar]
- Silva-Gomez AB, Rojas D, Juarez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain research. 2003;983(1–2):128–136. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature. 2002;419(6906):475–480. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- Singh KK, Miller FD. Activity regulates positive and negative neurotrophin-derived signals to determine axon competition. Neuron. 2005;45(6):837–845. doi: 10.1016/j.neuron.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Singh KK, Park KJ, Hong EJ, Kramer BM, Greenberg ME, Kaplan DR, Miller FD. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nature neuroscience. 2008;11(6):649–658. doi: 10.1038/nn.2114. [DOI] [PubMed] [Google Scholar]
- Tolias KF, Bikoff JB, Burette A, Paradis S, Harrar D, Tavazoie S, Weinberg RJ, Greenberg ME. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45(4):525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Tolwani RJ, Buckmaster PS, Varma S, Cosgaya JM, Wu Y, Suri C, Shooter EM. BDNF overexpression increases dendrite complexity in hippocampal dentate gyrus. Neuroscience. 2002;114(3):795–805. doi: 10.1016/s0306-4522(02)00301-9. [DOI] [PubMed] [Google Scholar]
- Ueda S, Fujimoto S, Hiramoto K, Negishi M, Katoh H. Dock4 regulates dendritic development in hippocampal neurons. J Neurosci Res. 2008;86(14):3052–3061. doi: 10.1002/jnr.21763. [DOI] [PubMed] [Google Scholar]
- Valverde F. Structure of the cerebral cortex. Intrinsic organization and comparative analysis of the neocortex. Rev Neurol. 2002;34(8):758–780. [PubMed] [Google Scholar]
- Waimey KE, Cheng HJ. Axon pruning and synaptic development: how are they per-plexin? Neuroscientist. 2006;12(5):398–409. doi: 10.1177/1073858406292631. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50(6):897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Wirth MJ, Brun A, Grabert J, Patz S, Wahle P. Accelerated dendritic development of rat cortical pyramidal cells and interneurons after biolistic transfection with BDNF and NT4/5. Development. 2003;130(23):5827–5838. doi: 10.1242/dev.00826. [DOI] [PubMed] [Google Scholar]
- Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3(10):803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- Woronowicz A, Koshimizu H, Chang SY, Cawley NX, Hill JM, Rodriguiz RM, Abebe D, Dorfman C, Senatorov V, Zhou A, Xiong ZG, Wetsel WC, Loh YP. Absence of carboxypeptidase E leads to adult hippocampal neuronal degeneration and memory deficits. Hippocampus. 2008;18(10):1051–1063. doi: 10.1002/hipo.20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JI, Lessard J, Olave IA, Qiu Z, Ghosh A, Graef IA, Crabtree GR. Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron. 2007;56(1):94–108. doi: 10.1016/j.neuron.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Zhu XWK, Rife L, Cawley NX, Brown B, Adams T, Teofilo K, Lillo C, Williams DS, Loh YP, Craft CM. Carboxypeptidase E is required for normal synaptic transmission from photoreceptors to the inner retina. J Neurochem. 2005;95(5):1351–1362. doi: 10.1111/j.1471-4159.2005.03460.x. (5):1351–1362. [DOI] [PubMed] [Google Scholar]