Abstract
Spontaneous remission is a well known characteristic of idiopathic membranous nephropathy, but contemporary studies describing predictors of remission and long-term outcomes are lacking. We conducted a retrospective, multicenter cohort study of 328 patients with nephrotic syndrome resulting from idiopathic membranous nephropathy that initially received conservative therapy. Spontaneous remission occurred in 104 (32%) patients: proteinuria progressively declined after diagnosis until remission of disease at 14.7 ± 11.4 months. Although spontaneous remission was more frequent with lower levels of baseline proteinuria, it also frequently occurred in patients with massive proteinuria: 26% among those with baseline proteinuria 8 to 12 g/24 h and 22% among those with proteinuria >12 g/24 h. Baseline serum creatinine and proteinuria, treatment with angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists, and a >50% decline of proteinuria from baseline during the first year of follow-up were significant independent predictors for spontaneous remission. Only six patients (5.7%) experienced a relapse of nephrotic syndrome. The incidence of death and ESRD were significantly lower among patients with spontaneous remission. In conclusion, spontaneous remission is common among patients with nephrotic syndrome resulting from membranous nephropathy and carries a favorable long-term outcome with a low incidence of relapse. A decrease in proteinuria >50% from baseline during the first year predicts spontaneous remission.
Idiopathic membranous nephropathy (IMN) is one of the most common causes of nephrotic syndrome in adults.1,2 Treatment with several immunosuppressive agents has shown beneficial effect on the course of this disease3–11; however, controversy persists regarding the proper timing of immunosuppression and the best therapeutic regimen.12–15
The appearance of spontaneous remission (SR) in IMN not induced by immunosuppressive therapy is a well known characteristic of the disease. Classic studies about the natural history of IMN report a SR incidence ranging from 30% to 60%.16–20 Age at presentation <50 years old and female sex are predictors of SR, whereas SR is reported as very unusual in patients presenting with proteinuria >8 g/d. Nevertheless, these studies were performed 2 or 3 decades ago, when supportive treatment of nephrotic syndrome was less well established and efficient than in present times. In particular, treatment with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II type 1 receptor antagonists (ARBs) in patients with nephrotic syndrome was uncommon, whereas they are now widely prescribed in patients with proteinuric nephropathies. Furthermore, some of the studies reporting SR in untreated IMN patients included an important number of patients presenting with non-nephrotic proteinuria,21 a clinical presentation with an inherently good prognosis,22 and recent data about clinical characteristics, predicting factors, and long-term outcome in IMN are scanty. The aim of the present retrospective study was to report the clinical features and outcome of 328 patients with biopsy-proven IMN, in whom an initially conservative therapeutic approach, without corticosteroids or other immunosuppressive agents, was followed. The primary outcome was the appearance of SR, partial or complete, and major secondary outcomes included relapses, progression to ESRD, and mortality.
Results
Baseline Characteristics
Baseline characteristics of the 328 patients are listed in Table 1. Most patients had a preserved renal function [estimated GFR (eGFR) >60 ml/min/1.73 m2 in 71.3%]. ACEI/ARB treatment was started at baseline or thereafter in 219 patients (66.7%). Of them, 149 (68%) received ACEIs, 53 (24%) received ARBs, and 17 (8%) were treated with an ACEI/ARB combination. Initial doses of these drugs were relatively low, adjusted for blood pressure values, and tolerance was good. Thirty-eight (11.5%) patients were lost to follow-up; they were censored at last visit and their data included for the analysis.
Table 1.
Characteristics of patients at baseline
| Characteristic | Patients with SR (n = 104) | Patients without SR (n = 224) | P Value | All Patients (n = 328) |
|---|---|---|---|---|
| Age at diagnosis, years | 50 ± 17 | 51 ± 16 | 0.38 | 50 ± 16 |
| Male gender, n (%) | 60 (58) | 161 (71.8) | 0.008 | 221 (67.3) |
| Proteinuria, g/24 h | 6.6 (3.5 to 21) | 8 (3.5 to 32.6) | <0.003 | 7.4 (3.5 to 32.6) |
| Serum creatinine, mg/dl | 1 ± 0.4 | 1.3 ± 0.8 | <0.001 | 1.2 ± 0.7 |
| eGFR, ml/min/1.73 m2 | 84 ± 30 | 73 ± 32 | <0.001 | 77 ± 32 |
| ACEI/ARB treatment, n (%) | 83 (79.8) | 136 (60.7) | 0.009 | 219 (66.7) |
Patients with SR
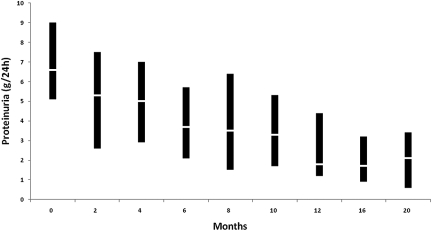
One hundred and four patients (31.7%) developed SR. Time to achieve partial remission (PR) was 14.7 ± 11.4 months, ranging from 1 to 66 months. Fifty-two of these 104 patients (50%) persisted with PR, whereas the remaining 52 patients (50%) progressed into complete remission (CR). Time to achieve CR was 38.5 ± 25.2 months, ranging from 4 to 120 mo. As shown in Figure 1, the reduction of proteinuria was gradually progressive: it had decreased from 6.6 (3.5 to 21) g/24 h at baseline to 3.8 (0.2 to 11) g/24 h at 6 months (P < 0.0001 with respect to baseline) and to 1.8 (0 to 7.2) g/24 h at 12 months (P < 0.0001 with respect to 6 months).
Figure 1.
Evolution of proteinuria in patients with SR. The line within the box denotes the median and the box spans the interquartile range (25th to 75th percentiles).
In comparison with patients who did not develop SR, patients with SR included a higher number of women and showed better renal function and lower proteinuria at baseline, as shown in Table 1. As shown in Table 2, SR was more frequent with lower baseline proteinuria: 37.1% among patients with baseline proteinuria <8 g/24 h, 26.3% among those with proteinuria 8 to 12 g/24 h, and 21.5% among those with proteinuria >12 g/24 h. There was no correlation between baseline proteinuria and time to remission, as shown in Table 2.
Table 2.
Number of SR (partial and complete) and time to achieve partial and complete SR according to baseline proteinuriaa
| Baseline Proteinuria (g/24 h) | SR (n = 104) |
Partial SR (n = 52) |
Complete SR (n = 52) |
No SR (n = 224) |
||||
|---|---|---|---|---|---|---|---|---|
| n (%) | ACEI/ARB Treatment n (%) | n (%) | Time to PR | n (%) | Time to CR | n (%) | ACEI/ARB Treatment (%) | |
| 3.5 to 8 (n = 186) | 69 (37.1) | 59 (85.5) | 37 (19.9) | 15.6 ± 12 | 32 (17.2) | 41.1 ± 27.6 | 117 (62.9) | 70 (59.8)b |
| 8 to 12 (n = 91) | 24 (26.3) | 17 (70.8) | 11 (12.1) | 14.9 ± 17.4 | 13 (14.3) | 39.3 ± 20.1 | 67 (73.7) | 42 (62.7) |
| >12 (n = 51) | 11 (21.5) | 7 (63.6) | 4 (7.8) | 20.5 ± 15 | 7 (13.7) | 24.8 ± 18 | 40 (78.5) | 24 (60) |
aAlso shown are the number and percentage of patients treated with ACEIs/ARBs by different values of baseline proteinuria.
bP = 0.007 with respect to patients with SR.
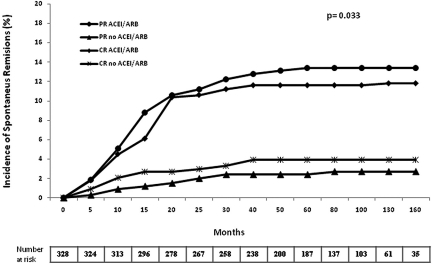
The number of patients treated with ACEIs or ARBs was significantly higher among patients with SR (79.8% versus 60.7%, P = 0.009; Table 1), although this difference was restricted to patients with baseline proteinuria <8 g/24 h (Table 2). As shown in Figure 2, the probability of SR (CR and PR) was significantly higher among patients treated with ACEIs/ARBs. The probability of SR was 22.3%, 32.8%, and 36% among ACEI/ARB-treated patients and 11%, 13.8%, and 19.3% among those who did not receive such treatment, 1, 2, and 3 years after baseline, respectively (P = 0.009).
Figure 2.
Probability of SR in patients treated with ACEIs/ARBs and in patients who did not receive this treatment.
On multivariate analysis (Table 3), baseline serum creatinine, the amount of baseline proteinuria, treatment with ACEIs/ARBs, and an spontaneous proteinuria decrease >50% of baseline during the first year of follow-up emerged as significant independent predictor factors for SR.
Table 3.
Results of univariate and multivariate analyses of independent prognostic factors for the appearance of SR
| Factor | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|
| Hazard Ratio for SR (95% CI) | P Value | Hazard Ratio for SR (95% CI | P Value | |
| Female gender | 1.8 (1.10 to 3) | 0.008 | 1.45 (0.68 to 3.10) | 0.33 |
| Baseline serum creatinine (mg/dl) | 0.35 (0.18 to 0.66) | <0.001 | 0.40 (0.19 to 0.85) | 0.018 |
| Baseline proteinuria (g/24 h) | 0.92 (0.86 to 0.98) | <0.003 | 0.85 (0.77 to 0.94) | <0.002 |
| Proteinuria decrease >50% in the first year of follow-up | 7.08 (3.59 to 13.9) | <0.0001 | 12.6 (5.2 to 30.5) | <0.0001 |
| ACEI/ARB treatment | 2 (1.1 to 3.5) | 0.009 | 2.36 (1.09 to 5.12) | 0.029 |
Patients without SR
Nephrotic syndrome persisted without SR in 224 patients (68.2%). They initially received, as did patients who later developed SR, only symptomatic treatment for nephrotic syndrome. However, along their clinical course 176 of 224 patients (78.5%) initiated immunosuppressive treatment for the following reasons: rapid worsening of renal function (n = 39), nephrotic syndrome complications or severe edema (n = 53), and excessive persistence of nephrotic syndrome (according to each participating group criteria) (n = 84). In the remaining 48 patients no immunosuppressive treatment was given.
Immunosuppresive regimens were not uniform, according to the different therapeutic protocols of the participating groups. The most frequently used regimen was corticosteroids plus chlorambucil (43%), followed by corticosteroids plus tacrolimus (25%), corticosteroids as single therapy (14%), corticosteroids plus cyclosporin (11%), and corticosteroids plus mycophenolate mofetil (7%). The interval between diagnosis and the onset of immunosuppressive treatment in those patients who received it was 10.6 ± 18.4 months, ranging from 2 to 144 months.
Long-Term Follow-Up and Outcome
The mean duration of follow-up was 91 ± 61 months in the group of patients who develop SR and 69 ± 51 months in those who did not. As shown in Table 4, 42 of 224 patients (18.7%) without SR started chronic dialysis throughout follow-up, whereas no patient among those developing SR developed ESRD (P < 0.0001).
Table 4A.
Final outcome
| Parameter | Patients with SR (n = 104) | Patients without SR (n = 224) | P Value |
|---|---|---|---|
| Immunosuppressive treatment, n (%) | 0 (0) | 176 (78.5) | <0.0001 |
| Chronic dialysis, n (%) | 0 (0) | 42 (18.7) | <0.0001 |
| Deaths, n (%) | 2 (1.9%) | 24 (10.7) | 0.008 |
| Follow-up (months) | 91 ± 61 | 69 ± 51 | 0.003 |
Table 4B.
Final outcome compared with baseline values in patients with and without SR
| Parameter | Patients with SR (n = 104) |
Patients without SR (n = 224) |
P Valueb | ||||
|---|---|---|---|---|---|---|---|
| Baseline | P Valuea | Final | Baseline | P Valuea | Final | ||
| Proteinuria, g/24 h | 6.6 (3.5 to 21) | <0.0001 | 0.4 (0 to 3.3) | 8 (3.5 to 32.6) | <0.0001 | 2 (0 to 20) | 0.06 |
| Serum creatinine, mg/dl | 1 ± 0.4 | 0.03 | 1.1 ± 0.4 | 1.3 ± 0.8 | <0.0001 | 2.4 ± 2.2 | <0.0001 |
| eGFR, ml/min/1.73m2 | 84 ± 30 | <0.001 | 79 ± 28 | 73 ± 32 | <0.0001 | 53 ± 35 | <0.001 |
aWithin-group comparisons.
bBetween-group comparisons.
Table 4C.
Final outcome in patients with complete and partial SR
| Parameter | Patients with Complete SR (n = 52) |
Patients with Partial SR (n = 52) |
P Valueb | ||||
|---|---|---|---|---|---|---|---|
| Baseline | P Valuea | Final | Baseline | P Valuea | Final | ||
| Serum creatinine, mg/dl | 1 ± 0.3 | 0.74 | 1 ± 0.2 | 0.9 ± 0.4 | 0.01 | 1.1 ± 0.2 | 0.09 |
| eGFR, ml/min/1.73 m2 | 77 ± 21 | 0.12 | 72 ± 25 | 91 ± 32 | 0.007 | 79 ± 30 | 0.22 |
| Proteinuria, g/24 hc | 0.2 (0 to 2.7) | 1.6 (0.3 to 0.2) | 0.03 | ||||
| >50% increase of baseline serum creatinine, n (%) | 6 (11.5) | 5 (9.6) | 0.07 | ||||
| Follow-up (months) | 104 ± 57 | 79 ± 64 | 0.03 | ||||
aWithin-group comparisons.
bBetween-group comparisons.
cMean proteinuria value after the appearance of SR.
The number of deaths during follow-up was also significantly higher among patients without SR: 24 (10.7%) versus 2 (1.9%) (P = 0.002). Deaths among patients without SR occurred 62.2 ± 45.3 months after diagnosis (ranging from 5 to 146 months) and were due to cardiovascular complications (n = 9), tumors (n = 6), and unknown causes (n = 9). Of them, only four patients died during the first 12 months; their baseline proteinuria was <8 g/24 h in three patients and 10 g/24 h in the remaining patient. Deaths among patients with SR (n = 2) occurred 36 and 240 months after diagnosis because of a cardiovascular complication (n = 1) and unknown cause (n = 1). Their baseline proteinuria was 8.1 and 7.4 g/24 h, respectively.
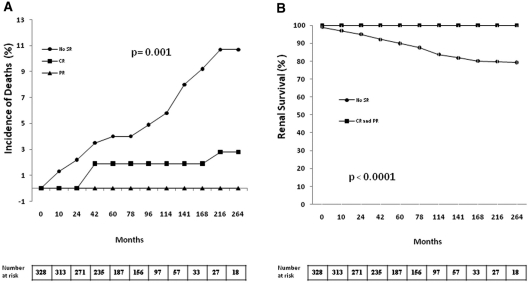
As shown in Figure 3, the probability of survival without death or chronic dialysis was significantly higher among patients who developed SR, both CR or PR.
Figure 3.
(A) Incidence of deaths and (B) renal survival in patients with or without SR.
As shown in Table 4 and Figure 3, long-term outcome of patients with SR was excellent. Their mean loss of renal function, estimated by eGFR, was of 8.6 ± 26 ml/min/1.73 m2 during a mean follow-up of 91 ± 61 months, representing a renal function loss of 1.8 ± 13.1 ml/min/yr.
Comparison between patients with CR and PR is shown in Table 4. Despite (by definition) a higher proteinuria value in the latter, there were no significant differences in the mean eGFR decrease between patients with CR or PR, and the number of patients with a 50% increase of baseline serum creatinine was similar.
Patients with Baseline Proteinuria >12 g/24 h
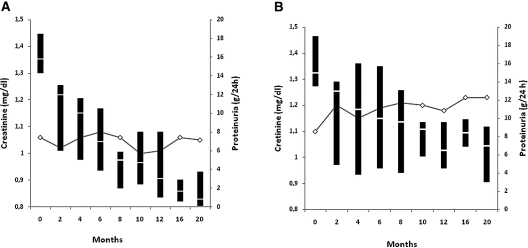
As shown in Table 2, 11 of 51 patients with baseline proteinuria >12 g/24 h (21.5%) developed SR (CR in 7 and PR in 4). As shown in Figure 4, proteinuria decrease was gradually progressive and renal function remained stable. No patient showed relapse of nephrotic syndrome after SR. Long-term outcome was excellent with 100% of renal and patient's survival.
Figure 4.
Evolution of serum creatinine (lines) and proteinuria (box plots) in patients with baseline proteinuria >12 g/24 h for patients (A) with and (B) without SR. For proteinuria, the line within the box denotes the median and the box spans the interquartile range (25th to 75th percentiles).
In the remaining 40 patients (78.5%), immunosuppressive treatment was started 7.9 ± 9.9 months (1 to 42) after diagnosis because of rapid worsening of renal function in 11 patients or severe edema and clinical intolerance to nephrotic syndrome in 29. The evolution of renal function and proteinuria in these patients during the first 20 months is shown in Figure 4. Six patients (15%) died 59.2 ± 42.8 months after diagnosis (12 to 108 months) because of cardiovascular causes (n = 2), tumors (n = 2), and nonidentified causes (n = 2). Of them, one patient died 12 months after diagnosis while receiving immunosuppressive treatment. The five remaining deaths occurred 48 to 108 months after diagnosis, 28 to 84 months after the end of immunosuppressive therapy. Eleven patients (27.5%) developed end-stage renal failure.
Relapses
Six (5.7%) of the 104 patients who developed SR presented a nephrotic syndrome relapse. These relapses appeared in patients showing a CR (three patients) and PR (three patients). Mean interval between the appearance of SR and the relapse of nephrotic syndrome was 132 ± 78 months. Of the six patients, four entered again into remission after the reintroduction of ACEI/ARB agents (that the patients had withdrawn previously) and one patient after immunosuppressive treatment. The remaining patient persisted with nephrotic syndrome despite immunosuppressive therapy.
Discussion
The most worldwide accepted model to decide therapeutic interventions in patients with IMN is based on the algorithm proposed by the Toronto Glomerulonephritis Registry.2,16 This model stratifies patients in low risk (normal renal function and proteinuria <4 g/d), medium risk (normal renal function, proteinuria >4 to <8 g/d) and high risk (proteinuria >8 g/d with or without renal insufficiency) categories for progression.23 Following this scheme, immunosuppressive therapy is advised without delay for high-risk patients and recommended for medium-risk patients when nephrotic-range proteinuria persists for >6 months.16
SR is considered to be very uncommon among patients presenting with heavy proteinuria.1,2 On the contrary, our study shows that an important proportion of patients with massive proteinuria developed SR: 26.3% among those with baseline proteinuria 8 to 12 g/24 h and 21.5% among those with proteinuria >12 g/24 h. Even more, the time to achieve SR among these patients was not longer than that of patients with a lower proteinuria at presentation, as shown in Table 2. The total number of SR (104 of 328, 31.7%) is also remarkable, considering that we only included in the study patients with nephrotic syndrome. Some of the previous studies reporting SR in untreated IMN patients included an important number of patients presenting with non-nephrotic proteinuria,21 a clinical presentation with an inherently good prognosis.1,13,22
Our study also provides interesting and previously not well characterized features of SR. Confirming previous studies16–20 most SR appeared during the first 2 years after diagnosis. However, our data show that proteinuria in SR did not decrease abruptly from nephrotic to non-nephrotic ranges, but it experienced a gradually progressive decrease, as shown in Figure 1. In fact, a proteinuria decrease >50% of baseline values during the first year of follow-up was one of the factors significantly predicting the appearance of SR. These data altogether would suggest that a conservative approach should be maintained in patients showing a progressive decline in proteinuria during the first year of follow-up, provided that renal function continues to be normal and that such rule could also be applicable to patients with massive proteinuria. However, because most patients with high-grade proteinuria (>8 g/24 h) did not achieve SR, it is very important to emphasize the need for a close and careful monitoring of these patients during the first 12 to 18 months of the disease. A conservative approach should be recommended only for those patients showing a clear and progressive proteinuria decrease during the first year, together with a stable renal function. On the other hand, immunosuppression should be considered for patients with massive proteinuria (>12 g/24 h) at baseline who do not show a significant proteinuria reduction within the first 6 months (Figure 4).
Another interesting finding of our study not previously reported is that ACEI/ARB treatment was associated with a significantly increased probability of SR appearance. The antiproteinuric effect of ACEIs/ARBs in diabetic and nondiabetic nephropathies is well known,24 but its efficacy in IMN appears to be poorer than in other types of primary glomerular disorders.25,26 However, it could be possible that the rather modest and slow proteinuria reduction induced by ACEI/ARB treatment in patients with IMN would facilitate the appearance of SR through mechanisms currently unknown. Nevertheless, as shown in Table 2, the association between ACEI/ARB treatment and SR was restricted to patients with baseline proteinuria <8 g/24 h.
The long-term follow-up of our IMN cohort allowed us to demonstrate the excellent long-term outcome of patients who developed SR. In comparison with patients without SR, they showed a significantly lower mortality and a better renal survival. No patient with SR developed ESRD needing maintenance dialysis and only 11 patients (10.5%) increased their serum creatinine >50% of baseline values throughout follow-up. Interestingly, this excellent outcome was shared by patients with CR and PR, despite the persistently higher values of proteinuria in the latter. Previous studies27,28 have stressed the importance of a PR, spontaneous or induced by immunosuppression, as a valid therapeutic goal associated with a good prognosis.
The number of relapses after SR (5.7%) was relatively low and their prognosis favorable. Most of patients entered into SR again after the reintroduction of ACEI/ARB treatment that had been previously withdrawn.
The remarkable percentage of SR and the excellent long-term outcome shown in our study by no means should be interpreted as a global favorable prognosis for patients with IMN. In most of the remaining 224 cohort patients (68.2%) who did not develop SR immunosuppressive therapy had to be started because of nephrotic syndrome persistence, complications of the nephrotic syndrome, or rapid worsening of renal function. Despite this aggressive therapy, 18.7% of the patients who did not show SR started chronic dialysis and another 10.7% died.
Our study has important limitations inherent to its retrospective design. The number of patients in the different periods of the study was not homogeneous because of the different ages of the hospitals participating in the study. We cannot exclude unmeasured confounding factors that would influence the appearance of SR such as statin treatment. In addition, given the observational retrospective design of the study, the association between ACEIs/ARBs and SR should be interpreted cautiously. On the other hand, the number of included patients and their careful long-term follow-up allow analysis of several important clinical questions that hardly could be studied in a prospective design.
In conclusion, a significant number of IMN patients with nephrotic syndrome (31.7%) develop SR and their long-term outcome is excellent, with a low incidence of relapses, a renal survival of 100%, and a lower mortality than patients without SR. Almost one-quarter of patients with proteinuria >8 g/d at baseline developed SR in our study. On the basis of our findings, we recommend a close monitoring and a conservative therapy (that should include an ACEI or an ARB) for all patients with IMN during the first 12 or 18 months of follow-up independent of their baseline proteinuria and provided that renal function continues to be normal and proteinuria shows a progressive decline. This latter point needs being emphasized because a proteinuria decrease >50% of baseline during the first year significantly predicts the appearance of SR. On the contrary, immunosuppressive therapy should be considered without delay for patients with deteriorating renal function29,30 and for those without a significant proteinuria decrease during this period, particularly if the baseline proteinuria was >8 g/24 h. This policy would allow better identification of patients who develop SR and avoid the use of potentially dangerous immunosuppressive agents in an important number of patients.
Concise Methods
The study was an initiative of the Scientific Committee of the Spanish Group for the Study of Glomerular Diseases. All participating centers followed an initially conservative therapeutic approach for patients with IMN, defined by avoidance of corticosteroids or any other immunosuppressive agent as initial treatment for all patients with IMN, supportive treatment of nephrotic syndrome, and regular follow-up after diagnosis. Immunosuppressive treatment was started during the follow-up at the treating physician's discretion because of complications or an unsatisfactory evolution, and the reasons to take this decision were registered. Fourteen centers in Spain that followed this conservative therapeutic approach agreed to participate in the study.
Patients
Participating centers were required to include all patients with nephrotic syndrome and a biopsy-proven diagnosis of membranous nephropathy. Exclusion criteria included the diagnosis of diabetes mellitus, systemic lupus erythematosus, malignancy or any other systemic disease known to be associated with secondary membranous nephropathy, and immunosuppressive therapy before hospital admission. Three hundred and twenty-eight patients meeting these criteria and admitted to the participating centers during the period 1975 to 2007 were included in the study. For each patient, the date of renal biopsy was established as the baseline point. All patients were followed at regular intervals. Thirty-eight patients (11.5%) were lost to follow-up and censored at last visit.
Data Collection
Baseline data at the time of renal biopsy were compiled from medical records at each participating center using a uniform protocol. The average of the first three 24-hour urinary protein excretion measurements after the performance of renal biopsy was used as baseline proteinuria. eGFR was calculated by the Modifications in Diet and Renal Disease four-variable equation.
Events occurring during follow-up that were recorded and analyzed included the appearance of SR (partial or complete), relapse of nephrotic syndrome after SR, death, onset of chronic dialysis, transplantation, onset of ACEI/ARB treatment, and timing and type of immunosuppressive therapy. Reasons for immunosuppressive therapy were grouped in three different categories: deterioration of renal function, persistent edema or complications of the nephrotic syndrome, and protracted duration of nephrotic syndrome at treating physician's discretion. Patients were censored at the start of renal replacement therapy, inmunosupression, or lost to follow-up.
Outcome
The primary outcome was the appearance of SR, partial or complete. Secondary outcomes included appearance of relapses after a SR, end-stage renal failure, and all-cause mortality.
End-Point Definitions
Nephrotic syndrome was defined by a proteinuria value >3.5 g/d along with hypoalbuminemia (serum albumin <3 g/dl). PR was defined by a proteinuria value <3.5 g/24 h along with normal serum albumin in the absence of immunosuppressive therapy or concomitant renal function worsening. CR was defined by a proteinuria value <0.3 g/24 h in at least three consecutive visits and in the absence of immunosuppressive therapy or renal function worsening.
A relapse was defined by the reappearance of proteinuria >3.5 g/24 h in at least three consecutive visits in those who previously presented a partial or complete SR.
Renal function was evaluated by means of serum creatinine values and eGFR, calculated by Modifications in Diet and Renal Disease four-variable equation. Renal survival was defined by the absence of chronic dialysis or renal transplantation. The interval between baseline (renal biopsy performance) and immunosuppressive treatment was registered. ACEI/ARB treatment was defined by the treatment with any agent belonging to these drug classes that was started after the onset of the disease and before the appearance of SR or before the onset of immunosuppressive therapy. Interval between baseline and the onset of ACEI/ARB treatment was registered in every case.
Statistical Analysis
Normally distributed variables are displayed as mean ± SD or 95% confidence interval and compared using t test, one-way ANOVA or Pearson correlation coefficients. Proteinuria is expressed as median (range). Categorical variables are expressed as percentage and compared with χ2 test. The cumulative probability of SR was calculated by means of Kaplan–Meier analysis and curves were compared with use of the log-rank test. Influence of ACEI/ARB treatment on the probability of SR appearance and comparisons of renal survival and death between patients with or without SR were calculated by Kaplan–Meier analysis. Survival time for each patient was computed from baseline evaluation to last follow-up. The Cox proportional hazard model was performed to explore the influence of several variables on the occurrence of SR. Only variables significant in univariate analysis were included by forward stepwise entry into the multivariate model. Loss of renal function (eGFR), expressed in ml/min/yr, was calculated in every patient.
Changes in proteinuria, serum creatinine, and renal function (measured by eGFR) between baseline and last follow-up were compared in patients with and without SR and in patients with partial or complete SR by means of t test (within-group analysis) or Kruskal–Wallis and Mann–Whitney tests for between-group comparisons.
Comparisons were made between mean renal function loss for patients with or without SR and complete or partial SR. To evaluate renal function changes in patients with SR more subtle than advanced renal failure, the number of patients with a >50% increase in baseline serum creatinine was calculated and comparisons were made between patients with a complete or partial SR.
A P value <0.05 was considered significant. Statistical analysis was performed with SPSS software (version 15.0 for Windows).
Disclosures
None.
Acknowledgments
This study was partially supported by the Asociación para el Estudio y Tratamiento de las Enfermedades Renales.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Idiopathic Membranous Nephropathy: Getting Better by Itself,” on pages 551–552.
References
- 1. Fervenza FC, Sethi S, Specks U: Idiopathic membranous nephropathy: Diagnosis and treatment. Clin J Am Soc Nephrol 3: 905– 919, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Cattran DC: Idiopathic membranous nephropathy. Kidney Int 59: 1983– 1994, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Rivera F, López-Gómez JM, Pérez García R: Clinicopathological correlations of renal pathology in Spain. Kidney Int 66: 898– 904, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Coggins CH. for the Collaborative Study of the Adult Idiopathic Nephrotic Syndrome A controlled study of short-term prednisone treatment in adults with membranous nephropathy. N Engl J Med 301: 1301– 1306, 1979 [DOI] [PubMed] [Google Scholar]
- 5. Ponticelli C, Zucchelli P, Passerini P, Cagnoli L, Cesana B, Pozzi C, Pasquali S, Imbasciati E, Grassi C, Redaelli B, Sasdelli M, Locatelli F: A randomized trial of methylprednisone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med 320: 8– 13, 1989 [DOI] [PubMed] [Google Scholar]
- 6. Jha V, Ganquli A, Saha TK, Kohli HS, Sud K, Gupta KL, Joshi K, Sakhuja V: A randomized controlled trial of steroids and cyclophosphamide in adults with idiopathic membranous nephropathy. J Am Soc Nephrol 18: 1899– 1904, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Brown JH, Alistair FD, Murphy BG, Hill CM, McNamee PT, Nelson WE, Doherty CC: Treatment of renal failure in idiopathic membranous nephropathy with azathioprine and prednisolone. Nephrol Dial Transplant 13: 443– 448, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL. for the North American Nephrotic Syndrome Study Group Cyclosporine in patients with steroid-resistant membranous nephropathy: A randomized trial. Kidney Int 59: 1484– 1490, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Praga M, Barrio V, Fernández-Juárez G, Luño J. for the Grupo Español de Estudio de la Nefropatía Membranosa Tacrolimus monotherapy in membranous nephropathy: A randomized controlled trial. Kidney Int 71: 924– 930, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Segarra A, Amoedo ML, Martínez García JM, Pons S, Praga M, Garcia EI, Alonso JC, Gascó JM, Pou L, Piera L: Efficacy and safety of “rescue teraphy” with mycophenolate mofetil in resistant primary glomerulonephritis-A multicenter study. Nephrol Dial Transplant 22: 1351– 1360, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Ponticelli C, Passerini P, Salvadori M, Manno C, Viola BF, Pasquali S, Mandolfo S, Messa P: A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropin hormone in idiopathic membranous nephropathy. Am J Kidney Dis 47: 233– 240, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Remuzzi G, Chiurchiu C, Abbate M, Brusegan V, Bontempelli M, Ruggenenti P: Rituximab for idiopathic membranous nephropathy. Lancet 360: 923– 924, 2002 [DOI] [PubMed] [Google Scholar]
- 13. du Buf-Vereijken PWG, Branten AJW, Wetzels JFM: Idiopathic membranous nephropathy: Outline and rationale of a treatment strategy. Am J Kidney Dis 46: 1012– 1029, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Perna A, Schieppati A, Zamora J, Giuliano GA, Braun N, Remuzzi G: Immunosuppressive treatment for idiopathic membranous nephropathy: A systematic review. Am J Kidney Dis 44: 385– 401, 2004 [PubMed] [Google Scholar]
- 15. Glassock RJ: The treatment of idiopathic membranous nephropathy: A dilemma or a conundrum? Am J Kidney Dis 44: 562– 566, 2004 [PubMed] [Google Scholar]
- 16. Cattran DC: Management of membranous nephropathy: When and what for treatment. J Am Soc Nephrol 16: 1188– 1194, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Noel LH, Zanetti M, Droz D, Barbanel C: Long-term prognosis of idiopathic membranous glomerulonephritis. Am J Med 66: 82– 90, 1979 [DOI] [PubMed] [Google Scholar]
- 18. Davison AM, Cameron JS, Kerr DN, Ogg CS, Wilkinson RW: The natural history of renal function in untreated idiopathic membranous glomerulonephritis in adults. Clin Nephrol 22: 61– 67, 1984 [PubMed] [Google Scholar]
- 19. MacTier R, Boulton-Jones JM, Payton CD, McLay A: The natural history of membranous nephropathy in the west of Scotland. Q J Med 60: 793– 802, 1986 [PubMed] [Google Scholar]
- 20. Donadio JJV, Torres VE, Velosa JA, Wagoner RD, Holley KE, Okamura M, Ilstrup DM, Chu CP: Idiopathic membranous nephropathy: The natural history of untreated patients. Kidney Int 33: 708– 715, 1988 [DOI] [PubMed] [Google Scholar]
- 21. Schieppati A, Mosconi L, Perna A, Mecca G, Bertani T, Garattini S, Remuzzi G: Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med 329: 85– 89, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Hladunewich MA, Troyanov S, Calafati J, for the Metropolitan Toronto Glomerulonephritis Registry. Cattran DC: The natural history of the non-nephrotic membranous nephropathy patient. Clin J Am Soc Nephrol 4: 1417– 1422, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cattran DC, Pei Y, Greenwood CMT, Ponticelli C, Passerini P, Honkanen E: Validation of predictive model of idiopathic membranous nephropathy: Its clinical and research implications. Kidney Int 51: 901– 907, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, Maschio G, Brenner BM, Kamper A, Zucchelli P, Becker G, Himmelmann A, Bannister K, Landais P, Shahinfar S, de Jong PE, de Zeeuw D, Lau J, Levey AS. and the ACE Inhibition in Progressive Renal Disease Study Group Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 135: 73– 87, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Praga M, Hernández E, Montoyo C, Andres A, Ruilope LM, Rodicio JL: Long-term beneficial effects of ACE inhibition in patients with nephrotic proteinuria. Am J Kidney Dis 20: 240– 248, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Rostoker G, Maadi AB, Remy P, Lang P, Weil B: Low-dose angiotensin-converting enzyme inhibitor captopril to reduce proteinuria in adult idiopathic membranous nephropathy: A prospective study of long-term treatment. Nephrol Dial Transplant 10: 25– 29, 1995 [PubMed] [Google Scholar]
- 27. Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC. for the Toronto Glomerulonephritis Registry Group Idiopathic membranous nephropathy: Definition and relevance of a partial remission. Kidney Int 66: 1199– 1205, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Ponticelli C, Passerini P, Altieri P, Locatelli F, Pappalettera M. Remissions and relapses in idiopathic membranous nephropathy. Nephrol Dial Transplant 7[ Suppl 1]: 85– 90, 1992 [PubMed] [Google Scholar]
- 29. Torres A, Domínguez-Gil B, Carreño A, Hernández E, Morales E, Segura J, González E, Praga M: Conservative versus inmunosuppresive treatment of patients with idiopathic membranous nephropathy and deteriorating renal function. Kidney Int 61: 219– 227, 2002 [DOI] [PubMed] [Google Scholar]
- 30. de Buf-Vereijken PWG, Branten AJW, Wetzels JFM. for the Membranous Nephropathy Study Group Cytotoxic therapy for membranous nephropathy and renal insufficiency: Improved renal survival but high relapse rate. Nephrol Dial Transplant 19: 1142– 1148, 2004 [DOI] [PubMed] [Google Scholar]