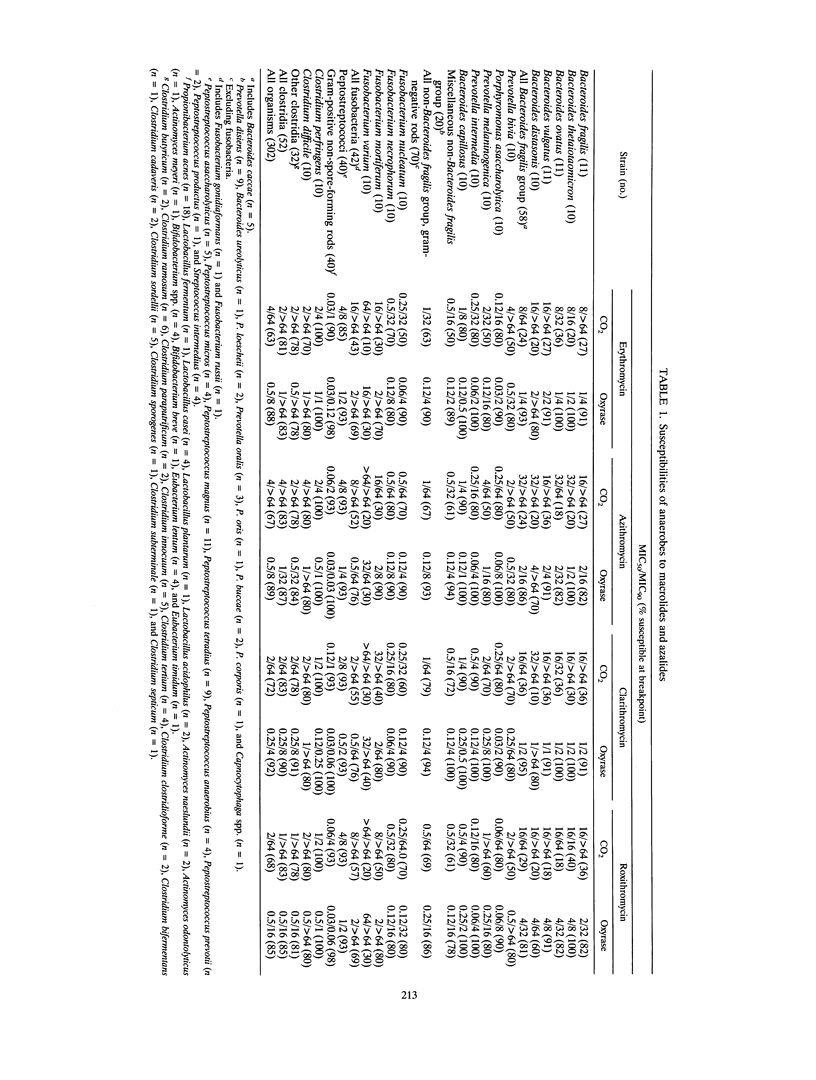
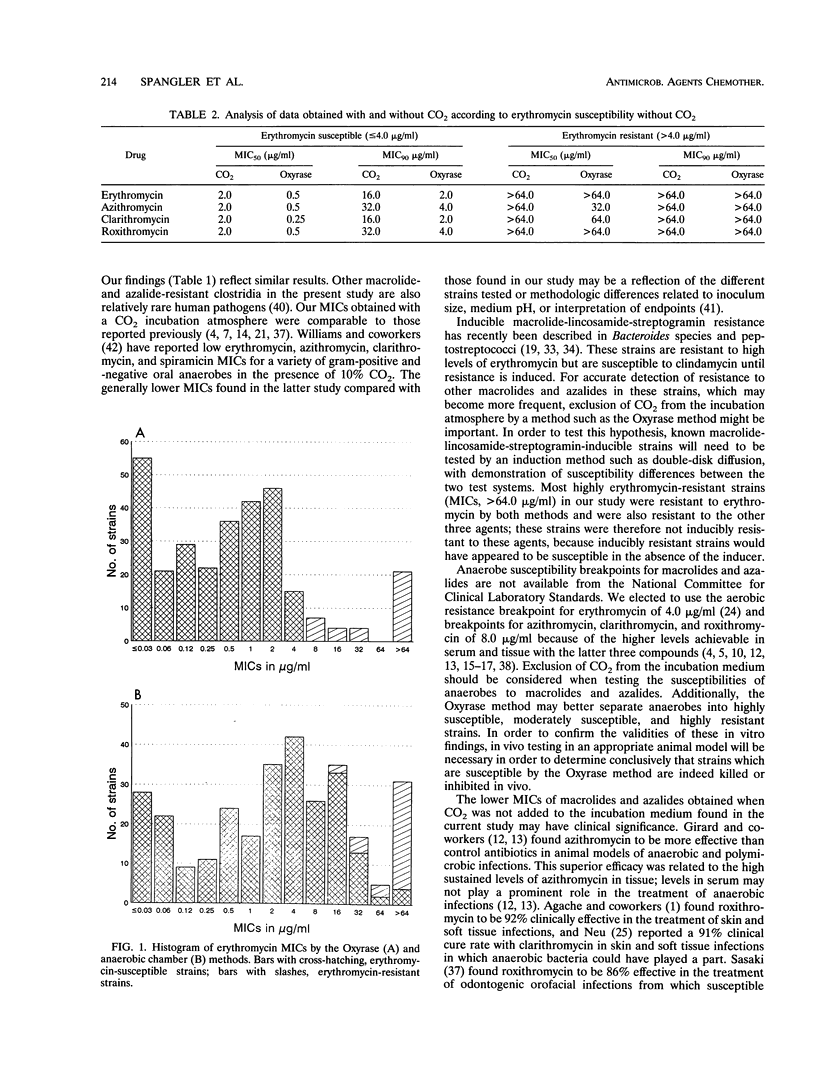
Abstract
The Oxyrase agar dilution method (Oxyrase, Inc., Mansfield, Ohio), which provides an anaerobic environment without added CO2, was compared with the reference agar dilution method recommended by the National Committee for Clinical Laboratory Standards (anaerobic chamber with 10% CO2) to test the susceptibilities of 302 gram-negative and gram-positive anaerobes to erythromycin, azithromycin, clarithromycin, and roxithromycin. For erythromycin, the overall MIC for 50% of isolates tested (MIC50) was 0.5 micrograms/ml and the MIC90 was 8.0 micrograms/ml by the Oxyrase method, whereas they were 4.0 and 64.0 micrograms/ml, respectively, under standard anaerobic conditions with CO2. At a breakpoint of 4.0 micrograms/ml, 88% of strains were susceptible to erythromycin by the Oxyrase method, whereas 63% were susceptible in the chamber. The corresponding MIC50s and MIC90s of azithromycin, clarithromycin, and roxithromycin by the Oxyrase method were 0.5 and 8.0, 0.25 and 4.0, and 0.5 and 16.0 micrograms/ml, respectively, whereas in the chamber they were 4.0 and > 64.0, 2.0 and 64.0, and 2.0 and 64.0 micrograms/ml, respectively. At a breakpoint of 8.0 micrograms/ml for these three drugs, 89, 92, and 85% of the isolates, respectively, were susceptible by the Oxyrase method, whereas 67%, 72, and 68% of the isolates, respectively, were susceptible in the chamber. Most strains resistant to all four compounds by both methods were Bacteroides distasonis, Fusobacterium mortiferum, Fusobacterium varium and non-Clostridium perfringens Clostridium species. Results of the study may lead to a reappraisal of the role played by macrolides and azalides in the treatment of anaerobic infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agache P., Amblard P., Moulin G., Barrière H., Texier L., Beylot C., Bergoend H. Roxithromycin in skin and soft tissue infections. J Antimicrob Chemother. 1987 Nov;20 (Suppl B):153–156. doi: 10.1093/jac/20.suppl_b.153. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Fuchs P. C. In-vitro potency of azithromycin against gram-negative bacilli is method-dependent. J Antimicrob Chemother. 1991 Oct;28(4):607–610. doi: 10.1093/jac/28.4.607. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Fuchs P. C. Influence of the test medium on azithromycin and erythromycin regression statistics. Eur J Clin Microbiol Infect Dis. 1991 Oct;10(10):846–849. doi: 10.1007/BF01975838. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N., Thornsberry C. In vitro activities of azithromycin (CP 62,993), clarithromycin (A-56268; TE-031), erythromycin, roxithromycin, and clindamycin. Antimicrob Agents Chemother. 1988 May;32(5):752–754. doi: 10.1128/aac.32.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergogne-Bérézin E. Tissue distribution of roxithromycin. J Antimicrob Chemother. 1987 Nov;20 (Suppl B):113–120. doi: 10.1093/jac/20.suppl_b.113. [DOI] [PubMed] [Google Scholar]
- Dubreuil L. In-vitro comparison of roxithromycin and erythromycin against 900 anaerobic bacterial strains. J Antimicrob Chemother. 1987 Nov;20 (Suppl B):13–19. doi: 10.1093/jac/20.suppl_b.13. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Bailer R., Swanson R., Hanson C. W., McDonald E., Ramer N., Hardy D., Shipkowitz N., Bower R. R., Gade E. In vitro and in vivo evaluation of A-56268 (TE-031), a new macrolide. Antimicrob Agents Chemother. 1986 Dec;30(6):865–873. doi: 10.1128/aac.30.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds G., Shepard R. M., Johnson R. B. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- George W. L., Kirby B. D., Sutter V. L., Citron D. M., Finegold S. M. Gram-negative anaerobic bacilli: Their role in infection and patterns of susceptibility to antimicrobial agents. II. Little-known Fusobacterium species and miscellaneous genera. Rev Infect Dis. 1981 May-Jun;3(3):599–626. doi: 10.1093/clinids/3.3.599. [DOI] [PubMed] [Google Scholar]
- Girard A. E., Girard D., English A. R., Gootz T. D., Cimochowski C. R., Faiella J. A., Haskell S. L., Retsema J. A. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987 Dec;31(12):1948–1954. doi: 10.1128/aac.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard A. E., Girard D., Retsema J. A. Correlation of the extravascular pharmacokinetics of azithromycin with in-vivo efficacy in models of localized infection. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):61–71. doi: 10.1093/jac/25.suppl_a.61. [DOI] [PubMed] [Google Scholar]
- Hansen S. L., Swomley P., Drusano G. Effect of carbon dioxide and pH on susceptibility of Bacteroides fragilis group to erythromycin. Antimicrob Agents Chemother. 1981 Feb;19(2):335–336. doi: 10.1128/aac.19.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson C. W., Bailer R., Gade E., Rode R. A., Fernandes P. B. Regression analysis, proposed interpretative zone size standards, and quality control guidelines for a new macrolide antimicrobial agent, A-56268 (TE-031). J Clin Microbiol. 1987 Jun;25(6):1079–1082. doi: 10.1128/jcm.25.6.1079-1082.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D. J., Guay D. R., Jones R. N. Clarithromycin, a unique macrolide. A pharmacokinetic, microbiological, and clinical overview. Diagn Microbiol Infect Dis. 1992 Jan;15(1):39–53. doi: 10.1016/0732-8893(92)90055-x. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Fuchs P. C., Thornsberry C. Disk diffusion susceptibility testing of two macrolide antimicrobial agents: revised interpretive criteria for erythromycin and preliminary guidelines for roxithromycin (RU 965). J Clin Microbiol. 1986 Aug;24(2):233–239. doi: 10.1128/jcm.24.2.233-239.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzis M. D., Goldstein F. W., Miégi M., Acar J. F. In-vitro activity of azithromycin against various Gram-negative bacilli and anaerobic bacteria. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):15–18. doi: 10.1093/jac/25.suppl_a.15. [DOI] [PubMed] [Google Scholar]
- Leclercq R., Courvalin P. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 1991 Jul;35(7):1267–1272. doi: 10.1128/aac.35.7.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan M. N., Ashby J. P., Andrews J. M., Wise R. The in-vitro and disc susceptibility testing of clarithromycin and its 14-hydroxy metabolite. J Antimicrob Chemother. 1991 Feb;27(2):161–170. doi: 10.1093/jac/27.2.161. [DOI] [PubMed] [Google Scholar]
- Maskell J. P., Sefton A. M., Williams J. D. Comparative in-vitro activity of azithromycin and erythromycin against Gram-positive cocci, Haemophilus influenzae and anaerobes. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):19–24. doi: 10.1093/jac/25.suppl_a.19. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The development of macrolides: clarithromycin in perspective. J Antimicrob Chemother. 1991 Feb;27 (Suppl A):1–9. doi: 10.1093/jac/27.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- Nilsen O. G., Aamo T., Zahlsen K., Svarva P. Macrolide pharmacokinetics and dose scheduling of roxithromycin. Diagn Microbiol Infect Dis. 1992 May-Jun;15(4 Suppl):71S–76S. doi: 10.1016/0732-8893(92)90130-l. [DOI] [PubMed] [Google Scholar]
- Nilsen O. G. Comparative pharmacokinetics of macrolides. J Antimicrob Chemother. 1987 Nov;20 (Suppl B):81–88. doi: 10.1093/jac/20.suppl_b.81. [DOI] [PubMed] [Google Scholar]
- Osono T., Umezawa H. Pharmacokinetics of macrolides, lincosamides and streptogramins. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):151–166. doi: 10.1093/jac/16.suppl_a.151. [DOI] [PubMed] [Google Scholar]
- Pechére J. C., Auckenthaler R. In-vitro activity of roxithromycin against respiratory and skin pathogens. J Antimicrob Chemother. 1987 Nov;20 (Suppl B):1–5. doi: 10.1093/jac/20.suppl_b.1. [DOI] [PubMed] [Google Scholar]
- Puri S. K., Lassman H. B. Roxithromycin: a pharmacokinetic review of a macrolide. J Antimicrob Chemother. 1987 Nov;20 (Suppl B):89–100. doi: 10.1093/jac/20.suppl_b.89. [DOI] [PubMed] [Google Scholar]
- Reig M., Campello M. G., Baquero F. Inducible macrolides-lincosamides-streptogramin B resistance in Bacteroides species. Antimicrob Agents Chemother. 1987 Apr;31(4):665–666. doi: 10.1128/aac.31.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig M., Moreno A., Baquero F. Resistance of Peptostreptococcus spp. to macrolides and lincosamides: inducible and constitutive phenotypes. Antimicrob Agents Chemother. 1992 Mar;36(3):662–664. doi: 10.1128/aac.36.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retsema J., Girard A., Schelkly W., Manousos M., Anderson M., Bright G., Borovoy R., Brennan L., Mason R. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother. 1987 Dec;31(12):1939–1947. doi: 10.1128/aac.31.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki J. Clinical evaluation of roxithromycin in odontogenic orofacial infections. J Antimicrob Chemother. 1987 Nov;20 (Suppl B):167–170. doi: 10.1093/jac/20.suppl_b.167. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Ballow C. H. Tissue-directed pharmacokinetics. Am J Med. 1991 Sep 12;91(3A):5S–11S. doi: 10.1016/0002-9343(91)90394-d. [DOI] [PubMed] [Google Scholar]
- Spangler S. K., Appelbaum P. C. Oxyrase, a method which avoids CO2 in the incubation atmosphere for anaerobic susceptibility testing of antibiotics affected by CO2. J Clin Microbiol. 1993 Feb;31(2):460–462. doi: 10.1128/jcm.31.2.460-462.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. D., Maskell J. P., Shain H., Chrysos G., Sefton A. M., Fraser H. Y., Hardie J. M. Comparative in-vitro activity of azithromycin, macrolides (erythromycin, clarithromycin and spiramycin) and streptogramin RP 59500 against oral organisms. J Antimicrob Chemother. 1992 Jul;30(1):27–37. doi: 10.1093/jac/30.1.27. [DOI] [PubMed] [Google Scholar]



