Abstract
There is no established modality to repair kidney damage resulting from ischemia-reperfusion injury (IRI). Early responses to IRI involve lymphocytes, but the role of B cells in tissue repair after IRI is unknown. Here, we examined B cell trafficking into postischemic mouse kidneys and compared the repair response between control (wild-type) and μMT (B cell-deficient) mice with and without adoptive transfer of B cells. B cells infiltrated postischemic kidneys and subsequently activated and differentiated to plasma cells during the repair phase. Plasma cells expressing CD126 increased and B-1 B cells trafficked into postischemic kidneys with distinct kinetics. An increase in B lymphocyte chemoattractant in the kidney preceded B cell trafficking. Postischemic kidneys of μMT mice expressed higher IL-10 and vascular endothelial growth factor and exhibited more tubular proliferation and less tubular atrophy. Adoptive transfer of B cells into μMT mice reduced tubular proliferation and increased tubular atrophy. Treatment with anti-CD126 antibody increased tubular proliferation and reduced tubular atrophy in the late repair phase. These results demonstrate that B cells may limit the repair process after kidney IRI. Targeting B cells could have therapeutic potential to improve repair after IRI.
Ischemia is a leading cause of acute kidney injury (AKI) in both native kidneys and allografts. In allografts, ischemic AKI frequently results in delayed graft function.1 Many studies have demonstrated that both innate and adaptive immune responses are involved in the pathogenesis of renal injury after renal ischemia-reperfusion injury (IRI).2,3 On the basis of traditional concepts of adaptive immunity, lymphocytes were not expected to play an important role in the early renal injury after IRI; however, T cells were found to mediate the early phase of IRI in kidney and in other organs, both directly and indirectly.4–6 B cells also seem to participate in the early injury response of renal IRI,7 and B cell products are also important in early IRI response in skeletal muscle.8
B cells have been identified as important mediators of various autoimmune diseases, such as experimental allergic encephalomyelitis (EAE), collagen-induced arthritis, and inflammatory bowel disease.9–11 In EAE, B cells seem to function as antigen-presenting cells during the initiation phase.12,13 In a recent report, B cells were involved in both initiation and progression of EAE.14 Clinical trials using mAb to CD20 expressed on B cells have suggested beneficial effects in autoimmune diseases such as rheumatoid arthritis, lupus erythematosus, and multiple sclerosis.15–18 Although ischemic AKI and autoimmune disease are traditionally viewed as different disease categories, they share a crucial feature: A prominent immune/inflammatory response.
It was previously shown that B cells traffic into chronically inflamed organs, activate and form ectopic germinal centers, and locally differentiate to plasma cells.19,20 A number of studies have demonstrated that B cells infiltrate into renal allografts and contribute to rejection21,22; however, the exact role of B cells that have infiltrated into renal allografts is still unclear. Some studies reported that B cells could cause transplant acute cellular rejection as well as humoral rejection and increase the risk for graft failure independent of C4d peritubular deposition,23,24 whereas other studies have not shown this clinical correlation.25,26 One recent article characterized intragraft B cells during renal allograft rejection: Both mature B cells and interstitial plasmablasts correlated with circulating donor-specific antibody concentration and poor response to steroid therapy during rejection.27 The presence of mature B cells was associated with reduced graft survival.
On the basis of recent advances in studies of B cells in auto- and alloimmune diseases, the increasingly recognized pathogenic role for lymphocytes in IRI, and lack of treatment to augment repair, we tested the hypothesis that B cells modulate the repair process after kidney IRI. We analyzed the numbers and phenotypes of kidney-infiltrating B cells and the expression of B lymphocyte chemoattractant (BLC) during the repair phase. We found marked trafficking of B cells into the postischemic kidney during repair, with a distinct phenotype at different time points, along with increased BLC expression. We then evaluated the renal repair status of control (wild-type) mice, mature B cell–deficient (μMT) mice, μMT mice with adoptive B cell transfer, and μMT mice with serum transfer. We found that B cells modify tubular repair and proliferation. Finally, we targeted CD126-expressing plasma cells with an anti-CD126 antibody and found a significant improvement in tissue repair after IRI.
Results
B Cells Trafficked into the Postischemic Kidneys and Differentiated into Plasma Cells
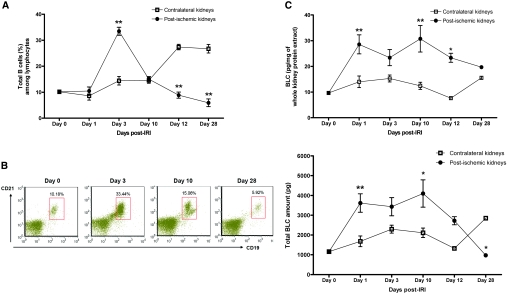
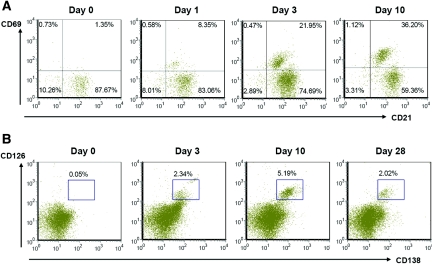
The number of total kidney mononuclear cells (KMNCs) was highest in the postischemic kidneys on day 3 after IRI (contralateral versus postischemic kidneys, 0.59 ± 0.04 versus 1.01 ± 0.05 × 106 per pair of kidneys; P < 0.05). The number of total KMNCs did not differ between contralateral and postischemic kidneys on days 1, 10, 12, and 28 after IRI (data not shown); however, an increased number of mature B cells (expressing both CD19 and CD21) trafficked into the postischemic kidneys during the repair phase (Table 1). The percentage of B cells in the postischemic kidneys was the highest on day 3 after IRI and decreased over time (Figure 1). BLC, measured in kidney protein extract, was increased on day 1 after IRI. Postischemic kidneys expressed higher BLC protein than contralateral kidneys on days 1 and 10 after IRI (Figure 1). Both maturation and activation status of infiltrating B cells were evaluated with several surface markers including IgM, MHC class II, CD21, CD38, CD40, CD69, CD126, and CD138. MHC class II expression on B cells trafficked into the postischemic kidneys was highest on day 3 after IRI, whereas CD69 (activation marker) expression on B cells remained elevated throughout the repair phase (Figure 2, Table 1). We used anti-CD138 antibody and anti-CD126 antibody to identify plasma cells. Plasma cells appeared on day 3 and reached peak numbers on day 10 after IRI, then decreased over time (Figure 2). There was no difference in the expression of CD38 (surface marker for plasmablasts) and CD40 (surface marker reacting with CD40L on T cells) on B cells trafficking into postischemic kidneys at various time points of the repair phase (data not shown). The percentage of total T cells among KMNCs markedly increased over time in the postischemic kidneys (Table 1).
Table 1.
Postischemic kidney B cell and T cell populations in the repair phase of renal IRI in wild-type control mice
| % of Cells | Day 3 after IRI |
Day 10 after IRI |
Day 28 after IRI |
|||
|---|---|---|---|---|---|---|
| CT | IRI | CT | IRI | CT | IRI | |
| Total B cells | 14.41 ± 1.63 | 33.44 ± 1.54a | 14.57 ± 1.12 | 15.06 ± 0.98b | 26.71 ± 1.64 | 5.92 ± 1.49a,c |
| MHCII+ B cells | 9.42 ± 1.31 | 23.60 ± 1.76d | 10.39 ± 0.64 | 14.71 ± 0.76b,d | 13.58 ± 2.65 | 4.65 ± 0.42c,d |
| CD69+ B cells | 4.68 ± 0.54 | 8.67 ± 1.25d | 4.46 ± 0.53 | 17.32 ± 3.03b,d | 4.05 ± 0.21 | 9.92 ± 1.15d,e |
| B-1 B cells | 0.54 ± 0.11 | 0.58 ± 0.11 | 0.65 ± 0.14 | 5.62 ± 1.16a,b | 0.11 ± 0.02 | 2.69 ± 0.36a,c |
| CD126+ plasma cells | 0.07 ± 0.02 | 2.34 ± 0.33a | 0.04 ± 0.01 | 5.19 ± 0.69a,b | 0.07 ± 0.01 | 2.02 ± 0.19a,e |
| Total T cells | 25.80 ± 0.14 | 29.38 ± 0.69 | 22.78 ± 1.01 | 48.60 ± 5.27a,b | 27.35 ± 0.74 | 66.61 ± 2.65a,c |
Data are means ± SEM (n = 12 per group). CT, contralateral kidneys; IRI, postischemic kidneys; total B cells, mature B cells expressing CD19 and CD21 among total KMNCs; MHCII+ B cells, MHC class II expressing B cells among total KMNCs; CD69+ B cells, CD69 (early activation marker) expressing B cells among total KMNCs; B-1 B cells, B-1 B cell subset expressing CD5 and IgM among total B cells; plasma cells, plasma cells expressing CD138 and CD126 among total KMNCs; total T cells, T cells expressing T cell receptor β.
aP < 0.001 versus contralateral kidneys at the same time point.
bP < 0.05 versus postischemic kidneys on day 3 after IRI.
cP < 0.05 versus postischemic kidneys on day 3 and day 10 after IRI.
dP < 0.05 versus contralateral kidneys at the same time point.
eP < 0.05 versus postischemic kidneys on day 10 after IRI.
Figure 1.
Trafficking mature B cells into the postischemic kidneys in response to BLC (CXCL13). (A) Trafficking of mature B cells into the postischemic kidneys. Mature B cell trafficking into the postischemic kidneys peaked on day 3 after IRI and decreased over time (n = 12 per group). (B) B cell trafficking into the postischemic kidneys was maximum on day 3 after IRI. Gated cells indicate CD19 and CD21 double-positive B cells among total kidney mononuclear cells (n = 12 per group). (C) Changes in BLC expression in postischemic kidneys. Total BLC amount was obtained by multiplying BLC concentration with kidney weight. Postischemic kidneys expressed higher BLC than contralateral control kidneys on days 1 and 10 after IRI (n = 6 to 12 per group). *P < 0.05 versus contralateral kidneys; **P < 0.001 versus contralateral kidneys.
Figure 2.
B cells activate and differentiate to plasma cells with time in the postischemic kidneys. (A) Activated mature B cells increased over time in the postischemic kidneys during the repair phase. Upper right quadrants of dot plots indicate CD21 and CD69 double-positive B cells among total B cells expressing CD19 (n = 12 per group). (B) Plasma cells appeared at the early repair phase and increased with time, then decreased at day 28 after IRI. Gated cells indicate CD138 and CD126 double-positive plasma cells among total KMNCs (n = 12 per group).
B-1 B Cells Trafficked into the Postischemic Kidneys
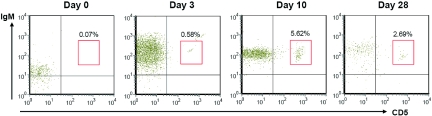
B-1 B cells, a minor subset of B cells abundant in the peritoneal cavity, were examined with anti-CD5 antibody and anti-IgM antibody. The percentage of B-1 B cells among total B cells in the kidneys was very low before IRI and remained low until day 3 after IRI; however, the percentage of B-1 B cells markedly increased in the postischemic kidneys on day 10, then decreased over time (Figure 3, Table 1).
Figure 3.
B-1 B cells infiltrate into the postischemic kidneys during the repair phase. B-1 B cells expressing IgM and CD5 started trafficking at day 3 with maximal infiltration at day 10 (n = 12 per group).
B Cells Transferred into the μMT Mice Trafficked into the Postischemic Kidneys
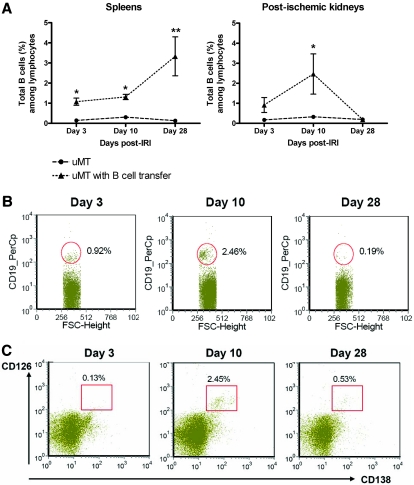
B cells isolated from spleens of control mice were transferred to the μMT mice on day 1 after renal IRI to evaluate the effect of B cells on repair and avoid affecting the early 24-hour injury phase. Transferred B cells trafficked into both postischemic kidneys and spleens of μMT mice. The number of B cells trafficked into the postischemic kidneys in B cell–transferred μMT mice was highest on day 10 and barely detectable on day 28 after IRI (Figure 4). This pattern was similar to B cell trafficking into the postischemic kidneys of control mice, whereas trafficking of B cells into spleens increased over time.
Figure 4.
B cells adoptively transfer into μMT mice. B cells were isolated from spleens of control mice and transferred into μMT mice on day 1 after IRI. (A) B cells transferred into μMT mice trafficked into both spleens and postischemic kidneys (n = 5 to 6 per group). (B) Postischemic kidneys of B cell–transferred μMT mice showed the highest number of B cells on day 10 after IRI. Gated cells indicate CD19+ B cells (n = 5 to 6 per group). (C) B cells trafficked into the postischemic kidneys of B cell–transferred μMT mice differentiated into plasma cells. Gated cells indicate plasma cells expressing CD138 and CD126 (n = 5 to 6 per group). *P < 0.05 versus μMT mice; **P < 0.001 versus μMT mice.
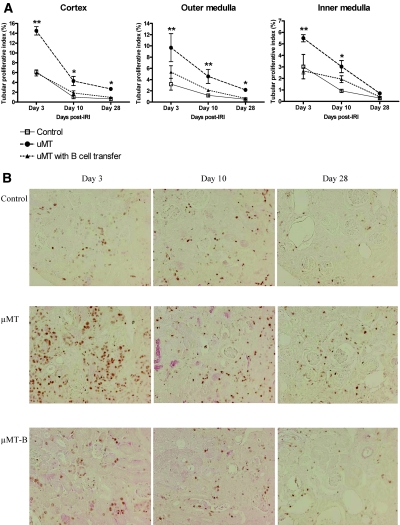
B Cell Deficiency Resulted in Increased Epithelial Cell Proliferation and Reduced Tubular Atrophy in Postischemic Kidneys during the Repair Phase of IRI
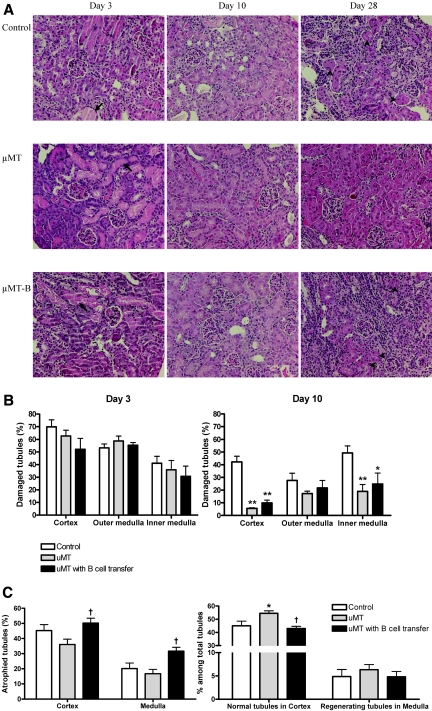
The tubular proliferative index was highest in the wild-type postischemic kidneys on day 3 after IRI, which decreased with time afterward. The tubular proliferative index was significantly higher in the postischemic kidneys of μMT mice compared with both control and B cell–transferred μMT mice throughout the repair phase, and the difference of the tubular proliferative index was most prominent in the cortex on day 3 after IRI (Figure 5). Adoptive transfer of B cells into μMT mice led to reduced tubular proliferation compared with μMT mice. Tubular damage evaluated with hematoxylin and eosin (H&E) staining was similar between groups on day 3; however, on day 10, postischemic kidneys of μMT mice had significantly less tubular damage than control mice (Figure 6). On day 28, tubular atrophy and regeneration were evaluated on the postischemic kidneys with three different stains: H&E, Masson's trichrome, and periodic acid-Schiff. Postischemic kidneys of μMT mice had reduced tubular atrophy and a higher number of normal tubules compared with control mice. B cell transfer led to more tubular atrophy and a reduced number of normal tubules in μMT mice (Figure 6).
Figure 5.
B cell deficiency increases tubular proliferation in postischemic kidneys during the repair phase. (A) Tubular proliferative index in postischemic kidneys. The tubular proliferative index of postischemic kidneys was highest on day 3 after IRI in all groups and decreased over time (n = 5 to 8 per group). (B) Ki-67 immunohistochemistry of renal cortex. The tubular proliferative index of μMT mice was higher than both control and B cell–transferred μMT mice in the cortex throughout the repair phase and also in the medulla on days 3 and 10 after IRI. The cells stained reddish-brown represent Ki-67–positive cells, which reflect the proliferation process. μMT-B mice were μMT mice that underwent adoptive B cell transfer on day 1 after renal IRI. *P < 0.05 versus control mice; **P < 0.001 versus control mice. Magnification, ×200.
Figure 6.
B cell deficiency decreases tubular damage and atrophy in postischemic kidneys during the repair phase. (A) H&E staining of renal cortex. Arrows indicate dilated tubules with casts, and arrowheads indicate atrophied tubules. (B) Extent of tubular damage on days 3 and 10 after renal IRI. On day 3 after IRI, there was no difference in tubular damage among the three groups. On day 10 after IRI, postischemic kidneys of μMT mice showed significantly less tubular damage than control mice (n = 7 to 9 per group). (C) Tubular atrophy on day 28 after renal IRI. On day 28, μMT mice showed less tubular atrophy and more normal tubules, whereas B cell–transferred μMT mice exhibited more tubular atrophy and fewer normal tubules compared with μMT mice (n = 5 to 10 per group). μMT-B mice were μMT mice that underwent adoptive B cell transfer on day 1 after renal IRI. *P < 0.01 versus control mice; **P < 0.001 versus control mice; †P < 0.05 versus μMT mice. Magnification, ×200.
B Cell Transfer into μMT Mice Changed Renal IL-10 Expression but not T Cell Trafficking in the Postischemic Kidneys of μMT Mice
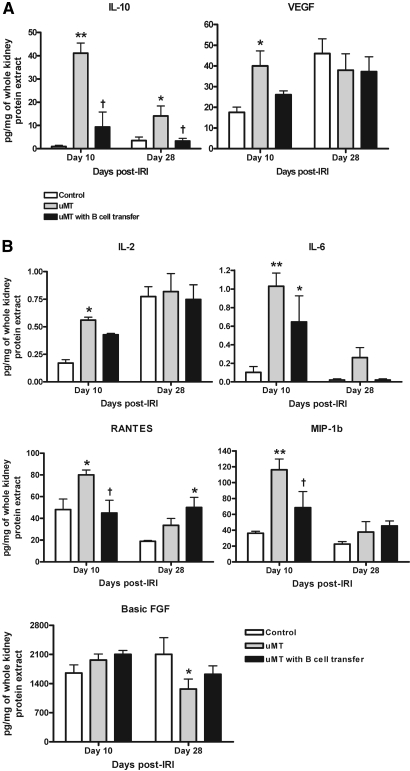
Postischemic kidneys of μMT mice expressed significantly higher IL-10 and vascular endothelial growth factor (VEGF) compared with wild-type control mice on day 10 after IRI (Figure 7). On day 28, IL-10 expression was still higher in the postischemic kidneys of μMT mice. The expression of IL-2, IL-6, RANTES (CCL5), and macrophage inflammatory protein 1b (MIP-1b) was higher on day 10, whereas the expression of basic fibroblast growth factor was lower on day 28 in the postischemic kidneys of μMT mice compared with control mice. Adoptive transfer of B cells decreased the expression of IL-10, RANTES, and MIP-1b in the postischemic kidneys of μMT mice (Figure 7).
Figure 7.
Cytokines are expressed in postischemic kidneys. (A) Postischemic kidneys of μMT mice expressed higher IL-10 and VEGF on day 10 after IRI than wild-type controls. B cell transfer reduced the expression of IL-10 and VEGF in the postischemic kidneys of μMT mice (n = 5 per group). (B) Postischemic kidneys of μMT mice expressed higher IL-2, IL-6, RANTES, and MIP-1b on day 10 but lower basic fibroblast growth factor (FGF) on day 28 compared with control mice. B cell transfer reduced the expression of RANTES and MIP-1b in the postischemic kidneys of μMT mice (n = 5 per group). *P < 0.05 versus control mice; **P < 0.001 versus control mice; †P < 0.05 versus μMT mice.
The total number of KMNCs was not different between μMT and B cell–transferred μMT mice (data not shown). The percentages of total T cells, CD4 T cells, and CD8 T cells were similar between μMT and B cell–transferred μMT mice (Table 2).
Table 2.
Postischemic kidney T cell populations of μMT and B cell–transferred μMT mice
| % of Cells | Day 3 after IRI |
Day 10 after IRI |
Day 28 after IRI |
||||||
|---|---|---|---|---|---|---|---|---|---|
| CT of μMT | IRI of μMT | IRI of μMT-B | CT of μMT | IRI of μMT | IRI of μMT-B | CT of μMT | IRI of μMT | IRI of μMT-B | |
| Total T cells | 50.95 ± 5.26 | 53.47 ± 3.70 | 53.86 ± 2.29 | 49.30 ± 2.65 | 61.80 ± 0.27a,b | 61.93 ± 1.67a,b | 54.66 ± 3.10 | 70.98 ± 2.17a,c | 76.99 ± 1.03a,c |
| CD4 T cells | 54.18 ± 4.61 | 55.36 ± 3.23 | 49.35 ± 3.64 | 57.83 ± 0.78 | 58.93 ± 1.37 | 56.57 ± 2.87 | 57.25 ± 0.53 | 57.87 ± 1.92 | 57.37 ± 3.87 |
| Activated CD4 T cells | 15.06 ± 1.19 | 13.85 ± 2.75 | 9.26 ± 1.10 | 20.30 ± 2.38 | 29.57 ± 1.44b | 26.90 ± 2.86b | 25.22 ± 1.83 | 36.41 ± 1.75a,c | 28.18 ± 2.55b |
| CD8 T cells | 28.64 ± 2.45 | 26.78 ± 2.87 | 29.15 ± 3.55 | 32.64 ± 0.54 | 35.61 ± 1.30b | 36.99 ± 2.42 | 34.39 ± 2.19 | 35.90 ± 1.98b | 39.58 ± 3.68 |
| Activated CD8 T cells | 7.53 ± 0.26 | 4.96 ± 0.12 | 4.34 ± 0.75 | 10.77 ± 0.85 | 17.02 ± 0.77a,b | 19.61 ± 0.57a,b | 11.84 ± 0.11 | 28.49 ± 1.37a,c | 29.41 ± 2.92a,c |
Data are means ± SEM (n = 5 to 6 per group). T cell population and proportion of contralateral kidneys of B cell-transferred μMT mice were not different from μMT mice (data not shown). CT, contralateral kidneys; IRI, postischemic kidneys; μMT-B, μMT mice that underwent adoptive B cell transfer on day 1 after renal IRI; total T cells, T cells expressing T cell receptor β among total KMNCs; CD4 T cells, CD4 expressing T cells among total T cells; activated CD4 T cells, CD69 (early activation marker) expressing CD4 T cells among total T cells; CD8 T cells, CD8 expressing T cells among total T cells; activated CD8 T cells, CD69 (early activation marker) expressing CD8 T cells among total T cells.
aP < 0.05 versus contralateral kidneys of μMT mice at the same time point.
bP < 0.05 versus postischemic kidneys of the same group on day 3 after IRI.
cP < 0.05 versus postischemic kidneys of the same group on days 3 and 10 after IRI.
Anti-mouse CD126 Antibody–Treated Mice Had Less Tubular Atrophy on Day 28 after Renal IRI
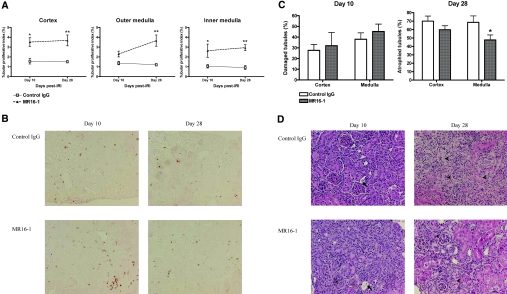
Given that CD126-expressing plasma cells were infiltrating into postischemic kidney during repair, we hypothesized that targeting plasma cells could alter the repair process. On day 10 after IRI, there was no difference in tubular damage between anti-mouse CD126 antibody (MR16-1) and control Ig (rat IgG)-treated mice; however, postischemic kidneys of MR16-1–treated mice had a higher rate of tubular proliferation on day 10 and the tubular proliferative index of MR16-1–treated mice remained higher than control IgG treated mice on day 28 after IRI. Importantly, postischemic kidneys of MR16-1–treated mice exhibited reduced tubular atrophy compared with control IgG-treated mice on day 28 after IRI (Figure 8).
Figure 8.
MR16-1 induced greater tubular proliferation and resulted in reduced tubular atrophy on the postischemic kidneys. MR16-1 and control IgG treatments were initiated on day 1 after renal IRI and continued until day 28. (A) Tubular proliferative index in anti-CD126 antibody–treated mice. Postischemic kidneys of MR16-1–treated mice showed more tubular proliferation than control IgG-treated mice on days 10 and 28 after IRI (n = 6 to 8 per group). (B) Ki-67 immunohistochemistry of renal cortex. The cells stained reddish-brown represent Ki-67–positive cells. (C) Tubular damage and atrophy after anti-CD126 antibody treatment. Postischemic kidneys of MR16-1–treated mice showed less tubular atrophy on day 28 after IRI (n = 6 to 8 per group). (D) H&E staining of renal cortex. Arrows indicate dilated tubules with casts, and arrowheads indicate atrophied tubules. *P < 0.05 versus control IgG-treated mice; **P < 0.001 versus control IgG-treated mice. Magnification, ×200.
Adoptive B Cell Transfer and MR16-1 Treatment Influenced Renal Function on Day 28 after Renal IRI
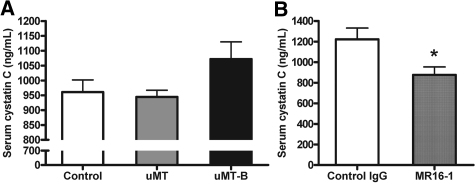
We measured serum cystatin C level to assess renal functional impairment on day 28 after left renal IRI. Mouse serum cystatin C has been reported as a reliable marker for renal function in mouse AKI models that could be better than serum creatinine levels.28,29 B cell–transferred μMT mice showed a tendency toward increased serum cystatin C level compared with μMT mice (control versus μMT mice versus B cell–transferred μMT mice, 961.1 ± 40.6 versus 944.3 ± 22.6 versus 1072.0 ± 57.9 ng/ml; P = 0.083 between μMT mice and B cell–transferred μMT mice). MR16-1–treated mice had lower serum cystatin C level than control IgG-treated mice (control IgG versus MR16-1–treated mice, 1222.4 ± 109.4 versus 876.2 ± 77.8 ng/ml; P < 0.05; Figure 9).
Figure 9.
Serum cystatin C levels varied on day 28 after IRI. (A) Serum cystatin C level tended to be higher in B cell–transferred μMT mice compared with μMT mice on day 28 after left renal IRI (n = 4 per group). (B) MR16-1–treated mice revealed lower serum cystatin C than control IgG-treated mice on day 28 after left renal IRI (n = 6 per group). μMT-B mice were μMT mice that underwent adoptive B cell transfer on day 1 after renal IRI. *P < 0.05 versus control IgG-treated mice.
Serum IgM Level Increased with Time after Renal IRI
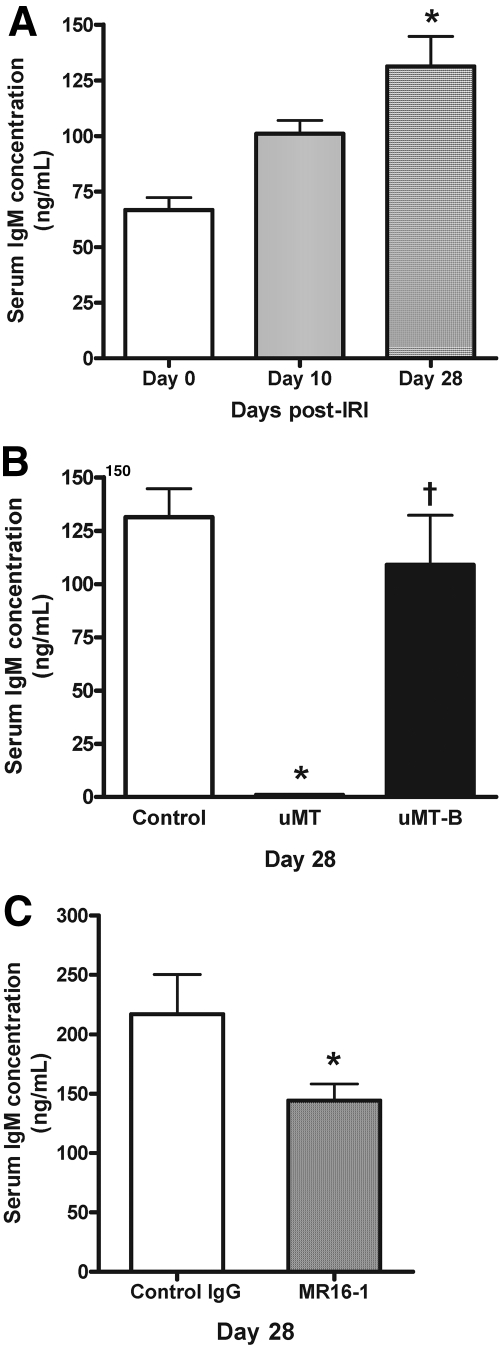
Serum IgM levels were significantly higher on day 28 after left renal IRI. In μMT mice, serum IgM was undetectable on day 28 after IRI, and adoptive B cell transfer restored serum IgM (Figure 10). MR16-1 treatment significantly reduced serum IgM production after IRI.
Figure 10.
Serum IgM levels varied after IRI. (A) In the control (wild-type) mice that underwent renal IRI, serum samples obtained on day 28 after renal IRI contained significantly higher IgM compared with serum samples obtained on days 0 and 10 (n = 5 to 12 per group). *P < 0.05 versus both days 0 and 10. (B) On day 28 after renal IRI, serum IgM was not detected in μMT mice. B cell–transferred μMT mice had significantly higher IgM in their serum than μMT mice but a comparable level of IgM to control mice (n = 7 to 12 per group). *P < 0.001 versus control; †P < 0.001 versus μMT. (C) On day 28 after renal IRI, MR16-1–treated mice revealed a significantly reduced level of serum IgM compared with control IgG-treated mice (n = 6 per group). *P < 0.01 versus control IgG.
Heat-inactivated serum extracted from mice that underwent sham operation (sham serum) and from mice that underwent renal IRI (IRI serum) was transferred into μMT mice on day 1 after IRI. Both μMT mice with sham serum and μMT mice with IRI serum showed increased tubular atrophy in medulla of postischemic kidneys on day 28 compared with μMT mice without serum transfer (atrophied tubules; μMT versus μMT with sham serum versus μMT with IRI serum, 26.2 ± 7.2 versus 47.1 ± 9.5 versus 44.7 ± 6.8% in cortex [P > 0.05 between groups]; 28.8 ± 10.5 versus 74.0 ± 5.6 versus 67.0 ± 5.8% in medulla; P < 0.001 between μMT mice and μMT mice with sham serum, P < 0.01 between μMT mice and μMT mice with IRI serum).
Discussion
Our study demonstrates that B cells modulate kidney repair after IRI. B cells migrate into the postischemic kidney during the repair phase, with specific changes in populations and activation status with time. B cell deficiency reduces renal tubular atrophy by enhancing tubular proliferation. Adoptive transfer of B cells into μMT mice increases renal tubular atrophy and reduces tubular proliferation in μMT mice, demonstrating that the altered tubular atrophy and proliferation in μMT mice were indeed due to B cell deficiency. Targeting infiltrating CD126-expressing plasma cells using anti-CD126 antibody led to reduced tubular atrophy with enhanced tubular proliferation and reduced functional impairment. These data are the first demonstration of a role for B cells in repair from kidney warm IRI and reveal the promise of targeting B cells to improve clinical outcomes in this common condition.
We initially studied B cell infiltration into the postischemic kidneys to look for evidence supporting a role for B cells in the repair process. The maximum increase in mature infiltrating B cells occurred at day 3 and returned to control levels by day 10. T cell trafficking into the postischemic kidneys was markedly increased at day 10 and remained increased during the late repair phase up to day 28. That B cell trafficking preceded T cell trafficking during repair suggests that B cells could be involved in regulating T cell infiltration and expansion in postischemic kidney. An important role of B cells as antigen-presenting or co-stimulatory cells was also reported in animal models of autoimmune disease30; however, adoptive B cell transfer into μMT mice did not affect T cell trafficking into the postischemic kidneys, suggesting that B cells could be acting on tubular repair independent of T cell trafficking.
On day 10, there was a peak in plasma cells and B-1 B cells in the postischemic kidneys. Intragraft plasma cells were found to correlate with circulating donor-specific antibodies in a recent clinical study27; however, our IRI model was alloantigen independent. There is evidence that B-1 B cells are involved in initiation of early injury in a murine intestinal IRI model but not in repair.31 A recent clinical report also suggested an important role of B cells expressing both CD19 and CD5 (expressed on B-1 B cells) in the pathogenesis of primary IgA nephropathy.32
After finding B cell trafficking in postischemic kidney during repair, we examined the expression of a BLC that could be involved in recruiting the B cells. BLC is a 16- to 18-kD member of the α- or CXC family of chemokine and induces migration of both naive B cells and B-1 B cells.33,34 The important role of BLC as a B cell chemoattractant is supported by a report demonstrating that overexpression of CXCR5, the receptor for BLC, was sufficient to overcome antigen-induced B cell movement to the T cell zone.33 BLC expression in the postischemic kidneys was significantly increased after renal IRI, remained elevated during the repair phase, and could have mediated the B cell trafficking.
Given that B cells were trafficking into postischemic kidney, with a distinct phenotypic change with time and an upregulation of BLC, we hypothesized that B cells directly modulate the repair process from kidney IRI. We therefore examined repair from kidney IRI in μMT mice. Ki-67 immunostaining was used to evaluate tubular proliferation; this staining was previously reported as a useful and accurate tool in assessing renal tubular proliferation.35,36 At day 3, there was no difference in the tubular injury of postischemic kidneys between wild-type control and μMT mice; however, at day 10, there was greater tubular proliferation and reduced tubular injury in μMT mice. To test whether these changes in the μMT mice were due to the absence of B cells, we adoptively transferred B cells into the μMT mice. With adoptive transfer of B cells, the μMT mice regained tubular proliferation and tubular injury similar to wild-type control mice, demonstrating that it was indeed the B cells that were mediating this effect on postischemic kidney repair. Renal tubular cells in the cortex of postischemic kidneys after blood flow has returned to near normal levels have been suggested as an early site of repair processes during AKI.37 In μMT mice, the tubular proliferative index of postischemic kidneys was significantly higher than control mice on day 3, and this may have led to less tubular damage of postischemic kidneys compared with control mice on day 10 after IRI. On day 28 after renal IRI, postischemic kidneys of μMT mice had reduced tubular atrophy and more normal tubules than control mice, which may have resulted from higher tubular proliferation of μMT mice on day 10. B cell deficiency has also been reported to attenuate liver fibrosis in a murine CCl4-induced liver fibrosis model.38 Adoptive transfer of B cells into μMT mice in our study resulted in a marked decrease of the tubular proliferative index of postischemic kidneys, and tubular damage and atrophy were comparable to control mice. Adoptively transferred B cells trafficked into the postischemic kidneys and differentiated into plasma cells. B cell transfer also affected intrarenal cytokine expression. Postischemic kidneys of μMT mice expressed higher IL-10 on days 10 and 28 and higher VEGF on day 10 after IRI. A protective role of IL-10 in renal IRI has been suggested in several studies,39–42 and a beneficial effect of VEGF on renal IRI has also been reported.43,44 After adoptive B cell transfer into μMT mice, renal expression of these cytokines was reduced to a level comparable to control mice. We also investigated the degree of fibrosis using Masson's trichrome staining on day 28 and found discordant effects of B cells on fibrosis compared with tubular atrophy. Future studies that examine fibrosis more carefully at various and later time points would be useful in evaluating the influence of B cell manipulation on fibrosis after AKI.
One of the unexpected findings was the increase in kidney-infiltrating plasma cells expressing CD138 and CD126 (IL-6 receptor) during repair, particularly at day 10. Intrarenal plasma cells were recently implicated in humoral rejection.27 We tested the functional significance of plasma cells during repair from IRI using MR16-1 given at day 1 after IRI (rather than before or at the time of ischemia so as not to interfere with early injury mechanisms). MR16-1 is known to bind and block both soluble and membrane-bound types of IL-6 receptor.45 The postischemic kidneys of MR16-1–treated mice did not show any difference in tubular injury on day 10; however, there was less tubular atrophy on day 28 after renal IRI with MR16-1 treatment compared with control mice. The tubular proliferative index of postischemic kidneys of MR16-1–treated mice was already higher than control mice on day 10 and remained so on day 28 after IRI. The beneficial effect of MR16-1 has been reported in other disease models in which B cells contribute to the pathogenesis, such as collagen-induced arthritis and Castleman disease, and in a murine autoimmune kidney disease model.46–48 Although CD126 is not entirely specific for plasma cells, CD126 is strongly expressed on plasma cells and relatively weakly expressed on other leukocytes.49 Plasma cells may function in renal IRI in a manner distinct from their role in secreting Igs; however, we cannot exclude the possibility that CD126 antibody had a beneficial effect independent of its effects on plasma cells.
We measured serum IgM to check the possibility of humoral effects of B cells during the repair phase of ischemic AKI and found that serum IgM level increased with time after unilateral IRI. MR16-1 treatment significantly reduced serum IgM production in wild-type control mice after unilateral IRI. Our data suggest that IRI, even unilateral, induces humoral responses well into the repair phase. Heat-inactivated serum from the wild-type control mice that underwent IRI or sham operation was transferred into μMT mice on day 1 after IRI to assess the effects of soluble factors generated by B cells on repair status. Serum-transferred μMT mice, either from sham-operated mice or from mice that underwent IRI, had more tubular atrophy than μMT mice in medulla. These data suggest that humoral components may enhance tubular atrophy after IRI. Further study is required to elucidate the exact nature of the humoral factor during the repair phase after kidney IRI and, if it turns out to be an antibody, how this antibody works.
These studies demonstrate that B cells traffic into the postischemic kidneys in the absence of alloantigen stimulation and modulate the repair process after IRI. B cells influence tubular atrophy and regeneration, and blocking CD126 improves repair from IRI. It already has been reported that B cell infiltration is involved in allograft rejection and poor graft survival.22–24 Our data demonstrate that warm kidney IRI, independent of alloresponses, can cause B cell trafficking to the renal allograft and alter tubular repair process. Furthermore, modulating B cells and targeting CD126 represent a novel way to improve clinical outcomes from kidney IRI.
Concise Methods
Mice
C57BL/6J and μMT (B cell–deficient) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). μMT mice were generated from homozygous mutation of the Igh-6tm1Cgn and lack mature B cells expressing IgM. All mice were approximately 7- to 10-week-old males and were housed in a specific pathogen-free barrier facility. The Johns Hopkins University Animal Care and Use Committee approved all studies.
Renal Ischemia-Reperfusion Model
Mice were anesthetized with an intraperitoneal injection of sodium pentobarbital (75 mg/kg). After abdominal midline incision, left renal pedicle was bluntly dissected and clamped with a microvascular clamp (Roboz Surgical Instrument, Gaithersburg, MD) for 45 minutes. This ischemic time was chosen after preliminary experiments, in which 30 minutes of ischemia led to too mild histologic response on days 10 and 28 and 60 minutes of ischemia led to too severe histologic injury. During the procedures, mice were kept well hydrated with warm sterile saline at a constant temperature (37°C). After the clamps were removed, the wounds were sutured and the mice were allowed to recover with free access to food and water. Cohorts of mice were killed on days 1, 3, 10, 12, and 28 after surgery. Both postischemic kidneys and contralateral (right, untouched) kidneys were collected and compared.
Flow Cytometry Analysis of KMNCs
KMNCs were isolated according to the method previously described.50 Briefly, decapsulated kidneys were immersed in RPMI buffer (Mediatech, Manassas, VA) containing 5% FBS and disrupted mechanically using Stomacher 80 Biomaster (Seward, Worthing, West Sussex, UK). Samples were strained, washed, and resuspended in 36% Percoll (Amersham Pharmacia Biotech, Piscataway, NJ) followed by gentle overlaying onto 72% Percoll. After centrifugation at 1000 × g for 30 minutes at room temperature, KMNCs were collected from the Percoll interface, washed twice, and counted on a hemocytometer using trypan blue exclusion.
Isolated KMNCs were preincubated with anti-CD16/CD32 Fc receptor blocking antibody for 10 minutes to minimize nonspecific antibody binding. Cells were then incubated with anti-mouse anti-CD19, CD21, CD38, CD40, CD69, CD126, CD138, IgM, and MHC class II (all from BD Biosciences except for anti-mouse FITC-conjugated anti-IgM antibody from eBioscience, San Diego, CA) for 25 minutes at 4°C, washed with FACS buffer, and fixed in 1% paraformaldehyde solution. Four-color immunofluorescence staining was acquired and analyzed using a FACSCalibur instrument (BD Biosciences, San Jose, CA) and FCS Express V3 (De Novo Software, Los Angeles, CA). Each assay included at least 10,000 gated events.
Mouse BLC (CXCL13) Immunoassay
Mouse BLC (CXCL13) was measured on kidney protein samples extracted from whole-kidney tissues using mouse BLC immunoassay kit (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol. BLC concentration (pg/ml) of all samples was divided by protein concentration (mg/ml) for normalization.
Isolation of B Cells and Adoptive Transfer into μMT Mice
Mature B cells were isolated from spleens of C57BL/6J mice using a mouse B cell enrichment kit (StemCell Technologies, Vancouver, British Columbia, Canada). Briefly, spleens were macerated in RPMI buffer containing 5% FBS and centrifuged, followed by red blood cell lysis using 1× red blood cell lysis buffer (eBioscience). The splenocyte pellet was resuspended in PBS with 2% normal rat serum. The cell suspension was incubated with SpinSep mouse B cell enrichment cocktail, biotin selection cocktail, and dense particles sequentially. Then samples were diluted and layered on top of the SpinSep density medium. After centrifugation at 1200 × g for 10 minutes at room temperature, B cells were collected from the top of the density medium. B cells were resuspended in sterile normal saline and injected into μMT mice on day 1 after renal IRI. We used a B cell isolation method based on negative selection to avoid any disruption of B cell integrity. Mean purity of CD19-expressing mature B cells analyzed with flow cytometry was 95%. Approximately 1.5 × 107 cells were injected into the tail vein and 4.5 × 107 cells were injected into peritoneal cavity in each mouse. The amount of B cells injected into the tail vein was similar to a recent report using the same strain of B cell–deficient mice.51 Mice were killed on days 3, 10, and 28 after IRI.
Administration of Anti-CD126 Antibody
MR16-1 was provided by Chugai Pharmaceutical Co. (Tokyo, Japan). Control Ig (rat IgG) was purchased from Innovative Research (Novi, MI). Both MR16-1 and control IgG were diluted with sterile normal saline, and 50 mg/kg of each was administered into the peritoneal cavity every 5 days starting at day 1 after renal IRI. Mice that received either MR16-1 or control IgG were killed on days 10 and 28 after IRI.
Tissue Histologic Analysis
Kidneys were harvested after exsanguination. Tissue sections were fixed with 10% buffered formalin followed by paraffin embedding and stained with H&E, periodic acid-Schiff, or Masson's trichrome. Renal tubular damage, atrophy, and regeneration were scored by a renal pathologist who was blinded to the experimental groups.
Immunohistochemistry of Postischemic Kidneys with Ki-67
Immunohistochemistry staining with Ki-67 was performed on formalin-fixed kidney tissues. Sections (4 μm) were deparaffinized with xylene and rehydrated in a graded alcohol series, then placed in citrate buffer solution (pH 6.0). Slides were placed in a pressure cooker and heated with microwave for 10 minutes to enhance antigen retrieval. After cooling, sections were immersed in 3% hydrogen peroxide for 10 minutes to block endogenous peroxidase, then treated with avidin/biotin block and protein block serum-free (DAKO, Carpinteria, CA) sequentially and incubated overnight at 4°C with 1:50 diluted monoclonal rat anti-mouse antibody to Ki-67 (clone TEC-3; DAKO), a 360-kD nuclear protein, expressed by proliferating cells in all phases of the active cell cycle but absent in resting cells. The next day, the slides were incubated with a biotin-conjugated goat anti-rat IgG secondary antibody (Jackson ImmunoResearch, West Grove, PA) for 30 minutes at room temperature, followed by an avidin-biotin horseradish peroxidase complex (ABC peroxidase; Vector Laboratories, Burlingame, CA). 3,3′-Diaminobenzidene tetrahydrochloride (Vector Laboratories) was applied to the slides for developing brown color. Counterstaining was done with eosin.
For calculation of the tubular proliferative index, Ki-67–incubated slides were also incubated with Höechst Dye solution (Invitrogen, Carlsbad, CA) for 5 minutes in the dark to counterstain total nuclei. Light and fluorescence microscopy of the same field were performed at ×200 magnification (Eclipse E600 microscope; Nikon, Melville, NY). Three different areas of cortex, outer medulla, and inner medulla were evaluated for each slide. Images were captured and digitized using Digital Still Camera DXM1200 and ACT-1 software (Nikon) and then analyzed for cell counting using ImageJ software (National Institutes of Health, Bethesda, MD). For each animal, the tubular proliferative index in cortex, outer medulla, and inner medulla was expressed as ratio between the tubular Ki-67–positive cells and total tubular cell nuclei of the examined fields.36
Bioplex Protein Array
A panel of cytokines were measured in whole-kidney protein extracts with Bioplex Protein Array system (Bio-Rad, Hercules, CA) according to the manufacturer's method. This is a multiplexed, particle-based, flow cytometric assay that uses anti-cytokine mAbs linked to microspheres incorporating distinct properties of two fluorescence dyes. Our assay was designed to detect and quantify IL-2, IL-6, IL-10, RANTES (CCL5), VEGF, MIP-1b, and basic fibroblast growth factor. Each cytokine value was normalized by dividing the raw cytokine concentration (pg/ml) with kidney protein concentration (mg/ml) measured by the Bradford method.
Measurement of Serum Cystatin C Level
Cystatin C level was measured with serum samples obtained on day 28 after left renal IRI using ELISA kit (Biovendor, Candler, NC) according to the manufacturer's recommended method.
Measurement of Serum IgM Level
IgM level was measured with serum samples obtained on days 0, 10, and 28 after left renal IRI using ELISA kit (Immunology Consultants Laboratory, Inc., Newberg, OR) according to the manufacturer's recommended method.
Serum Transfer into μMT Mice
Serum was extracted from the control (C57BL/6J) mice that underwent left renal IRI or sham operation on day 10. All serum was heated at 56°C for 30 minutes before being transferred to inactivate complement. A total of 0.5 ml of heat-inactivated serum (half into the tail vein and half into the peritoneal cavity) was injected into each μMT mice on day 1 after renal IRI. Mice were killed on day 28 after IRI.
Statistical Analysis
All data are expressed as mean ± SEM. Group means were compared using Mann-Whitney test using SPSS 12.0K or ANOVA followed by Newman-Keuls post hoc analysis using GraphPad Prism 4. Statistical significance was determined at P < 0.05.
Disclosures
None.
Acknowledgments
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK054770 to H.R. H.R.J. was supported by the Korea Research Foundation.
We thank Chaitali Sarkar and Priya Kesari for technical assistance and Dr. Yanfei Huang for helpful advice.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1. Perico N, Cattaneo D, Sayegh MH, Remuzzi G: Delayed graft function in kidney transplantation. Lancet 364: 1814– 1827, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Jang HR, Rabb H: The innate immune response in ischemic acute kidney injury. Clin Immunol 130: 41– 50, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okusa MD: The inflammatory cascade in acute ischemic renal failure. Nephron 90: 133– 138, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Rabb H, Daniels F, O'Donnell M, Haq M, Saba SR, Keane W, Tang WW: Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol 279: F525– F531, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O'Donnell MP, Rabb H: Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 108: 1283– 1290, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Day YJ, Huang L, Ye H, Li L, Linden J, Okusa MD: Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: The role of CD4+ T cells and IFN-gamma. J Immunol 176: 3108– 3114, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H: B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol 171: 3210– 3215, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Weiser MR, Williams JP, Moore FD, Jr, Kobzik L, Ma M, Hechtman HB, Carroll MC: Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med 183: 2343– 2348, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM: B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3: 944– 950, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Mauri C, Gray D, Mushtaq N, Londei M: Prevention of arthritis by interleukin 10-producing B cells. J Exp Med 197: 489– 501, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK: Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16: 219– 230, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Bettelli E, Baeten D, Jager A, Sobel RA, Kuchroo VK: Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest 116: 2393– 2402, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krishnamoorthy G, Lassmann H, Wekerle H, Holz A: Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J Clin Invest 116: 2385– 2392, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF: Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest 118: 3420– 3430, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edwards JC, Cambridge G: Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology (Oxford) 40: 205– 211, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Anolik JH, Barnard J, Cappione A, Pugh-Bernard AE, Felgar RE, Looney RJ, Sanz I: Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum 50: 3580– 3590, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, Sloand JA, Rosenblatt J, Sanz I: B cell depletion as a novel treatment for systemic lupus erythematosus: A phase I/II dose-escalation trial of rituximab. Arthritis Rheum 50: 2580– 2589, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH: B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 358: 676– 688, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Cassese G, Lindenau S, de Boer B, Arce S, Hauser A, Riemekasten G, Berek C, Hiepe F, Krenn V, Radbruch A, Manz RA: Inflamed kidneys of NZB/W mice are a major site for the homeostasis of plasma cells. Eur J Immunol 31: 2726– 2732, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Magalhaes R, Stiehl P, Morawietz L, Berek C, Krenn V: Morphological and molecular pathology of the B cell response in synovitis of rheumatoid arthritis. Virchows Arch 441: 415– 427, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Sarwal M, Chua MS, Kambham N, Hsieh SC, Satterwhite T, Masek M, Salvatierra O, Jr: Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med 349: 125– 138, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Martins HL, Silva C, Martini D, Noronha IL: Detection of B lymphocytes (CD20+) in renal allograft biopsy specimens. Transplant Proc 39: 432– 434, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Hippen BE, DeMattos A, Cook WJ, Kew CE, 2nd, Gaston RS: Association of CD20+ infiltrates with poorer clinical outcomes in acute cellular rejection of renal allografts. Am J Transplant 5: 2248– 2252, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Tsai EW, Rianthavorn P, Gjertson DW, Wallace WD, Reed EF, Ettenger RB: CD20+ lymphocytes in renal allografts are associated with poor graft survival in pediatric patients. Transplantation 82: 1769– 1773, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Bagnasco SM, Tsai W, Rahman MH, Kraus ES, Barisoni L, Vega R, Racusen LC, Haas M, Mohammed BS, Zachary AA, Montgomery RA: CD20-positive infiltrates in renal allograft biopsies with acute cellular rejection are not associated with worse graft survival. Am J Transplant 7: 1968– 1973, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Doria C, di Francesco F, Ramirez CB, Frank A, Iaria M, Francos G, Marino IR, Farber JL: The presence of B-cell nodules does not necessarily portend a less favorable outcome to therapy in patients with acute cellular rejection of a renal allograft. Transplant Proc 38: 3441– 3444, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Zarkhin V, Kambham N, Li L, Kwok S, Hsieh SC, Salvatierra O, Sarwal MM: Characterization of intra-graft B cells during renal allograft rejection. Kidney Int 74: 664– 673, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Song S, Meyer M, Turk TR, Wilde B, Feldkamp T, Assert R, Wu K, Kribben A, Witzke O: Serum cystatin C in mouse models: A reliable and precise marker for renal function and superior to serum creatinine. Nephrol Dial Transplant 24: 1157– 1161, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Sahsivar MO, Narin C, Kiyici A, Toy H, Ege E, Sarigul A: The effect of iloprost on renal dysfunction following renal ischemia reperfusion using cystatin C and beta2-microglobulin monitoring. Shock 32: 498– 502, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Bouaziz JD, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC, Tedder TF: Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci U S A 104: 20878– 20883, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fleming SD, Shea-Donohue T, Guthridge JM, Kulik L, Waldschmidt TJ, Gipson MG, Tsokos GC, Holers VM: Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J Immunol 169: 2126– 2133, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Yuling H, Ruijing X, Xiang J, Yanping J, Lang C, Li L, Dingping Y, Xinti T, Jingyi L, Zhiqing T, Yongyi B, Bing X, Xinxing W, Youxin J, Fox DA, Lundy SK, Guohua D, Jinquan T: CD19+CD5+ B Cells in primary IgA nephropathy. J Am Soc Nephrol 19: 2130– 2139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Forster R, Cyster JG: Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature 416: 94– 99, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Brandes M, Legler DF, Spoerri B, Schaerli P, Moser B: Activation-dependent modulation of B lymphocyte migration to chemokines. Int Immunol 12: 1285– 1292, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Hall PA, Greenwood RA, d'Ardenne AJ, Levison DA: In situ demonstration of renal tubular regeneration using the monoclonal antibody Ki67. Nephron 49: 122– 125, 1988 [DOI] [PubMed] [Google Scholar]
- 36. Nadasdy T, Laszik Z, Blick KE, Johnson LD, Silva FG: Proliferative activity of intrinsic cell populations in the normal human kidney. J Am Soc Nephrol 4: 2032– 2039, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Molitoris BA, Sutton TA: Endothelial injury and dysfunction: Role in the extension phase of acute renal failure. Kidney Int 66: 496– 499, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Novobrantseva TI, Majeau GR, Amatucci A, Kogan S, Brenner I, Casola S, Shlomchik MJ, Koteliansky V, Hochman PS, Ibraghimov A: Attenuated liver fibrosis in the absence of B cells. J Clin Invest 115: 3072– 3082, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Daemen MA, van de Ven MW, Heineman E, Buurman WA: Involvement of endogenous interleukin-10 and tumor necrosis factor-alpha in renal ischemia-reperfusion injury. Transplantation 67: 792– 800, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA: Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int 60: 2118– 2128, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Koken T, Serteser M, Kahraman A, Akbulut G, Dilek ON: Which is more effective in the prevention of renal ischemia-reperfusion-induced oxidative injury in the early period in mice: Interleukin (IL)-10 or anti-IL-12? Clin Biochem 37: 50– 55, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Godet C, Goujon JM, Petit I, Lecron JC, Hauet T, Mauco G, Carretier M, Robert R: Endotoxin tolerance enhances interleukin-10 renal expression and decreases ischemia-reperfusion renal injury in rats. Shock 25: 384– 388, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Lemos FB, Ijzermans JN, Zondervan PE, Peeters AM, van den Engel S, Mol WM, Weimar W, Baan CC: Differential expression of heme oxygenase-1 and vascular endothelial growth factor in cadaveric and living donor kidneys after ischemia-reperfusion. J Am Soc Nephrol 14: 3278– 3287, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Faleo G, Neto JS, Kohmoto J, Tomiyama K, Shimizu H, Takahashi T, Wang Y, Sugimoto R, Choi AM, Stolz DB, Carrieri G, McCurry KR, Murase N, Nakao A: Carbon monoxide ameliorates renal cold ischemia-reperfusion injury with an upregulation of vascular endothelial growth factor by activation of hypoxia-inducible factor. Transplantation 85: 1833– 1840, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Okazaki M, Yamada Y, Nishimoto N, Yoshizaki K, Mihara M: Characterization of anti-mouse interleukin-6 receptor antibody. Immunol Lett 84: 231– 240, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Takagi N, Mihara M, Moriya Y, Nishimoto N, Yoshizaki K, Kishimoto T, Takeda Y, Ohsugi Y: Blockage of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum 41: 2117– 2121, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Katsume A, Saito H, Yamada Y, Yorozu K, Ueda O, Akamatsu K, Nishimoto N, Kishimoto T, Yoshizaki K, Ohsugi Y: Anti-interleukin 6 (IL-6) receptor antibody suppresses Castleman's disease like symptoms emerged in IL-6 transgenic mice. Cytokine 20: 304– 311, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Mihara M, Takagi N, Takeda Y, Ohsugi Y: IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clin Exp Immunol 112: 397– 402, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Janeway C: CD antigens. In: Immunobiology, 6th Ed., New York, Garland Science Publishing, 2005, pp 731– 746 [Google Scholar]
- 50. Ascon DB, Lopez-Briones S, Liu M, Ascon M, Savransky V, Colvin RB, Soloski MJ, Rabb H: Phenotypic and functional characterization of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J Immunol 177: 3380– 3387, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Sun JB, Flach CF, Czerkinsky C, Holmgren J: B lymphocytes promote expansion of regulatory T cells in oral tolerance: Powerful induction by antigen coupled to cholera toxin B subunit. J Immunol 181: 8278– 8287, 2008 [DOI] [PubMed] [Google Scholar]