Abstract
RNA interference by short interfering RNAs (siRNAs) holds promise as a therapeutic strategy, but use of siRNAs in vivo remains limited. Here, we developed a system to target delivery of siRNAs to glomeruli via poly(ethylene glycol)-poly(l-lysine)-based vehicles. The siRNA/nanocarrier complex was approximately 10 to 20 nm in diameter, a size that would allow it to move across the fenestrated endothelium to access to the mesangium. After intraperitoneal injection of fluorescence-labeled siRNA/nanocarrier complexes, we detected siRNAs in the blood circulation for a prolonged time. Repeated intraperitoneal administration of a mitogen-activated protein kinase 1 (MAPK1) siRNA/nanocarrier complex suppressed glomerular MAPK1 mRNA and protein expression in a mouse model of glomerulonephritis; this improved kidney function, reduced proteinuria, and ameliorated glomerular sclerosis. Furthermore, this therapy reduced the expression of the profibrotic markers TGF-β1, plasminogen activator inhibitor-1, and fibronectin. In conclusion, we successfully silenced intraglomerular genes with siRNA using nanocarriers. This technique could aid the investigation of molecular mechanisms of renal disease and has potential as a molecular therapy of glomerular diseases.
RNA interference by double-stranded short interfering RNAs (siRNAs) has great potential as a research tool and also as a therapeutic strategy, because of its powerful gene-silencing ability.1–4 However, in vivo application of siRNAs is still limited to local delivery.5–8 This limitation is attributed to the low stability of siRNAs in vivo due to enzymatic degradation and/or to their low permeability across the cell membrane. Thus, development of delivery vehicles is necessary for introduction of siRNAs to the target tissues by systemic administration. In the case of renal disease, only a few delivery systems have been reported to show any efficacy in delivery of siRNAs to renal tissues. The siRNAs conjugated with lipids9 or encapsulated in liposomes10 have been shown to accumulate in the liver and to silence target genes, but their distribution in the kidneys might merely reflect the process of degradation in tubular cells or their excretion into the tubular lumen. The glomeruli contain cells that secrete various pathogenic factors in response to hemodynamic or immunological derangements, making these cells reasonable targets for molecular therapy. However, siRNA-mediated gene silencing in glomeruli has proven difficult.
In the present study, we provide evidence for efficient targeting of siRNAs to the murine glomeruli using poly(ethylene glycol) (PEG)-poly(l-lysine) (PLL) copolymer-based delivery vehicles, also termed polyion complex (PIC) nanocarriers. Using this delivery system, MAPK1 siRNAs were targeted to the glomeruli in the murine lupus nephritis model. In this model, these siRNAs actually inhibited the glomerular expression of MAPK1 mRNA and MAPK1 protein, resulting in the amelioration of pathologic changes in the glomeruli.
Results
Characteristics of the Polyion Complex Nanocarriers
In this study, siRNAs complexed with PIC nanocarriers were prepared by simply mixing PEG-PLL copolymers (12 to 73) with siRNA solution at an N/P ratio of 1.4. PIC nanocarriers are formed through the electrostatic interaction between positively charged poly(l-lysine) segment of PEG-PLL and negatively charged siRNA. siRNA is not covalently conjugated to PEG-PLL (Figure 1A). Size distribution in the dynamic light scattering (DLS) measurement revealed the formation of complexes about 10 nm in size (Figure 1B). Fluorescence correlation spectroscopy (FCS) analysis confirmed the complexes to be 13.7 ± 0.1 nm in size. These nanocarriers are therefore small compared with Hemaglutinating virus of Japan (HVJ) envelope (HVJ-E) vectors, which measured 297.3 nm in diameter by DLS analysis. The diameter of the naked siRNA was shown to be 6.56 ± 0.07 nm by FCS analysis. Therefore, this complex might exist as a unit composed of one siRNA and a smaller number of PEG-PLL copolymers.
Figure 1.
Characteristics of the PIC nanocarriers. (A) Chemical structures of PEG-PLL block copolymers and P(Asp) homopolymers. (B) Size distribution of the PIC nanocarriers was measured by DLS.
In Vitro Transfection of Mesangial Cells with siRNAs Complexed with Nanocarriers
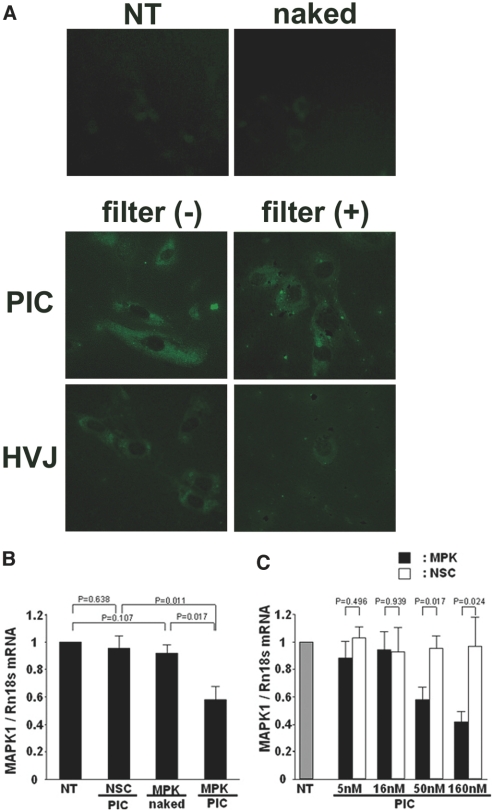
The in vitro transfection efficiency of PIC nanocarriers was examined with FITC-labeled nonsilencing control siRNAs complexed with PIC nanocarriers, with FITC-labeled NSC siRNAs encapsulated in HVJ-E, or with FITC-labeled naked nonsilencing control siRNAs using cultured mouse mesangial cells. The cells treated with the siRNA/PIC nanocarrier complex showed strong fluorescence (Figure 2A), whereas almost no fluorescence was seen in control cells or the cells treated with naked siRNAs.
Figure 2.
In vitro transfection of siRNAs complexed with PIC nanocarriers into mesangial cells. (A) Fluorescence photomicrograph of the cultured mesangial cells treated with the FITC-labeled nonsilencing control siRNAs (naked), FITC-labeled siRNAs complexed with the nanocarriers (PIC), FITC-labeled siRNAs encapsulated in HVJ-E (HVJ), or nontreated (NT) cells. The PIC nanocarriers, in contrast to HVJ-E, were shown to pass through the 0.2-μm-sized filter. Figures are representative of three independent experiments. Original magnification ×200. (B and C) Q-RT-PCR analysis of the MAPK1 expression in the cultured mesangial cells. The transfection of the MAPK1 siRNAs complexed with the nanocarriers significantly suppressed the MAPK1 mRNA expression at a concentration of more than 50 nM MAPK1 siRNAs in a dose-dependent manner. P values were calculated by ANOVA, mean ± SEM, n = 5. NSC/PIC, nonsilencing control siRNAs complexed with PIC nanocarriers; MPK/naked, naked MAPK1 siRNAs; MPK/PIC, MAPK1 siRNAs complexed with PIC nanocarriers.
To mimic the size-selective barrier of the fenestrated glomerular endothelium, transfection into mesangial cells was performed after the samples were filtered through 0.2-μm filters. In this case, the fluorescence almost disappeared in the siRNA/HVJ-E group pretreated with the filter, whereas the cells treated with filtrated siRNA/nanocarrier complexes retained significant fluorescence. This indicated that the siRNA/nanocarrier complex could pass through a 0.2-μm pore (Figure 2A).
Next, we investigated the gene-silencing effect of the MAPK1 siRNA/nanocarrier complex in mesangial cells. Quantitative real-time (Q-RT) PCR analysis revealed that MAPK1 mRNA expression was significantly suppressed at 50 nM or higher concentrations of MAPK1 siRNAs (Figure 2, B and C).
In Vivo and Ex Vivo Optical Imaging Analysis
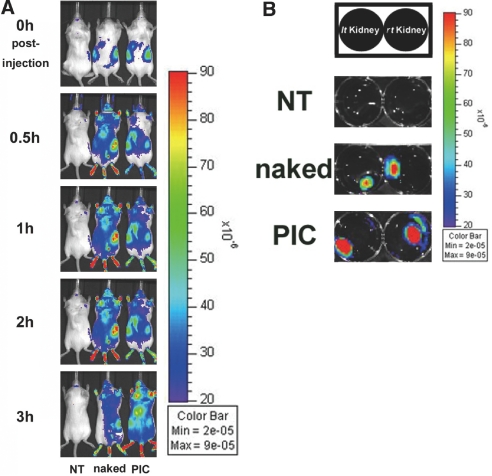
We used an IVIS system to investigate the systemic distribution of the siRNAs complexed with nanocarriers. This system can detect the fluorescence intensity of the distributed fluorescence substance of intact or degraded siRNAs in two dimensions, indicated by color graduation. Whole-body images of mice showed strong fluorescence in both kidneys immediately after peritoneal injection of 5 nmol of Cy5-labeled siRNA/nanocarrier complexes. A high-fluorescence signal could be significantly visualized in kidneys with minimal contrast within 3 hours, unlike kidneys treated with naked siRNAs (Figure 3A). We also performed ex vivo imaging analysis of the major organs 3.5 hours after intraperitoneal administration of naked siRNAs or siRNA/nanocarrier complexes. The fluorescence signal was detected in the liver, lungs, and kidneys in mice treated with either naked siRNAs or siRNA/nanocarrier complexes. However, the entire kidney showed high fluorescence in the mice treated with siRNA/nanocarrier complexes. Kidneys treated with naked siRNAs showed fluorescence only in the central region, suggesting that much of the degraded product was excreted in urine, although some of the siRNAs might be intact. The siRNA/nanocarrier complexes apparently had a persistent distribution to the cortical area where the glomeruli are located (Figure 3B).
Figure 3.
In vivo and ex vivo optical imaging analysis. (A) Cy5-labeled control siRNAs complexed with the PIC nanocarriers accumulated in kidneys immediately after intraperitoneal injection and retained high fluorescence signals in kidneys for more than 3 hours. (B) Ex vivo imaging for kidneys 3.5 hours postinjection. Prolonged signal was detected in the whole kidneys, including cortical area of the mouse treated with siRNA/nanocarriers complex, compared with naked siRNAs. Figures are representative of three independent experiments. NT, nontreatment; naked, naked siRNAs; PIC, siRNAs complexed with the PIC nanocarriers.
Intraglomerular Accumulation and Localization of the Nanocarriers
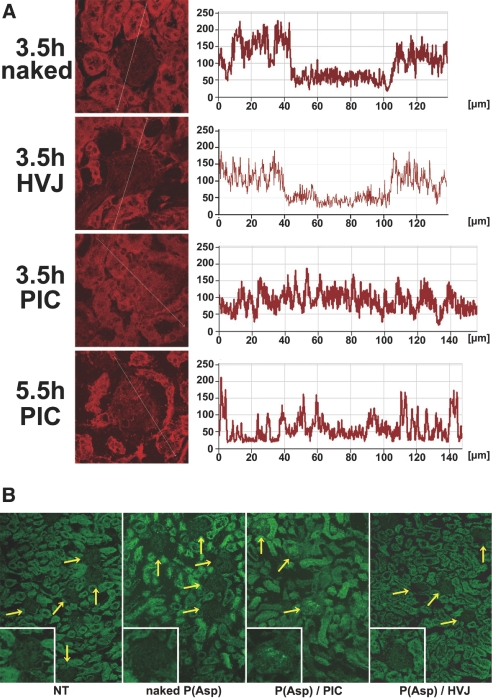
Cross-sectional analysis by fluorescence intensity profiling revealed higher fluorescence signals at 3.5 hours in the glomeruli of the mice treated with the siRNA/nanocarrier complexes than in those treated with naked siRNAs or with siRNA/HVJ-E (Figure 4A). An autofluorescence of the tubular region was found in all groups. A high-fluorescence intensity of a P(Asp)/nanocarrier complex could also be demonstrated in almost all glomeruli with fluorescence microscopy (Figure 4B) when FITC-labeled poly(α,β-aspartic acid) [P(Asp)] was used as a model polyanion, in place of siRNAs. Staining for OX-7, a mesangial marker, was used to further examine the cellular localization of the P(Asp)/nanocarrier complexes injected into Wistar rats. FITC-positive areas in the glomeruli were almost all overlaid with OX-7 staining, suggesting that the complexes were localized primarily in the mesangium (Supplemental Figure 1).
Figure 4.
The PIC nanocarrier complex accumulates in glomeruli. (A) Confocal microscopy analysis and cross-sectional intensity measurements showing that Cy5-labeled siRNAs complexed with the PIC nanocarriers were taken up in the glomeruli at 3.5 or 5.5 hours after intraperitoneal injection. naked, naked siRNAs; PIC, siRNAs complexed with the PIC nanocarriers; HVJ, siRNAs encapsulated in HVJ-E. (B) Fluorescence photomicrograph of the kidney section at 6 hours after intraperitoneal injection of FITC-labeled P(Asp) complexed with the PIC nanocarriers (P(Asp)/PIC), compared with nontreatment (NT), naked P(Asp), and P(Asp) encapsulated in HVJ-E (P(Asp)/HVJ). The high-fluorescence intensity was detected in almost all glomeruli (yellow arrows and inset) of the mice treated with P(Asp)/PIC. Autofluorescence was found in tubular region. Figures are representative of three independent experiments. Original magnification ×200.
Prolonged Blood Circulation of siRNAs Complexed with the Nanocarriers
Polyacrylamide gel electrophoresis of siRNAs was performed to evaluate the stability of siRNAs complexed with PIC nanocarriers in circulation. Ethidium bromide (EtBr) staining showed that the naked siRNAs injected intraperitoneally were rapidly eliminated from the blood within 10 minutes, whereas siRNA/nanocarrier complexes prolonged the circulation of siRNAs for 2 hours beyond intraperitoneal injection (Supplemental Figure 2A). We also directly measured the fluorescence intensity in mouse plasma at various time points after injection of Alexa Fluor 647-labeled siRNAs complexed with PIC nanocarriers or of Alexa Fluor 647-labeled naked siRNAs (Supplemental Figure 2B). The Alexa Fluor 647-labeled siRNA/nanocarrier complexes showed persistent fluorescence for 2 hours, unlike the naked siRNAs. For urinary excretion of siRNAs, we measured fluorescence intensity of urine spots. The naked siRNAs were excreted quickly after injection, and only a trace amount of fluorescence was detected in urine samples after 210 minutes, whereas the excretion of siRNA/nanocarrier complexes into urine was delayed by about 60 minutes (Supplemental Figure 3, A and B).
Intraglomerular MAPK1 Silencing by MAPK1 siRNAs Complexed with Nanocarriers in Glomerulonephritic Mice
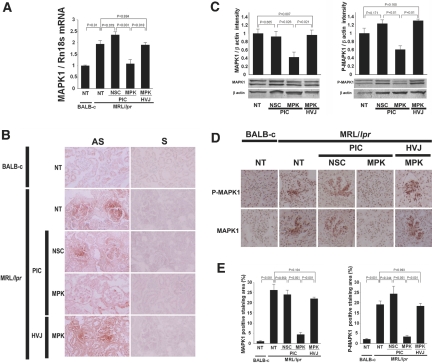
To investigate whether MAPK1 siRNAs complexed with PIC nanocarriers could exert an in vivo gene-silencing effect in the glomeruli, we used MRL/lpr mice, lupus nephritis models. Repeated intraperitoneal injections of MAPK1 siRNAs complexed with nanocarriers, control siRNAs complexed with nanocarriers, or MAPK1 siRNAs encapsulated in HVJ-E were carried out twice weekly between 12 and 16 weeks of age. The mice were killed at 17 weeks of age. Q-RT-PCR analysis showed that MAPK1 mRNA expression in isolated glomeruli was significantly suppressed in the mice treated with the MAPK1 siRNA/nanocarrier complexes, whereas control siRNA/nanocarrier complexes or MAPK1 siRNA/HVJ-E had no effect on expression (Figure 5A). In situ hybridization of MAPK1 mRNA in kidney sections directly indicated the silencing of MAPK1 in glomeruli when MAPK1 siRNA/nanocarrier complex was used, whereas no silencing was observed with control siRNA/nanocarrier complexes or in untreated controls (Figure 5B). Western blot analysis of the sieved glomerular fractions revealed that total MAPK1 and phosphorylated MAPK1 protein levels were reduced in the mice treated with the MAPK1 siRNA/nanocarrier complex (Figure 5C). Immunohistochemical analysis showed that both MAPK1 and phosphorylated MAPK1 in glomeruli were almost completely suppressed in the group treated with MAPK1 siRNA/nanocarrier complexes compared with the group treated with control siRNA/nanocarrier complex or the untreated group (Figure 5, D and E). Treatment with MAPK1 siRNAs encapsulated in HVJ-E did not suppress the glomerular expression of MAPK1 mRNA and MAPK1 protein (Figure 5). We also performed immunohistochemical study for glomerular synaptopodin to rule out the nonspecific global knockdown of gene expression in glomeruli. No suppression of synaptopodin was seen in the group treated with MAPK1 siRNAs complexed with the PIC nanocarriers (Supplemental Figure 4).
Figure 5.
Silencing of intraglomerular MAPK1 by MAPK1 siRNAs complexed with the PIC nanocarriers. (A) Q-RT-PCR analysis of MAPK1 mRNA expression in isolated glomeruli of MRL/lpr mice. NT, nontreatment; NSC/PIC, nonsilencing control siRNAs complexed with PIC nanocarriers; MPK/PIC, MAPK1 siRNAs complexed with PIC nanocarriers; MPK/HVJ, MAPK1 siRNAs encapsulated in HVJ-E. Nontreated BALB-c mice were used as control. P values were calculated by ANOVA, mean ± SEM, n = 5. (B) In situ hybridization for MAPK1 mRNA in the kidney sections using antisense (AS) and sense (S) probes. Original magnification ×200. (C) Western blots and densitometry analysis of the results. MAPK1 siRNAs complexed with the PIC nanocarriers suppressed the expression of both MAPK1 protein (left) and P-MAPK1 protein (right). P values were calculated by ANOVA, mean ± SEM, n = 4. (D) MAPK1 and P-MAPK1 immunostaining of the kidney sections. Original magnification ×200. (E) Densitometry analysis in panel D. Data were expressed as the positive-stained area of MAPK1 (left) or P-MAPK1 (right) in a glomerulus (%).
siRNA-Mediated Intraglomerular MAPK1 Silencing by Nanocarriers Ameliorates the Glomerular Histology and Laboratory Data in Glomerulonephritic Mice
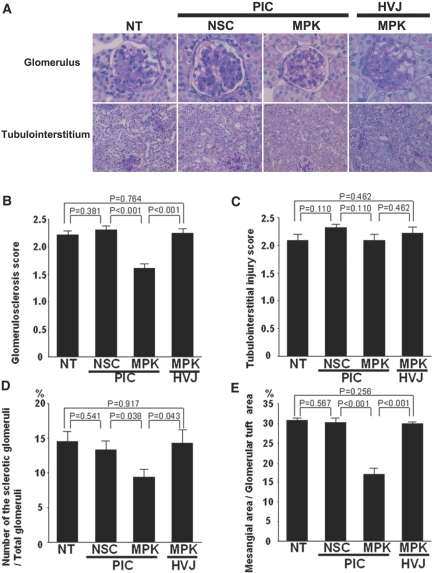
Control MRL/lpr mice showed an elevation of blood urea nitrogen (BUN) at 17 weeks of age, which was consistent with a previous report.11 After repeated intraperitoneal injections of MAPK1 siRNA/nanocarrier complexes between 12 and 16 weeks of age, BUN and urinary protein levels were significantly reduced compared with untreated mice, or those treated with control siRNA/nanocarrier complexes or MAPK1 siRNA/HVJ-E (Table 1). The histopathological analysis of the kidney was performed by periodic acid-Schiff (PAS) staining (Figure 6). The glomerular sclerosis score (Figure 6B), the number of the glomeruli falling into global sclerosis (Figure 6D), and the percentage of PAS-positive glomerular lesions (Figure 6E) were all decreased significantly in the group receiving the MAPK1 siRNA/nanocarrier complexes, although tubulointerstitial lesions (Figure 6C) were unchanged.
Table 1.
Characteristics of study populations
| NT | NSC/PIC | MPK/PIC | MPK/HVJ | Pa | Pb | |
|---|---|---|---|---|---|---|
| Body weight, g | 33.28 ± 6.115 | 34.66 ± 2.074 | 36.20 ± 2.078 | 30.31 ± 7.700 | 0.291 | 0.228 |
| Spleen, g | 0.45 ± 0.149 | 0.59 ± 0.210 | 0.52 ± 0.185 | 0.52 ± 0.155 | 0.427 | 0.511 |
| Kidney, g | 0.21 ± 0.039 | 0.22 ± 0.022 | 0.22 ± 0.018 | 0.23 ± 0.059 | 0.357 | 0.518 |
| BUN, mg/dl | 29.66 ± 2.171 | 26.90 ± 4.057 | 19.18 ± 1.199 | 24.77 ± 1.350 | <0.001 | <0.01 |
| Proteinuria, mg/mg creatinine | 1.97 ± 0.270 | 2.15 ± 0.509 | 0.88 ± 0.135 | 1.97 ± 0.315 | <0.05 | <0.05 |
NT, not treated; NSC/PIC, nonsilencing control siRNAs complexed with PIC nanocarriers; MPK/PIC, MAPK1 siRNAs complexed with PIC nanocarriers; MPK/HVJ, MAPK1 siRNAs encapsulated in HVJ-E.
aNT versus MPK/PIC.
bNSC/PIC versus MPK/PIC.
Figure 6.
Amelioration of the glomerular lesions by MAPK1 siRNAs complexed with the PIC nanocarriers. (A) Representative glomeruli (upper) and tubulointerstitial regions (lower) are shown. NT, nontreatment; NSC/PIC, nonsilencing control siRNAs complexed with PIC nanocarriers; MPK/PIC, MAPK1 siRNAs complexed with PIC nanocarriers; MPK/HVJ, MAPK1 siRNAs encapsulated in HVJ-E. Original magnification ×200. (B–E) Histologic analysis of the kidney sections. The glomerular sclerosis score (B), the number of glomeruli with global sclerosis (D), and the PAS-positive glomerular lesions (%) (E) were significantly decreased in the group treated with MAPK1 sRNAs complexed with the nanocarriers. Semiquantitative scoring of tubulointerstitial injury (C) showed no significant differences between the four groups. P values were calculated by ANOVA, mean ± SEM, n = 6.
Modulation of the TGF-β Signaling Pathway by Intraglomerular MAPK1 Silencing
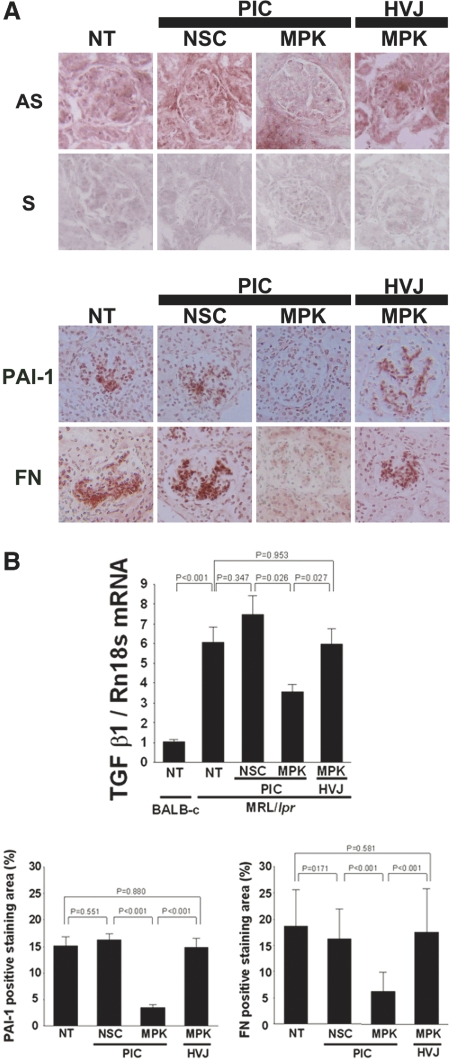
Q-RT-PCR analysis of isolated glomeruli revealed that the expression of TGF-β1 mRNA was significantly suppressed in the group treated with MAPK1 siRNA/nanocarrier complexes (Figure 7B). In situ hybridization also showed that TGF-β1 mRNA expression was significantly inhibited in the glomeruli of the mice treated with these complexes (Figure 7A). This suggested a role of MAPK1 as an upstream regulator for TGF-β signaling.
Figure 7.
TGF-β1, PAI-1, and fibronectin (FN) expression are suppressed by intraglomerular MAPK1 silencing. (A) Representative photomicrographs. Upper: In situ hybridization for TGF-β1 mRNA in the kidney sections. Lower: Immunohistochemistry for PAI-1 and FN expression in the glomeruli. NT, nontreatment; NSC/PIC, nonsilencing control siRNAs complexed with PIC nanocarriers; MPK/PIC, MAPK1 siRNAs complexed with PIC nanocarriers; MPK/HVJ, MAPK1 siRNAs encapsulated in HVJ-E; AS, antisense probe; S, sense probe. Original magnification ×200. (B) Q-RT-PCR analysis (upper) revealed that the treatment with MAPK1 siRNAs complexed with the PIC nanocarriers suppressed the expression of the TGF-β1 mRNA in the glomeruli of MRL/lpr mice. Nontreated BALB-c mice were used as controls. P values were calculated by ANOVA, mean ± SEM, n = 5. Densitometry analysis of PAI-1 and FN staining in glomeruli is shown in lower panel. Data were expressed as the positive staining area of PAI-1 or FN in each glomerulus (%). P values were calculated by ANOVA, mean ± SEM, n = 3.
Immunohistochemical analysis of extracellular matrix proteins and plasminogen activator inhibitor-1 (PAI-1) showed that these expressions were clearly reduced in the group treated with the MAPK1 siRNA/nanocarrier complex (Figure 7, A and B). Therefore, in the lupus nephritis model mice, the increased MAPK1 may have stimulated TGF-β1 expression, leading to the accumulation of extracellular matrix proteins and glomerulosclerosis.
Discussion
In this study, we effectively delivered siRNA to glomeruli using PEG-PLL copolymer-based delivery vehicles. MAPK1 siRNAs targeted to glomeruli inhibited MAPK1 expression in glomeruli at both the mRNA and the protein levels and ameliorated pathologic changes in the glomerular disease of MRL/lpr mice.
Fenestrated endothelium without covering by diaphragma is a characteristic of the glomerular capillary and is different from the fenestrated capillary in intestinal villi and endocrine glands. In addition to anatomical differences, there are differences in physiologic aspects as well. For example, the blood flow rate in the kidney, at approximately 400 ml/100 g of tissue per minute, is much greater than that of other organs (e.g., 100 ml/100 g per minute in the liver). The hydraulic pressure in the glomerular capillary is estimated at 45 to 70 mmHg, which is higher than that of peripheral capillary or portal venules. These differences might suggest that efficient drug delivery to glomeruli, avoiding other tissues, may be feasible. In this regard, our delivery vehicles, the PIC nanocarriers, have a number of significant advantages that would favor in vivo targeted delivery to glomeruli or mesangial cells.
One advantage may reside in the size of the nanocarriers carrying the siRNAs. The PIC nanocarriers are extremely small compared with conventional liposomes or HVJ-E vectors (10 to 20 nm versus 200 to 500 nm), as shown by DLS measurement and fluorescence correlation spectroscopy analysis (Figure 1B). Our nanocarriers can permeate through the capillary walls across the endothelial fenestrations of about 100 nm and yet be still restricted in passing through the glomerular basement membrane (GBM), which generates a size-selective filtration barrier with a pore size of 4 nm, equal to that of an albumin molecule. Therefore, the nanocarriers may come into contact with mesangial cells because the mesangium directly adjoins the endothelium without intervening GBM (Supplemental Figure 5).12 Indeed, accumulation of the nanocarrier complex in glomeruli, particularly in the mesangium, was clearly shown with confocal and fluorescence microscopy (Figure 4, A and B). In general, early treatment of glomerular diseases is recommended before pathologic changes, including endothelial injury, occur. Our nanocarriers may be clinically advantageous, especially compared with liposomes that are too large to pass through the intact endothelium.13–18 Conjugated siRNAs, mostly bound to albumin, are also unfavorable for the treatment of nephrotic diseases because of their filtration through the injured GBM.
Second, the prolonged circulation time of our nanocarriers is also of importance. The outstanding biocompatibility of PEG, including water solubility, enzymatic tolerance, and minimized nonspecific interaction with plasma protein and blood cells,19–22 largely contributes to this feature. The siRNA/nanocarriers complex had a prolonged circulation time compared with naked siRNAs, as indicated by polyacrylamide gel electrophoresis and the fluorescence intensity measurement of mouse plasma (Supplemental Figure 2). The longevity in circulation should contribute to an efficient accumulation in the glomeruli.
Third, the pharmaceutical merits of our nanocarriers include ease of preparation. The PLL segment can form a polyion complex with siRNAs through the electrostatic interaction in the buffer solution just by simple mixing.23
The PEG-PLL-based construct in our system may also be safer for clinical use, compared with virus vectors.24 PLL is approved by the Food and Drug Administration (FDA) as a substance “generally recognized as safe.” PEG, also approved by the FDA, has already been used for sustained-action drugs such as pegylated IFN. All of these characteristics of our nanocarriers may enable their clinical application as drug delivery systems for the treatment of glomerular diseases.
The MRL/lpr strain is a murine model of human systemic lupus erythematosus caused by mutation of fas encoding Fas.25 MRL/lpr mice spontaneously develop an autoimmune syndrome characterized by elevated levels of Ig, a variety of autoantibodies, and development of nephritis and vasculitis accompanied by massive lymphoproliferation.26,27 Renal manifestations of MRL/lpr mice are known to be mesangial proliferative glomerulonephritis at disease onset, followed by diffuse proliferative glomerulonephritis with crescent formation later in the course, and finally leading to death due to the irreversible progression of renal failure.28 Therefore, the treatment between 12 and 16 weeks of age could allow us to assess the effect of our nanocarrier system on pathologic changes in the developing stages of glomerulosclerosis.
Mitogen-activated protein kinases have a variety of functions in regulating cell proliferation and apoptosis in inflammatory processes, including kidney disease.29,30 Recent studies have revealed that activation of MAPK1, c-Jun NH2 terminal kinase, and p38 MAPK might be involved in the glomerular injury in experimental nephritis.31,32 In MRL/lpr mice, the activation of p38 MAPK also may contribute to the pathogenesis of autoimmune renal diseases.33 In our studies, we have found the up-regulation of MAPK1 in MRL/lpr mice. The intraglomerular MAPK1 silencing probably led to the histologic improvement of glomerular lesions, as well as the amelioration of renal function and proteinuria.
TGF-β is a well-known profibrotic factor in progressive kidney diseases. The signaling pathways of MAPKs have also been found to participate in the TGF-β signaling cascades in various types of cells, although the intracellular signaling of TGF-β superfamily is mediated by a series of Smad proteins.34–36 MAPK1 is activated by TGF-β1 in mesangial cells.34,37 Synergistic roles between TGF-β1-stimulated MAPK1 and Smad signaling are suggested in vitro, but the in vivo interaction of these signaling cascades remains to be determined. On the other hand, MAPK1 is known to mediate or precede the expression of TGF-β1 in cultured mesangial cells, stimulated by various factors including angiotensin II,38 renin,39 mechanical stretch,40,41 and high glucose.42,43 In the present study, we showed that MAPK1 plays a role as an upstream regulator of TGF-β1 in glomeruli and a consequent modulator of glomerulosclerosis via the expression of extracellular matrix components and PAI-1.
In summary, we successfully silenced intraglomerular genes using siRNA complexed with PEG-PLL-based nanocarriers. This system is useful as a tool for investigating the molecular mechanisms of glomerular diseases. In addition, it also is a potential candidate strategy for future treatment of chronic kidney or glomerular diseases.
Concise Methods
Experimental Animal
Six-week-old female BALB-c mice and 4-week-old male Wistar rats were purchased from Charles River Laboratories (Kanagawa, Japan). Eight-week-old female MRL/lpr mice were purchased from Japan SLC (Shizuoka, Japan). All mice and rats were housed with ad libitum access to autoclaved food and sterile water. All animal studies were performed according to the principles of the Guide for Animal Experimentation at the University of Tokyo.
Materials
Alpha-methoxy-omega-amino PEG (molecular weight = 12000) was obtained from Nippon Oil and Fats Co. Ltd. (Tokyo, Japan). The PEG-PLL block copolymers (polymerization degree of PLL: 72) were synthesized by ring-opening polymerization of N-carboxyl anhydride of amino acid derivatives, as described previously.44 HVJ-E was purchased from Ishihara Sangyo Kaisha Ltd. (Osaka, Japan). FITC-labeled poly(α,β-aspartic acid) (FITC-P(Asp)) homopolymers (polymerization degree of P(Asp): 26) were prepared by simple conjugation of FITC to the N-terminal primary amino group of P(Asp), as described previously.45 MAPK1 siRNAs and nonsilencing control (scrambled) siRNAs were purchased from Qiagen. The siRNA sequences were as follows:
Mm/Hs_MAPK1 (common to human and mouse):
5′-UGCUGACUCCAAAGCUCUGdTdT-3′ (forward),
3′-dTdTACGACUGAGGUUUCGAGAC-5′ (reverse);
nonsilencing control siRNA:
5′-UUCUCCGAACGUGUCACGUdTdT-3′ (forward),
3′-dTdTAAGAGGCUUGCACAGUGCA-5′ (reverse).
For some experiments, nonsilencing control siRNAs were labeled with FITC, Alexa Fluor® 647, Cy3, or Cy5.
Preparation of PIC Nanocarriers
FITC-P(Asp) or siRNA and PEG-PLL were separately dissolved in 10 mM phosphate-buffered saline (pH 7.4) and mixed at an N/P ratio [= (primary amino groups in PLL)/(carboxyl groups in P(Asp) or phosphate units in siRNA)] = 1.4 to form PIC nanocarriers.46,47
Preparation of HVJ-E Vector
We prepared high-efficiency transfection HVJ-E vector according to the manufacturer's instructions. HVJ-E is one of the most available vehicles for the delivery to the kidney,14,48 and we used it for comparison.
Characterization of the PIC Nanocarriers
The size and polydispersity were evaluated by DLS using a Zetasizer Nanoseries (Malvern Insturements Ltd., United Kingdom) equipped with an He-Ne laser (633 nm). FCS experiments were carried out for size determination of the complex between PEG-PLL and siRNA using LSM510 (Carl Zeiss, Germany) with a 40× objective (C-Apochromat; Carl Zeiss) and the ConfoCor3 module. The illumination source was an Ar laser (488 nm) for Cy3-siRNA. Samples were prepared from a mixture of Cy3-siRNA and nonlabeled siRNA (1:200; final Cy3-siRNA concentration, 50 nM) in eight-well Laboratory-Tek chambers (Nalgene Nunc International, Rochester, NY). The diffusion coefficient (D) was calculated based on a reference of rhodamine 6G (50 nM). The hydrodynamic diameter (d) was calculated from the Stokes-Einstein equation (d = kBT/3πηD, where η is the solvent viscosity, kB is the Boltzmann constant, and T is the temperature).
Cell Culture and In Vitro Assessment
Mouse mesangial cells were obtained by culturing glomeruli isolated from kidneys of 8-week-old female MRL/lpr mice, as described previously49,50 and were maintained in DMEM/F12 (50/50) medium containing 15% FCS, streptomycin (100 μg/ml), penicillin (100 U/ml), and l-glutamine (2 mM). The cells exhibited typical morphologic characteristics of mesangial cells and showed uniformly positiveness for smooth muscle α actin staining. Cells between passages 5 and 10 were used for subsequent experimental procedures. To examine the cellular uptake of PIC nanocarriers by mesangial cells, 3 μl/well of PIC nanocarriers carrying FITC-labeled nonsilencing control (scrambled) siRNAs or naked FITC-labeled siRNAs at a concentration of 50 nM were added to mouse mesangial cells passaged in 0.4 ml/well of serum-containing media on chamber slides (eight-well Lab-Tek™ Chamber Slides™; Nunc). Filtration through 0.2-μm-sized Acrodisc syringe filters (Pall Corporation, NY) was used to examine the effect of size barriers on the delivery vehicles, comparing siRNAs complexed with PIC nanocarriers and siRNAs encapsulated in HVJ-E. To assess the gene-silencing effect of siRNAs, siRNAs complexed with PIC nanocarriers (MAPK1 and nonsilencing control siRNAs) or native MAPK1 siRNAs were added to the cultured mouse mesangial cells (1.0 × 105 cells per well) at various concentrations (5, 16, 50 or 160 nM). Twenty-four hours later, the cells were used in further experiments.
Accumulation of PIC Nanocarriers in Kidneys
To investigate the possible accumulation of the PIC nanocarriers in the kidney, FITC-P(Asp) complexed with PIC nanocarriers (0.5 ml, 0.7 mg of P(Asp) content), naked FITC-P(Asp) (0.5 ml, 0.7 mg), or FITC-P(Asp) encapsulated in HVJ-E (0.5 ml, 0.7 mg of P(Asp) content) was administered to BALB-c mice by intraperitoneal injection. Each mouse was killed 6 hours after injection, and tissues were snap-frozen in OCT compound (Lab-Tek Products; Miles Laboratories, Naperville, IL). Frozen sections (4-μm thickness) were observed using a fluorescence microscope (model BX51; Olympus, Tokyo, Japan).
For the investigation of siRNA accumulation, Cy5-labeled siRNAs complexed with PIC nanocarriers (0.5 ml, 5 nmol of siRNA content), naked Cy5-labeled siRNAs (0.5 ml, 5 nmol), or Cy5-labeled siRNAs encapsulated in HVJ-E (0.5 ml, 5 nmol of siRNA content) were administrated to BALB-c mice. Each mouse was killed 3.5 or 5.5 hours after intraperitoneal injection. The tissues were snap-frozen in OCT compound, and 4-μm frozen sections were observed under a confocal laser scanning microscope (LSM 510; Carl Zeiss).
To examine the cellular localization of the injected PIC nanocarriers in glomeruli, 1 ml of FITC-P(Asp) complexed with PIC nanocarriers was administered to 4-week-old male Wistar rats. The kidney tissues were obtained at 120 minutes after tail vein injection and snap-frozen. Frozen sections (4 μm) were stained with antibodies to Thy1.1, a mesangial cell-specific antigen (clone MRC OX-7; Serotec Ltd., Oxford, England), which were then detected by biotinylated antimouse IgG (secondary antibody; Dako Corp.) and Rhodamine/Neutralite Avidin (Southern Biotec, Birmingham, AL).
Stability of PIC Nanocarriers in Mouse Plasma and Urinary Excretion
To examine the siRNA stability in blood circulation, naked Alexa Fluor 647-labeled nonsilencing siRNAs, Alexa Fluor 647-labeled nonsilencing siRNAs complexed with PIC nanocarriers, or Alexa Fluor 647-labeled nonsilencing siRNAs encapsulated in HVJ-E were administered by intraperitoneal injection at various time periods. The same amount of siRNA was used in each group (0.5 ml, 5 nmol of siRNA content). Thereafter, blood samples were collected from the mice. Relative fluorescence units in plasma of the samples were measured with a Nanodrop ND-3300 Fluorospectrometer (Nanodrop Technologies Inc., DE), and the percentage of the injected dose was calculated. 100% of the injection dose was estimated using a total blood volume based on mouse weight. Otherwise, stability of siRNA in plasma samples was directly examined by electrophoresis and EtBr staining. In brief, we obtained 50 μl of fresh mouse plasma and mixed this with 350 μl of Tris-EDTA buffer. The siRNAs were extracted from the reaction mixtures with phenol/chroroform/isoamyl alcohol (25:24:1). The siRNAs were electrophoresed on 5% to 20% polyacrylamide gel (Wako Pure Chemical Industries Ltd., Osaka, Japan) and visualized under UV irradiation after EtBr (0.1 μg/L) staining. For siRNA excretion into urine, we examined the relative fluorescence units of a urine spot at various time points using the Nanodrop ND-3300.
In Vivo and Ex Vivo Imaging
To examine the biodistribution of siRNAs complexed with or without PIC nanocarriers, we used an IVIS 200 Imaging System composed of a highly sensitive, cooled CCD camera mounted in a light-tight specimen box (Xenogen, CA). Images and measurements of fluorescence signals were acquired and analyzed using Living Image software (Xenogen). Six-week-old female BALB-c mice were anesthetized using 1% to 3% isoflurane (Abbott Laboratories, IL), and then Cy5-labeled siRNAs complexed with PIC nanocarriers (0.5 ml, 5 nmol of siRNA content) or naked Cy5-labeled siRNAs (0.5 ml, 5 nmol) were injected intraperitoneally. Mice were placed onto a warmed stage inside the camera box and received continuous exposure of 1% to 2% isoflurane to sustain sedation. After imaging living mice, mice were euthanized, organs of interest were removed, and the organs were imaged ex vivo within 30 minutes.
Evaluation of the Silencing Effect of MAPK1 siRNAs Complexed with PIC Nanocarriers on Chronic Glomerulonephritis
To evaluate the in vivo knockdown effect of MAPK1 siRNAs complexed with PIC nanocarriers in glomeruli, we used MRL/lpr mice. The mice were randomly divided into four groups (n = 6); those treated with (1) MAPK1 siRNAs complexed with PIC nanocarriers, (2) nonsilencing control siRNAs complexed with the nanocarriers, (3) MAPK1 siRNAs encapsulated in HVJ-E, and (4) untreated controls. Twice-weekly intraperitoneal injections were initiated at 12 weeks of age at the dose of 2 nmol of siRNA per week. At 17 weeks of age, urine and blood samples were collected, and all mice were killed to obtain the tissues.
Q-RT-PCR
We isolated total cellular RNA using an RNeasy® kit (Qiagen) and synthesized the first-strand cDNA using RNAse H+ reverse transcriptase and random primers (Qiagen).
Then, we performed duplex, real-time PCR assay using the probes labeled with FAM or Yakima Yellow dye (Qiagen). The expressions of a housekeeping gene Rn18s and the target gene (MAPK1 and TGF-β1) were quantified simultaneously in the same wells. The expression of the target gene was then normalized to that of the Rn18s. PCR was carried out on ABI PRISM 7000 (Applied Biosystems, CA). Relative quantification was accomplished with measurement of the threshold cycle and use of a standard curve. All PCR experiments were done in triplicate.
Western Blot Analysis
Western blotting was performed using antibodies against phospho-ERK1/2 (Cell Signaling Technology, Beverly, MA) and pan-ERK1/2 antibody (Cell Signaling Technology) to evaluate MAPK1/2 (ERK1/2) phosphorylation and total MAPK1/2 protein expression, respectively. Anti-β-actin antibody was from Cell Signaling Technology. Glomerular fractions were solubilized for 30 minutes at room temperature in cell-solubilizing buffer, and the protein concentration of the samples was measured by the DC protein assay (Bio-Rad, Hercules, CA). Twenty micrograms of protein were loaded in each lane, and the SDS-PAGE was performed under nonreducing conditions. The bound antibody was detected using 1 μg/ml alkaline phosphatase-conjugated antimouse IgG (Promega, Madison, WI). 5-Bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium tablets (Sigma Fast; Sigma Chemical Co., St. Louis, MO) were used as a substrate.
Immunohistochemistry
Frozen sections were stained for MAPK-1/2, phospho-MAPK-1/2, PAI-1 (American Diagnostics Inc., Stamford, CT), FN (BD Biosciences, CA), and synaptopodin (Progen Biotechnik GmbH, Heidelberg, Germany) using the Vectastain elite ABC Kit (Vector Laboratories Inc., Burlingame, CA) and peroxidase substrate kit, diaminobenzidine (DAB; Vector Laboratories). For quantitative analysis, DAB-positive area was calculated densitometrically using image-analyzing software (Molecular Devices Corp., Downingtown, PA) in more than 20 randomly selected glomeruli of each section.
Histologic Assessment of Glomerular and Tubulointerstitial Lesions
To examine the renal histology, 4-μm sections of paraffin-embedded tissues were stained with PAS reagent. Glomerular lesions were scored semiquantitatively by a blinded observer who examined at least 30 randomly selected glomeruli in each specimen using glomerular sclerosis score.51,52 Glomerular sclerosis was defined as synechiae formation by PAS staining with focal or global obliteration of capillary loops, and then graded (0 to 4) as follows: 0, normal; 1, 0% to 25% of glomerular area was affected; 2, 25% to 50% affected; 3, 50% to 75% affected; and 4, 75% to 100% affected. Tubular lesions were scored semiquantitatively by a blinded observer who examined at least 20 randomly selected cortical fields in each specimen.51,53 Tubulointerstitial injury was graded (0 to 5) on the basis of the percentage of tubular cellularity, basement membrane thickening, cell infiltration, dilation, atrophy, sloughing, or interstitial widening as follows: 0, no change; 1, <10% tubulointerstitial injury; 2, 10% to 25%; 3, 25% to 50%; 4, 50% to 75%; and 5, 75% to 100%. Glomerular lesions were analyzed by the ratio of the number of glomeruli with global sclerosis to the total number of glomeruli in each cross-section and also by the semiquantification for PAS-positive area in glomeruli using image-analyzing software.
In Situ Hybridization
For determining the sequences of the probes, we searched databases using Megablast (Optimize for highly similar sequences) and selected a 50-base sequence complementary to mouse MAPK1 mRNA and TGF-β1 mRNA, without significant similarity with any other known sequences. Fifty picomoles of the oligonucleotide probe were labeled using a DIG oligonucleotide tailing kit (Roche Diagnostics, Mannheim, Germany). Free DIG was removed by ethanol precipitation and dissolved in diethylpyrocarbonate-treated water. In situ hybridization was performed according to the protocol as described previously.54,55 Briefly, frozen sections (4 μm thick) were fixed in 4% paraformaldehyde in PBS, deproteinized by HCl, and digested with proteinase K (Sigma Chemical Co.). The specimens were prehybridized in a prehybridization buffer, drained, and hybridized overnight with digoxigenin (DIG)-labeled oligonucleotide probe in the prehybridization buffer. After hybridization, the DIG-labeled probe was visualized using a sheep polyclonal anti-DIG antibody (Roche Diagnostics, Mannheim, Germany), horseradish peroxidase-conjugated rabbit anti-sheep antibody (Dako), and horseradish peroxidase-conjugated swine anti-rabbit antibody (Dako). Color was developed with diaminobenzidine tetrahydrochloride (DAB Chromogen; Dako) in 0.05 mol/L Tris-HCl, pH 7.6, and 0.03% H2O2. Sections were counterstained with Methyl Green (Vector Laboratories). To evaluate the specificity of the reaction, a sense probe corresponding to the sequence was used in place of the antisense probe.
Measurement of Proteinuria and Blood Urea Nitrogen
Urinary protein concentration was measured by Pyrogallol Red method; the concentration of creatinine was measured by enzymatic assay. BUN was measured according to the urease-glutamate dehydrogenase method.
Statistical Analysis
All values are presented as mean ± SEM. ANOVA was used to test for significant differences between groups. A P value of less than 0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (30376446) given to Y.H.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “siRNA Therapy for Glomerulonephritis,” on pages 549–551.
References
- 1. Gewirtz AM: On future's doorstep: RNA interference and the pharmacopeia of tomorrow. J Clin Invest 117: 3612– 3614, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akhtar S, Benter IF: Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest 117: 3623– 3632, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blow N: Small RNAs: Delivering the future. Nature 450: 1117– 1120, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Kim DH, Rossi JJ: Strategies for silencing human disease using RNA interference. Nat Rev Genet 8: 173– 184, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Savaskan NE, Heckel A, Hahnen E, Enquelhorn E, Doerfler A, Nimsky C, Buchfelfer M, Eyüpoglu IY: Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 14: 629– 632, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Palliser D, Chowdhury D, Wang QY, Lee SJ, Bronson RT, Knipe DM, Lieberman J: An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature 439: 89– 94, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Thakker DR, Natt F, Hüsken D, Maier R, Müller M, Hüsken D, van der Putten H, Hoyer D, Cryan JF: Neurochemical and behavioral consequences of widespread gene knockdown in the adult mouse brain by using nonviral RNA interference. Proc Natl Acad Sci U S A 101: 17270– 17275, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bitko V, Musiyenko A, Shulyayeva O, Barik S: Inhibition of respiratory viruses by nasally administered siRNA. Nat Med 11: 50– 55, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Soutschek JJ, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Röhl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP: Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432: 173– 178, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Röhl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachalan I: RNAi-mediated gene silencing in non-human primates. Nature 441: 111– 114, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Prez de Lema G, Maier H, Nieto E, Vielhauer V, Luckow B, Mampaso F, Schlonforff D: Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J Am Soc Nephrol 12: 1369– 1382, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Kriz W, Elger M, Lemley K, Sakai T: Structure of the glomerular mesangium: A biomechanical interpretation. Kidney Int 38[ suppl 30]: S2– S9, 1990 [PubMed] [Google Scholar]
- 13. Imai E, Takabatake Y, Mizui M, Isaka Y: Gene therapy in renal diseases. Kidney Int 65: 1551– 1555, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Wada J, Hashimoto I, Eguchi J, Yasuhara A, Kanwar YS, Shikata K, Makino H: Therapeutic approach for diabetic nephropathy using gene delivery of translocase of inner mitochondrial membrane 44 by reducing mitochondrial superoxide production. J Am Soc Nephrol 17: 1090– 1101, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Isaka Y, Fujiwara Y, Ueda N, Kaneda Y, Kamada T, Imai E: Glomerulosclerosis induced by in vivo transfection of transforming growth factor-beta or platelet-derived growth factor gene into the rat kidney. J Clin Invest 92: 2597– 2601, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maeshima Y, Kashihara N, Yasuda T, Sugiyama H, Sekikawa T, Okamoto K, Kanao K, Watanabe Y, Kanwar YS, Makino H: Inhibition of mesangial cell proliferation by E2F decoy oligodeoxynucleotide in vitro and in vivo. J Clin Invest 101: 2589– 2597, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomita N, Morishita R, Lan HY, Yamamoto K, Hashizume M, Notake M, Toyosawa K, Fujitani B, Mu W, Nikolic-Paterson DJ, Atkins RC, Kaneda Y, Higaki J, Ogihara T: In vivo administration of a nuclear transcription factor-kappaB decoy suppresses experimental crescentic glomerulonephritis. J Am Soc Nephrol 11: 1244– 1252, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Tuffin G, Waelti E, Huwyler J, Hammer C, Marti HP: Immunoliposome targeting to mesangial cells: A promising strategy for specific drug delivery to the kidney. J Am Soc Nephrol 16: 3295– 3305, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Harada-Shiba M, Yamauchi K, Harada A, Takamisawa I, Shimokado K, Kataoka K: Polyion complex micelles as vectors in gene therapy–pharmacokinetics and in vivo gene transfer. Gene Ther 9: 407– 414, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Itaka K, Yamauchi K, Harada A, Nakamura K, Kawaguchi H, Kataoka K: Polyion complex micelles from plasmid DNA and poly(ethylene glycol)-poly(L-lysine) block copolymer as serum-tolerable polyplex system: Physicochemical properties of micelles relevant to gene transfection efficiency. Biomaterials 24: 4495– 4506, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Harada A, Togawa H, Kataoka K: Physicochemical properties and nuclease resistance of antisense-oligodeoxynucleotides entrapped in the core of polyion complex micelles composed of poly(ethylene glycol)-poly(L-lysine) block copolymers. Eur J Pharm Sci 13: 35– 42, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Akagi D, Oba M, Koyama H, Nishiyama N, Fukushima S, Miyata T, Nagawa H, Kataoka K: Biocompatible micellar nanovectors achieve efficient gene transfer to vascular lesions without cytotoxicity and thrombus formation. Gene Ther 14: 1029– 1038, 2007 [DOI] [PubMed] [Google Scholar]
- 23. DeRouchey J, Schmidt C, Walker GF, Koch C, Plank C, Wagner E, Rädler JO: Monomolecular assembly of siRNA and poly(ethyleneglycol)-peptide copolymers. Biomacromolecules 9: 724– 732, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Wang X, Skelley L, Cade R, Sun Z: AAV delivery of mineralocorticoid receptor shRNA prevents progression of cold-induced hypertension and attenuates renal damage. Gene Ther 13: 1097– 1103, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S: Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356: 314– 317, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Theofilopoulos AN, Dixon FJ: Murine models of systemic lupus erythematosus. Adv Immunol 37: 269– 390, 1985 [DOI] [PubMed] [Google Scholar]
- 27. Cohen PL, Eisenberg RA: Lpr and gld: Single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol 9: 243– 269, 1991 [DOI] [PubMed] [Google Scholar]
- 28. Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ: Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med 1148: 1198– 1215, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herlaar E, Brown Z: p38 MAPK signaling cascades in inflammatory disease. Mol Med Today 5: 439– 447, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Tian W, Zhang Z, Cohen DM: MAPK signaling and the kidney. Am J Physiol Renal Physiol 279: F593– F604, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Bokemeyer D, Ostendorf T, Kunter U, Lindemann M, Kramer HJ, Floege J: Differential activation of mitogen-activated protein kinase in experimental mesangioproliferative glomerulonephritis. J Am Soc Nephrol 11: 232– 240, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Bokemeyer D, Guglielmi KE, McGinty A, Sorokin A, Lianos EA, Dunn MJ: Activation of extracellular signal-regulated kinase in proliferative glomerulonephritis in rats. J Clin Invest 100: 582– 588, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iwata Y, Wada T, Furuichi K, Sakai N, Matsushima K, Yokoyama H, Kobayashi K: p38 Mitogen-activated protein kinase contributes to autoimmune renal injury in MRL-Fas lpr mice. J Am Soc Nephrol 14: 57– 67, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Huwiler A, Pfeilschifter J: Transforming growth factor beta 2 stimulates acute and chronic activation of the mitogen-activated protein kinase cascade in rat renal mesangial cells. FEBS Lett 354: 255– 258, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Hartsough MT, Mulder KM: Transforming growth factor β activation of p44mapk in proliferating cultures of epithelial cells. J Biol Chem 270: 7117– 7124, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Frey RS, Mulder KM: Involvement of extracellular signal-regulated kinase 2 and stress-activated protein kinase/Jun N-terminal kinase activation by transforming growth factor β in the negative growth control of breast cancer cells. Cancer Res 57: 628– 633, 1997 [PubMed] [Google Scholar]
- 37. Hayashida T, Decaestecker M, Schnaper HW: Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J 17: 1576– 1578, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Uchiyama-Tanaka Y, Matsubara H, Nozawa Y, Murasawa S, Mori Y, Kosaki A, Maruyama K, Masaki H, Shibasaki Y, Fujiyama S, Nose A, Iba O, Hasagawa T, Tateishi E, Higashiyama S, Iwasaka T: Angiotensin II signaling and HB-EGF shedding via metalloproteinase in glomerular mesangial cells. Kidney Int 60: 2153– 2163, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Huang Y, Noble NA, Zhang J, Xu C, Border WA: Renin-stimulated TGF-beta1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int 72: 45– 52, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Ingram AJ, Ly H, Thai K, Kang M, Scholey JW: Activation of mesangial cell signaling cascades in response to mechanical strain. Kidney Int 55: 476– 485, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Ishida T, Haneda M, Maeda S, Koya D, Kikkawa R: Stretch-induced overproduction of fibronectin in mesangial cells is mediated by the activation of mitogen-activated protein kinase. Diabetes 48: 595– 602, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Isono M, Cruz MC, Chen S, Hong SW, Ziyadeh FN: Extracellular signal-regulated kinase mediates stimulation of TGF-beta1 and matrix by high glucose in mesangial cells. J Am Soc Nephrol 11: 2222– 2230, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Hayashida T, Schnaper HW: High ambient glucose enhances sensitivity to TGF-beta1 via extracellular signal–regulated kinase and protein kinase Cdelta activities in human mesangial cells. J Am Soc Nephrol 15: 2032– 2041, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Harada A, Cammas S, Kataoka K: Stabilized α-helix structure of poly(L-lysine)-block-poly(ethylene glycol) in aqueous medium through supramolecular assembly. Macromolecules 29: 6183– 6188, 1996 [Google Scholar]
- 45. Nishiyama N, Kataoka K: Preparation and characterization of size-controlled polymeric micelle containing cis-dichlorodiammineplatinum(II) in the core. J Control Release 74: 83– 94, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Itaka K, Kanayama N, Nishiyama N, Jang WD, Yamasaki Y, Nakamura K, Kawaguchi H, Kataoka K: Supramolecular nanocarrier of siRNA from PEG-based block catiomer carrying diamine side chain with distinctive pKa directed to enhance intracellular gene silencing. J Am Chem Soc 126: 13612– 13613, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Ideta R, Yanagi Y, Tamaki Y, Tasaka F, Harada A, Kataoka K: Effective accumulation of polyion complexmicelle to experimental choroidal neovascularization in rats. FEBS Lett 557: 21– 25, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A: IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med 12: 99– 106, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Okuda T, Yamashita N, Ogata E, Kurokawa K: Angiotensin II and vasopressin stimulate calcium-activated chloride conductance in rat mesangial cells. J Clin Invest 78: 1443– 1448, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kaname S, Uchida S, Ogata E, Kurokawa K: Autocrine secretion of transforming growth factor-β in cultured rat mesangial cells. Kidney Int 42: 1319– 1327, 1992 [DOI] [PubMed] [Google Scholar]
- 51. Tanaka T, Kojima I, Ohse T, Ingelfinger JR, Adler S, Fujita T, Nangaku M: Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab Invest 85: 1292– 1307, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Raij L, Azar S, Keane W: Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26: 137– 143, 1984 [DOI] [PubMed] [Google Scholar]
- 53. Pichler RH, Franceschini N, Young BA, Hugo C, Andoh TF, Burdmann EA, Shankland SJ, Alpers CE, Bennett WM, Couser WG, Johnson RJ: Pathogenesis of cyclosporine nephropathy: Roles of angiotensin II and osteopontin. J Am Soc Nephrol 6: 1186– 1196, 1995 [DOI] [PubMed] [Google Scholar]
- 54. Miyazaki M, Nikolic-Paterson DJ, Endoh M, Nomoto Y, Sakai H, Atkins RC, Koji T: A sensitive method of non-radioactive in situ hybridization for mRNA localization within human renal biopsy specimens: use of digoxigenin labeled oligonucleotides. Intern Med 33: 87– 91, 1994 [DOI] [PubMed] [Google Scholar]
- 55. Yamada K, Hori Y, Hanafusa N, Okuda T, Nagano N, Choi-Miura NH, Couser WG, Miyata T, Kurokawa K, Fujita T, Nangaku M: Clusterin is up-regulated in glomerular mesangial cells in complement-mediated injury. Kidney Int 59: 137– 146, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.