Abstract
GABAergic neurons in the caudal ventrolateral medulla (CVLM) are driven by baroreceptor inputs relayed via the nucleus tractus solitarius (NTS), and they inhibit neurons in rostral ventrolateral medulla to reduce sympathetic nerve activity (SNA) and arterial pressure (AP). After arterial baroreceptor denervation or lesions of the NTS, inhibition of the CVLM continues to increase AP, suggesting additional inputs also tonically activate the CVLM. This study examined whether the NTS contributes to baroreceptor-independent drive to the CVLM and whether glutamate promotes baroreceptor- and NTS-independent activation of the CVLM to tonically reduce SNA. In addition, we evaluated whether altering central respiratory drive, a baroreceptor-independent regulator of CVLM neurons, influences glutamatergic inputs to the CVLM. Splanchnic SNA and AP were measured in chloralose-anesthetized, ventilated, paralyzed rats. The infusion of nitroprusside decreased AP below threshold for baroreceptor afferent firing (<50 mmHg) and increased SNA to 209 ± 22% (P < 0.05), but the subsequent inhibition of the NTS by microinjection of the GABAA agonist muscimol did not further increase SNA. In contrast, after inhibition of the NTS, blockade of glutamatergic inputs to CVLM by microinjection of kynurenate increased SNA (274 ± 54%; P < 0.05; n = 7). In vagotomized rats with baroreceptors unloaded, inhibition of glutamatergic inputs to CVLM evoked a larger rise in SNA when central respiratory drive was increased (219 ± 16% vs. 271 ± 17%; n = 5; P < 0.05). These data suggest that baroreceptor inputs provide the major drive for the NTS-mediated excitation of the CVLM. Furthermore, glutamate tonically activates the CVLM to reduce SNA independent of the NTS, and this excitatory input appears to be affected by the strength of central respiratory drive.
Keywords: splanchnic sympathetic nerve activity, baroreceptor, muscimol, kynurenate, respiratory, caudal ventrolateral medulla, nucleus tractus solitarius
neurons in the caudal ventrolateral medulla (CVLM) exert a powerful, tonic inhibitory influence on sympathetic nerve activity (SNA) and arterial pressure (AP) by releasing GABA onto spinally projecting, presympathetic neurons in the rostral ventrolateral medulla (RVLM) (18). Acute stimulation of cell bodies within the CVLM silences presympathetic RVLM neurons to reduce sympathetic vasomotor tone and AP (1, 11). Conversely, inhibition of CVLM neuronal activity or blockade of glutamatergic inputs to the region significantly increases RVLM neuronal activity, SNA, and AP (2, 3, 15, 20). The CVLM is an essential relay for many sympathoinhibitory reflexes evoked by activation of the nucleus tractus solitarius (NTS). Accordingly, blockade of glutamatergic inputs to the CVLM eliminates the decrease in RVLM activity and SNA evoked by stimulation of arterial baroreceptors (1, 8).
Although the CVLM is clearly driven by arterial baroreceptor inputs to the NTS, the CVLM tonically restrains SNA independent of these inputs. For example, inhibition of the CVLM continues to increase SNA and AP after acute transection of baroreceptor afferent nerves (4). In addition, after acute lesions of the NTS, inhibition of the CVLM or blockade of GABAergic receptors in the RVLM continues to increase AP (5, 6). The baroreceptor-related and -independent regulation of the CVLM is observed in extracellular recordings of individual GABAergic neurons in the region (18, 19). These CVLM neurons are activated by elevations in AP and have reduced activity when AP is decreased to unload baroreceptor inputs (19). Nevertheless, many baroactivated CVLM neurons still fire when AP is reduced below the threshold for baroreceptor afferent nerve activity (10, 17, 18), suggesting these CVLM neurons are also driven by other inputs. The sources and nature of these baroreceptor-independent inputs to the CVLM are not known.
Central respiratory neurons appear to provide baroreceptor-independent inputs to CVLM neurons that regulate SNA. Sympathetic vasomotor nerves display respiratory-related activity, and the strength of this modulation is enhanced by elevated central respiratory drive. Likewise, baroactivated GABAergic CVLM neurons also fire in relation to the central respiratory cycle, and this respiratory-related activity in CVLM neurons and SNA persists after baroreceptor unloading by hypotension (9, 10). Furthermore, central respiratory neurons appear to be essential for normal CVLM function, because lesions of glutamatergic central respiratory neurons attenuate the ability of the CVLM to decrease AP (28). Whether central respiratory neurons make direct connections to baroactivated CVLM neurons or modulate CVLM activity through the NTS is not known.
In the present study, we determined whether the NTS may promote excitation of the CVLM in the acute absence of baroreceptor inputs. We also examined the hypothesis that the baroreceptor- and NTS-independent drive to the CVLM is primarily glutamatergic. To examine the hypothesis that altering the strength of central respiratory drive affects the baroreceptor-independent, glutamatergic drive to the CVLM, we measured sympathetic responses to the blockade of glutamatergic receptors in the CVLM under conditions of reduced and elevated Pco2.
MATERIALS AND METHODS
Animals.
Procedures were performed on adult male Sprague-Dawley rats (275–350 g; Harlan, Indianapolis, IN). Animals were pair-housed in the temperature and humidity-controlled Medical College of Georgia animal facility and were provided ad libitum access to Purina Rat Chow and tap water. A standard 12-h light cycle was observed. Experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the approval of the Medical College of Georgia Institutional Animal Care and Use Committee.
Surgical procedures.
Rats were initially anesthetized with 2.5% isoflurane to cannulate a femoral artery and vein to measure AP and deliver drugs, respectively. Rectal temperature was maintained at 37°C (TC-1000 Temperature Controller; Charles Ward Electronics). Rats were artificially ventilated with 100% oxygen (Rodent Ventilator Model 683; Harvard), and end tidal CO2 was maintained at 3.5–4.5% (Capstar-100; Charles Ward Electronics). In experiments where the influence of central respiratory drive on inputs to the CVLM was evaluated, ventilation rate was altered to increase or decrease central respiratory drive. To focus on changes in central respiratory drive, the cervical vagus nerves were cut distal to the junction with the superior laryngeal nerve to eliminate potentially altered vagal signals due to changes in the ventilation rate. Rats were placed into a stereotaxic frame (David Kopf Instruments) with the bite bar set at −11 mm. The left splanchnic nerve was isolated, and activity was recorded from a pair of Teflon-coated silver wires bared at the tips and embedded in dental impression material (polyvinylsiloxane; Super-Dent) as previously described (10). The dorsal surface of the brainstem was exposed by removing the occipital bone and retracting the underlying dura mater. Isoflurane was slowly withdrawn as rats were infused with α-chloralose (60 mg/kg iv; Fisher Scientific). Rats were allowed to recover for 30 min following surgical procedures and change of anesthesia before experimental protocols began. After adequate anesthesia (<10 mmHg change in AP with firm toe pinch) was ensured, the rats were paralyzed with 1 mg/kg pancuronium (Hospira). Supplemental doses of chloralose and pancuronium (1/3 original dose) were administered hourly.
Microinjections.
Calibrated glass pipettes (5 μl; VWR) were pulled, broken to a tip diameter of 20–30 μm, mounted on a stereotaxic arm, and connected to a pressure microinjection apparatus (Picospritzer III; Parker Instrumentation) (12). Microinjections into the NTS were performed bilaterally using previously described coordinates (0.5 mm rostral, 0.5 mm lateral, and 0.5 mm ventral to calamus scriptorius and the surface of the brain stem) (21). Microinjections into the CVLM were performed bilaterally as previously described (1.0 mm rostral, 1.8 mm lateral, and 2.6 mm ventral from calamus scriptorius and the dorsal brain stem surface) (20). The sites for NTS and CVLM were functionally verified by visualization depressor responses to microinjection of glutamate (1 nmol; >15 mmHg). Subsequent microinjections of muscimol (100 pmol) or kynurenate (5 nmol) contained 5% red or green latex microspheres (Lumiphore) for histological confirmation of the injection sites. All injection volumes were 100 nl, and drugs were diluted in artificial cerebrospinal fluid. The absence of a sympathoinhibitory response to phenyl biguanide (20 μg/kg iv) was used to verify the efficacy of the blockade in the NTS (21). In rats where the vagi were cut, the anterior end of the right vagus nerve was stimulated (5 Hz, 5 V, 10 s) to verify the functional blockade of the CVLM. In control states, these stimulation parameters evoked decreases in SNA and AP as seen previously (23), and these responses were eliminated after the inhibition of the CVLM.
In a subset of rats, physiological responses were examined after the unloading of the baroreceptors by a slow infusion of sodium nitroprusside (50 μl/min of a 1 mg/ml solution iv). In some of these rats (n = 2), a second catheter was inserted into the contralateral femoral vein to allow for testing responses to phenyl biguanide after the inhibition of the NTS during the nitroprusside infusion.
Blood gas measurements.
Blood gases were analyzed in animals with altered central respiratory drive using the i-STAT portable clinical analyzer (Heska). i-STAT CG8+ cartridges were used to determine Pco2, Po2, and pH. Blood was withdrawn (0.1 ml) from the arterial catheter 5–10 min before experimental protocols.
Histological processing.
Rats were perfused with 4% formaldehyde, and brains were stored in the same fixative for 48 h. The brain stems were sectioned coronally at 50 μm using a Vibratome. Sections were mounted onto glass slides, and coverslips were affixed with Krystalon (VWR). Injection sites were visualized under epifluorescence using an Olympus microscope (BX60). With the use of the Neurolucida system and a Ludl motor-driven stage, the outlines of the sections containing representative injection sites were drawn along with the injection sites and nearby landmarks as previously described (19).
Data analysis.
The raw SNA was amplified, filtered (Differential AC Amplifier Model 1700; A-M Systems), and integrated into 1-s bins as previously described (10, 12). The changes in SNA were evaluated at a percent change from baseline (100%) with the zero value determined at the end of the experiment by an intravenous injection of clonidine (50 μg/kg) (10, 12). Physiological variables were amplified with the Neurolog system (Digitimer Limited), digitized (Micro 1401l; Cambridge Electronic Design), and visualized using Spike2 software (CED).
For microinjection experiments, the initial baseline AP and SNA reflect a 60-s average immediately preceding any manipulations. To measure cumulative effects of the microinjections upon SNA, the 100% baseline before any manipulation was maintained throughout the experiment, and the SNA level following each set of bilateral microinjections was measured. To measure the effect of each blockade within the NTS or CVLM, the 100% baseline SNA was reset immediately before each set of bilateral microinjections. The maximum change in SNA within 1 min following the second microinjection was estimated with a 30-s window. Because the physiological variables lag behind the neural changes (see Fig. 2), the maximum changes in AP and HR were recorded within the first 5 min after the second microinjection. To evaluate the time course of changes in SNA and AP following bilateral microinjections of kynurenate into the CVLM, the original baseline 100% was used. The initial SNA value (time 0) was estimated immediately preceding the microinjections of kynurenate, and the next values were taken every 30 s for 10 min following the second microinjection of kynurenate into the CVLM.
Maximum changes from 100% baseline SNA were assessed by Wilcoxon signed rank tests. Differences in the change in AP or SNA due to serial injections were compared by paired t-tests with repeated measures or by one-way ANOVA with repeated measures followed by Newman-Keuls post hoc tests. To assess the time course of changes in SNA and AP, data were analyzed by a one-way ANOVA with repeated measures followed by Newman-Keuls post hoc tests. Significance was set at P < 0.05 for all statistical comparisons.
RESULTS
Functional and histological verification of injection sites.
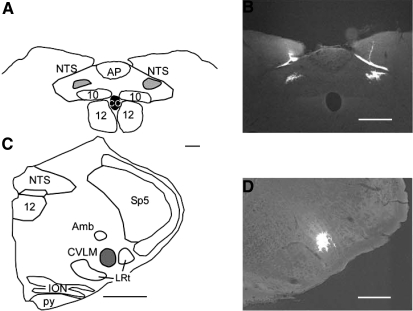
The locations of the NTS and CVLM were confirmed functionally using a discrete microinjection of glutamate into the specified stereotaxic coordinates. Microinjection of glutamate into the NTS decreased SNA by 81 ± 5% (range, 40–100%) and mean AP by 34 ± 4 mmHg (range, 15–58 mmHg). Likewise, microinjection of glutamate into the CVLM decreased SNA by 88 ± 4% change (range, 62–100%) and mean AP by 25 ± 2 mmHg (range, 15–44 mmHg). Injected fluorescent beads were found where expected within the regions of the intermediate NTS at the rostro-caudal level of area postrema and in the CVLM at the rostro-caudal level of the rostral lateral reticular nucleus (Fig. 1) (16). Since the beads did not travel very far within these regions, they were considered to be an indication of the center of the injection site as opposed to a realistic representation of the functional spread of the neuroactive drugs.
Fig. 1.
Representative histological confirmation of microinjections into the intermediate nucleus tractus solitarius (NTS) and caudal ventrolateral medulla (CVLM). A: example of a reconstruction of typical injection sites into the NTS confirmed by the deposition of fluorescent beads, shown on the dorsal portion of a coronal section through the medulla oblongata at bregma −13.7 mm. B: photomicrograph of typical injection sites into the NTS. In some rats, beads were found along the top of the NTS separate from the injection deposit. This was considered to be a deposit due to leakage during the insertion or withdrawal of the pipette and was not included in the drawing of the microinjection deposit shown in A. C: example of a typical microinjection into the CVLM confirmed by the deposition of fluorescent beads, shown on a medullary hemisection in the coronal plane at bregma −13.0 mm. D: photomicrograph of a typical injection site into the CVLM. Amb, nucleus ambiguus; AP, area postrema; CC, central canal; ION, inferior olivary nucleus; LRt, lateral reticular nucleus; Py, pyramidal tracts; Sp5, spinal trigeminal nucleus; 10, dorsal vagal motor nucleus; 12, hypoglossal nucleus. Scale bars are 500 μm in A and C and 250 μm in B and D.
Effects of serial inhibition of the NTS and CVLM during baroreceptor unloading by hypotension.
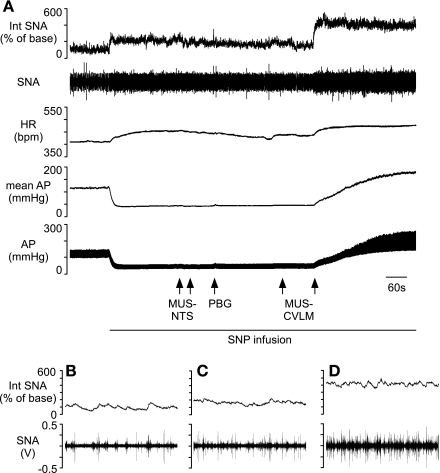
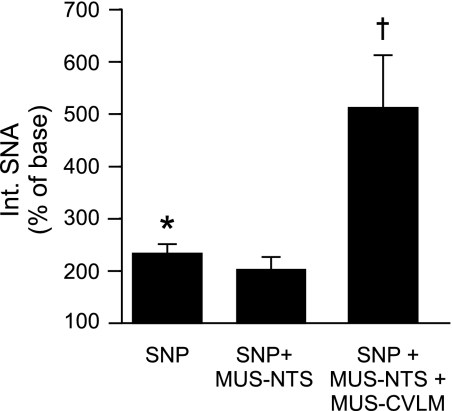
To determine whether the NTS contributes a significant baroreceptor-independent influence upon sympathetic vasomotor tone, the NTS was inhibited while baroreceptor inputs were minimized by hypotension. In this group of five rats, baseline mean AP was 124 ± 7 mmHg and heart rate (HR) was 368 ± 9 beats/min. Nitroprusside was infused slowly to decrease and maintain mean AP below baroreceptor threshold (Table 1 and Fig. 2) (17, 25). Nitroprusside-induced hypotension evoked significant increases in SNA and HR (Table 1 and Figs. 2 and 3). The subsequent inhibition of the NTS by bilateral microinjection of the GABAA agonist muscimol produced no further changes in SNA, HR, or mean AP (Table 1 and Figs. 2 and 3). The reflexive inhibition of SNA in response to an intravenous injection of phenyl biguanide was reversed or abolished in all rats tested, indicating a functional inhibition of the NTS (see Fig. 2). To ensure that it was possible to further increase SNA, the CVLM was subsequently inhibited with muscimol during the combined nitroprusside infusion and blockade of neuronal activity within the NTS (Figs. 2 and 3). Under these conditions, bilateral microinjection of muscimol into the CVLM evoked considerable further increases in SNA, HR, and mean AP (Table 1 and Figs. 2 and 3).
Table 1.
Effects of infusion of sodium nitroprusside during microinjections of muscimol into the NTS and CVLM on splanchnic SNA, HR, and mean AP
| Change in Int SNA, % | Change in HR, beats/min | Change in Mean AP, mmHg | |
|---|---|---|---|
| SNP | 209±22* | 26±3* | −82±7* |
| Mus in NTS | 3±2 | 0±3 | 1±1 |
| Mus in CVLM | 291±56* | 40±4* | 50±23* |
Values are means ± SE and reflect immediate changes in integrated (Int) splanchnic sympathetic nerve activity (SNA), heart rate (HR), and mean arterial pressure (AP) with each manipulation; n = 5 rats/group. During this protocol, sodium nitroprusside (SNP) infusion was maintained during the microinjections of muscimol (Mus) into the nucleus tractus solitarius (NTS) and caudal ventrolateral medulla (CVLM). In addition, the microinjections of Mus into the CVLM were performed during the steady-state responses to both SNP and Mus in the NTS. The cumulative effects of these three manipulations on SNA are shown in Figs. 2 and 3.
P < 0.05, significant change from 100% baseline value preceding each set of bilateral microinjections.
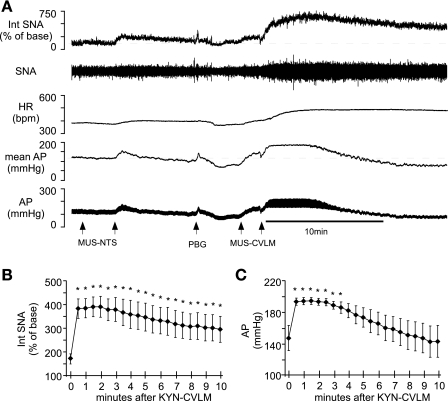
Fig. 2.
Representative responses to bilateral inhibition of the NTS and CVLM during infusion of sodium nitroprusside (SNP). A: infusion of SNP throughout the protocol reduced AP below baroreceptor threshold and increased sympathetic nerve activity (SNA) and heart rate (HR). Inhibition of the NTS by bilateral microinjection of muscimol (Mus; at arrowheads) during infusion of SNP did not change SNA, HR, or AP. Inhibition of the NTS abolished the sympathoinhibitory response to phenyl biguanide (PBG; at arrowhead). Subsequent inhibition of CVLM by bilateral microinjection of Mus during infusion of SNP significantly raised SNA, HR, and AP. Ten-second windows of SNA at baseline (B), after Mus-NTS (C), and after Mus-CVLM (D) show the increase in splanchnic nerve burst frequency with each manipulation. bpm, Beats/minute; Int, integrated.
Fig. 3.
Cumulative effects of serial inhibition of NTS and CVLM during infusion of SNP on SNA. Infusion of SNP to unload baroreceptors significantly raised SNA (*P < 0.05; n = 5). Under this condition, inhibition of the NTS with Mus did not alter SNA, but subsequent inhibition of CVLM with Mus significantly increased SNA above the level achieved by unloading baroreceptors with infusion of SNP. †P < 0.05, significantly different from SNA during SNP infusion alone. To measure the cumulative effects upon SNA, the initial baseline SNA was used for all measures.
Sympathetic and cardiovascular responses to inhibition of the NTS and CVLM.
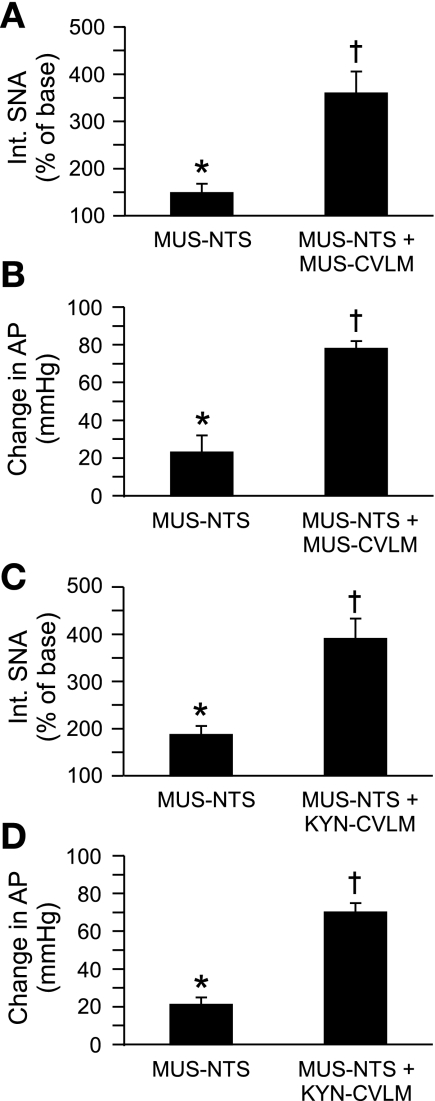
To assess the ability of the CVLM to alter SNA and AP after an acute inhibition of neurons in the intermediate NTS, the CVLM was inhibited by microinjection of muscimol after the confirmed inhibition of the NTS without the influence of nitroprusside. In this group (n = 6), baseline mean AP was 112 ± 12 mmHg and HR was 374 ± 22 beats/min. Inhibition of neuronal activity in the NTS by the bilateral microinjection of muscimol increased SNA, HR, and mean AP, as expected (Table 2 and Fig. 4) (14, 21). In each of these rats, the sympathoinhibitory response to phenyl biguanide was abolished. Subsequent microinjection of muscimol into the CVLM evoked significant increases in SNA, HR, and mean AP (Table 2 and Fig. 4). Because a recent report observing depressor responses to the serial inhibition of the NTS and CVLM used rats ventilated on room air (13), we examined these responses in a subset of rats (n = 2) ventilated on room air. The physiological responses to the inhibition of the NTS and CVLM were not different from those ventilated with 100% oxygen (n = 4), so the rats were pooled for group analyses.
Table 2.
Effects of serial inhibition of the NTS and CVLM on splanchnic SNA, HR, and mean AP
| Change in Int SNA, % | Change in HR, beats/min | Change in Mean AP, mmHg | |
|---|---|---|---|
| Group 1 | |||
| Mus in NTS | 148±19* | 10±4* | 23±8* |
| Mus in CVLM | 208±16* | 40±4* | 48±10* |
| Group 2 | |||
| Mus in NTS | 187±17* | 10±6* | 23±4* |
| Kyn in CVLM | 274±54* | 46±18* | 50±16* |
Values are means ± SE and reflect immediate changes in Int SNA, HR, and mean AP with each manipulation; n, no. of rats/group. The microinjections of Mus (n = 6) or kynurenate (Kyn; n = 7) into the CVLM were performed during the steady state of responses to Mus in the NTS. The cumulative effects of these two manipulations on SNA and mean AP are shown in Figs. 4, 5, and 6.
P < 0.05, significant change from 100% baseline value preceding each set of bilateral microinjections.
Fig. 4.
Cumulative effects of inhibition of NTS and inhibition or blockade of glutamatergic inputs to the CVLM on SNA and mean AP. Inhibition of the NTS by bilateral microinjection of Mus significantly increased SNA and AP in all rats. Subsequent inhibition of the CVLM by bilateral microinjection of Mus (n = 6) significantly increased SNA (A) and mean AP (B) above the levels achieved by inhibition of the NTS alone. Likewise, after inhibition of the NTS, antagonism of glutamatergic receptors in the CVLM (n = 7) by bilateral microinjection of kynurenate (Kyn) produced an additional increase in SNA (C) and mean AP (D). *P < 0.05, significant increase from 100% baseline; †P < 0.05, significantly different from SNA and mean AP values after inhibition of the NTS.
To determine whether the NTS-independent drive to the CVLM was primarily glutamatergic, the ionotropic glutamatergic receptor antagonist kynurenate was bilaterally microinjected into the CVLM after the confirmed inhibition of the NTS with microinjections of muscimol. In this group of seven rats, baseline mean AP was 126 ± 3 mmHg and HR was 372 ± 19 beats/min. The inhibition of neuronal activity in the NTS by a bilateral microinjection of muscimol increased SNA, HR, and AP (Table 2 and Fig. 4). After microinjection of muscimol into the NTS, microinjection of kynurenate into the CVLM evoked substantial increases in SNA, HR, and mean AP that were comparable with those achieved by the inhibition of the CVLM with muscimol (Table 2 and Fig. 4).
An examination of the time course of the changes in SNA and AP after microinjection of kynurenate into the CVLM revealed a divergence in the kinetics of the responses. Mean AP increased initially but returned to preinjection levels within 5 min (Fig. 5, A and C). In two of these animals, AP plummeted to levels well below the initial baseline (Fig. 5A). In contrast, the increase in SNA peaked within 1 min following the bilateral microinjection of kynurenate into the CVLM and remained above preinjection levels for 10 min.
Fig. 5.
Time courses of changes in SNA and mean AP after blockade of glutamatergic inputs to the CVLM in rats with NTS inhibited. Bilateral microinjection of Kyn into the CVLM after inhibition of the NTS evoked initial significant rises in SNA and AP (A, B, and C). Although the increase in SNA was maintained for at least 10 min (A and B), the increase in mean AP was transient with a restoration to preinjection levels within 5 min (C) and sometimes a fall well below baseline levels (see A). *P < 0.05, significant change in SNA or mean AP due to microinjection of Kyn into the CVLM. For the integrated SNA values in B, 100% is the baseline SNA before microinjections into the NTS. In B and C, the initial values (time 0) reflect the SNA and AP immediately before injection of Kyn into the CVLM.
Effect of altering central respiratory drive on sympathetic responses to glutamatergic blockade in the CVLM during baroreceptor unloading.
To determine whether the strength of central respiratory drive influences baroreceptor-independent, glutamatergic inputs to the CVLM, the glutamate antagonist kynurenate was microinjected into the CVLM during sodium nitroprusside infusion in vagotomized rats with reduced or increased Pco2. Reducing the ventilation rate consistently raised end tidal and Pco2 levels and reduced blood pH (Table 3). The Po2 levels remained high due to ventilation with 100% oxygen (Table 3). Before the experimental protocols, rats with diminished respiratory drive had a mean AP of 112 ± 10 mmHg and rats with enhanced central respiratory drive had a mean AP of 121 ± 16 mmHg (not significant; P > 0.05). An analysis of the raw voltage produced from the splanchnic nerve revealed no difference in SNA in rats with high or low central respiratory drive (10 ± 4 mV vs. 14 ± 2 mV).
Table 3.
Effects of altered rate of artificial ventilation rate to reduce or increase central respiratory drive in vagotomized rats
| Ventilation Rate, breaths/min | End Tidal CO2, % | pH | Pco2, mmHg | Po2, mmHg | |
|---|---|---|---|---|---|
| Central respiratory drive | |||||
| Reduced | 77±1 | 2.4±0.1 | 7.57±0.02 | 31.6±1.9 | 487±35 |
| Increased | 54±1* | 5.7±0.1* | 7.32±0.03* | 65.0±3.1* | 447±46 |
Values are means ± SE.
P < 0.05, significantly different from value in rats with reduced central respiratory drive.
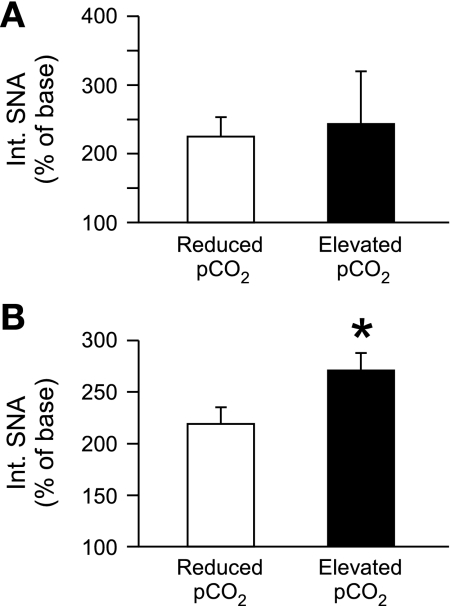
Baroreceptor unloading decreased AP to similar values in rats with reduced and elevated and reduced respiratory drive (39 ± 3 mmHg vs. 43 ± 2 mmHg) and promoted a comparable increase in SNA (Fig. 6A). In contrast, the subsequent blockade of glutamatergic inputs to the CVLM in rats with increased Pco2 promoted a larger increase in SNA compared with rats with decreased Pco2 (Fig. 6B). At baseline and during sodium nitroprusside infusion, vagal stimulation consistently decreased SNA and AP. After microinjection of kynurenate into the CVLM, stimulation of the vagus nerve increased SNA in all rats, confirming the blockade of glutamate receptors in the CVLM.
Fig. 6.
The strength of central respiratory drive influences the effect of glutamate receptor antagonism in the CVLM. A: rats with diminished central respiratory drive (reduced Pco2; n = 5) and rats with enhanced central respiratory drive (elevated Pco2; n = 5) experience equivalent increases in SNA after unloading the baroreceptors by infusion of SNP. B: microinjection of Kyn into the CVLM during SNP infusion results in a larger increase in SNA in animals with elevated Pco2. Baseline SNA was determined during SNP infusion, immediately before Kyn injection into the CVLM. *P < 0.05, significant difference between groups with reduced and elevated Pco2.
DISCUSSION
GABAergic neurons in the CVLM provide the major tonic inhibitory influence for presympathetic neurons in the RVLM that generate sympathetic vasomotor tone (2, 15, 20, 29). Although the CVLM has been traditionally regarded as an inhibitory relay nucleus for the generation of sympathoinhibitory reflexes, the CVLM is clearly regulated by inputs that are independent of baroreceptors and the NTS to tonically reduce the SNA that maintains AP. We aimed to begin to elucidate the nature and sources of these unknown influences upon the CVLM that impact SNA and AP. The present study reports three principle novel observations. First, in the acute absence of tonic baroreceptor inputs, inhibition of the NTS does not alter SNA or AP. Second, after acute inhibition of the NTS, inhibition of the CVLM or blockade of glutamatergic inputs continues to increase SNA and AP. Finally, the antagonism of glutamatergic inputs to the CVLM produces larger increases in SNA and AP when central respiratory drive is enhanced by hypoventilation.
The most straightforward explanation of these data is that microinjection of kynurenate into the CVLM blocks a baroreceptor-independent glutamatergic drive to neurons that tonically inhibit SNA. An assumption of the present study is that observed changes in SNA after microinjections into the CVLM are due to effects upon baroactivated GABAergic neurons in the region. Extracellular recordings in the CVLM reveal a population of neurons in which activity is tightly and inversely correlated with fluctuations in SNA (18, 19). These neurons are exquisitely sensitive to changes in AP and other stimuli that affect SNA, such as intravenous phenyl biguanide or cholecystokinin (12, 19). Inhibition of this region of the CVLM abolishes the sympathoinhibitory responses to stimuli, such low-frequency stimulation of the vagus nerve, intravenous injection of phenyl biguanide or cholecystokinin, and stimulation of the arterial baroreflex (7, 12, 26), suggesting the baroactivated GABAergic CVLM neurons are inhibited by the microinjections. Many baroactivated GABAergic CVLM neurons are still active after the tonic influence of baroreceptors has been removed (10, 18), suggesting they could provide the baroreceptor-independent regulation of SNA that is observed by manipulations of the CVLM. Nevertheless, increases in SNA after the inhibition of the CVLM could also be due to effects upon unidentified adjacent neurons of this heterogeneous region. Using a microinjection volume (100 nl) large enough to cover the intended target (18) could influence the activity of nearby nuclei such as the rostral ventral respiratory group, pre-Bötzinger nucleus, and nucleus ambiguus. Although inhibition of pre-Bötzinger neurons appears to affect the ability of the CVLM to decrease AP (28), it is likely that this occurs via a connection to baroactivated GABAergic CVLM neurons.
Our finding that the inhibition of the NTS does not alter SNA after nitroprusside-induced hypotension suggests that baroreceptors are the major driver for the NTS-mediated regulation of the CVLM in anesthetized rats. This novel observation is in agreement with a previous study showing that inhibition of neuronal activity in the NTS does not produce a change in AP in rats after chronic sinoaortic denervation (21). These data suggest that lack of drive from the NTS in chronic baroreceptor denervated rats is not due to a long-term adaptation to the loss of baroreceptor inputs. In agreement with the notion that the primary tonic drive through the NTS derives from baroreceptors, inhibition of neurons in the NTS and unloading of baroreceptors by nitroprusside-induced hypotension produce comparable rises in SNA. Although several sympathoinhibitory reflexes that are mediated by the activation of vagal afferents require activation of the NTS and CVLM to alter SNA, such as the von Bezold-Jarisch reflex or systemic cholecystokinin, these vagally initiated reflexes are only likely to be active under specific physiological conditions. The NTS also receives inputs from peripheral chemoreceptors to activate SNA, but ventilation with 100% oxygen would minimize their activity (27). In addition, these data suggest that the NTS is not a relay for the tonic activation of the CVLM by sources within the brain, such as central respiratory neurons or the hypothalamus (30).
After acute inhibition of the activity of neuronal cell bodies in the intermediate NTS, the present study showed that inhibition of the depressor region of the CVLM evoked substantial increases in SNA, HR, and AP. These data concur with previous studies suggesting an NTS-independent drive to the inhibitory CVLM neurons that influences cardiovascular function. In rabbits, after acute electrolytic lesions of the NTS, blockade of GABA receptors in the RVLM continues to increase AP (5), and this response is mimicked by the inhibition of the CVLM (6). Similarly, in rats with chronic lesions of the NTS, inhibition of the CVLM evokes a substantial rise in AP that is mimicked by blockade of GABAergic receptors in the RVLM (20). However, it has also been reported that after acute blockade of glutamatergic inputs to the NTS, inhibition of the CVLM paradoxically decreases AP (13). These results are not readily reconciled with the present study or others using lesions of the NTS but may be the result of subtle differences in injection sites. Alternatively, a measurement of changes in AP without concomitant measures of SNA may provide misleading notions regarding the output of the central nervous system. Indeed, in the present study, the serial inhibition of the NTS and CVLM produced a sustained, intense rise in SNA that was sometimes accompanied by a collapse in AP within minutes, likely due to neurogenic escape mechanisms. This observation highlights the importance of direct sympathetic measures and the evaluation of immediate responses to gain insights into the regulation of sympathetic vasomotor tone.
The NTS-independent drive to the CVLM neurons that regulate SNA appears to be largely glutamatergic. In the present study, we observed that antagonism of ionotropic glutamate receptors by microinjection of kynurenate into the CVLM after acute inhibition of the NTS evoked increases in SNA and AP that were comparable with those observed after inhibition of the CVLM with muscimol. These data are in accordance with analogous experiments performed in rats with chronic lesions of the NTS (20). Although the sources of these glutamatergic inputs are not known, they may be linked to central respiratory neurons. After baroreceptor inputs were minimized, the present study showed that the blockade of glutamatergic inputs to the CVLM evokes a larger increase in SNA when central respiratory drive is enhanced. These data suggest that the neural network that regulates central respiratory drive influences the ability of the CVLM to reduce SNA. In support of this finding, it has been previously shown that the loss of glutamatergic pre-Bötzinger neurons, which are critical to the regulation of central respiratory drive, decreases the ability of the CVLM to reduce SNA and AP (28). Further experiments will be necessary to determine the origin(s) and timing of glutamatergic inputs to the CVLM from central respiratory regulatory neurons.
Perspectives
Once considered a simple relay nucleus to convey reflexively mediated inhibition to presympathetic RVLM neurons, the CVLM is now known to possess complex regulatory influences upon the RVLM and sympathetic vasomotor tone. In addition to the profound ability of the CVLM to acutely alter SNA, chronic changes in CVLM activity may also contribute to the elevated SNA observed with hypertension (22, 24). Baroreceptor-driven, glutamatergic inputs from the NTS are clearly a major force for CVLM neuronal activity, but the sources and functional significance of baroreceptor-independent inputs to the CVLM remain to be clarified. In the present study, we determined that baroreceptor-independent drive to the CVLM is not likely derived from the NTS and propose that neurons involved in the regulation of central respiratory drive may influence SNA via glutamatergic inputs to the CVLM. In the future, more precise methodologies will be required to unravel the function of this intricate and influential region within the CVLM.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-075174 (to A. M. Schreihofer) and American Heart Association Predoctoral Fellowship 0615194B (to D. A. Mandel).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agarwal SK, Gelsema AJ, Calaresu FR. Neurons in rostral VLM are inhibited by chemical stimulation of caudal VLM in rats. Am J Physiol Regul Integr Comp Physiol 257: R265–R270, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal SK, Gelsema AJ, Calaresu FR. Inhibition of rostral VLM by baroreceptor activation is relayed through caudal VLM. Am J Physiol Regul Integr Comp Physiol 258: R1271–R1278, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Blessing WW Depressor neurons in rabbit caudal medulla act via GABA receptors in rostral medulla. Am J Physiol Heart Circ Physiol 254: H686–H692, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Cravo SL, Morrison SF. The caudal ventrolateral medulla is a source of tonic sympathoinhibition. Brain Res 621: 133–136, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Dampney RA, Blessing WW, Tan E. Origin of tonic GABAergic inputs to vasopressor neurons in the subretrofacial nucleus of the rabbit. J Auton Nerv Syst 24: 227–239, 1988. [DOI] [PubMed] [Google Scholar]
- 6.Gieroba ZJ, Blessing WW. Effect of nucleus tractus solitarius lesions on cardiovascular responses elicited from the caudal ventrolateral medulla. J Auton Nerv Syst 39: 97–104, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Gordon FJ Aortic baroreceptor reflexes are mediated by NMDA receptors in caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 252: R628–R633, 1987. [DOI] [PubMed] [Google Scholar]
- 8.Guyenet PG, Filtz TM, Donaldson SR. Role of excitatory amino acids in rat vagal and sympathetic reflexes. Brain Res 407: 272–284, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Haselton JR, Guyenet PG. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am J Physiol Regul Integr Comp Physiol 256: R739–R750, 1989. [DOI] [PubMed] [Google Scholar]
- 10.Mandel DA, Schreihofer AM. Central respiratory modulation of barosensitive neurones in rat caudal ventrolateral medulla. J Physiol 572: 881–896, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masuda N, Terui N, Koshiya N, Kumada M. Neurons in the caudal ventrolateral medulla mediate the arterial baroreceptor reflex by inhibiting barosensitive reticulospinal neurons in the rostral ventrolateral medulla in rabbits. J Auton Nerv Syst 34: 103–117, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Mobley SC, Mandel DA, Schreihofer AM. Systemic cholecystokinin differentially affects baro-activated GABAergic neurons in rat caudal ventrolateral medulla. J Neurophysiol 96: 2760–2768, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Moreira TS, Sato MA, Takakura AC, Menani JV, Colombari E. Role of pressor mechanisms from the NTS and CVLM in control of arterial pressure. Am J Physiol Regul Integr Comp Physiol 289: R1416–R1425, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Mueller PJ, Hasser EM. Putative role of the NTS in alterations in neural control of the circulation following exercise training in rats. Am J Physiol Regul Integr Comp Physiol 290: R383–R392, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Natarajan M, Morrison SF. Adrenal epinephrine secretion is not regulated by sympathoinhibitory neurons in the caudal ventrolateral medulla. Brain Res 827: 169–175, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (6th ed.). Amsterdam: Elsevier, 2007.
- 17.Sapru HN, Wang SC. Modification of aortic baroreceptor resetting in the spontaneously hypertensive rat. Am J Physiol 230: 664–674, 1976. [DOI] [PubMed] [Google Scholar]
- 18.Schreihofer AM, Guyenet PG. The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol 29: 514–521, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Schreihofer AM, Guyenet PG. Baro-activated neurons with pulse-modulated activity in the rat caudal ventrolateral medulla express GAD67 mRNA. J Neurophysiol 89: 1265–1277, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Schreihofer AM, Ito S, Sved AF. Brain stem control of arterial pressure in chronic arterial baroreceptor-denervated rats. Am J Physiol Regul Integr Comp Physiol 289: R1746–R1755, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Schreihofer AM, Sved AF. Nucleus tractus solitarius and control of blood pressure in chronic sinoaortic denervated rats. Am J Physiol Regul Integr Comp Physiol 263: R258–R266, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Smith JK, Barron KW. Cardiovascular effects of L-glutamate and tetrodotoxin microinjected into the rostral and caudal ventrolateral medulla in normotensive and spontaneously hypertensive rats. Brain Res 506: 1–8, 1990. [PubMed] [Google Scholar]
- 23.Sun MK, Guyenet PG. Arterial baroreceptor and vagal inputs to sympathoexcitatory neurons in rat medulla. Am J Physiol Regul Integr Comp Physiol 252: R699–R709, 1987. [DOI] [PubMed] [Google Scholar]
- 24.Sved AF, Ito S, Madden CJ. Baroreflex dependent and independent roles of the caudal ventrolateral medulla in cardiovascular regulation. Brain Res Bull 51: 129–133, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Thorén P, Saum WR, Brown AM. Characteristics of rat aortic baroreceptors with nonmedullated afferent nerve fibers. Circ Res 40: 231–237, 1977. [DOI] [PubMed] [Google Scholar]
- 26.Verberne AJ, Guyenet PG. Medullary pathway of the Bezold-Jarisch reflex in the rat. Am J Physiol Regul Integr Comp Physiol 263: R1195–R1202, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Von Euler C, Lagererantz H. Neurobiology of the Control of Breathing. New York: Raven Press, 1987.
- 28.Wang H, Weston MC, McQuiston TJ, Stornetta RL, Guyenet PG. Neurokinin-1 receptor-expressing cells regulate depressor region of rat ventrolateral medulla. Am J Physiol Heart Circ Physiol 285: H2757–H2769, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Willette RN, Punnen S, Krieger AJ, Sapru HN. Interdependence of rostral and caudal ventrolateral medullary areas in the control of blood pressure. Brain Res 321: 169–174, 1984. [DOI] [PubMed] [Google Scholar]
- 30.Yang Z, Coote JH. The influence of the paraventricular nucleus on baroreceptor dependent caudal ventrolateral medullary neurones of the rat. Pflügers Arch 438: 47–52, 1999. [DOI] [PubMed] [Google Scholar]