Abstract
Vasopressin antagonists increase the serum sodium concentration in patients who have euvolemia and hypervolemia with hyponatremia in the short term (≤30 days), but their safety and efficacy with longer term administration is unknown. SALTWATER was a multicenter, open-label extension of the Study of Ascending Levels of Tolvaptan in Hyponatremia (SALT-1 and SALT-2). In total, 111 patients with hyponatremia received oral tolvaptan for a mean follow-up of 701 days, providing 77,369 patient-days of exposure. All patients had hyponatremia at randomization in SALT-1 and SALT-2, and 85% continued to have hyponatremia at entry in SALTWATER. The most common adverse effects attributed to tolvaptan were pollakiuria, thirst, fatigue, dry mouth, polydipsia, and polyuria. Six drug-related adverse effects led to study discontinuation. The increase in serum sodium exceeded the desired 1 mmol/L per h at initiation in five patients. Hypernatremia (>145 mmol/L) led to discontinuation in one patient. Mean serum sodium increased from 130.8 mmol/L at baseline to >135 mmol/L throughout the observation period (P < 0.001 versus baseline at most points). Responses were comparable between patients with euvolemia and those with heart failure but more modest in patients with cirrhosis. In conclusion, prolonged administration of tolvaptan maintains an increased serum sodium with an acceptable margin of safety.
Orally active vasopressin antagonists, “vaptans,” provide a potentially effective method to treat chronic water-retaining disorders.1 Several members of this drug family, conivaptan,2–4 satavaptan,5,6 tolvaptan,7,8 and lixivaptan,9 have been reported to increase serum sodium in patients with hyponatremia. These studies established primarily short-term efficacy and safety: The drugs were given for 5 days,2,4 14 days,5 28 to 30 days,7,8 or 84 days.3 The study of satavaptan6 had a longer term, 12-month open-label extension, but only 10 patients were followed for 1 year, providing limited long-term efficacy and safety information. Tolvaptan was administered to a large number of patients who were hospitalized with heart failure for a median follow-up of 9.9 months in the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) trial.10 That trial provided reassuring data as to the long-term safety of the drug, but <10% of the patients had hyponatremia, and limited conclusions could be drawn regarding long-term efficacy to maintain normonatremia.
The Safety and sodium Assessment of Long-term Tolvaptan With hyponatremia: A year-long, open-label Trial to gain Experience under Real-world conditions (SALTWATER) was a 4-year sequential, open-label extension of the randomized, placebo-controlled, double-blind Study of Ascending Levels of Tolvaptan in Hyponatremia (SALT-1 and SALT-2).8 The objective of SALTWATER was to assess whether tolvaptan maintained its safety and efficacy over a prolonged period in a substantial number of patients who had hyponatremia and were treated with a flexible-dosage regimen. Preliminary results of SALTWATER were provided to US and European regulatory authorities in their consideration of the eventual approval of tolvaptan. These results revealed that improvements in serum sodium concentration were well maintained over longer periods with an acceptable adverse event (AE) profile.
Results
Patients
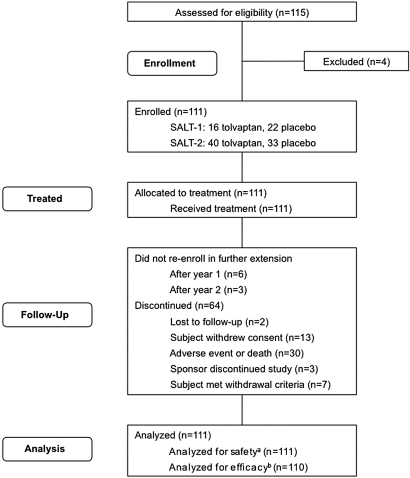
A total of 154 of 325 patients who had hyponatremia and completed the 30-day treatment phase and 7-day follow-up period in SALT-1 and SALT-28 were at sites participating in SALTWATER. Of these, 111 (38 patients from SALT-1 and 73 from SALT-2) enrolled in the open-label SALTWATER extension and received treatment (Figure 1). The median length of time between completion of the SALT-1 and SALT-2 and enrollment in SALTWATER was 30 days per patient (minimum 0 days; maximum 804 days). Sixty-four of the enrolled patients discontinued during the follow-up period for a variety of reasons (Figure 1). Of these, only two were lost to follow-up, three were withdrawn at sites closed by the sponsor, seven met withdrawal criteria (e.g., “subject received liver transplant,” “subject's sodium did not drop when off study drug,” “subject was not taking [noncompliant with] investigative medication”), nine did not re-enroll after either the first- or the second-year extension, 13 withdrew consent, and 30 discontinued because of an AE or death. All 111 patients were analyzed for safety, and 110 patients were analyzed for efficacy.
Figure 1.
SALTWATER flow diagram shows progression of study. aReceived at least one dose of tolvaptan; breceived at least one dose of tolvaptan and had a serum sodium assessment at baseline and at least one postbaseline time point.
Because patients who had been on active drug and placebo during the SALT-1 and SALT-2s did not differ significantly from one another, they were analyzed together in the open-label trial. Baseline demographics of the study patients in SALTWATER are presented in Table 1. Fifty-two (46.8%) patients had had mild hyponatremia (serum sodium ≥130 and <135 mmol/L), and 59 (53.2%) had had marked hyponatremia (<130 mmol/L) in SALT-1 and SALT-2 at baseline. At entry to SALTWATER, the number of patients who had normonatremia, mild hyponatremia, and more marked hyponatremia were 17 (15.3%), 59 (53.2%), and 35 (31.5%), respectively. Patients were distributed by their original causes of hyponatremia (congestive heart failure [CHF] 29.7%, cirrhosis 18.0%, and syndrome of inappropriate antidiuretic hormone secretion [SIADH]/other 52.3%; Table 1). Mean dosage of tolvaptan increased to approximately 30 mg/d during the first 16 to 20 weeks and remained at that level throughout the remainder of the study (data not shown).
Table 1.
Demographics and patient characteristics
| Parameter | Value |
|---|---|
| Total patients | 111 |
| Male (n [%]) | 55 (49.5) |
| Age (years; mean ± SD) | 64.6 ± 15.0 |
| Weight (kg; mean ± SD) | 73.45 ± 18.9 |
| Race (n [%]) | |
| white | 104 (94.0) |
| black | 6 (5.0) |
| Hispanic | 1 (1.0) |
| Baseline serum sodium (mmol/L; n [%])a | |
| <130 | 59 (53.2) |
| ≥130 and <135 | 52 (46.8) |
| Hyponatremia cause (n [%])b | |
| CHF | 33 (29.7) |
| cirrhosis | 20 (18.0) |
| SIADH/other | 58 (52.3) |
aFrequencies in SALT-1 and SALT-2.
bInvestigator-assessed.
Safety
Mean follow-up time on tolvaptan therapy during SALTWATER was 701 days (1.9 years; median 639 days [1.7 years]) per patient, for a total exposure of 77,369 patient-days (212 patient-years; Table 2). During this time, 105 of 111 patients experienced an AE. AEs that occurred in >10% of patients (drug-related or -unrelated) included peripheral edema (25 patients), hyponatremia (23 patients), anemia (20 patients), diarrhea (19 patients), urinary tract infection (18 patients), nausea (17 patients), fatigue (15 patients), hypokalemia (14 patients), headache (14 patients), ascites (13 patients), hypotension (13 patients), pneumonia (13 patients), cardiac failure (12 patients), thirst (12 patients), and dizziness (12 patients). Among the 23 patients with reports of hyponatremia, 11 experienced their episodes during the treatment period (three during interruptions in tolvaptan therapy) and 13 during the posttreatment period. The most common AEs assessed by the investigator as being potentially related to tolvaptan use were pollakiuria (11 patients); thirst (10 patients); fatigue (six patients); and dry mouth, polydipsia, polyuria, hypotension, hypernatremia, dizziness, headache, peripheral edema, and acute renal failure (four patients each; Table 2).
Table 2.
Investigator-assessed treatment-emergent adverse events
| Parameter | Valuea |
|---|---|
| Treated patients | 111 |
| Total exposure (patient-days) | 77,369 |
| Exposure per patient (days) | |
| mean | 701 |
| median | 639 |
| Total AEs (all causes; n [%]) | 105 (94.6) |
| Total AEs leading to discontinuation or death (all causes; n [%]) | 30 (27.0) |
| AEs leading to discontinuation | 19 (17.1) |
| AEs leading to death before withdrawal | 11 (9.9) |
| Drug-related AEs (n [%]) | 52 (46.8) |
| most common drug-related AEsb | |
| pollakiuria | 11 (9.9) |
| thirst | 10 (9.0) |
| fatigue | 6 (5.4) |
| dry mouth | 4 (3.6) |
| polydipsia | 4 (3.6) |
| polyuria | 4 (3.6) |
| hypotension | 4 (3.6) |
| hypernatremia | 4 (3.6) |
| dizziness | 4 (3.6) |
| headache | 4 (3.6) |
| peripheral edema | 4 (3.6) |
| acute renal failure | 4 (3.6) |
| Drug-related AEs leading to discontinuation | 6 (5.4) |
| ventricular tachycardia | 1 (0.9) |
| irritability | 1 (0.9) |
| blood sodium increase | 1 (0.9) |
| anorexia | 1 (0.9) |
| blood creatinine increase | 1 (0.9) |
| pruritus | 1 (0.9) |
AEs that started during the tolvaptan treatment phase or were continuous from baseline and were serious; drug-related; or resulted in death, discontinuation, interruption, or reduction in tolvaptan dosage.
aPatients counted once per AE for the most severe of multiple occurrences.
bReported in >3% of patients.
Of the 64 patients who withdrew from the study during the 214-week treatment period, 19 withdrew for a treatment-emergent AE of any cause (Table 2). The AEs in six of these 19 patients subsequently resulted in death (cardiac failure [two patients], esophageal varices, hepatic cirrhosis, cerebral hemorrhage, and gastrointestinal hemorrhage). The AEs in the remaining 13 patients led to discontinuation but not death (ventricular tachycardia, vertigo, gastrointestinal hemorrhage, vomiting, gait disturbance, irritability, serum creatinine increase, serum sodium increase, anorexia, bladder cancer, dysphasia, myocardial infarction, psychotic disorder, renal failure, and pruritus). An additional 13 patients died as an outcome of an AE without being withdrawn from the study as a result of the event (cardiac failure [three patients], renal failure [two patients], hepatorenal syndrome, cardiorespiratory arrest, cardiac arrest, pneumonia, cerebral hemorrhage, respiratory failure, sepsis, and urosepsis). Thus, a total of 19 patients died during the 212 patient-years of exposure: nine deaths per 100 patient-years of exposure. The death rate during SALTWATER was lower than that observed for SALT (86.9 deaths per 100 patient-years of exposure).
Six AEs leading to drug discontinuation were assessed by the investigator as possibly or probably related to tolvaptan therapy and included severe ventricular tachycardia (on day 3), severe irritability (day 14), mild serum sodium increase (day 15), mild anorexia (day 22), severe serum creatinine increase (day 329), and moderate pruritus (day 513; Table 2). Ten patients experienced at least one serious AE assessed by the investigator as potentially related to study treatment. The events were not adjudicated by a review committee and are presented as described by the investigator in Table 3. Of the 19 patients who died during the study, one case of severe hepatorenal syndrome resulting in death occurred in a 65-year-old woman on day 53 and was judged by the investigator to be possibly related to study medication use (Table 3).
Table 3.
Investigator-assessed serious AEs potentially related to tolvaptan use
| Description | Cause | Days | Relationship | Action | Outcome |
|---|---|---|---|---|---|
| Ventricular tachycardia in a 75-year-old man | CHF | 3 | Possible | Discontinued | Resolved |
| ARF in a 53-year-old man with decompensated heart failure, atrial tachycardia, gastrointestinal hemorrhage, and catheter site infection | CHF | 14, 30, 459 (three events) | Possible | Interrupted use | Resolved |
| Hyponatremia and convulsions in a 76-year-old woman | SIADH | 16, 168 | Probable | Dosage increase | Resolved |
| Hepatorenal syndrome in a 65-year-old woman | Cirrhosis | 53 | Possible | Interrupted use | Fatal |
| Clostridium difficile colitis in an 86-year-old woman with CHF, peripheral ischemia, gastrointestinal hemorrhage, and pneumonia | CHF | 173 | Possible | None | Recovered |
| ARF in a 48-year-old man with decompensated and worsening heart failure | CHF | 218 | Possible | Interrupted use | Resolved |
| Severe dehydration, hypoglycemia, and therapeutic agent toxicity in an 87-year-old woman with recurrent atrial fibrillation | SIADH | 238, 238, 238 | Possible | Interrupted use | Resolved |
| Nausea, vomiting, and increased blood creatinine in a 79-year-old man with transient ischemic attack | CHF | 329, 315, 315 | Possible | Discontinued | Resolved |
| ARF in a 33-year-old man with heart failure, cardiac tamponade, and respiratory distress | CHF | 540 | Possible | Interrupted use | Resolved |
| Dehydration in a 72-year-old woman with depression and gastroenteritis | SIADH | 709 | Possible | None | Resolved |
ARF, acute renal failure.
No consistent and clinically meaningful changes were observed in vital signs, electrocardiogram, serum chemistries (other than serum sodium and chloride), or hematology panels during the follow-up period. Serum sodium correction exceeded the desirable rate of 1 mmol/L per h at the 8-hour time point in five patients. Eighteen patients had assessed serum sodium levels >145 mmol/L at individual time points. One of the latter patients was withdrawn from the trial for mild serum sodium increase; the hypernatremia resolved in the other 17 patients.
Efficacy
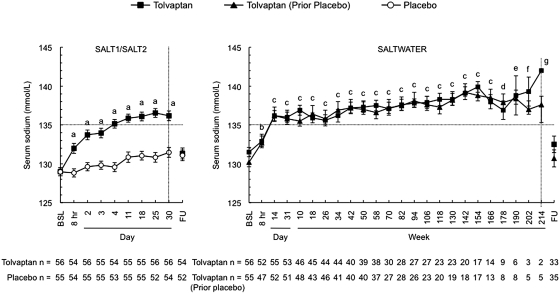
The mean serum sodium concentration in all 111 patients at baseline in SALTWATER was 130.8 ± 4.4 mmol/L (range 114 to 141 mmol/L), compared with 129.0 ± 3.8 mmol/L (range 114 to 136 mmol/L) for the same patients in SALT-1 and SALT-2. Correction of serum sodium levels during the first 8 hours of therapy occurred at similar rates in SALTWATER and the SALT-1 and SALT-2; moreover, by day 14 in both trials, serum sodium levels had reached similar plateaus (Figure 2). Previous treatment with tolvaptan or placebo in SALT-1 and SALT-2 did not alter the long-term efficacy of tolvaptan in SALTWATER. After the initial titration period, mean serum sodium levels remained within the normal range throughout the subsequent >4-year treatment period.
Figure 2.
Serum sodium levels obtained in the course of the SALT-1, SALT-2 and SALTWATER trials. Rate of correction of serum sodium levels and the plateaus reached afterwards were similar in both studies. The left-hand figure includes only patients in the SALT-1 and SALT-2 who continued into SALTWATER. Error bars are ± SE. BSL, baseline; FU, follow-up visit 7 days after early withdrawal or trial completion. aP < 0.001, tolvaptan versus placebo; bP ≤ 0.0011, tolvaptan and tolvaptan (before placebo) versus baseline; cP < 0.001, tolvaptan and tolvaptan (before placebo) versus baseline; dP = 0.021, tolvaptan versus baseline and P < 0.001 tolvaptan (before placebo) versus baseline; eP < 0.001, tolvaptan (before placebo) versus baseline; fP = 0.005, tolvaptan (before placebo) versus baseline; gP = 0.016 tolvaptan (before placebo) versus baseline.
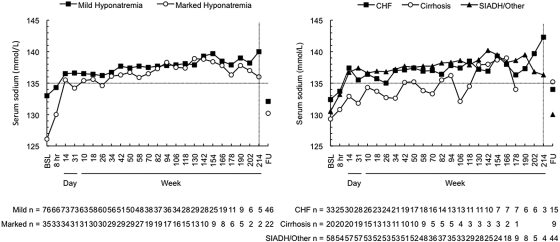
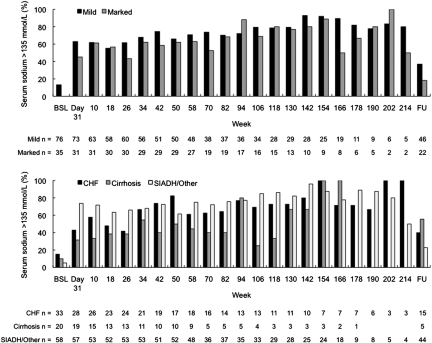
Serum sodium correction rate was initially greater for the marked hyponatremia subgroup but overall had similar kinetics in the mild and marked hyponatremia groups (Figure 3A) and in the CHF and SIADH/other cause groups (Figure 3B). In patients with cirrhosis, there seemed to be a trend toward lower final serum sodium levels. The normalization rate (percentage of patients with serum sodium levels >135 mmol/L) over time according to hyponatremia severity and cause is shown in Figure 4. More than 60 and 45% of patients in the mild and marked hyponatremia groups, respectively, exhibited normal serum sodium levels by week 4. Correction rates seemed to be generally similar among patients with CHF and SIADH/other but may have been somewhat lower among patients with cirrhosis. In all patient subgroups, serum sodium levels declined by 7 days of withholding tolvaptan, indicating a need for continued tolvaptan therapy to maintain serum sodium normalization in many patients (Figures 2 and 3). On drug discontinuation, the proportion of patients who declined by at least 3 mEq/L was 68%, and an equal proportion fell from ≥135 mEq/L to below this threshold of normal.
Figure 3.
Serum sodium measured according to severity of hyponatremia (left) and underlying cause (right). The correction rate had similar kinetics for all groups, except a steeper initial response for the marked hyponatremia subgroup (left). Comparisons versus baseline were statistically significant (P < 0.05) for patients with mild hyponatremia at all time points but week 214 and the follow-up visit; for patients with marked hyponatremia at all visits but weeks 202 and 214; for patients with CHF at all time points but weeks 190, 202, and 214 and the follow-up visit; for patients with cirrhosis at 8 hours, day 31, weeks 10, 18, and 50, and the follow-up visit; and for patients with SIADH/other at all visits but week 214 and the follow-up visit.
Figure 4.
Percentage of patients who normalized their serum sodium according to severity of hyponatremia (top) and etiology (bottom). Approximately half of patients exhibited normal serum sodium levels by week 4. Correction rates seemed to be generally similar among patients with CHF and SIADH/other but may have been somewhat lower among patients with cirrhosis.
Mean time to first fluid restriction was 122.3 ± 184.3 and 162.5 ± 186 days in the mild and marked hyponatremia subgroups, respectively; 13.2% of patients in the mild hyponatremia group and 5.4% in the marked hyponatremia group required fluid restriction. One (0.9%) patient received urea, and no patient received demeclocycline.
Discussion
Because many of the therapies that are available to treat euvolemic and hypervolemic hyponatremic disorders are fraught with adverse effects and/or limited effectiveness, the advent of orally active inhibitors of the vasopressin 2 receptor represent a welcome addition to other therapies. Whether given orally2–9 or intravenously,11 the drugs significantly increase serum sodium in the majority of patients who are treated with these vasopressin antagonists. The drugs seem to have, in their short term, an acceptable adverse effect profile, most AEs being related to the expected effect of the drug to increase urine flow8; however, all studies involved very short follow-ups, and those with somewhat longer monitoring had a very limited number of patients (≤10).3,6 Thus, after the completion of SALT-1 and SALT-2, we undertook this open-label long-term extension. The extension, initiated for 111 patients, provided 212 patient-years of observation and monitoring for both potential drug-related adverse effects and maintenance of efficacy. The patients were approximately equally balanced between those with mild hyponatremia (sodium between 130 and 135 mmol/L) and those with more marked decrements (<130 mmol/L) at the time of their initial study. Likewise, the study population was approximately equally balanced between those who had hypervolemia and euvolemia.
The number of reported AEs seems to be high at first analysis. It must be noted, however, that the trial did not have a comparator group of an equally ill cohort treated with placebo over the prolonged period of observation. Because most patients with hyponatremia with CHF and cirrhosis have severe underlying disease and because neoplastic disease is relatively common in patients with euvolemia, it is not surprising that nearly all patients at some time or another were found to have an AE. In the published results of the EVEREST trial of patients with decompensated heart failure, no overall effects on mortality or cardiovascular morbidity were noted in the population with low serum sodium (<137 mEq/L)10; however, the AEs attributed by the investigators to tolvaptan in our study were reminiscent of those reported in both EVEREST and shorter term studies, which would be expected from an aquaretic agent. More important, no new consistent AE or laboratory abnormality emerged from the more prolonged use of the drug. Notably, only six occasions of adverse effects potentially related to the administration of tolvaptan led to its discontinuation. Table 3 lists the 10 serious AEs that were deemed to be drug related. Most of these occurred at a time remote from the initiation of the drug; moreover, the relationship was thought to be possible rather than probable, because most of the patients had multiple associated medical problems that could have accounted for the event. On nine occasions, the AEs were reversible. The AEs and their interpretation were at the discretion of individual investigators who were unblinded.
Excessively rapid correction or overcorrection of hyponatremia at the initiation of treatment was likewise uncommon, but early monitoring of serum sodium during the initial 24 hours may nonetheless be appropriate. After the initial dose of 15 mg, only five patients had serum sodium increases >1.0 mmol/L per h in the first 8 hours (range 1.08 to 1.47 mmol/L per h), and no patient developed a clinical picture of osmotic demyelination. Hypernatremia (>145 mmol/L) occurred in 12 patients but led to drug withdrawal in only one and was readily reversible in all others. The percentage of patients who reached and maintained normonatremia was 57%, which again was comparable to the experience in the placebo-controlled trials. Although the prescription to drink in response to their physiologic thirst drive probably helped prevent overly rapid correction, it also may have limited the maximum potential effect of the drug. Similarly, the development of de novo hyponatremia in this susceptible study population was almost absent. Long-term tolvaptan use therefore seems to be well tolerated and associated with few AEs. Whether some unexpected adverse effects will emerge as the drug is used in more patients and for longer treatment time cannot be entirely excluded and will require further postmarketing surveillance.
The increases in serum sodium observed in our short-term trial8 were again observed in SALTWATER. Furthermore, the effect was sustained for the duration of the observation period. Because there was no comparator group, the differences were assessed relative to baseline serum sodium, and, in the great majority of the patients, serum sodium remained >135 mmol/L. The mean change in serum sodium concentration for those who had mild and more marked hyponatremia were comparable. The patients with cirrhosis had a less robust response, as was previously seen in several short-term trials involving vasopressin antagonists.8,9 The mechanisms for this difference have yet to be fully explored. The return of serum sodium toward baseline 7 days after study discontinuation reveals that most (68%) of the patients had an irreversible defect in water excretion and that the requirement for the drug may be open-ended. Conversely, 17 (15.3%) patients had serum sodium within the normal range at entry to the extension study.
Hyponatremia is common in hospitalized patients12 and not uncommon in the ambulatory care setting as well.13 It is a well-established predictor of high mortality in patients with cirrhosis,14 heart failure,15 and neurologic disorders.16 Accumulating data suggest that in addition to being a marker of severe disease, hyponatremia is associated with increasing risk for cognitive dysfunction and falls,17 as well as increased risk for fractures.18 It may therefore be desirable for the abnormality to be corrected, particularly in the susceptible elderly population.19 The availability of oral vasopressin antagonists provides an effective way to increase serum sodium over the long term. Whether the correction of the hyponatremia will result in decreased hospitalizations and translate into an increase in long-term survival awaits the completion of further studies.
Concise Methods
Study Design
SALTWATER was an international, multicenter, nonrandomized, open-label extension of SALT-1 and SALT-2.8 Patients were eligible when they participated successfully in the original trials and evidenced a continued need and desire for therapy. Patients were not eligible when they had a current medical condition for which long-term treatment with an aquaretic agent posed an undue risk (e.g., pregnancy, psychogenic polydipsia, hydrophobia, anorexia, urinary flow obstruction, hypotension); hyponatremia that was acute, reversible (e.g., hypothyroidism, hypocortisolism), artifactual, or due to conditions not associated with arginine vasopressin excess or likely to respond to aquaretic therapy; severe renal impairment (creatinine >3.5 mg/dl [310 μmol/L]); or a clinical condition with potential to confound results (e.g., poorly controlled diabetes).
Patients were enrolled to receive oral tolvaptan tablets once daily for up to 58 weeks initially, later amended to a maximum of 214 weeks. Entrance into SALTWATER occurred at least 7 days (and up to 2 to 3 years) after the final tolvaptan dose received in SALT-1 and SALT-2. The initial tolvaptan dosage was 15 mg, which was increased to 30 and 60 mg when the patient continued to have hyponatremia and the change in serum sodium was <5 mmol/L relative to a measurement at least 22 to 24 hours earlier. Dosages were maintained when the patient's serum sodium was consistently >135 mEq/L or the change in serum sodium was >5 mmol/L relative to a previous measurement. Down-titration was mandated when the serum sodium was consistently >145 mmol/L or when the measured increase in serum sodium was >8 mmol/L per 8 hours or >12 mmol/L per d. There were no restrictions on concomitant medications with the exception of specific agonists or antagonists of arginine vasopressin. Patients were allowed to receive standard therapies for hyponatremia, including fluid restriction, demeclocycline, and urea. When possible, fluid restriction was avoided during the first 24 hours of tolvaptan treatment, but patients who entered the study with a serum sodium level <130 mmol/L could be fluid restricted during the first day.
The institutional review board or ethics committee at each site approved the study protocol and ensured that written informed consent was obtained from all patients. The study adhered to the Ethical Principles for Medical Research Involving Human Subjects, World Medical Association Declaration of Helsinki.20
Assessments
Study assessments were to occur at clinic visits on day 1 (baseline and 8 hours after first dose), days 2 through 14 (to end of titration), and day 31; every 8 weeks from weeks 10 through 58; every 12 weeks from weeks 70 through 214; and a follow-up visit occurring 7 days after the last dose of tolvaptan (either after final visit or after early withdrawal).
Safety of long-term tolvaptan was assessed at all visits by AEs, vital signs, and physical examinations. AEs were defined as any new medical problem or exacerbation of an existing medical problem according to the Medical Dictionary for Regulatory Activities (MedDRA).21 Patients could spontaneously report such events to an investigator. Investigators were required to assess and report to the sponsor the seriousness and severity of each event and whether the event was associated with the study drug.
Additional safety assessments included hematology and coagulation panels, serum chemistries (routine metabolic profile including electrolytes, glucose, and tests of hepatic and renal function), pregnancy testing, and electrocardiograms. At a minimum, blood panels were conducted on days 1 and 14; at weeks 58, 106, 154, and 214 (or early withdrawal date); and at the 7-day follow-up visit. Serum sodium determinations occurred at every scheduled visit and often more frequently during the dosage titration phase.
The secondary objective of SALTWATER was to determine the long-term efficacy of tolvaptan therapy. Efficacy was assessed at all visits by serum sodium levels, percentage of patients with normalized serum sodium (>135 mmol/L), and percentage of patients who required other hyponatremia treatments.
Statistical Analysis
All safety and efficacy variables were assessed descriptively. Because there was no placebo or drug comparator, all statistical comparisons were with baseline values, with changes evaluated in the context of the patient's underlying clinical condition. Protocol development and data analyses were undertaken jointly by the sponsor, the investigators, and the authors. The sponsor holds the data, which were freely available to the authors.
Disclosures
Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan), owns the rights to tolvaptan (brand name SAMSCA), the therapeutic agent described in this article. T.B., J.G.V., R.W.S., and D.G.B. have received research funding and consultancy fees from Otsuka Pharmaceutical Development and Commercialization (Princeton, NJ); J.O. and F.S.C. are employees of Otsuka Pharmaceutical Development and Commercialization.
Acknowledgments
SALTWATER investigators were as follows: North America: Nezam Afdhal (Boston, MA), Tomas Berl (Aurora, CO), Barry Cabuay (Iowa City, IA), Samuel Castillo (Chicago, IL), Morris Goldman (Chicago, IL), Dinesh Gupta (Tullahoma, TN), Terrence C. Hack (Ayer, MA), Richard C. Josiassen (Norristown, PA), Michael Koren (Jacksonville, FL), Wayne Leimbach (Tulsa, OK), Barton S. Levine (Los Angeles, CA), Salem Malik (Thunder Bay, Ontario, Canada), Ricardo Martinez (Port Charlotte, FL), Douglas J. Pearce (Nashville, TN), Joseph Verbalis (Washington, DC), Nampalli Vijay (Denver, CO), Lynne Wagoner (Cincinnati, OH), and Frederick Wood (Fallbrook, CA). Europe: Guy Decaux (Anderlecht, Belgium), Mihaly Dudas (Gyula, Hungary), Jan Filipovsky (Pizen-Bory, Czech Republic), Miguel Garcia (Madrid, Spain), Lubor Golan (Praha, Czech Republic), Peter Gross (Dresden, Germany), Johannes Hensen (Hannover, Germany), Maciej Kozina (Wroclaw, Poland), František Pad'our (Prague, Czech Republic), Alfredo Pontecorvi (Rome, Italy), Miroslav Ryba (Liberec, Czech Republic), Walter Sehnert (Dortmund, Germany), Petr Svoboda (Brno, Czech Republic), and Maciej Zarebinski (Warsaw, Poland).
On behalf of the SALTWATER study investigators, we thank participating patients, subinvestigators, sponsor study managers (especially Holly Krasa, Mary Hobart, Keren Kedzierski, Joanne Piccione, and Terry Goldberg), study monitors and data managers, and programmers (especially Svetlana Ivanova). We also thank David Norris, PhD, for editorial assistance provided during the preparation of the manuscript. This assistance was paid for through funding supplied by Otsuka America Pharmaceutical, Inc., to Ecosse Medical Communications, LLC (Princeton, NJ). T.B. assumes responsibility for the overall preparation, content, and integrity of the article, with substantial contributions from the coauthors. All authors vouch for the accuracy and completeness of the reported data. R.W.S. and D.G.B. served on the SALTWATER Safety Committee.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Treatment of Chronic Hyponatremia: Now We Know How, but Do We Know When or If?” on pages 552–555.
References
- 1. Greenberg A, Verbalis JG: Vasopressin receptor antagonists. Kidney Int 69: 2124– 2130, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Annane D, Decaux G, Smith N: Efficacy and safety of oral conivaptan, a vasopressin-receptor antagonist, evaluated in a randomized, controlled trial in patients with euvolemic or hypervolemic hyponatremia. Am J Med Sci 337: 28– 36, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Decaux G: Long-term treatment of patients with inappropriate secretion of antidiuretic hormone by the vasopressin receptor antagonist conivaptan, urea, or furosemide. Am J Med 110: 582– 584, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Ghali JK, Koren MJ, Taylor JR, Brooks-Asplund E, Fan K, Long WA, Smith N: Efficacy and safety of oral conivaptan: A V1A/V2 vasopressin receptor antagonist, assessed in a randomized, placebo-controlled trial in patients with euvolemic or hypervolemic hyponatremia. J Clin Endocrinol Metab 91: 2145– 2152, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Gines P, Wong F, Watson H, Milutinovic S, del Arbol LR, Olteanu D: Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: A randomized trial. Hepatology 48: 204– 213, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Soupart A, Gross P, Legros JJ, Alfoldi S, Annane D, Heshmati HM, Decaux G: Successful long-term treatment of hyponatremia in syndrome of inappropriate antidiuretic hormone secretion with satavaptan (SR121463B), an orally active nonpeptide vasopressin V2-receptor antagonist. Clin J Am Soc Nephrol 1: 1154– 1160, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Gheorghiade M, Gottlieb SS, Udelson JE, Konstam MA, Czerwiec F, Ouyang J, Orlandi C: Vasopressin V(2) receptor blockade with tolvaptan versus fluid restriction in the treatment of hyponatremia. Am J Cardiol 97: 1064– 1067, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec F, Orlandi C: Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 355: 2099– 2112, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Wong F, Blei AT, Blendis LM, Thuluvath PJ: A vasopressin receptor antagonist (VPA-985) improves serum sodium concentration in patients with hyponatremia: A multicenter, randomized, placebo-controlled trial. Hepatology 37: 182– 191, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C: Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST Outcome Trial. JAMA 297: 1319– 1331, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Zeltser D, Rosansky S, van Rensburg H, Verbalis JG, Smith N: Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am J Nephrol 27: 447– 457, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Janicic N, Verbalis JG: Evaluation and management of hypo-osmolality in hospitalized patients. Endocrinol Metab Clin North Am 32: 459– 481, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Hawkins RC: Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta 337: 169– 172, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Wu CC, Yeung LK, Tsai WS, Tseng CF, Chu P, Huang TY, Lin YF, Lu KC: Incidence and factors predictive of acute renal failure in patients with advanced liver cirrhosis. Clin Nephrol 65: 28– 33, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Klein L, O'Connor CM, Leimberger JD, Gattis-Stough W, Pina IL, Felker GM, Adams KF, Jr, Califf RM, Gheorghiade M: Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: Results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation 111: 2454– 2460, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Bhardwaj A: Neurological impact of vasopressin dysregulation and hyponatremia. Ann Neurol 59: 229– 236, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G: Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med 119: 71.e1– 71.e8, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Gankam Kengne F, Andres C, Sattar L, Melot C, Decaux G: Mild hyponatremia and risk of fracture in the ambulatory elderly. QJM 101: 583– 588, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Miller M, Morley JE, Rubenstein LZ: Hyponatremia in a nursing home population. J Am Geriatr Soc 43: 1410– 1413, 1995 [DOI] [PubMed] [Google Scholar]
- 20.World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. 2008. [Accessed February 2010]. Available at: http://www.wma.net/en/30publications/10policies/b3/index.html.
- 21. MedDRA MSSO. Available at: http://www.meddramsso.com/files_acrobat/Browser_30_User_Manual.pdf Accessed February 2010