Abstract
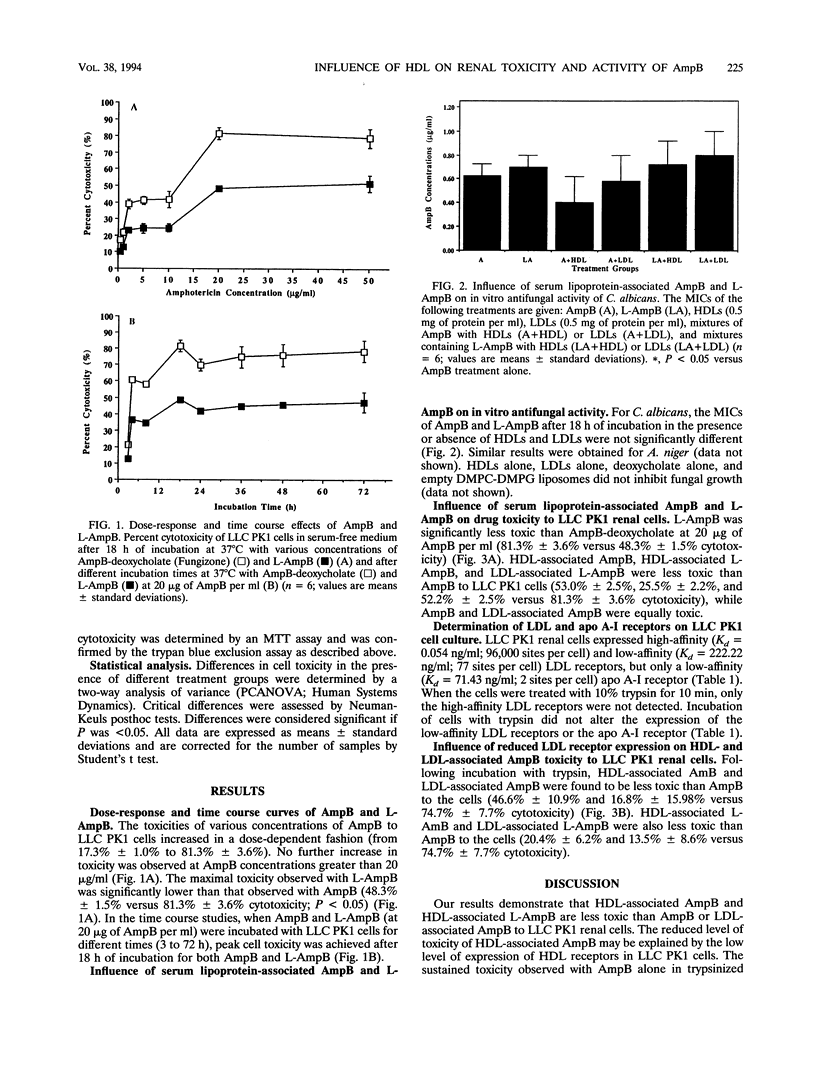
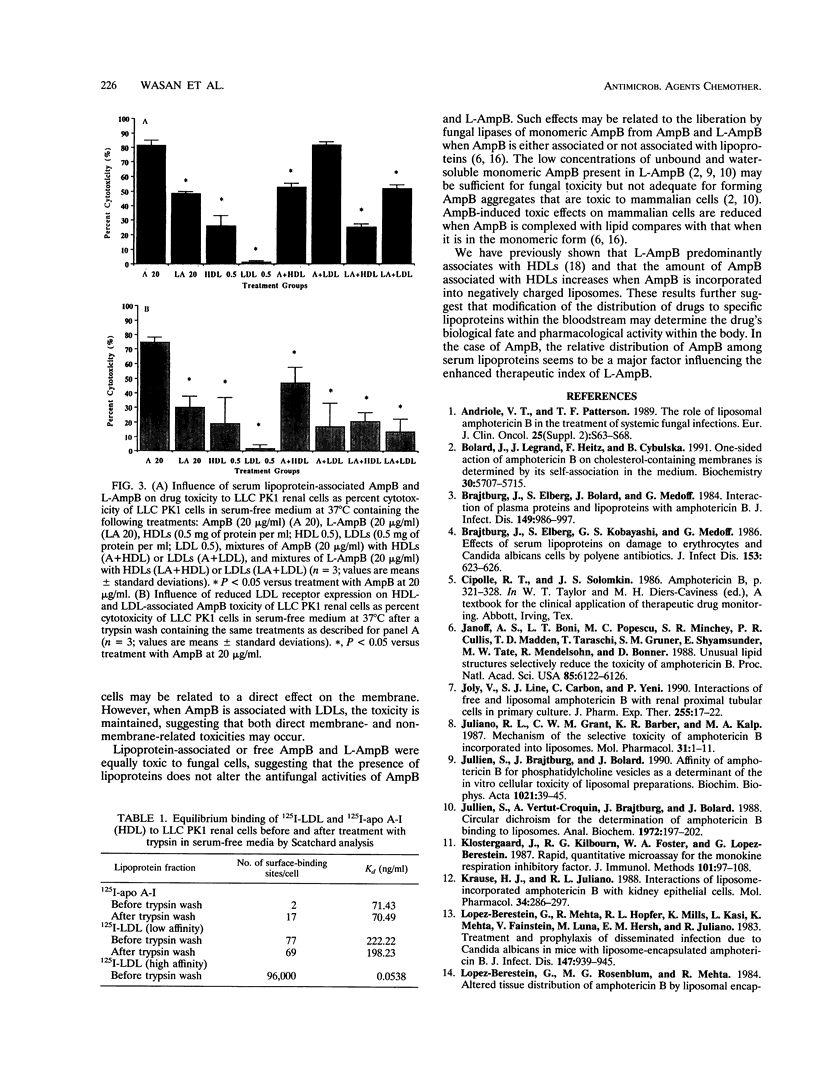
We examined the influence of high-density lipoproteins (HDLs) and low-density lipoproteins (LDLs) on the toxicity of amphotericin B (AmpB) to fungal and renal cells. Candida albicans was incubated for 18 h at 37 degrees C with AmpB and deoxycholate (Fungizone) or liposomal AmpB (L-AmpB) (0.1 to 2.0 micrograms of AmpB per ml) in the presence or absence of HDLs or LDLs (0.5 mg of protein per ml). The MICs of AmpB and L-AmpB, whether or not HDLs or LDLs were present, were similar. LLC PK1 renal cells, derived from primary cultures of pig proximal tubular cells, were incubated for 18 h at 37 degrees C in serum-free medium that contained AmpB and deoxycholate or L-AmpB at 20 micrograms of AmpB per ml, HDLs or LDLs at 0.5 mg of protein per ml, mixtures of AmpB with HDLs or LDLs, and mixtures of L-AmpB with HDLs or LDLs. HDL-associated AmpB was less toxic than AmB to LLC PK1 cells (53.0% +/- 2.5% versus 81.3% +/- 3.6% cytotoxicity; P = 0.01), while LDL-associated AmpB was as toxic as AmpB. L-AmpB, HDL-associated L-AmpB, and LDL-associated L-AmpB were less toxic to LLC PK1 cells than was AmpB (48.3% +/- 1.5%, 25.5% +/- 2.2%, and 52.2% +/- 2.5% versus 81.3% +/- 3.6% cytotoxicity; P = 0.02). To further understand why HDL-associated AmpB reduced renal cytotoxic effects, the LLC PK1 cells were examined for the presence of HDL and LDL receptors. LLC PK1 cells expressed high-affinity (K(d) = 0.0538 nanograms/ml; 96,000 sites per cell) and low-affinity (K(d) = 222.22 nanograms/ml; 77 sites per cell) LDL receptors but only a low-affinity HDL receptor (K(d) = 71.43 nanograms/ml; 2 sites per cell). HDL-associated AmpB and LDL-associated AmpB were less toxic than AmpB to trypsinized LLC PK1 cells (46.6% +/- 10.9% and 16.8% +/- 15.98% versus 74.7% +/- 7.7% cytotoxicity; P = 0.02). HDL-associated AmB and LDL-associated L-AmpB were also less toxic than AmpB to the cells (20.4% +/- 6.2% and 13.5% +/- 8.6% versus 74.7% cytotoxicity; P = 0.01). The antifungal activities of AmpB and L-AmpB were not altered in the presence of HDLs or LDLs. We conclude that the reduced nephrotoxicity associated with the use of L-AmpB is related to a decreased uptake of AmpB by renal cells when AmpB is associated with HDLs because of the low level of expression of HDL receptors in these cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolard J., Legrand P., Heitz F., Cybulska B. One-sided action of amphotericin B on cholesterol-containing membranes is determined by its self-association in the medium. Biochemistry. 1991 Jun 11;30(23):5707–5715. doi: 10.1021/bi00237a011. [DOI] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Bolard J., Kobayashi G. S., Levy R. A., Ostlund R. E., Jr, Schlessinger D., Medoff G. Interaction of plasma proteins and lipoproteins with amphotericin B. J Infect Dis. 1984 Jun;149(6):986–997. doi: 10.1093/infdis/149.6.986. [DOI] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Kobayashi G. S., Medoff G. Effects of serum lipoproteins on damage to erythrocytes and Candida albicans cells by polyene antibiotics. J Infect Dis. 1986 Mar;153(3):623–626. doi: 10.1093/infdis/153.3.623. [DOI] [PubMed] [Google Scholar]
- Janoff A. S., Boni L. T., Popescu M. C., Minchey S. R., Cullis P. R., Madden T. D., Taraschi T., Gruner S. M., Shyamsunder E., Tate M. W. Unusual lipid structures selectively reduce the toxicity of amphotericin B. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6122–6126. doi: 10.1073/pnas.85.16.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly V., Saint-Julien L., Carbon C., Yeni P. Interactions of free and liposomal amphotericin B with renal proximal tubular cells in primary culture. J Pharmacol Exp Ther. 1990 Oct;255(1):17–22. [PubMed] [Google Scholar]
- Juliano R. L., Grant C. W., Barber K. R., Kalp M. A. Mechanism of the selective toxicity of amphotericin B incorporated into liposomes. Mol Pharmacol. 1987 Jan;31(1):1–11. [PubMed] [Google Scholar]
- Jullien S., Brajtburg J., Bolard J. Affinity of amphotericin B for phosphatidylcholine vesicles as a determinant of the in vitro cellular toxicity of liposomal preparations. Biochim Biophys Acta. 1990 Jan 15;1021(1):39–45. doi: 10.1016/0005-2736(90)90381-w. [DOI] [PubMed] [Google Scholar]
- Jullien S., Vertut-Croquin A., Brajtburg J., Bolard J. Circular dichroism for the determination of amphotericin B binding to liposomes. Anal Biochem. 1988 Jul;172(1):197–202. doi: 10.1016/0003-2697(88)90432-0. [DOI] [PubMed] [Google Scholar]
- Klostergaard J., Kilbourn R. G., Foster W. A., Lopez-Berestein G. Rapid, quantitative microassay for the monokine respiration inhibitory factor. J Immunol Methods. 1987 Jul 16;101(1):97–108. doi: 10.1016/0022-1759(87)90222-5. [DOI] [PubMed] [Google Scholar]
- Krause H. J., Juliano R. L. Interactions of liposome-incorporated amphotericin B with kidney epithelial cell cultures. Mol Pharmacol. 1988 Sep;34(3):286–297. [PubMed] [Google Scholar]
- Lopez-Berestein G., Mehta R., Hopfer R. L., Mills K., Kasi L., Mehta K., Fainstein V., Luna M., Hersh E. M., Juliano R. Treatment and prophylaxis of disseminated infection due to Candida albicans in mice with liposome-encapsulated amphotericin B. J Infect Dis. 1983 May;147(5):939–945. doi: 10.1093/infdis/147.5.939. [DOI] [PubMed] [Google Scholar]
- Patterson T. F., Andriole V. T. The role of liposomal amphotericin B in the treatment of systemic fungal infections. Eur J Cancer Clin Oncol. 1989;25 (Suppl 2):S63–S68. [PubMed] [Google Scholar]
- Perkins W. R., Minchey S. R., Boni L. T., Swenson C. E., Popescu M. C., Pasternack R. F., Janoff A. S. Amphotericin B-phospholipid interactions responsible for reduced mammalian cell toxicity. Biochim Biophys Acta. 1992 Jun 30;1107(2):271–282. doi: 10.1016/0005-2736(92)90414-h. [DOI] [PubMed] [Google Scholar]
- Wasan K. M., Brazeau G. A., Keyhani A., Hayman A. C., Lopez-Berestein G. Roles of liposome composition and temperature in distribution of amphotericin B in serum lipoproteins. Antimicrob Agents Chemother. 1993 Feb;37(2):246–250. doi: 10.1128/aac.37.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]



