Abstract
Adenine phosphoribosyltransferase (APRT) deficiency is a rare autosomal recessive disorder causing 2,8-dihydroxyadenine stones and renal failure secondary to intratubular crystalline precipitation. Little is known regarding the clinical presentation of APRT deficiency, especially in the white population. We retrospectively reviewed all 53 cases of APRT deficiency (from 43 families) identified at a single institution between 1978 and 2009. The median age at diagnosis was 36.3 years (range 0.5 to 78.0 years). In many patients, a several-year delay separated the onset of symptoms and diagnosis. Of the 40 patients from 33 families with full clinical data available, 14 (35%) had decreased renal function at diagnosis. Diagnosis occurred in six (15%) patients after reaching ESRD, with five diagnoses made at the time of disease recurrence in a renal allograft. Eight (20%) patients reached ESRD during a median follow-up of 74 months. Thirty-one families underwent APRT sequencing, which identified 54 (87%) mutant alleles on the 62 chromosomes analyzed. We identified 18 distinct mutations. A single T insertion in a splice donor site in intron 4 (IVS4 + 2insT), which produces a truncated protein, accounted for 40.3% of the mutations. We detected the IVS4 + 2insT mutation in two (0.98%) of 204 chromosomes of healthy newborns. This report, which is the largest published series of APRT deficiency to date, highlights the underdiagnosis and potential severity of this disease. Early diagnosis is crucial for initiation of effective treatment with allopurinol and for prevention of renal complications.
Adenine phosphoribosyltransferase (APRT) is a purine salvage enzyme that catalyzes the formation of 5′-AMP and pyrophosphate from adenine and 5-phosphoribosyl-1-pyrophosphate (Figure 1A). In APRT deficiency,1–3 adenine is oxidized by xanthine oxydase to 2,8-dihydroxyadenine (2,8-DHA), a highly insoluble compound that crystallizes in urine.2,4 APRT deficiency is an autosomal recessive disorder, and patients with homozygous or compound heterozygous APRT mutation produce large amounts of 2,8-DHA, leading to urolithiasis and renal failure. Tools for diagnosis include stone analysis, identification of typical 2,8-DHA crystals in urine or renal biopsy, and measurement of APRT activity in erythrocytes. Early diagnosis of the disease is critical because patients may develop renal failure5–7 that may be efficiently prevented by allopurinol, a xanthine oxydase inhibitor.8
Figure 1.
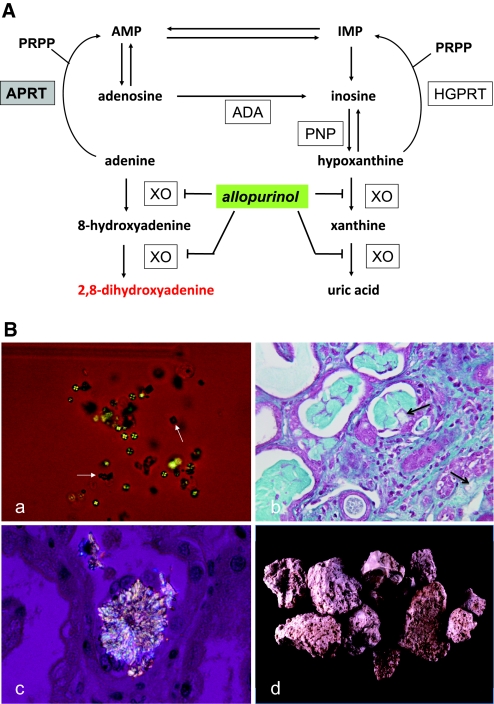
APRT deficiency causes 2,8-DHA accumulation, leading to urolithiasis and crystalline nephropathy. (A) Metabolic pathways for the disposal of adenine in human show, in the absence of APRT activity, the alternative route of oxidation by xanthine oxydase (XO) to 2,8-DHA via the 8-hydroxy-intermediate in a manner analogous to the production of uric acid from hypoxanthine via xanthine. In human, adenine cannot be converted to adenosine as hypoxanthine to inosine by purine nucleoside phosphorylase (PNP); the only alternative pathway is oxidation. The site of inhibitory effect of allopurinol on 2,8-DHA synthesis is also indicated. ADA, adenosine deaminase; AMP, adenosine monophosphate; HGPRT, hypoxanthine-guanine phosphoribosyltransferase; IMP, inosine monophosphate; PRPP, 5-phosphoribosyl-1-pyrophosphate. (B) Morphologic features of 2,8-DHA crystals and stones. (a) Crystalluria study by polarized microscopy revealing typical 2,8-DHA crystals appearing round and reddish-brown with characteristic central Maltese cross pattern. Note the presence of few crystals of calcium oxalate dihydrate (white arrows) in addition to 2,8-DHA crystals. All crystalluria examined in our patients with APRT deficiency were positive for 2,8-DHA crystals. (b) Light microscopy study of kidney allograft biopsy, showing severe tubulointerstitial injury secondary to precipitation of crystals (arrows; Masson's Trichrome staining). (c) Kidney allograft biopsy examined by polarized microscopy showing precipitation of 2,8-DHA crystals within tubular lumen and in renal interstitium. (d) 2,8-DHA stones. Surface of stones are typically rough, humpy, soft, and friable. Color is reddish-brown turning grey when drying. Stone sections are disorganized with porosities and beige to brown color. Magnifications: ×200 in Ba; ×400 in B, b and c.
Two different types of APRT deficiency have been described2 with similar clinical expression and complete APRT deficiency in vivo, but they have been distinguished on the level of enzyme activity in cell extracts in vitro. Type I (complete deficiency in vivo and in vitro) has been reported in various ethnic groups and predominantly affects the white population.9,10 By contrast, type II (complete deficiency in vivo but partial deficiency in vitro) has been observed almost exclusively in the Japanese population.11 The aprt gene located on chromosome 16q24 is approximately 2.6 kb long and contains five exons.12 Mutant alleles responsible for the disease have been classified as APRT*Q0 for type I and APRT*J for type II. APRT*Q0 represents a heterogeneous collection of mutations,13–15 and patients with type I deficiency are either homozygous or compound heterozygous for these mutations. APRT*J is a single-mutant allele with a missense mutation in exon 5 (Met136Thr),11,16 and patients with type II deficiency have the genotype APRT*J/ APRT*J or, more rare, APRT*J/APRT*Q0.17
Data on clinical presentation and diagnosis of APRT deficiency are scarce and limited to case reports and one small series,10 especially in the white population. We present here the results of a study undertaken with the aim of describing clinical and diagnostic features, genotype, and follow-up of patients with APRT deficiency in a large French cohort.
Results
Patients with Diagnosis of APRT Deficiency
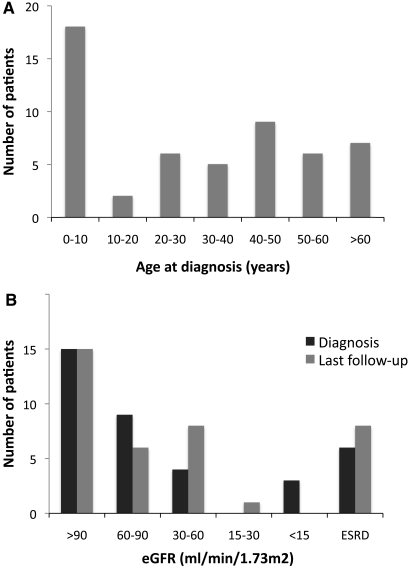
APRT deficiency was found in 53 patients from 43 families during the studied period (Table 1). Age at diagnosis ranged from 0.5 to 78.0 years, and median age was 36.3 years (range 6.4 to 50.5 years). Thirty-three (62.3%) patients were older than 16 years at diagnosis (Figure 2A). Tests leading to diagnosis are detailed in Table 1. For some patients, several of these tests were simultaneously performed and led to the diagnosis (e.g., crystalluria and stone analysis). For five (9.4%) patients, diagnosis was directly made by APRT activity measurement in the setting of familial screening.
Table 1.
Characteristics of patients identified with APRT deficiency and tests used for diagnosis (alone or in combination)
| Characteristic | Value |
|---|---|
| No. of families | 43 |
| No. of patients | 53 |
| <16 years (n [%]) | 20 (37.7) |
| >16 years (n [%]) | 33 (62.3) |
| Male gender (n [%]) | 34 (64.1) |
| Age at diagnosis (years; median [IQR]) | 36.3 (6.4 to 50.5) |
| Tests leading to diagnosis (n [%]) | |
| stone analysis | 31 (58.5) |
| crystalluria | 15 (28.3) |
| renal biopsy | 6 (11.3) |
| native kidney | 2 (3.8) |
| transplant | 3 (5.7) |
| both | 1 (1.9) |
| APRT activity (overall) | 41 (77.4) |
| APRT activity in asymptomatic | 2 (3.8) |
For some patients, several diagnostic tests were simultaneously performed (e.g., crystalluria and stone analysis). For five patients, diagnosis was made directly by APRT activity measurement in the setting of familial screening. IQR, interquartile ratio.
Figure 2.
Diagnosis of APRT deficiency was often made late with impaired renal function. (A) Repartition of patients depending on their age when diagnosis of APRT deficiency was made. Data are provided for the 53 patients described in Table 1. (B) Repartition of patients depending on renal function at diagnosis and last follow-up. Data are provided for the 38 patients described in Table 2. eGFR, estimated GFR by MDRD formula.
At time of diagnosis, all urine samples examined were positive for 2,8-DHA crystals. In most crystalluria samples, 2,8-DHA crystals appeared typically round and reddish-brown and showed characteristic central Maltese cross pattern on polarized light microscopy (Figure 1Ba). The number of 2,8-DHA crystals/mm3 in untreated patients was 1177 ± 384. Renal biopsies performed in patients with renal failure showed that 2,8-DHA crystals precipitated, causing severe tubulointerstitial injury (Figure 1B, b and c). By morphologic examination, 2,8-DHA stones were reddish-brown turning gray when drying and were friable (Figure 1Bd). Infrared spectroscopy confirmed their composition. APRT activity in erythrocytes was measured in 40 (76.9%) individuals, all demonstrating complete deficiency. Of note, two (3.9%) of these individuals were totally asymptomatic.
Clinical Presentation at Diagnosis of APRT Deficiency
Full clinical data were available for 40 patients from 33 families of our cohort (Table 2). All were found with null APRT enzyme activity, and molecular study of aprt gene was performed for 38 patients (31 families).
Table 2.
Clinical presentation at diagnosis of APRT deficiency
| Parameter | Value |
|---|---|
| No. of families | 33 |
| No. of patients | 40 |
| <16 years (n [%]) | 15 (37.5) |
| >16 years (n [%]) | 25 (62.5) |
| Male gender (n [%]) | 24 (60.0) |
| Parental consanguinity (n = 34; n [%]) | 5 (14.7) |
| Age at diagnosis (years; median [IQR]) | 28.9 (5.6 to 51.0) |
| Familial screening (asymptomatic; n [%]) | 2 (5.0) |
| History of urolithiasis (n [%]) | 36 (90.0) |
| Age at first lithiasis (n = 31; years; median [IQR]) | 12.5 (3.1 to 35.0) |
| Delay from first lithiasis to diagnosis (years; median [IQR]) | 1.5 (0.0 to 17.2) |
| Lithiasis episodes (n [%]) | |
| 0 | 4 (10.0) |
| 1 to 2 | 17 (42.5) |
| 3 to 5 | 10 (25.0) |
| >5 | 9 (22.5) |
| Urologic procedures (n [%]) | |
| 0 | 23 (57.5) |
| 1 to 2 | 12 (30.0) |
| 3 to 5 | 4 (10.0) |
| >5 | 1 (2.5) |
| Type of urologic procedures (n [%]) | |
| extracorporeal shock waves lithotripsy | 12 (30.0) |
| ureteroscopy | 5 (12.5) |
| percutaneous nephrolithotomy | 3 (7.5) |
| surgery | 5 (12.5) |
| nephrectomy | 1 (2.5) |
| Acute renal failure (n [%]) | 1 (2.5) |
| Chronic renal failure (n [%]) | 13 (32.5) |
| ESRD reached before diagnosis (n [%]) | 6 (15.0) |
| Renal transplant recipients not on dialysis (n [%]) | 4 (10.0) |
| Renal transplant recipients on dialysis (n [%]) | 2 (5.0) |
Full clinical data for 40 patients from 33 families are provided. (n indicates the number of patients for whom data was available when the information was lacking for some patients).
Median age was 28.9 years (range 5.6 to 51.0 years), and 25 (62.5%) were older than 16 years at diagnosis. History of consanguinity was reported in five (15.1%) families. Thirty-six (90%) patients had a history of urolithiasis at diagnosis. Median age at first episode of urolithiasis (known for 32 patients) was 12.5 years (3.1 to 35.0 years). Number of episodes of urolithiasis that occurred before diagnosis was highly variable, and 17 (42.5%) patients had undergone urologic procedures (detailed in Table 2). Delay from first episode of urolithiasis to diagnosis was extremely variable, ranging from 0 to 43 years, with a median time of 1.5 years (0.0 to 17.2 years).
One (2.5%) patient presented with acute renal failure, and 13 (32.5%) had chronic renal failure. Six (15%) patients had reached ESRD requiring dialysis or renal transplantation before diagnosis of APRT deficiency was made. Serum creatinine level (SCr) at diagnosis, known for 31 of 34 patients without ESRD, was 70 μmol/L (range 47 to 112 μmol/L). Distribution of patients by renal function stage is summarized in Figure 2B.
In five of six patients who had reached ESRD and underwent transplantation before the diagnosis was made, APRT deficiency was diagnosed in the setting of severe renal allograft dysfunction caused by intratubular and interstitial precipitation of 2,8-DHA crystals. In four of them, identification of 2,8-DHA crystals by Fourier transform infrared microscopy in renal graft biopsy led to diagnosis. Interestingly, the review of native kidney biopsy performed before ESRD revealed the presence of 2,8-DHA crystals, which had been misinterpreted as unspecific findings, in one of these patients. In another patient who underwent transplantation, APRT deficiency was detected through APRT activity assay only once the disease was diagnosed in his brother. Of these five patients who underwent transplantation and had severely impaired renal function at time of diagnosis, two returned to dialysis and the other three patients had SCr level ranging between 400 and 450 μmol/L. In the sixth patient who underwent transplantation, 2,8-DHA crystals were detected by crystalluria at day 3 after transplantation, whereas SCr level was 123 μmol/L. Allopurinol therapy was started, and renal function remained stable.
APRT deficiency was diagnosed in two (5%) asymptomatic individuals. In one individual, 2,8-DHA crystals were fortuitously discovered in urine of a 7-year-old girl. The other individual was a 15-year-boy who had a 6-year-old brother with 2,8-DHA urolithiasis and was found with null APRT activity in the setting of familial screening. Of note, he presented a first episode of urolithiasis a few weeks after diagnosis and before he was started on allopurinol.
Follow-up after Diagnosis of APRT Deficiency
Data on follow-up after diagnosis of APRT deficiency were analyzed for all 40 patients described (Table 3). Median duration of follow-up was 74 months (range 14 to 112 months). Thirty-five (87.5%) patients received long-term allopurinol therapy. Two patients were prescribed allopurinol but rapidly stopped taking this drug of their own. In three patients, diagnosis was made only recently and allopurinol was not started yet. Allopurinol dosages given (known for 22 adults and 11 children) were relatively similar for all patients. Median dosage was 300 mg/d (200 to 300 mg/d) in adults and 10 mg/kg per d (9 to 10 mg/kg per d) in children. Stone recurrence occurred in six (15%) patients, five of whom were receiving allopurinol. Crystalluria studies repeated during follow-up revealed a marked decrease in the number of crystals (67 ± 8 versus 1177 ± 384/mm3; P < 0.0001) and even crystalluria disappearance in 39 (60.9%) of 64 urine samples. Thirty (75%) patients required urologic procedures, in most cases for treating stones that had formed before diagnosis rather than new stones (Table 3).
Table 3.
Follow-up after diagnosis of APRT deficiency
| Parameter | Value |
|---|---|
| Follow-up duration (months; median [IQR]) | 74 (14 to 112) |
| Allopurinol therapy (n [%]) | 35 (87.5) |
| Allopurinol dosage (median [IQR]) | |
| adults (n = 22; mg/d) | 300 (200 to 300) |
| children (n = 11; mg/kg per d) | 10 (9 to 10) |
| Stone recurrence (n [%]) | 6 (15.0) |
| treated by allopurinol (n = 35) | 5 (14.3) |
| untreated (n = 5) | 1 (20.0) |
| Urologic procedures (n [%]) | |
| 0 | 30 (75.0) |
| 1 to 2 | 8 (20.0) |
| >3 | 2 (5.0) |
| Type of urologic procedures (n [%]) | |
| extracorporeal shock waves | 7 (17.5) |
| ureteroscopy | 3 (7.5) |
| percutaneous nephrolithotomy | 1 (2.5) |
| surgery | 2 (5.0) |
| nephrectomy | 2 (5.0) |
| Reaching ESRD during follow-up (n [%]) | 2 (5.0) |
| Renal transplantation during follow-up (n [%]) | 2 (5.0) |
| Total ESRD before or during follow-up (n [%]) | 8 (20.0) |
Data regarding follow-up for the 40 patients described in Table 2 are provided here.
Two (5%) patients with ESRD at diagnosis underwent renal transplantation during follow-up, and allopurinol therapy prevented recurrence of crystalline nephropathy in both. Two (5%) patients reached ESRD during follow-up, raising the total number of patients with ESRD to eight (20%). One of these had severe renal failure when diagnosis was made (SCr 500 μmol/L) and reached ESRD 159 months later despite allopurinol therapy. The other had SCr of 70 μmol/L at diagnosis but developed ESRD 101 months later (no data were available regarding adherence to allopurinol therapy). In the other patients, renal function remained stable or even improved during follow-up.
Median SCr level at last follow-up in patients without ESRD (available for 30 of 32 patients) was 80 μmol/L (54 to 119 μmol/L). Distribution of patients according to renal function is summarized in Figure 2B.
Molecular Study
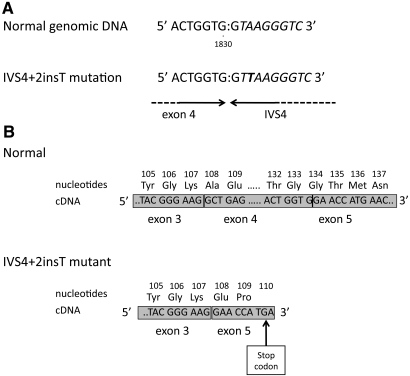
The five exons and flanking regions of aprt gene were amplified by PCR and then sequenced in 38 patients belonging to 31 of 33 families with detailed phenotype. Mutations at the genomic level and their expected effect on protein and the geographic origin of families are summarized in Table 4. In all, 54 (87%) mutated chromosomes were found on the 62 chromosomes analyzed. Eighteen different mutations were identified (Table 4). Of these, 14 are not yet described. Two mutated alleles were detected in 24 families. Of these, 13 families were carrying a homozygous mutation and 11 families had compound heterozygous mutations. The most prevalent mutation, a single T insertion in intron 4 splice donor site named IVS4 + 2insT (Figure 3), accounted for 25 (40.3%) chromosomes. Aberrant splicing at this site causes a deletion of exon 4 from mRNA, leading to a frame shift and a truncated protein of 109 amino acids instead of 180 named Ala108GluX3 (Figure 3). To study the prevalence of IVS4 + 2insT mutant allele in the general population, we performed molecular study of 102 healthy newborns. IVS4 + 2insT mutation was found in two (0.98%) of 204 chromosomes examined. Other mutations were not found in 20 chromosomes analyzed from a control population.
Table 4.
Mutations in aprt gene
| Family | Geographic Origin | No. of Cases | Gene Region | Nucleotide Change | Effect on Coding Sequence |
|---|---|---|---|---|---|
| 1 | Unknown | 1 | Intron 4 | IVS4 + 2insT15,25,26,28 | Ala108GluX3 |
| Intron 4 | IVS4 + 2insT | Ala108GluX3 | |||
| 2 | Metropolitan France | 2 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| Intron 4 | IVS4 + 2insT | Ala108GluX3 | |||
| 3 | Metropolitan France | 2 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| Exon 5 | 2185C→T | Leu176Phe | |||
| 4 | Turkey | 1 | Exon 1 | 3G→A | no protein |
| Exon 1 | 3G→A | no protein | |||
| 5 | Metropolitan France | 1 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| Exon 3 | 1443_1444 delCT or 1445_1446delCT | Thr96ThrfsX13 or Leu97ValfsX12 | |||
| 6 | Metropolitan France | 1 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| Exon 1 | 1A→G18,26 | No protein | |||
| 7 | Metropolitan France | 1 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| Exon 5 | 2176_2178delTTC or 2179_2181 delTTC14,15,18 | ΔPhe173 or ΔPhe174 | |||
| 8 | Metropolitan France | 1 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| Intron 4 | IVS4 + 2insT | Ala108GluX3 | |||
| 9 | Metropolitan France | 1 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| Exon 1 | 1A→G | No protein | |||
| 10 | Metropolitan France | 2 | Exon 4 | 1801T→G | Val124Gly |
| Exon 4 | 1801T→G | Val124Gly | |||
| 11 | Metropolitan France | 3 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| Intron 4 | IVS4 + 2insT | Ala108GluX3 | |||
| 12 | Metropolitan France | 1 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| Exon 5 | 2183C→T | Ser175Phe | |||
| 13 | Poland | 1 | Exon 3 | 1443_1444 delCT or 1445_1446delCT | Thr96fsX13 or Leu97ValfsX12 |
| Exon 3 | 1443_1444 delCT or 1445_1446delCT | Thr96fsX13 or Leu97ValfsX12 | |||
| 14 | Metropolitan France | 1 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| Exon 3 | 1355C→T | Arg67X | |||
| 15 | Metropolitan France | 1 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| Exon 5 | 2176_2178delTTC or 2179_2181 delTTC | ΔPhe173 or ΔPhe174 | |||
| 16 | Metropolitan France | 1 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| Exon 5 | 2131_2133delGAG | ΔGlu158 | |||
| 17 | Italy/Metropolitan France | 1 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| Intron 4 | IVS4 + 2insT | Ala108GluX3 | |||
| 18 | Metropolitan France | 3 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| Exon 3 | 1443_1444 delCT or 1445_1446delCT | Thr96ThrfsX13 or Leu97ValfsX12 | |||
| 19 | Morocco | 1 | Exon 2 | 282G→C | Arg40Pro |
| Exon 2 | 282G→C | Arg40Pro | |||
| 20 | Metropolitan France | 1 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| Exon 5 | 2087T→C | Leu143Pro | |||
| 21 | Senegal | 1 | Exon 3 | 1467A→G | Glu104Gly |
| Exon 3 | 1467A→G | Glu104Gly | |||
| 22 | Spain | 1 | Exon 3 | 1350A→T13,18 | Asp65Val |
| Exon 3 | 1350A→T | Asp65Val | |||
| 23 | Lebanon | 1 | Exon 3 | 1344G→A | Gly63Asp |
| Exon 3 | 1344G→A | Gly63Asp | |||
| 24 | Portugal | 1 | Exon 3 | 1442_1443delAC | Thr96SerfsX13 |
| Exon 3 | 1442_1443delAC | Thr96SerfsX13 | |||
| 25 | Metropolitan France | 1 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| ND | ND | ND | |||
| 26 | Italy | 1 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| ND | ND | ND | |||
| 27 | Metropolitan France | 1 | Exon 5 | 2191C→T | Gln178X |
| ND | ND | ND | |||
| 28 | Metropolitan France | 1 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| ND | ND | ND | |||
| 29 | Metropolitan France | 1 | Intron 4 | IVS4 + 2insT | Ala108GluX3 |
| ND | ND | ND | |||
| 30 | Martinique | 1 | ND | ND | ND |
| ND | ND | ND | |||
| 31 | Metropolitan France | 1 | Exon 5 | 2200T→C | X181Arg |
| ND | ND | ND |
Molecular study was performed of 38 patients from 31 families. All cases were confirmed by APRT activity assay demonstrating null activity in erythrocyte lysates. Geographic origin of father and mother are indicated for each kindred. Gene region mutated and nucleotide changes in genomic DNA and their consequences on protein sequence for the two mutated alleles are provided for each family. ND, no mutation detected. References are indicated for the four previously reported mutations (IVS4 + 2insT, 1A→G, 1350A→T, and 2176–2178delTTC). See references12,13 for gene annotation.
Figure 3.
The IVS4+2insT nucleotide sequence at the exon 4–intron 4 junction shows the most prevalent mutation. (A and B) T insertion between nucleotides 1831 and 1832 or 1832 and 1833 in IVS4 splice donor site results in deletion of exon 4 in mRNA (A), leading to a premature termination at amino acid 110 (Ala108GluX3; B). Adapted from reference15.
Discussion
APRT deficiency was largely described in the Japanese population (type II deficiency) but rarely in other ethnic groups. The only series of cases in a non-Japanese population is the one reported by Edvardsson et al.,10 who described clinical features of 23 Icelandic patients (16 families) who all carried the same homozygous mutation (Asp65Val). Cases of APRT deficiency were only exceptionally reported in all other occidental countries, including US and European populations.18 More than 15 years ago, we drew attention to the underdiagnosis of APRT deficiency in the white population, considering the surprisingly small number of cases reported in contrast with a homozygosity at APRT locus estimated between one in 50,000 to one in 100,000.19 The most plausible explanation for this was that APRT deficiency may be largely unrecognized, which could be related to insufficient knowledge of the disease by clinicians involved in the treatment of patients with urolithiasis and/or renal failure.
Our report, which represents the largest series of APRT deficiency, highlights the potential severity of the disease and the crucial importance of early recognition to prompt treatment and prevent renal complications. Several diagnostic tools are helpful in identification of APRT deficiency. Stone analysis combining morphologic examination by stereomicroscope and infrared spectroscopy allows identification of 2,8-DHA in virtually all cases and should be systematically performed when a stone is available.20,21 Biochemical stone analysis should not be performed because this method does not differentiate 2,8-DHA from uric acid. Confusion between 2,8-DHA and uric acid stones is frequent, because both are radiolucent.
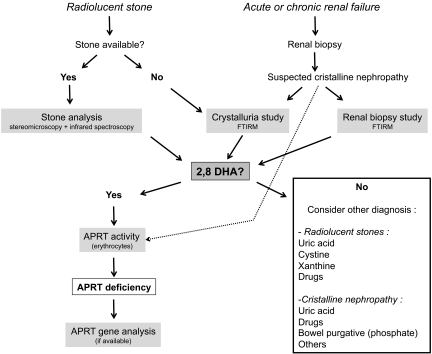
Whereas false-negative results have been exceptionally reported,10 in our experience, crystalluria revealing typical 2,8-DHA crystals is a highly sensitive (100%) and specific test. Therefore, crystalluria is a key diagnostic tool, as emphasized in the diagnostic algorithm (Figure 4).
Figure 4.
A diagnostic algorithm is proposed for diagnosis of complete APRT deficiency. Stone analysis (combining morphologic examination by stereomicroscope and infrared spectroscopy) allows identification of 2,8-DHA in virtually all cases. When observed by microscopy in urine samples or renal biopsy in patients with crystalline nephropathy, crystals should be studied by Fourier transformed infrared microscopy, which represents a highly specific and sensitive technique. In a second step, diagnosis of APRT deficiency must be confirmed by measure of APRT activity level in erythrocyte lysates. APRT activity assay may also be helpful in patients without analyzable stone, especially when crystalluria cannot be studied (e.g., patient with anuria; technique not available). Aprt gene analysis, although not necessary for diagnosis, may be performed to identify mutations.
APRT deficiency is an inborn error of metabolism often symptomatic during childhood. Despite this, the delay in diagnosis in many cases was one of the most striking of our findings. Half of our patients were older than 40 years at the time of diagnosis. These findings are in accordance with those reported by Edvardsson et al.10 For some patients, the reason for a late diagnosis was a delayed presentation. In our study, first stone occurred later than age 35 in 25% of patients. A minority of patients had no history of urolithiasis, and some of them had decreased renal function secondary to intratubular precipitation of crystals. This is consistent with previous reports indicating that APRT deficiency may be asymptomatic in up to 15 to 20% of patients.2,10,19
Diagnosis was also delayed in many patients who were symptomatic for years. Renal function was altered in 14 (35%) patients at time of diagnosis. Six (15%) patients reached ESRD before diagnosis, and APRT deficiency was detected only after renal transplantation. Recurrent crystalline nephritis, identified through graft biopsy, caused severe and irremediable allograft dysfunction in all except one case diagnosed early after transplantation. Similar cases in renal transplantation have been previously reported.5,7,22 Altogether, these catastrophic cases emphasize the underrecognition of APRT deficiency by clinicians despite the armamentarium available.
A few years ago, a study by our group estimated the overall proportion of urolithiasis-related ESRD to be 3.2%, hereditary diseases (including primary hyperoxaluria type 1 and cystinuria) accounting for 13.3% of cases.23 The proportion of ESRD cases related to APRT deficiency is unknown and usually considered to be negligible; however, this probably should be reconsidered in view of our results.
Treatment of APRT deficiency relies on allopurinol therapy, along with high fluid intake and low-purine diet. Alkalinization is useless, because 2,8-DHA is insoluble over a wide range of urinary pH. The majority of our patients were given allopurinol once the diagnosis of APRT deficiency was made. This treatment was well tolerated and seemed highly beneficial in most patients. A minority of patients experienced stone recurrence or renal failure under allopurinol therapy, but data regarding drug observance and adherence to dietary guidelines, namely water and purine intake, were not available. No firm guidelines can be drawn from our study about the amounts of fluid and purine that should be recommended. Our patients are usually advised to drink at least 2.5 L/d water and to avoid purine-rich food.
The strong decrease in crystal numbers observed under allopurinol suggests that crystalluria could be a valuable tool for treatment monitoring. In most patients, renal function remained stable or even improved under allopurinol therapy; however, overall, eight (20%) patients reached ESRD and only 60% of the patients had estimated GFR >60 ml/min per 1.73 m2 at last follow-up.
The factors underlying the high interfamilial but also intrafamilial variability observed in the disease severity remain unclear. Dietary habits, namely purine amount and water intake, are likely to be involved. In our experience, acute dehydration episodes (e.g., during gastroenteritis) can provoke oliguria, crystalline precipitation, and acute renal failure. Crystallization inhibitors, such as osteopontin, an inhibitor of 2,8-DHA crystal deposition, may modulate APRT deficiency severity as reported in an animal model.24
The aprt gene (16q24) encompasses 2.8 kb of DNA, contains five exons, and has a coding region of 540 bp.12,25 Various germline mutations reported include missense,13,16,25,26 nonsense,25,27 insertion or deletion,15,17,25 and mutation at the splice junction site leading to abnormal mRNA splicing.15,25,28
Approximately 90 families with type I defect, predominantly white individuals, have been reported from many different countries, including >30 from Japan. The type II deficiency has been identified in >70 other Japanese families.2 Two thirds of our families originated from metropolitan France; however, some families originated from Martinique, Poland, Italy, Spain, Turkey, Lebanon, Canada, and African countries, suggesting that APRT deficiency affects people worldwide.
In this study, molecular analysis identified 54 mutated alleles on 62 chromosomes analyzed. The most prevalent mutation was IVS4 + 2insT, resulting in aberrant splicing of exon 4 and a truncated protein Arg108GluX3. IVS4 + 2insT accounted for 40% of mutations and was found in heterozygous or homozygous state in 20 of the 31 families studied. IVS4 + 2insT was identified previously in several families from Europe.2,15,18,29,30 This mutation occurred on the aprt allele carrying the polymorphic TaqI site, suggesting a founder effect2,13 in some families but not all.30 In the white population, two common aprt mutations show uniform associations with highly polymorphic restriction sites for TaqI and SphI,31 namely a missense mutation in British and Icelandic patients13,30 and IVS4 + 2insT, which seems to be the most common cause of APRT deficiency among white individuals.14,15,25,29,30 Of note, IVS4 + 2insT mutation was detected only in families from metropolitan France and one Italian family. Families from other countries carried other mutations at homozygous state, suggesting consanguinity.
Interestingly, Asp65Val, which was found in homozygous state in all 16 families of the Icelandic cohort,10 was found in only one of our families (Spanish). By contrast, Met136Thr, the mutant allele responsible for type II phenotype in Japanese,32 was not found in our European population.
We failed to identify eight (13%) mutations in our families, whereas complete APRT deficiency was demonstrated by null APRT activity in all patients. We focused on coding regions and intron/exon junctions, and mutations not found could be located in promoter region or large deletions in one allele.
No clear correlation between phenotype and genotype was found in our study; however, such analysis was made difficult by the important heterogeneity in the treatment of patients, which strongly influenced potential indicators of severity (e.g., renal failure occurrence). Once APRT deficiency is diagnosed in a patient, screening of the kindred is recommended. Considering the autosomal recessive transmission, investigations should be focused on siblings. APRT activity, the most specific and sensitive test, should be performed. We found that first-degree relatives heterozygous for aprt mutation had residual APRT erythrocyte activity (20 to 25% of normal value) and were completely asymptomatic (data not shown), as previously reported.33
The APRT enzymatic deficiency has an estimated frequency of heterozygosity ranging from 0.4 to 1.2% in a healthy white population34,35; expected homozygosity should range from one in 50,000 to one in 100,000.2,19,36 IVS4 + 2insT, which accounted for 40% of mutations in our patients with APRT deficiency, was found in two (0.98%) of 204 healthy newborn chromosomes. This suggests that the prevalence of complete deficiency could be far higher in the French population than estimated from previous reports in other countries. Further studies will be necessary to confirm our findings.
In summary, our study shed light on clinical features associated with APRT deficiency. Delayed diagnosis can result in deterioration of renal function and even ESRD. We hope that our series, the largest yet published, will help clinicians in early detection of difficult cases by the mean of the different diagnostic tools available.
Concise Methods
Study Population
We retrospectively reviewed all cases of APRT deficiency identified between 1978 and 2009 in the biochemistry laboratories of the Necker Teaching Hospital (Paris, France), which are referral centers for urolithiasis and purine metabolism. Most patients were not followed at Necker Hospital, but blood and/or urine samples were sent from other centers. Patients were identified through the computerized records of the biochemistry laboratories. Diagnosis of APRT deficiency was made on the basis of one or several of the following items: (1) Identification of typical 2,8-DHA crystals in urine, (2) stone analysis, (3) identification of 2,8-DHA crystals in renal biopsy sample, or (4) null APRT activity in blood erythrocytes. When available, patients' medical charts were reviewed to collect data, such as age and clinical presentation at diagnosis and at the first clinical manifestation, ethnic group, familial history, renal function evolution during follow-up, medical therapy, and urologic procedures. For most patients, clinical data were collected through full review of medical charts. In other cases, clinical features were obtained through a detailed questionnaire. Episodes of urolithiasis were defined as one of the following: (1) Spontaneous stone expulsion, (2) renal colic, or (3) radiologic detection of a new stone. Renal function was estimated using the Modification of Diet in Renal Disease (MDRD) Study formula.37 Data were collected from time of diagnosis of APRT deficiency to last follow-up available. Two patients, one from family 738 and one for whom molecular study could not be performed,39 were previously reported.
Laboratory Tests
Crystalluria examination and renal biopsy studies were performed by light microscopy using a polarizing microscope, and stone analysis combined morphologic examination by stereomicroscope and infrared spectroscopy, as previously reported by our group.20,21 Crystals in renal biopsies were identified by polarizing microscopy from frozen biopsies or biopsies included in a paraffin matrix. Briefly, 5-μm tissue slices were spread out on a calcium fluoride plate and directly examined using a Fourier transform infrared microscopy.40 APRT enzyme activity was measured in erythrocyte lysates using radiolabeled 14C-adenine in a chromatographic assay using the same method as previously reported.41,42
Aprt Gene Analysis
Mutation analysis of the aprt gene was performed after written informed consent from the patient, using a PCR and sequencing. Genomic DNA was isolated from 5 ml of whole blood using a Wizzard Genomic DNA purification kit (Promega, Madison, WI). The coding region and flanking sequences of the five aprt exons were amplified with the PCR system using Platinum Pfx DNA polymerase (Invitrogen, Carlsbad, CA). The PCR was performed in a 25-μl volume containing 50 mM Tris-HCl (pH 8.0), 50 mM KCl, 1 mM dithiothreitol, 0.1 mM EDTA, 1 mM MgSO4, 300 μM of each dNTP, 0.6 μM of each primers (sequences of primers are given in Supplemental Table 1), 0.4 U of Pfx DNA polymerase, and 225 ng of genomic DNA. The conditions used for PCR amplification were an initial denaturation phase at 95°C for 2 minutes followed by 35 cycles at 95°C for 15 seconds, annealing at 59 to 69°C for 30 seconds (Supplemental Table 1), and extension at 70°C for 30 seconds, followed by a final step of 5 minutes at 70°C. The amplified fragments were purified using QIAquick PCR purification (Qiagen, Hilden, Germany) and sequenced directly using the same primers as for the PCR. Sequences were compared with the theoretical sequence of aprt gene using Serial Cloner software. The nomenclature for the description of sequence variants was as recommended by Den Dunnen and Antonarakis43 and the Human Genome Variation Society (http://www.hgvs.org/mutnomen/). The References for gene annotation were Broderick et al.12 and Chen et al.13
We evaluated the frequency of the IVS4 + 2insT in a control population from the newborn screening program for treatable genetic, endocrinologic, metabolic, and hematologic diseases performed at Necker Hospital by the Fédération Parisienne pour la Prévention et le Dépistage des Handicaps de l'Enfant (FPDPHE). The board of directors of FPDPHE, the Comité de Protection des Personnes, and the ethics committee of Necker Hospital gave their consent for the genetic testing of IVS4 + 2insT in this control population.
The screening of IVS4 + 2insT was performed from heel blood blotted onto filter paper. A total of 102 individuals were tested for the presence of this mutation corresponding to 204 chromosomes. For each individual tested, two dishes of 3 mm in diameter from the heel-blood samples on a filter paper card were punched. The dishes were washed three times with 1 ml of NaCl 0.9%. Then the dishes were treated with 150 μl of NaOH 10 mM and heated for 10 minutes at 100°C; after cooling, the PCR was performed for IVS4 + 2insT detection.
Statistical Analysis
Results were expressed as numerical values and percentages for categorical variables and as median and 25th and 75th centiles for continuous variables, except crystalluria results, which were expressed as mean crystal numbers ± SEM.
Disclosures
None.
Acknowledgments
This work was supported by the Fondation Louis D. Institut de France, GlaxoSmithKline laboratory, Association Lesch-Nyhan Action, Fondation Jérôme Lejeune, Association Malaury, Association pour l'Utilisation du Rein Artificiel, and Association pour l'Information et la Recherche sur les maladies rénales Génétiques.
We thank V. Droin (Necker Hospital, Paris, France) for technical assistance in enzymatic APRT determination and Dr. J.L. Pérignon (Necker Hospital, Paris, France) for advice in the process of testing IVS4 + 2insT in the newborn screening program. We are grateful for assistance provided by Dr. Daniel Dion (Sacre-Coeur Hospital, Montreal, Canada) to improve language.
We acknowledge Dr. Noël (Necker Hospital, Paris, France), who provided us pictures from renal biopsy. We are very grateful for excellent assistance provided by all physicians who sent us clinical data and blood samples of patients: Prof. Jacquot (HEGP Universitary Hospital, Paris, France), Dr. Snanoudj (Necker Hospital, Paris, France), Dr. Llanas (Bordeaux Universitary Hospital, France), Dr. Cozette (Nice Hospital, France), Dr. Cassuto (Nice Hospital, France), Dr. Bouvier (Draguignan, France), Dr. Azema (Trousseau Hospital, Paris, France), Dr. Demontis (Creil Hospital, France), Dr. Kernaonet (Le Mans Hospital, France), Dr. De Sagazan (Roubaix Hospital, France), Dr. Guest (Necker Hospital, Paris, France), Dr. Barrucand (Ugine, France), Dr. Medeira (Santa Maria Hospital, Lisbon, Portugal), Dr. Ferrando Monleon (La Ribeira Hospital, Valence, Spain), Dr. Garnier (Toulouse Universitary Hospital, France), Dr. Horen (Toulouse Universitary Hospital, France), Dr. Elhani (Arinthod, France), Dr. Boyer (Alpes du Sud Clinic, Gap, France), Dr. Gie (Rennes Universitary Hospital, France), Dr. Vende (Bichat Universitary Hospital, Paris, France), Dr. Rincé (Limoges Universitary Hospital, France), Dr. Leonetti (St. Brieuc Hospital, France), Dr. Rechke (Melun Hospital, France), Dr. Gaultier (CHICAS Hospital, Gap, France), Dr. Veau (Lignière, France), Dr. Palayret (Saint Calais, France), Dr. Kolb (Ste. Anne Clinic, Strasbourg, France), Dr. Airoldi (Maggiore Hospital, Novara, Italy), Dr. Cavanese (Maggiore Hospital, Novara, Italy), Dr. Stratta (Maggiore Hospital, Novara, Italy), Dr. Glachant (Bourg en Bresse Hospital, France), Dr. Maynard (Chamberry Hospital, France), Dr. Dheu (Strasbourg Universitary Hospital, France), Dr. Parent (Colmar Hospital, France), and Dr. Chauvet (Versailles Hospital, France).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1. Cartier P, Hamet M: Purine phosphoribosyltransferase activity of human erythrocytes. Technique of determination [in French]. Clin Chim Acta 20: 205– 214, 1968 [DOI] [PubMed] [Google Scholar]
- 2. Sahota AS, Tischfield JA, Kamatani N, Simmonds HA: Adenine phosporibosyltransferase deficiency and 2,8-dihydroxyadenine lithiasis. In: The Metabolic and Molecular Bases of Inherited Disease, 8th Ed., edited by Scriver CR, Baudet AL, Sly WS, Valle D. New York, McGraw-Hill Division, 2001, pp. 2571– 2584 [Google Scholar]
- 3. Cartier P, Hamet M: The normal metabolism of uric acid. Adv Nephrol Necker Hosp 3: 3– 28, 1974 [PubMed] [Google Scholar]
- 4. Hesse A, Miersch WD, Classen A, Thon A, Doppler W: 2,8-Dihydroxyadeninuria: Laboratory diagnosis and therapy control. Urol Int 43: 174– 178, 1988 [DOI] [PubMed] [Google Scholar]
- 5. Benedetto B, Madden R, Kurbanov A, Braden G, Freeman J, Lipkowitz GS: Adenine phosphoribosyltransferase deficiency and renal allograft dysfunction. Am J Kidney Dis 37: E37, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Fye KH, Sahota A, Hancock DC, Gelb AB, Chen J, Sparks JW, Sibley RK, Tischfield JA: Adenine phosphoribosyltransferase deficiency with renal deposition of 2,8-dihydroxyadenine leading to nephrolithiasis and chronic renal failure. Arch Intern Med 153: 767– 770, 1993 [PubMed] [Google Scholar]
- 7. Glicklich D, Gruber HE, Matas AJ, Tellis VA, Karwa G, Finley K, Salem C, Soberman R, Seegmiller JE: 2,8-Dihydroxyadenine urolithiasis: Report of a case first diagnosed after renal transplant. Q J Med 68: 785– 793, 1988 [PubMed] [Google Scholar]
- 8. Bouzidi H, Lacour B, Daudon M: 2,8-Dihydroxyadenine nephrolithiasis: From diagnosis to therapy [in French]. Ann Biol Clin (Paris) 65: 585– 592, 2007 [PubMed] [Google Scholar]
- 9. Doppler W, Hirsch-Kauffmann M, Schabel F, Schweiger M: Characterization of the biochemical basis of a complete deficiency of the adenine phosphoribosyl transferase (APRT). Hum Genet 57: 404– 410, 1981 [DOI] [PubMed] [Google Scholar]
- 10. Edvardsson V, Palsson R, Olafsson I, Hjaltadottir G, Laxdal T: Clinical features and genotype of adenine phosphoribosyltransferase deficiency in Iceland. Am J Kidney Dis 38: 473– 480, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Kamatani N, Terai C, Kuroshima S, Nishioka K, Mikanagi K: Genetic and clinical studies on 19 families with adenine phosphoribosyltransferase deficiencies. Hum Genet 75: 163– 168, 1987 [DOI] [PubMed] [Google Scholar]
- 12. Broderick TP, Schaff DA, Bertino AM, Dush MK, Tischfield JA, Stambrook PJ: Comparative anatomy of the human APRT gene and enzyme: nucleotide sequence divergence and conservation of a nonrandom CpG dinucleotide arrangement. Proc Natl Acad Sci U S A 84: 3349– 3353, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen J, Sahota A, Laxdal T, Scrine M, Bowman S, Cui C, Stambrook PJ, Tischfield JA: Identification of a single missense mutation in the adenine phosphoribosyltransferase (APRT) gene from five Icelandic patients and a British patient. Am J Hum Genet 49: 1306– 1311, 1991 [PMC free article] [PubMed] [Google Scholar]
- 14. Deng L, Yang M, Frund S, Wessel T, De Abreu RA, Tischfield JA, Sahota A: 2,8-Dihydroxyadenine urolithiasis in a patient with considerable residual adenine phosphoribosyltransferase activity in cell extracts but with mutations in both copies of APRT. Mol Genet Metab 72: 260– 264, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Hidaka Y, Palella TD, O'Toole TE, Tarle SA, Kelley WN: Human adenine phosphoribosyltransferase: Identification of allelic mutations at the nucleotide level as a cause of complete deficiency of the enzyme. J Clin Invest 80: 1409– 1415, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hidaka Y, Tarle SA, Fujimori S, Kamatani N, Kelley WN, Palella TD: Human adenine phosphoribosyltransferase deficiency: Demonstration of a single mutant allele common to the Japanese. J Clin Invest 81: 945– 950, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamatani N, Hakoda M, Otsuka S, Yoshikawa H, Kashiwazaki S: Only three mutations account for almost all defective alleles causing adenine phosphoribosyltransferase deficiency in Japanese patients. J Clin Invest 90: 130– 135, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sahota A, Chen J, Stambrook PJ, Tischfield JA: Mutational basis of adenine phosphoribosyltransferase deficiency. Adv Exp Med Biol 309B: 73– 76, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Ceballos-Picot I, Perignon JL, Hamet M, Daudon M, Kamoun P: 2,8-Dihydroxyadenine urolithiasis, an underdiagnosed disease. Lancet 339: 1050– 1051, 1992 [PubMed] [Google Scholar]
- 20. Daudon M, Bader CA, Jungers P. Urinary calculi: Review of classification methods and correlations with etiology. Scanning Microsc 7: 1081– 1104, discussion 1104–1106, 1993 [PubMed] [Google Scholar]
- 21. Daudon M, Jungers P: Clinical value of crystalluria and quantitative morphoconstitutional analysis of urinary calculi. Nephron Physiol 98: 31– 36, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Eller P, Rosenkranz AR, Mark W, Theurl I, Laufer J, Lhotta K: Four consecutive renal transplantations in a patient with adenine phosphoribosyltransferase deficiency. Clin Nephrol 61: 217– 221, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Jungers P, Joly D, Barbey F, Choukroun G, Daudon M: ESRD caused by nephrolithiasis: Prevalence, mechanisms, and prevention. Am J Kidney Dis 44: 799– 805, 2004 [PubMed] [Google Scholar]
- 24. Vernon HJ, Osborne C, Tzortzaki EG, Yang M, Chen J, Rittling SR, Denhardt DT, Buyske S, Bledsoe SB, Evan AP, Fairbanks L, Simmonds HA, Tischfield JA, Sahota A: Aprt/Opn double knockout mice: Osteopontin is a modifier of kidney stone disease severity. Kidney Int 68: 938– 947, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Chen J, Sahota A, Martin GF, Hakoda M, Kamatani N, Stambrook PJ, Tischfield JA: Analysis of germline and in vivo somatic mutations in the human adenine phosphoribosyltransferase gene: Mutational hot spots at the intron 4 splice donor site and at codon 87. Mutat Res 287: 217– 225, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Sahota A, Chen J, Boyadjiev SA, Gault MH, Tischfield JA: Missense mutation in the adenine phosphoribosyltransferase gene causing 2,8-dihydroxyadenine urolithiasis. Hum Mol Genet 3: 817– 818, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Mimori A, Hidaka Y, Wu VC, Tarle SA, Kamatani N, Kelley WN, Pallela TD: A mutant allele common to the type I adenine phosphoribosyltransferase deficiency in Japanese subjects. Am J Hum Genet 48: 103– 107, 1991 [PMC free article] [PubMed] [Google Scholar]
- 28. Gathof BS, Sahota A, Gresser U, Chen J, Stambrook PS, Tischfield JA, Zollner N: A splice mutation at the adenine phosphoribosyltransferase locus detected in a German family. Adv Exp Med Biol 309B: 83– 86, 1991 [DOI] [PubMed] [Google Scholar]
- 29. Gathof BS, Zollner N: The restriction enzyme Mse I applied for the detection of a possibly common mutation of the APRT locus. Clin Investig 70: 535, 1992 [DOI] [PubMed] [Google Scholar]
- 30. Menardi C, Schneider R, Neuschmid-Kaspar F, Klocker H, Hirsch-Kauffmann M, Auer B, Schweiger M: Human APRT deficiency: Indication for multiple origins of the most common Caucasian mutation and detection of a novel type of mutation involving intrastrand-templated repair. Hum Mutat 10: 251– 255, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Kamatani N, Kuroshima S, Hakoda M, Palella TD, Hidaka Y: Crossovers within a short DNA sequence indicate a long evolutionary history of the APRT*J mutation. Hum Genet 85: 600– 604, 1990 [DOI] [PubMed] [Google Scholar]
- 32. Kamatani N, Kuroshima S, Yamanaka H, Nakashe S, Take H, Hakoda M: Identification of a compound heterozygote for adenine phosphoribosyltransferase deficiency (APRT*J/APART*Q0) leading to 2,8-dihydroxyadenine urolithiasis. Hum Genet 85: 500– 504, 1990 [DOI] [PubMed] [Google Scholar]
- 33. Dean BM, Perrett D, Simmonds HA, Sahota A, Van Acker KJ: Adenine and adenosine metabolism in intact erythrocytes deficient in adenosine monophosphate-pyrophosphate phosphoribosyltransferase: A study of two families. Clin Sci Mol Med 55: 407– 412, 1978 [DOI] [PubMed] [Google Scholar]
- 34. Johnson LA, Gordon RB, Emmerson BT: Adenine phosphoribosyltransferase: A simple spectrophotometric assay and the incidence of mutation in the normal population. Biochem Genet 15: 265– 572, 1977 [DOI] [PubMed] [Google Scholar]
- 35. Srivastava SK, Villacorte D, Beutler E: Correlation between adenylate metabolizing enzymes and adenine nucleotide levels of erythrocytes during blood storage in various media. Transfusion 12: 190– 197, 1972 [DOI] [PubMed] [Google Scholar]
- 36. Simmonds H, Van Acker KJ, Sahota AS: 2,8-Dihydroxyadenine urolithiasis. Lancet 339: 1295– 1296, 1992 [DOI] [PubMed] [Google Scholar]
- 37. Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F: Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766– 772, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Hoffmann M, Talaszka A, Bocquet J, Le Monies de Sagazan H, Daudon M: Acute renal failure and 2,8-dihydroxyadeninuria [in French]. Néphrologie 25: 297– 300, 2004 [PubMed] [Google Scholar]
- 39. Gagne ER, Deland E, Daudon M, Noel LH, Nawar T: Chronic renal failure secondary to 2,8-dihydroxyadenine deposition: The first report of recurrence in a kidney transplant. Am J Kidney Dis 24: 104– 107, 1994 [DOI] [PubMed] [Google Scholar]
- 40. Estepa-Maurice L, Hennequin C, Marfisi C, Bader C, Lacour B, Daudon M: Fourier transform infrared microscopy identification of crystal deposits in tissues: Clinical importance in various pathologies. Am J Clin Pathol 105: 576– 582, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Ceballos-Picot I, Mockel L, Potier MC, Dauphinot L, Shirley TL, Torero-Ibad R, Fuchs J, Jinnah HA: Hypoxanthine-guanine phosphoribosyl transferase regulates early developmental programming of dopamine neurons: Implications for Lesch-Nyhan disease pathogenesis. Hum Mol Genet 18: 2317– 2327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ea HK, Bardin T, Jinnah HA, Aral B, Liote F, Ceballos-Picot I: Severe gouty arthritis and mild neurologic symptoms due to F199C, a newly identified variant of the hypoxanthine guanine phosphoribosyltransferase. Arthritis Rheum 60: 2201– 2204, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Den Dunnen JT, Antonarakis SE: Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat 15: 7– 12, 2000 [DOI] [PubMed] [Google Scholar]