Abstract
The peak prevalence of ESRD from glomerulosclerosis occurs at 70 to 79 years. To understand why old glomeruli are prone to failure, we analyzed the Fischer 344 rat model of aging under ad libitum-fed (rapid aging) and calorie-restricted (slowed aging) conditions. All glomerular cells contained genes whose expression changed “linearly” during adult life from 2 to 24 months: mesangial cells (e.g., MMP9), endothelial cells (e.g., ICAM and VCAM), parietal epithelial cells (e.g., ceruloplasmin), and podocytes (e.g., nephrin and prepronociceptin). Patterns of aging glomerular gene expression closely resembled atherosclerosis, including activation of endothelial cells, epithelial cells, and macrophages, as well as proinflammatory pathways related to cell adhesion, chemotaxis, blood coagulation, oxidoreductases, matrix metalloproteinases, and TGF-β activation. We used a nonbiased data-mining approach to identify NFκB as the likely transcriptional regulator of these events. We confirmed NFκB activation by two independent methods: translocation of NFκB p50 to glomerular nuclei and ChIP assays demonstrating NFκB p50 binding to the κB motif of target genes in old versus young glomeruli. These data suggest that old glomeruli exhibit NFκB-associated up-regulation of a proinflammatory, procoagulable, and profibrotic phenotype compared with young glomeruli; these distinctions could explain their enhanced susceptibility to failure. Furthermore, these results provide a potential mechanistic explanation for the close relationship between ESRD and atherosclerotic organ failure as two parallel arms of age-associated NFκB-driven processes.
The peak prevalence for treated end-stage kidney disease (ESKD) is 70 to 79 years of age.1 The major pathologic phenotype of ESKD of old age is glomerulosclerosis, and autopsy studies show an increasing proportion of sclerotic glomeruli after age 40 years in otherwise normal humans.2,3 Because ESKD is frequently associated with other common diseases of older age, such as diabetes and hypertension, it is often causally attributed to these conditions. However, it is likely that the aging process itself contributes to ESKD in ways that are not well understood.
Rat models of aging clearly demonstrate progressive glomerulosclerosis as an age-related phenomenon independent of diabetes and hypertension and accelerated by a high-calorie diet.4–10 The Fischer 344 rat, in which diabetes and hypertension are not features, is a well-established model system for aging.11,12 We defined changes in structure, function, and gene expression in the glomerulus in the Fischer 344 rat during aging, and compared rats that were fed an ad libitum diet (to promote age-related changes) to those that were calorie-restricted (to slow the rate of aging and onset of glomerulosclerosis).10 We found podocyte stress and depletion associated with age-associated glomerulosclerosis as in other forms of glomerulosclerosis.10,13
Aging is a genetically regulated process that can be modified by environmental factors. Mutations in genes controlling the insulin/IGF pathway alter life span throughout species ranging from yeast through worms and flies to mammals.14–16 Similarly, the Sir2 family of NAD+-dependent lysine deacetylases has a role in aging.17,18 Physiologic interventions, including calorie restriction and exercise, delay onset of the aging phenotype in the broad range of species.18–20
The NFκB pathway has previously been identified as a candidate activator of age-related transcriptional changes in human and mouse tissues.21–24 Genetic blockade of NFκB in mouse skin reversed the normal age-associated pattern of gene expression and the aging phenotype, thereby demonstrating that disruption of this single gene was sufficient to reverse features of aging, even in late life.22,24 In the nonactivated state, NFκB proteins are held in the cytoplasm by binding to IκB proteins. Stress states cause degradation of IκB, thereby releasing NFκB to translocate to the nucleus and bind to the consensus κB DNA motif in promoter and enhancer regions of genes involved in innate/adaptive immunity, cell adhesion, inflammation, cell stress responses, and apoptosis.24–26
To understand the molecular events associated with the aging process in the rat glomerulus, we used a statistical approach to identify genes whose expression changed “linearly” (incrementally in the same direction) over time from young adulthood to old age. An unbiased data-mining method identified NFκB as a common transcription factor that could drive changes in aging-associated glomerular gene expression. We then confirmed NFκB activation in old versus young glomeruli by demonstrating immunofluorescent translocation of NFκB to the nucleus as well as localization of NFκB protein on the regulatory regions of key genes by chromatin immunoprecipitation (ChIP) analysis. These data are consistent with the concept that the old glomerulus is different from the young glomerulus, and is in a proinflammatory, procoagulant, and profibrotic state that sets it up for failure.
Results
Fischer 344 male rats were maintained on either an ad libitum or a calorie-restricted diet from the age of 2 months until 24 months. We previously mapped the age-associated morphologic and functional changes.10 DNA microarrays were generated using RNA from isolated glomeruli at 2, 6, 17, and 24 months of age as described previously.10
Genes Whose Expression Changes “Linearly” with Aging
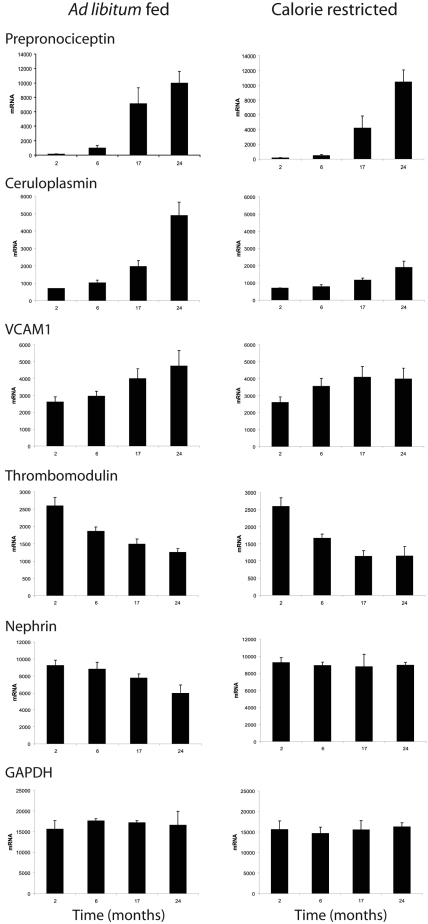
We observed that the level of expression of some genes changed either up or down throughout the period of adult aging from 2 to 24 months (Figure 1). We therefore used a statistical approach to quantify the linear component of the temporal variation in gene expression from young adulthood to old age to identify these genes (see Concise Methods). The 172 genes thus identified with a slope of 2 or greater in ad libitum and calorie-restricted conditions are shown in Supplemental Tables 1A (positive slope) and 1B (negative slope). Of the 92 genes shown as meeting criteria for increased expression with age in Supplemental Table 1A, there were 91 in the ad libitum-fed group and 34 in the calorie-restricted group. Of the 80 genes shown as meeting criteria for decreased expression with age in Supplemental Table 1B, 60 were in the ad libitum group and 20 were in the calorie-restricted group. Only six genes were identified as changing in the calorie-restricted group which did not also meet criteria for inclusion in the ad libitum group (one in Supplemental Table 1A and five in Supplemental Table 1B).
Figure 1.
Glomerular gene expression profiles linearly changed with aging. Rats were fed an ad libitum diet (left) or a calorie-restricted diet (right). Note that some gene expression profiles was linearly increased during aging (e.g., Pnoc, ceruloplasmin, or VCAM), and some were linearly decreased during aging (e.g., thrombomodulin or nephrin). In some cases, calorie restriction blunted or prevented changes in gene expression with aging (e.g., ceruloplasmin or nephrin). A control gene GAPDH, which does not change with age, is shown at the bottom. The gene lists for all genes identified as changing linearly with age are shown in Supplemental Tables 1A and 1B.
Identification of Glomerular Cell Types Involved in Age-Related Alterations in Glomerular Gene Expression
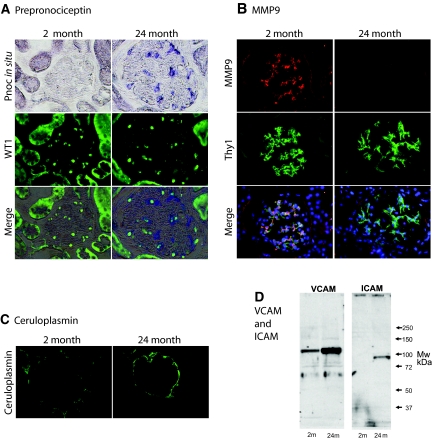
As reported previously and shown in Supplemental Table 1, nephrin mRNA and protein decreased and desmin mRNA and protein increased over time during glomerular aging in the Fischer 344 rat.10 However, genes whose expression changed throughout aging also included genes expressed by endothelial cells (e.g., vascular cell adhesion molecule (VCAM), intercellular adhesion molecule (ICAM), thrombomodulin, NOS3). To determine which cells might contribute to “linear” aging-associated gene expression, we examined tissue expression of the most prominently regulated transcripts (Supplemental Table 1). Prepronociceptin (Pnoc) is a neuropeptide that modulates pain sensation and whose expression in old glomeruli increases by >80-fold between 2 and 24 months. Pnoc has not previously been reported to be expressed in the glomerulus. In situ hybridization studies showed that glomerular Pnoc was expressed by podocytes in old glomeruli but was not detectable in podocytes (or other glomerular cells) in young glomeruli (Figure 2A). Thus, Pnoc is an example, along with nephrin (whose expression decreases) and desmin (whose expression increases), of altered podocyte gene expression with aging. Changes in gene expression with age were also present in other glomerular cells. MMP9 expression was markedly decreased in glomeruli as they aged and showed a mesangial expression pattern (Figure 2B). Ceruloplasmin mRNA expression was markedly increased during aging, as we have previously reported,27 and was expressed by the parietal epithelial cell (Figure 2C). Several endothelial cell proteins showed altered expression during aging. We confirmed by Western blot analysis that VCAM and ICAM protein were both increased in old glomeruli (Figure 2D). From these data we conclude that (1) old glomeruli are not the same as young glomeruli in terms of their gene expression profiles; (2) differences in gene expression can occur in a progressive “linear” manner from young adulthood to old age; (3) these changes in gene expression can be modulated in some cases by environmental factors, such as diet (see Figure 1 and Table 1); and (4) linear changes in glomerular gene expression during aging can occur in all glomerular cell types.
Figure 2.
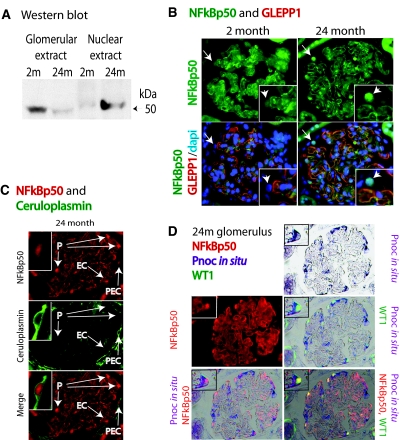
Glomerular cell types expressed altered gene expression with age. (A) Pnoc mRNA expression was not detectable in young (2-month) glomeruli but was detectable in old (24-month) glomeruli by in situ hybridization. Double-label studies using antibodies to WT1 to identify podocyte nuclei show that the cells that express Pnoc also have WT1-positive nuclei. Therefore, the Pnoc-expressing cells in old glomeruli are podocytes. (B) MMP9-expressing cells identified by immunofluorescence are detectable in young 2-month glomeruli but not in old 24-month glomeruli. Double-label studies using Thy1 as a mesangial cell marker demonstrate that the cells in young glomeruli that express MMP9 protein are mesangial cells. (C) The parietal epithelial cells lining Bowman capsule in old 24-month glomeruli express ceruloplasmin, whereas those of young 2-month glomeruli do not. (D) Western blots of glomerular extracts show that old 24-month glomerular extracts contain higher levels of both VCAM and ICAM than do glomerular extracts from young 2-month glomeruli. Mw, molecular weight; m, month. All photomicrograph images were made at ×200 magnification.
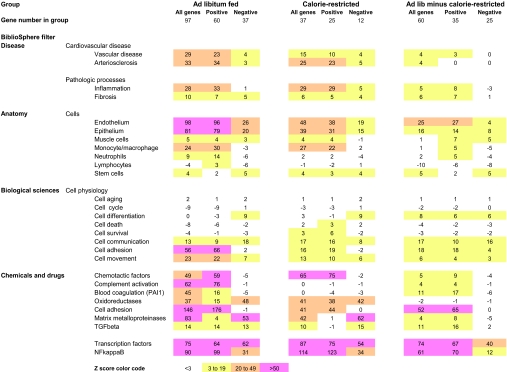
Table 1.
Z-scores for co-citation analysis of genes whose expression changes with age.
Co-citation analysis to identify patterns of gene expression using the Genomatix BiblioSphere™ Program. The gene lists used for co-citation analysis were from Supplemental Tables 1A and 1B. Not all genes were identified by the Genomatix Bioliosphere™ as co-cited. Therefore, the total genes evaluated in Table 1 are less than the totals shown in Supplemental Tables 1A and 1B. Analysis was done separately for genes in the ad libitum fed gene list (left columns), the calorie-restricted gene list (middle columns), and for genes in the ad libitum fed minus the calorie-restricted lists (right columns). In each case the analysis was done for all genes (all), or only those genes that increased (positive) or only those genes that decreased (negative). The Genomatix Bibliosphere™ filters are the designated filters that sort PubMed abstracts according to established criteria. The Z scores determine the extent to which a gene is expressed in excess of (+ value) or less than (− value) expected from a random distribution calculated by the Genomatix Bibliosphere™ program from the PubMed database. A Z score of 3 is statisically significant. A color code is used to visualize the patterns of alterations in Z score between the different groups. A Z score of <3 is designated uncolored, Z scores of 3-19 are designated yellow, 20-49 designated orange, and >50 designated pink, as illustrated at the bottom of the table.
Cocitation Analysis to Identify Mechanisms Involved in Glomerular Aging
An advantage of DNA array data is that they provide an unbiased accounting of relative gene expression in a tissue at any one point in time. A disadvantage is that it is difficult to weigh individual pieces of data within the array profile because investigator bias has an effect on how we think of a particular gene and its potential role in causing a phenotype. To circumvent this problem, it is useful to evaluate gene expression data using natural language processing (NLP) tools in the context of the complete PubMed database. This option is available through the Genomatix BiblioSphereTM program, in which computer-read abstracts are used to define associations between variables. This approach allows a statistical value (the Z score) to be assigned to the cumulative value of genes identified as activated or suppressed under a particular circumstance in relation to descriptors such as cell type, physiologic function, pathologic mechanism, disease process, etc. Using this approach, one can arrive at a relatively unbiased view of what a collection of genes expressed under a particular circumstance might represent in terms of known biologic process or mechanism. Of course, this type of analysis is always limited by the extent and bias of human knowledge, in this case defined as the limits of the PubMed database. Armed with this information, it is then possible to go back to the tissues and determine independently whether the hypothesis thus derived is or is not supported.
We used the Genomatix BiblioSphereTM program to tell us what processes this particular set of “linearly” expressed genes in glomeruli might represent. A Z score of 3 was considered to be statistically significant. Genomatix BibliosphereTM annotated 134 of the 163 genes in Supplemental Tables 1A and 1B to its internal database. As shown in Table 1 (left), the “disease” most resembling that observed in the glomerular “linear” aging gene profile is arteriosclerosis. The “pathologic processes” filter identified inflammation and fibrosis as major active processes. Under the filter “anatomy,” high Z scores indicated that gene expression changes with age occurred in epithelial and endothelial cells and macrophages. Under the filter “biologic sciences” and “cell physiology,” genes associated with cell communication, cell adhesion, and cell movement were increased. Interestingly, genes associated with cell aging, cell cycle, cell differentiation, cell death, and cell survival that might be expected to have an effect on the aging process were not identified in the data sets. Under the filter “chemicals and drugs,” genes associated with chemotaxis, cell adhesion, complement activation, blood coagulation, oxidoreductases, matrix metalloproteinases, and TGF-β pathways were identified by high Z scores. Thus the pattern of “linearly” altered gene expression associated with aging was proinflammatory, procoagulant, and profibrotic.
Do Calorie-Restricted Rats That Do Not Develop Glomerulosclerosis Demonstrate a Fundamentally Different Pattern of Gene Expression?
We previously demonstrated that calorie-restricted rats had smaller glomeruli, had less proteinuria, and did not develop glomerulosclerosis by 24 months of age.10 As shown in Table 1 (middle), the changes in gene expression that occurred in the calorie-restricted rat glomeruli were similar to those that occurred in ad libitum-fed rat glomeruli, albeit to a lesser extent. To expand on this point, we measured Z scores for the genes that were changed in ad libitum-fed rat glomeruli but not in calorie-restricted rat glomeruli (Table 1 right). These genes were therefore only significantly up-regulated or down-regulated in the ad libitum-fed rat glomeruli. Again, the same key pathways were identified, namely, epithelial and endothelial cell activation, leading to cell adhesion and communication, representing a proinflammatory and profibrotic phenotype. Thus, the ad libitum-fed rat glomeruli had more genes significantly changing than did calorie-restricted rats, but they represented activation of the same general pathways.
NFκB as a Potential Coordinating Mechanism for Altered Gene Expression with Age
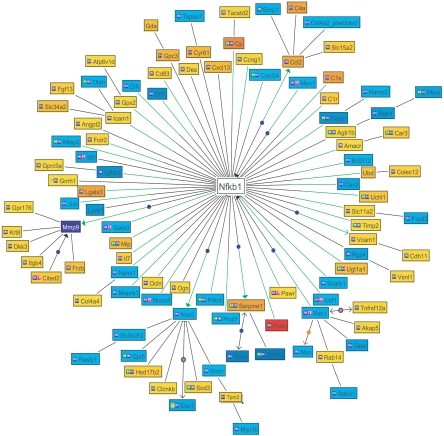
We next used the Genomatix BiblioSphereTM program to identify transcription factors that might play a coordinate role in producing the “linear” alterations in gene expression observed during aging (Table 1). The transcription factor NFκB was identified as by far the most likely candidate that might drive the age-associated “linear” alterations of gene expression observed (Z score 90 to 123). Figure 3 emphasizes diagrammatically the central position NFκB could play on the basis of Genomatix BiblioSphereTM NLP and automated promoter analysis for NFκB within the age-associated “linearly” altered gene network.
Figure 3.
The relationship of NFκB to genes identified as “linearly” changing with age on the basis of cocitation analysis (Genomatix BibliosphereTM) is shown as a Bibliosphere image. Of the probe sets with a slope of >2 in the ad libitum-fed group, 85 genes are cocited in the Genomatix BibliosphereTM database. At the level of B0 (cocitation in abstracts), 72 of the 85 genes are connected to NFκB in a network (when NFκB is included). Genes with negative slope with aging are displayed as blue nodes; a positive slope with aging is given as yellow/red nodes. The intensity of color denotes the degree of slope shown in Supplemental Tables 1A and 1B. Nodes are connected by black lines if they are cocited. A green line indicates an NFκB-binding site in the proximal promoter region of the gene (500 bp upstream to 100 bp downstream of transcription start site). IN indicates that the gene was part of the input gene set. TF indicates that the gene is a transcription factor. ST indicates that the gene is part of a signal transduction pathway. M indicates that the gene is part of a metabolic pathway. A closed arrowhead indicates activation. A dark blue node indicates a connection annotated by GenomatixTM. An orange node indicates that the connection is annotated by Molecular ConnectionsTM.
Nuclear NFκB Localization in Old Glomeruli
NFκB is generally considered not to be regulated in a major way at the messenger RNA level or at the protein translational level. Rather, NFκB pathway activation occurs via translocation of NFκB proteins from the cytoplasm to the nucleus as a consequence of IκB degradation.24–26 To determine whether NFκB had preferentially translocated into nuclei in old glomeruli, we therefore performed Western blot and immunofluorescence studies using antibodies against the NFκB p50 protein. We found that NFκB p50 protein was present preferentially in old glomerular nuclei by Western blot analysis and was demonstrable in nuclei from podocytes, parietal epithelial cells, and endothelial cells in glomeruli of old rats to a greater extent than for young rats (Figure 4). In old glomerular cells, NFκB nuclear localization could be demonstrated in association with genes that were up-regulated with age, including ceruloplasmin (in parietal epithelial cells) and Pnoc (in podocytes).
Figure 4.
NFκB p50 is translocated to nuclei in old glomeruli. (A) Western blot showing that whole-glomerular extracts had less NFκB p50 protein identified in old versus young glomeruli when equal amounts of protein were loaded. In contrast, the nuclear extracts of old glomeruli had increased detectable NFκB p50 protein when equal amounts of protein were loaded. (B) Immunofluorescent photomicrographs showing enhanced NFκB p50 (green) presence in glomerular nuclei of 24-month compared with 2-month rats (inset). The glomerular architecture is illustrated by double-label staining with GLEPP1 (red) to demonstrate podocyte foot process distribution along glomerular capillary loops. The arrows indicate podocyte nuclei identified on the glomerular surface based on GLEPP1 (red) and DAPI (blue) which also express NFκB p50 (green) in 24-month but not 2-month glomeruli. (C) A 24-month glomerulus showing nuclear NFκB p50 (red) staining in podocyte nuclei (P), a parietal epithelial cell nucleus (PEC), and a probable endothelial cell nucleus (EC) on the basis of its location within the capillary loop. Double-label staining of ceruloplasmin (green) of parietal epithelial cell cytoplasm demonstrates that the ceruloplasmin-containing cell also has an NFκB-stained nucleus (see inset). (D) A 24-month glomerulus demonstrating that the podocytes identified by having WT1-positive nuclei (green) also contain nuclear NFκB p50 protein (red) and are the same cells that express Pnoc mRNA as detected by in situ hybridization (purple). The insets demonstrate triple labeling of a podocyte to emphasize this point. m, month; dapi, 4′,6-diamidino-2-phenylindole. All photomicrograph images were made at ×200 magnification.
NFκB Binding to the DNA Regulatory Region of Genes
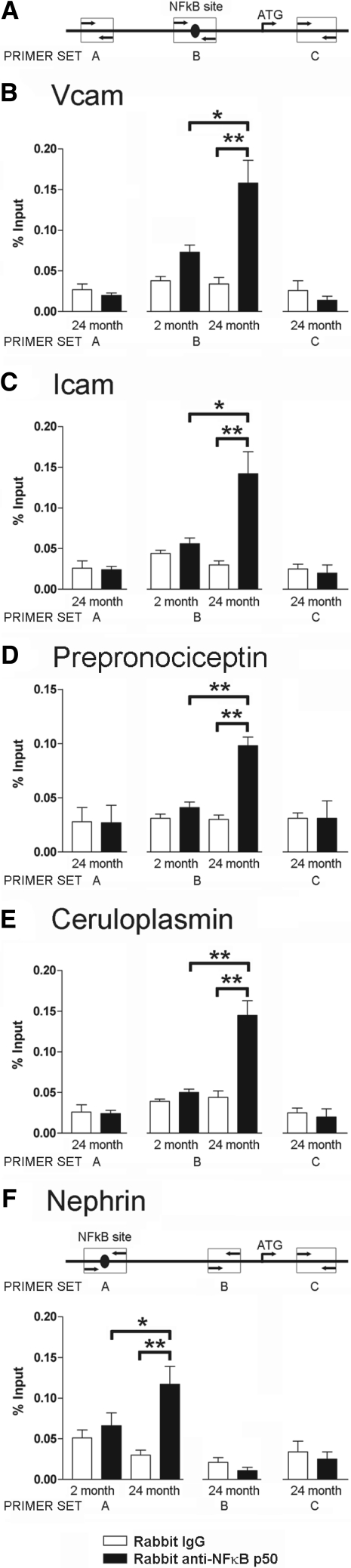
If NFκB was indeed translocated to the nucleus (where it would bind to κB motifs on DNA and thereby modulate expression of particular genes during aging), then it should be possible to demonstrate this through ChIP assays. In these assays, NFκB protein immunoprecipitated by antibodies should coprecipitate the DNA regulatory regions of specific genes. These DNA sequences can then be quantitated by qRT-PCR. We examined five examples from the gene lists shown in Supplemental Table 1 and chosen to represent different glomerular cells (ICAM and VCAM from endothelial cells, ceruloplasmin from parietal epithelial cells, and nephrin and Pnoc from podocytes). The ICAM, VCAM, ceruloplasmin, and Pnoc putative κB motifs were all within 400 bp of the ATG start site (these genes were all up-regulated during aging). The nephrin κB motif was 2.5 kb upstream of the nephrin ATG. Nephrin expression was down-regulated in glomeruli of ad libitum-fed rats during aging. The results shown in Figure 5 demonstrate that NFκB p50 antibody preferentially coprecipitated fragments of DNA containing the κB-binding region present in regulatory sequences identified for these genes from old versus young glomeruli. In contrast, upstream and downstream DNA fragments of old DNA that did not contain κB motifs were not preferentially coimmunoprecipitated. This result demonstrates that NFκB p50 is preferentially bound to the κB motifs in the regulatory regions of genes in old glomerular cells (podocytes, parietal epithelial cells, and endothelial cells), where it would be positioned to play a role in the observed altered gene expression of aging.
Figure 5.
ChIP assays compare NFκB immunoprecipitates of glomerular DNA from young and old rats. (A) Schematic illustrating primer set sites in relation to the putative NFκB site and the gene transcription start site for VCAM (B), ICAM (C), Pnoc (D), and ceruloplasmin (E). (F) For the nephrin κB site, which was 2.5 kb upstream of the start site, two control primer sets were chosen downstream of the putative κB site as shown in the schematic. NFκB p50 antibodies preferentially coimmunoprecipitated DNA fragments spanning the κB sites of genes from old (24 month) versus young (2-month) glomeruli for five examples of genes expressed in glomerular endothelial cells (VCAM and ICAM), podocytes (prepronociceptin and nephrin), and parietal epithelial cells (ceruloplasmin). Additional controls are shown to demonstrate that DNA sequences not containing κB sites upstream and downstream from the putative κB sites were not preferentially coimmunoprecipitated from old glomerular DNA. Data were analyzed by one-way ANOVA using a Bonferroni correction for multiple comparisons. *P < 0.01; **P < 0.001. The white bars refer to immunoprecipitation with nonimmune rabbit IgG. The closed bars refer to immunoprecipitation using rabbit anti-NFκB p50 IgG.
Discussion
To understand the biology of the aging glomerulus, we identified genes whose expression changed “linearly” throughout the aging process. We found that “linearly” changing genes were expressed not only in podocytes, but also in other glomerular cells, including endothelial cells, parietal epithelial cells, and mesangial cells. These data therefore support the concept of an aging process affecting all cell types in the glomerulus. This does not mean that the glomerulosclerosis that occurs in aging-associated ESKD is not due in significant part to podocyte failure, as previously suggested,9,10 but it does mean that a more general process affects all glomerular cell types throughout the aging process from young adulthood to old age.
We used NLP tools (Genomatix BiblioSphereTM) to identify biologic pathways represented by genes identified as changing “linearly” with age. Very high Z scores linked alterations in gene expression to both epithelial and endothelial cells and macrophages, as well as to inflammatory, procoagulant, and fibrotic pathways similar to those seen in atherosclerosis. These pathways included cell adhesion, chemotaxis, coagulation, oxidoreductases, matrix metalloproteinases, and TGF-β pathway activation. This result, therefore, supports the concept of a generalized process of proinflammatory, procoagulant, and profibrotic pathways becoming activated during aging that could predispose to accelerated glomerular failure. This is an important and novel concept.
Further analysis using the unbiased NLP strategy established that NFκB could be a major transcription factor that would directly (or indirectly) play a role in causing the patterns of “linearly” altered glomerular gene expression identified. On the basis of this information we then confirmed that NFκB p50 was indeed preferentially translocated to the nuclei in old glomeruli versus young glomeruli, particularly the nuclei of podocytes and parietal epithelial cells. As further proof of NFκB activation we used ChIP analysis to demonstrate that NFκB p50 protein was bound to the regulatory regions of genes identified as altered with aging. The examples used included VCAM and ICAM (genes expressed by endothelial cells), ceruloplasmin (expressed by parietal epithelial cells), and nephrin and Pnoc (expressed by podocytes). A similar result demonstrating age-associated NFκB nuclear translocation has recently been reported for human endothelial cells.28 These results imply that as glomeruli age, NFκB increasingly accumulates in nuclei, particularly in nuclei of long-lived cells such as the podocyte, where it collaborates with other transcription factors to modulate gene expression. Thus, the NFκB transcription factor may play a role in both up-regulating and down-regulating glomerular (including podocyte) gene expression during aging. The relationship between nephrin down-regulation and NFκB activation in podocytes has recently been reported by Kosiell and colleagues.29
As outlined in the introduction of this study, NFκB is now thought to play an important role in the aging process.22–24 NFκB activation by many stimuli can be blocked by antioxidants, thereby demonstrating a key role for redox regulation of NFκB activation, and reactive oxygen species can directly activate IκKα, a key regulator of NFκB nuclear localization and transcriptional activation.30–34 Zheng et al.35 previously reported that old female mouse glomeruli had up-regulated inflammatory pathways, which they attributed to NFκB activation. They found that cultured mesangial cells derived from these old mice continued to express activated NFκB. Therefore, the current report using an in vivo unbiased screening approach independently documents age-associated NFκB activation in the old glomerulus versus the young glomerulus.
This result is entirely consistent with other reports linking NFκB activation to aging,21–24 as well as to NFκB activation in age-associated conditions, including atherosclerosis, diabetic complications, and dementia.36–38 If NFκB activation is a marker of relatively accelerated aging in an individual, and glomerular NFκB activation causing a proinflammatory, procoagulant, and profibrotic state does indeed lead to glomerular failure and ESKD, one would predict that in an aging person there would be associations between various conditions reflected by parallel NFκB activation in other organs. This prediction is borne out by the fact that abnormal renal function and proteinuria are major risk factors for atherosclerotic cardiovascular disease,39,40 and that atherosclerotic cardiovascular disease is >20-fold increased and the major cause of death in ESKD.41 It may therefore not be necessary to look for reasons why abnormal renal function per se might trigger accelerated cardiovascular disease (or vice versa). Rather, mechanisms activating NFκB (such as oxidative stress) could explain the remarkably strong associations between ESKD and atherosclerosis, diabetic complications, and dementia that all result in part from parallel NFκB activation.39–42
Concise Methods
Rat Aging Model
Fischer 344 rats were purchased from the National Institute on Aging at 2, 5 to 7, 15 to 17, and 24 months of age either as calorie-restricted or in the ad libitum-fed state as described previously.10
Glomerular Isolation
Kidneys perfused with cold PBS at 4°C were excised, had the cortex removed, and were diced with a razor blade. The diced tissue was pushed through a 100-μm nylon sieve (Sefar, Briarcliff Manor, NY), and the material that passed through the sieve was collected on a second nylon 75-μm sieve. Glomerular preparations were checked for purity by counting the number of glomerular tufts and tubular fragments. The purity of the glomerular preparations was assessed as the percent of the elements present in the preparation that were glomeruli. The data (mean ± SEM) were as follows: 2-month group: 84% ± 3%; 6-month ad libitum group: 83% ± 1%; 17-month ad libitum group: 81% ± 2%; 24-month: ad libitum group: 81% ± 2%; 6-month calorie-restricted group: 83% ± 2%; 17-month calorie-restricted group: 85% ± 3%; and 24-month calorie-restricted group: 80% ± 3%. There were no statistical differences between these values.
RNA Purification
Isolated glomeruli were lysed in TRIZol (Invitrogen, Carlsbad, CA), followed by phenol extraction, and mRNA was purified using RNeasy kit (Qiagen, Valencia, CA). RNA preparations were evaluated by gel electrophoresis to assess the intactness of major RNA bands as assessed by agarose gel electrophoresis. All preparations showed intact, clear, well-defined RNA bands at 18S and 28S. The A260/A280 ratio was at least 1.8.
DNA Microarrays
Samples were processed (n = 4 for each group, including the common 2-month group, and then at 6, 17, and 24 months for the ad libitum-fed and calorie-restricted groups) by standard protocols using the Affymetrix rat RAE230A and RAE230B chips. These microarrays (Affymetrix, Santa Clara, CA) produce gene expression levels on 31,142 known genes and expressed sequence tags (both microarrays combined). Preparation of cRNA, and hybridization and scanning of the arrays were performed according to manufacturer's protocols and as described previously.10
Statistical Identification of Genes with “Linearly” Altered Expression during Aging
For each probe set on the array, the best linear fit between log scale expression and time (in months) was calculated using least squares. The R2, or proportion of variance explained (PVE), was used to quantify the quality of the linear fit. A PVE of 1 occurs when the data lie exactly on a line with no scatter, and a PVE of 0 occurs when the best-fitting line is a constant function of time. Probe sets of interest were defined as those in which the slope is greater than 2 in magnitude, and the PVE exceeds 0.5. This designation was made separately for the ad libitum-fed or calorie-restricted glomerular datasets. The gene expression data thus identified as monotonically changing over time were used for further analysis and are referred to in the text as “linearly” changing.
Immunohistochemical Analysis
Rats (n = 5 per time point) were anesthetized by ketamine/xylazine injection. They were perfusion fixed with paraformaldehyde/lysine/periodate fixative at 4°C for 8 minutes as described previously.10 Four-micrometer-thick, paraformaldehyde-perfused, formalin-fixed, paraffin-embedded sections were deparaffinized, hydrated, and treated with Retrieve-All-1 target unmasking fluid (Signet Labs, Dedham, MA) for 2 hours at 90°C. Sections were permeabilized with 1% SDS and blocked with 10% goat and 10% rat serum in PBS. Immunofluorescent staining was performed using goat polyclonal antibodies against rat NFκB (Santa Cruz Biotechnology, Santa Cruz, CA). An FITC-conjugated goat anti-mouse secondary antibody was used (Jackson ImmunoResearch, West Grove, PA). Sections were examined using an inverted Leica DM inverted microscope (Bannockburn, IL) and SPOT camera system (Diagnostic Instruments Inc., Sterling Heights, MI).
In Situ Hybridization for Pnoc
Rat cDNA Pnoc clone 99002 (Resgen, Huntsville, AL) was amplified by PCR (Expand High Fidelity; Roche, Indianapolis, IN) using a 5′ primer (with a BamHI restriction site CGCGGATCCATGAAAATCCTGTTTTGT) and a 3′ primer (with an EcoRI restriction site CCGGAATTCCTACACATTACCATTCTG). The digested PCR product was cloned into Bluescript vector (Stratagene, La Jolla, CA). Pnoc DNA probes (sense and antisense) were prepared using T7 and T3 priming sites and were labeled with digoxigenin. Six-micrometer paraformaldehyde/lysine/periodate-fixed cryostat sections mounted on vectabond-treated slides (Vector Labs Inc., Burlington, CA), rinsed twice with 2 × SSC for 3 minutes, treated with 0.25% acetic anhydride in 0.1 M triethanolamine (pH 7.2) for 10 minutes at room temperature, and rinsed again in 2× SSC for 3 minutes. Slides were incubated with 200μl of hybridization buffer containing 50% formamide, 0.3 M NaCl, 20 mM Tris (pH 7), 5 mM EDTA, 10% dextran sulfate, 1× Denhardt solution, and 500 μg/ml yeast tRNA. Slides were prehybridized for 2 hours at 60°C. Hybridization used 0.2 μg of digoxigenin-labeled sense or antisense RNA probe in 200μl of hybridization fluid overnight at 60 °C in a humidified box. Slides were washed (30 minutes) at room temperature in 4× SSC, then at 60°C in 2 × SSC with 50% formamide, 1 × SSC with 50% formamide, and then 0.5× SSC with 50% formamide. Anti-digoxigenin alkaline phosphatase-conjugated antibody was used to detect signal using 5-bromo-4-chloro-3-indolyl phosphate with nitro blue tetrazolium (Dig Nucleic Acid Detection Kit; Roche, Germany). In four consecutive experiments, the sense probe showed no signal, whereas antisense probes showed a clear podocyte cytoplasmic signal. After development of the in situ hybridization product, sections were prepared for superimposed immunofluorescence by treatment with Retrieve-All-1 target unmasking fluid (Signet Labs) for 2 hours at 90°C and processed for immunofluorescence as outlined above.
Nuclear Extracts
Nuclei from isolated glomeruli were isolated by sucrose step gradients as described.8 The nuclei were resuspended in elution buffer (25 mM HEPES (pH 7.8), 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, 25% glycerol, and protease inhibitors) and dounced 10 times with a tight-fitting Type B homogenizer. The samples were rotated for 30 minutes at 4°C and centrifuged at 14,000 × g for 20 minutes at 4°C. The supernatant was dialyzed against Z-buffer (25 mM HEPES (pH 7.8), 100 mM KCl, 12.5 mM MgCl2, 1 mM dithiothreitol, 0.1% NP-40, and 20% glycerol). Samples were aliquoted and stored at −80°C.
Glomerular Protein Extraction and Western Blotting
Protein extracts of glomeruli were made by suspending isolated glomeruli in PBS-containing inhibitors (Protease Inhibitor Cocktail; Roche Diagnostics Inc., Mannheim, Germany) at 10,000 glomeruli per milliliter, followed by homogenizing with RIPA buffer and centrifugation. Extracts were then aliquoted and stored at −80°C until use. Western blot analyses were performed on glomerular extracts (100 μg of protein loaded per lane) and nuclear extracts (5.6 μg loaded per lane) with appropriate antibodies.
ChIP Assays
ChIP was performed in triplicate by a modification of published protocols from Upstate Biotechnology and as described previously.43 Glomeruli were isolated from five 24-month and five 2-month rats. Isolated glomeruli were fixed for 10 minutes at 25°C with 1% formaldehyde in culture medium. Cross-linking was stopped by the addition of glycine to 0.125 M. Isolated glomeruli were washed twice with ice-cold PBS and harvested by centrifugation. The pellet was washed in PBS, resuspended in lysis buffer (5 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (pH 8.0), 85 mM KCl, 0.5% NP-40, and protease inhibitors), incubated at 4°C for 5 minutes, and centrifuged for 5 minutes at 3000 × g. The nuclei were resuspended in nuclear lysis buffer (50 mM Tris-HCl (pH 8.1), 10 mM EDTA, 1% SDS, protease inhibitors) and sonicated on ice with three 25-second pulses using a microtip probe sonicator (Branson Sonifier 250) with output control set to 3. Sonicated lysates were clarified by centrifugation at 4°C for 15 minutes. Twenty micrograms of chromatin was diluted in intraperitoneal dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl (pH 8.1), and 167 mM NaCl) and preclarified with 80 μl of protein A-agarose (Upstate Biotechnology). Each immunoprecipitation was performed with 5 μg of antibody (either rabbit anti-NFκB p50 IgG or nonimmune rabbit IgG as a control). After overnight incubation at 4°C, 60 μl of protein A-agarose was added, and the incubation was continued for 1 hour. The beads were sequentially washed two times in intraperitoneal dilution buffer, two times in TSE-500 wash buffer (0.1% SDS, 1% Triton X-100, 2 nM EDTA, 20 mM Tris-HCl (pH 8.1), and 500 mM NaCl), two times in LiCl buffer (100 mM Tris-HCl (pH 8.1), 500 mM LiCl, 1% NP-40, and 1% Na deoxycholate), and finally two times in Tris-EDTA. Bound complexes were eluted by vortexing beads twice for 15 minutes at 25°C in 250 μl of elution buffer (50 mM Na bicarbonate and 1% SDS). NaCl (5 M) was added to a final concentration of 0.2 M to the pooled eluates, and cross-links were reversed by incubating samples at 65°C overnight. The samples were digested with proteinase K for 1 hour at 56°C, were phenol-chloroform extracted, and DNA was precipitated with 100% ethanol. The precipitated DNA was reconstituted in sterile water, and real-time PCR quantitation of precipitated genomic DNA relative to inputs was performed in triplicate using IQ SYBR GREEN with ROX mastermix (Bio-Rad) in a real-time PCR machine from Applied Biosystems. The comparative CT method was used to determine relative expression compared with input, which was then averaged over four to seven independent experimental animals as described.43
NFκB-Binding Site Localization
GenomatixTM and P-Match on Gene-regulation.com were used to find consensus NFκB sites in the regulatory regions for five genes. The predicted regulatory regions so identified for rat were also evaluated for mouse and human genomes to identify conserved sites and to reduce the risk of false site identification. Sites meeting these criteria were identified in the first 1 kb upstream of the start codon in all four of five genes. The putative nephrin site was approximately 2.5 kb upstream of the start site. Primer pairs approximately 100 bp apart were designed to amplify these sites. ChIP analysis was then performed using the goat polyclonal p50 NFκB antibody. NFκB-binding sites were identified at −162 for VCAM, −179 for ICAM, −306 for ceruloplasmin, −2522 for nephrin, and −125 for Pnoc. PCR primer sets for the NFκB flanking regions and for the upstream and downstream regions from the κB-binding sites as shown in Figure 5 were as follows: Rat VCAM: primer set A, forward: ACTCCTCAACCACCCTTCCT; reverse: AGTGCAAGTTCCCAGACGTT; primer set B, forward: AAAGGGTCTTGCTGGAGAGG; reverse: AGGGTTAACGTGGGGACTTG; primer set C, forward: CCTGTACTCGACAATGAAATCAA; reverse: AGCACAGGACAGACCCAAAG. Rat ICAM: primer set A, forward: CCATAAATATGGGGGTGTGG; reverse: CCGGTGAACACACACTGAAG; primer set B, forward: CCCCCACATGTCATTACTTTCA; reverse: CGGAATGAGGGCTTCGGTA; and primer set C, forward: TGGGCCTCAGGTCTTTGATA; reverse: GAAGGCAGGAGAATCGTGAG. Rat ceruloplasmin: primer set A, forward: GAGGACAGAAATGCACACGA; reverse: GTCAAATGCAGTCCTGACGA; primer set B, forward: GTGCCTGGGATTGCTAGAAG; reverse: AGGGTTCTCTAACTACAAAAG; and primer set C, forward: CCTCCGGATGCAGTTCTTT; reverse: TTAGCATCACCCATTCACCA. Rat prepronociceptin: primer set A, forward: GGGATGCAACCTCCAGTCT; reverse: CATCCATGTCCTCCCTGTCT; primer set B, forward: ACAAAAGGGCCAAGCACCA; reverse: TGGTCTGCATTCTGGGAGAGCA; and primer set C, forward: GGGGCTGTCATCCTCAATAA; reverse: CATGAGCCACAGATGGAGAA. Rat nephrin: primer set A, forward, TCTCCACTCAGTCGTCCCCC; reverse: GCAAAGCAGGTGGGAGGGA; primer set B, forward: AAGGAGAGATGGAGGGGTTG; reverse: AGAGCATCGGGACAGTCAGT; and primer set C, forward: TGTCTCCCAACAATGCTTGA; reverse: AGGTGCTGGTTTTGCATCTC. Note that for VCAM, ICAM, prepronociceptin, and ceruloplasmin, primers A and C are upstream and downstream of the κB site, respectively. In contrast, for nephrin the κB site was 2.5 kb upstream from the start site. Therefore, nephrin primers B and C are both downstream of the κB site.
Antibody Sources
Antibodies were obtained from commercial sources as follows: Santa Cruz Biotechnology: MMP9 (C-20), subcutaneously-6840 affinity-purified goat polyclonal antibody; NFκB p50 (C-19), subcutaneously-1190 goat polyclonal antibody; NFκB p50, subcutaneously-7178 rabbit polyclonal IgG (H-119); VCAM (C-19), subcutaneously-1504, goat polyclonal antibody; ICAM (M-19), subcutaneously-1511 goat polyclonal antibody; and WT1 (F-6), subcutaneously-7385 mouse monoclonal IgG1 antibody. Ceruloplasmin mouse IgG1 monoclonal antibody and Thy1 (CD90) monoclonal antibody (IgG1) were from United States Biological (Swampscott, MA). Rabbit IgG was from Jackson Immunoresearch Laboratories (West Grove, PA).
Disclosures
None.
Acknowledgments
This work was supported by K08 funding from the National Institute for Aging (AG022019), National Institute of Diabetes and Digestive and Kidney Diseases grants P50 DK39253 and P30 DK081943 (to R.C.W., S.R.P., and M.K.) and R01 DK46073 (to R.C.W.), and the Ann Arbor Veterans' Administration Geriatric Research, Education and Clinical Center (to J.E.W.). We wish to acknowledge advice from Dr. Larry Holzman, M.D.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1. U.S. Renal Data System: USRDS Annual Data Report, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, 2007 [Google Scholar]
- 2. Kaplan C, Pasternack B, Shah H, Gallo G: Age-related incidence of sclerotic glomeruli in human kidneys. Am J Pathol 80: 227– 234, 1975 [PMC free article] [PubMed] [Google Scholar]
- 3. Kappel B, Olsen S: Cortical interstitial tissue and sclerosed glomeruli in the normal human kidney, related to age and sex. Virchows Arch A Pathol Anat Histol 387: 271– 277, 1980 [DOI] [PubMed] [Google Scholar]
- 4. Coleman GL, Barthold SW, Osbaldiston GW, Foster SJ, Jonas AM: Pathological changes during ageing in barrier-reared Fischer 344 male rats. J Gerontol 32: 258– 278, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brandis A, Bianchi G, Reale E, Helmchen U, Kuhn K: Age-dependant glomerulosclerosis and proteinuria occurring in rats of the Milan normotensive strain and not in rats of the Milan hypertensive strain. Lab Invest 55: 234– 243, 1986 [PubMed] [Google Scholar]
- 6. Kleinknecht C, Laouari D, Hinglais N, Habib R, Dodu C, Lacour B, Broyer M: Role of amount and nature of carbohydrates in the course of experimental renal failure. Kidney Int 30: 687– 693, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Tapp DC, Wortham WG, Addison JF, Hammonds DN, Barnes JL, Venkatachalam MA: Food restriction retards body growth and prevents end-stage renal pathology in remnant kidneys of rats regardless of protein intake. Lab Invest 60: 184– 195, 1989 [PubMed] [Google Scholar]
- 8. Keenan KP, Coleman JB, McCoy CL, Hoe CM, Soper KA, Laroque P: Chronic nephropathy in ad libitum overfed Sprague-Dawley rats and its early attenuation by increasing degrees of dietary (caloric) restriction to control growth. Toxicol Pathol 28: 788– 798, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Floege J, Hackman B, Kliem V, Kriz W, Alpers CE, Johnson RJ, Kuhn KW, Koch KM, Brunkhorst R: Age-related glomerulosclerosis and interstitial fibrosis in Milan normotensive rats: A podocyte disease. Kidney Int 51: 230– 243, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC: Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the ageing rat: Prevention by calorie restriction. J Am Soc Nephrol 16: 2953– 2966, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Yu P, Masoro E, McMahan A: Nutritional influences on ageing of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol 40: 657– 670, 1985 [DOI] [PubMed] [Google Scholar]
- 12. Maeda H, Gleiser CA, Masoro EJ, Murata I, McMahan CA, Yu BP: Nutritional influences on ageing of Fischer 344 rats: II. Pathology. J Gerontol 40: 671– 688, 1985 [DOI] [PubMed] [Google Scholar]
- 13. Wiggins R: The spectrum of podocytopathies. A unifying view of glomerular diseases. Kidney Int 71: 1205– 1214, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Kenyon C: The plasticity of aging: insights from long-lived mutants. Cell 120: 449– 460, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y: IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421: 182– 187, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Bluher M, Kahn B, Kahn C: Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299: 572– 574, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Longo V, Kennedy B: Sirtuins in aging and age-related disease. Cell 126: 257– 268, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Haigis M, Guarente L: Mammalian sirtuins-emerging roles in physiology, aging, and calorie restriction. Genes Dev 20: 2913– 2921, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Dhahbi J, Kim H, Mote P, Beaver R, Spindler S: Temporal linkage between the phenotypic and genotypic responses to calorie restriction. Proc Natl Acad Sci U S A 101: 5524– 5529, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Melov S, Tarnopolsky M, Beckman K, Felkey K, Hubbard A: Resistance exercise reverses aging in human skeletal muscle. PLoS ONE 2: 185– 190, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Helenius M, Hanninen M, Lehtinen S, Salminen A: Changes associated with aging and replicative senescence in the regulation of transcription factor nuclear factor kappa B. Biochem J 318: 603– 608, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adler A, Sinha S, Kawahara T, Zhang J, Segal E, Chang H: Motif module map reveals enforcement of aging by continual NFκB activity. Genes Dev 21: 3244– 3257, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brink T, Regenbrecht C, Demetrius L, Lehrach H, Adjaye J: Activation of the immune response is a key feature of aging in mice. Biogerentology, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adler A, Kawahara T, Segal E, Chang H: Reversal of aging by NFκB blockade. Cell Cycle 7: 556– 559, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Perkins ND: Integrating cell-signaling pathways with NFκB and IKK function. Nat Rev Mol Cell Biol 8: 49– 62, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Hoffmann A, Natoli G, Ghosh G: Transcriptional regulation via the NFκB signaling molecule. Oncogene 25: 6706– 6716, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Wiggins JE, Goyal M, Wharram B, Wiggins R: The anti-oxidant ceruloplasmin is expressed by glomerular parietal epithelial cells and secreted into the urine in association with glomerular aging and high calorie diet. J Am Soc Nephrol 17: 1382– 1387, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR: Aging is associated with greater nuclear NFkappaB, reduced IkappaBalpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7: 805– 812, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hussain S, Romio L, Saleem M, Mathieson P, Serrano M, Moscat J, Diaz-Meco M, Scambler P, Koziell A: Nephrin deficiency activates NF-kappaB and promotes glomerular injury. J Am Soc Nephrol 20: 1733– 1743, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bubici C, Papa S, Dean K, Franzoso G: Mutual cross-talk between reactive oxygen species and nuclear factor-kappa: Molecular basis and biological significance. Oncogene 25: 6731– 6748, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Scmidt K, Armstad P, Cerutti P, Baeuerle P: The roles of hydrogen peroxide and superoxide as messengers in the activation of transcription factor NFκB. Biol Chem 2: 13– 22, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Gloire G, Legrand-Poels S, Piette J: NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem Pharmacol 72: 1493– 1505, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Pineda-Molina E, Klatt P, Vasquez J, Marina A, de Lacoba M, Perez-Sala D, Lamas S: Glutathionylation of the p50 subunit of NFκB: A mechanism for redox-induced inhibition of DNA binding. Biochemistry 40: 14134– 14142, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Gloire G, Desjardin E, Piette J: Extending the nuclear roles of IκB kinase subunits. Biochem Pharmacol 72: 1081– 1089, 2006, [DOI] [PubMed] [Google Scholar]
- 35. Zheng F, Cheng QL, Plati AR, Ye SQ, Berho M, Banerjee A, Potier M, Jaimes EA, Yu H, Guan YF, Hao CM, Striker LJ, Striker GE: The glomerulosclerosis of aging females: Contribution of the proinflammatory mesangial cell phenotype to macrophage infiltration. Am J Pathol 165: 1789– 1798, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Monaco C, Paleolog E: Nuclear factor kappaB: A potential therapeutic target in atherosclerosis and thrombosis. Cardiovasc Res 61: 671– 682, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Patel JR, Brewer GJ: Age-related differences in NFkappaB translocation and Bcl-2/Bax ratio caused by TNFalpha and Abeta42 promote survival in middle-age neurons and death in old neurons. Exp Neurol 213: 93– 100, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cameron NE, Cotter MA: Pro-inflammatory mechanisms in diabetic neuropathy: Focus on the nuclear factor kappa B pathway. Curr Drug Targets 9: 60– 67, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA: Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 351: 1285– 1295, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Elsayed EF, Tighiouart H, Griffith J, Kurth T, Levey AS, Salem D, Sarnak MJ, Weiner DE: Cardiovascular disease and subsequent kidney disease. Arch Int Med 167: 1130– 1136, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Go A, Chertow G, Fan D, McCulloch C, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events and hospitalization. N Engl J Med 351: 1296– 1305, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Murray A, Tupper D, Knopman D, Gilbertson D, Pederson S, Li G, Smith E, Hochhalter A, Collins A, Kane R: Cognitive impairment in hemodialysis patients is common. Neurology 67: 216– 223, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Patel SR, Levitan I, Kim D, Dressler GR: The BRCT-domain containing protein PTIP links Pax2 to a histone H3, lysine 4 methyltransferase complex. Dev Cell 13: 580– 592, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]