Abstract
Although heat shock protein 72 (HSP72) ameliorates renal tubulointerstitial fibrosis by inhibiting epithelial-to-mesenchymal transition (EMT), the underlying mechanism is unknown. Because Smad proteins transduce TGF-β signaling from the cytosol to the nucleus and HSP72 assists in protein folding and facilitates nuclear translocation, we investigated whether HSP72 inhibits TGF-β-induced EMT by modulating Smad expression, activation, and nuclear translocation. To evaluate the roles of distinct HSP72 structural domains in these processes, we constructed vectors that expressed wild-type HSP72 or mutants lacking either the peptide-binding domain (HSP72-ΔPBD), which is responsible for substrate binding and refolding, or the nuclear localization signal (HSP72-ΔNLS). Overexpression of wild-type HSP72 or HSP72-ΔNLS inhibited TGF-β1-induced EMT, but HSP72-ΔPBD did not, suggesting a critical role for the PBD in this inhibition. HSP72 overexpression inhibited TGF-β1-induced phosphorylation and nuclear translocation of Smad3 and p-Smad3, but not Smad2; these inhibitory effects required the PBD but not the NLS. Coimmunoprecipitation assays suggested a physical interaction between Smad3 and the PBD. siRNA knockdown of endogenous HSP72 enhanced both TGF-β1-induced Smad3 phosphorylation and EMT and confirmed the interaction of HSP72 with both Smad3 and p-Smad3. In vivo, induction of HSP72 by geranylgeranylacetone suppressed Smad3 phosphorylation in renal tubular cells after unilateral ureteral obstruction. In conclusion, HSP72 inhibits EMT in renal epithelial cells primarily by exerting domain-specific effects on Smad3 activation and nuclear translocation.
Transforming growth factor β (TGF-β) and related factors regulate a wide variety of biologic activities.1 Furthermore, abnormalities in TGF-β signaling lead to various human diseases.2 Signals from TGF-β are mainly transduced by the Smad family of transcription factors. Upon TGF-β stimulation, Smad2 and Smad3 are phosphorylated at their carboxy-tails by the activated TGF-β type I receptor kinase,3 forming a stable complex with Smad4 in cytoplasm and then accumulating in the nucleus to regulate transcription of target genes. In contrast, Smad7 inhibits the TGF-β receptor type I-dependent Smad2/3 activation. It has been demonstrated that Smad3 phosphorylation plays a predominant role in guiding the complex through the nuclear pore.4 Moreover, there is compelling evidence that Smads actively shuttle between the nucleus and the cytoplasm.5 Blockade of TGF-β signaling events, specifically the phosphorylation of Smad2 and Smad3 and/or nuclear translocation, prevent epithelial-to-mesenchymal transition (EMT) and tissue fibrosis.6,7
Heat shock protein 72 (HSP72) exerts cytoprotective effects by assisting in protein folding, assembly/disassembly and translocation of client proteins across membranes, protein degradation, and signal transduction.8,9 However, some cytoprotective effects do not require the HSP72 chaperone function.10 HSP72 contains distinct domains: a C-terminal peptide-binding domain (PBD) responsible for substrate binding and refolding, and a nuclear localization signal (NLS) that regulates HSP72 nuclear accumulation under stress conditions.11,12 It is evident that the PBD of HSP72 prevents apoptosis by inhibiting Bax activation, preventing mitochondrial cytochrome c and apoptosis-inducing factor release and subsequent caspase activation.13–15 Although nuclear localization of HSP72 is associated with cytoprotection through interacting with stress-induced protein aggregates in the nucleus,11,16–19 mutation of the NLS alters nuclear accumulation of HSP72 with stress but does not reduce cell viability.11,18 Therefore, some cytoprotective functions of HSP72 are independent of its nuclear localization.
Recently, we reported that HSP72 ameliorates renal tubulointerstitial fibrosis in chronic obstructive nephropathy by inhibiting tubular epithelial cell apoptosis and EMT.10 However, the mechanisms by which HSP72 regulates EMT have not been determined. Because Smad3 transduces TGF-β signaling from the cytosol to the nucleus,6 and HSP72 acts as a chaperone that facilitates the movement of proteins across the nuclear membrane,13,19,20 we hypothesized that HSP72 inhibits TGF-β-induced EMT by interrupting Smad protein phosphorylation and its nuclear translocation and accumulation.
Results
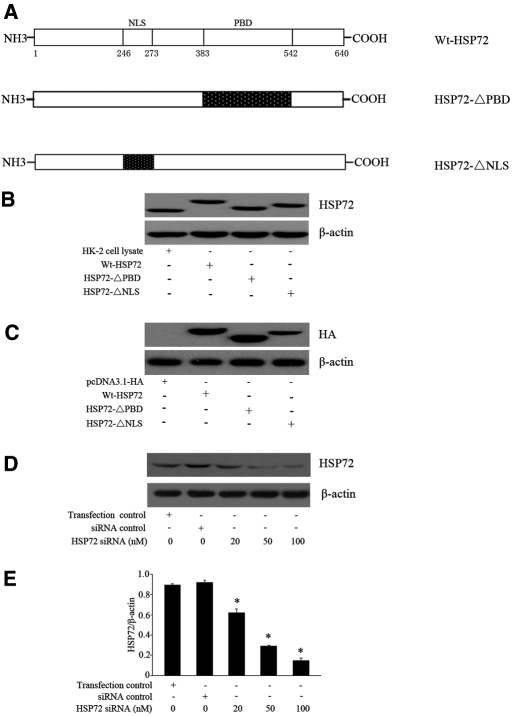
Expression of Wt-HSP72 and HSP72 Mutants and HSP72 siRNA Knockdown
Our previous studies showed that endogenous HSP72 could not be detected by routine immunoblot analysis in NRK-52E cells.10 To study the role of HSP72 in regulating EMT, NRK-52E cells were transiently and individually transfected with plasmid vectors encoding HA-epitope tagged-Wt-HSP72, HA-HSP72-ΔPBD, or HA-HSP72-ΔNLS (Figure 1A), and pcDNA3.1-HA empty vector was used as control. Previously characterized human kidney proximal tubule cells (HK-2) in which endogenous HSP72 is readily detected was used as an additional control.21 As shown in Figure 1B, transfection with Wt-HSP72, HSP72-ΔPBD, and HSP72-ΔNLS increased their respective contents in NRK-52E cells. As expected, these constructs migrated at a similar apparent molecular weight. Immunoblot analysis using antibodies directed against the HA-tag confirmed the presence of each HSP72 construct in NRK-52E cells (Figure 1C). To downregulate the overexpressed HSP72, NRK-52E cells were transfected with HA-Wt-HSP72 followed by treatment with a specific HSP72 small interfering RNA (siRNA). Consistent with our previous results,10 HSP72 siRNA treatment decreased HSP72 expression in a dose-dependent manner (Figure 1, D and E).
Figure 1.
Wt-HSP72, HSP72 domain mutant, and HSP72 siRNA expressed in NRK-52E cells. (A) Schematic representation of HSP72 functional domains. Western blot analysis of HSP72 content in cells transfected with empty-vector, wild-type HSP72, HSP72-ΔPBD, or HSP72-ΔNLS constructs using (B) an anti-HSP72 antibody, (C) an anti-HA antibody, or (D) after transfection with either scrambled siRNA or varying doses of HSP72 siRNA. (E) NRK-52E cells were transfected with HSP72 siRNA at 20, 50, or 100 nM. HSP72 levels were determined by Western blot analysis and expressed as mean ± SEM of three separate experiments performed in triplicate; *P < 0.01 versus siRNA or transfection control.
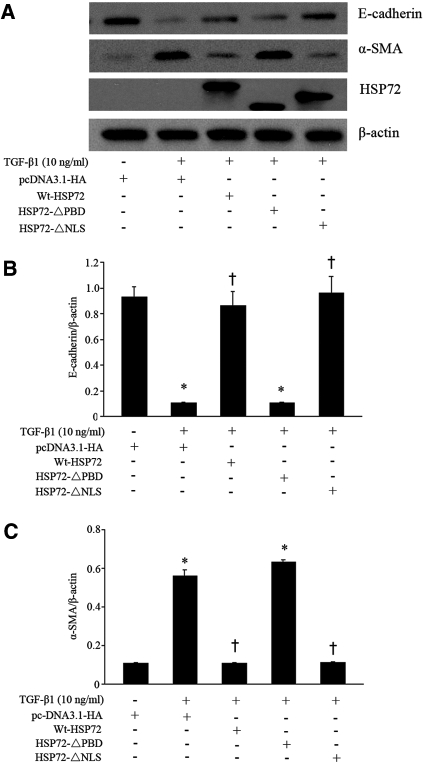
HSP72-ΔPBD Failed to Attenuate TGF-β1-induced EMT
Because the major function of HSP72 is to regulate protein binding and folding, it would be of interest to examine whether its chaperone function is required to inhibit EMT. Overexpressed Wt-HSP72 repressed TGF-β1-induced EMT in NRK-52E cells. Expression of the HSP72-ΔNLS mutant showed a similar degree of inhibitory effect on EMT as did Wt-HSP72 (Figure 2). Expression of HSP72-ΔPBD failed to inhibit TGF-β1-induced EMT. Notably, expression levels of HSP72 were similar in cells expressing wild-type or both HSP72 deletion mutants. Empty vector alone did not alter the expression of E-cadherin or α-smooth muscle actin (α-SMA) in TGF-β1 treated cells. In the absence of TGF-β1, neither Wt-HSP72 nor HSP72 siRNA changed the steady-state content of E-cadherin or α-SMA (data not shown). On the basis of these results, we propose that the functional HSP72 PBD inhibits TGF-β1-induced EMT.
Figure 2.
HSP72 abrogates EMT. (A) Wild-type or HSP72 mutants were overexpressed in NRK-52E cells before treatment with 10 ng/ml of TGF-β1 for 48 hours. Cell lysates were analyzed by immunoblotting with antibodies against E-cadherin, α-SMA, or HSP72. β-actin served as a loading control. (B, C) Densitometric analysis of the effect of HSP72 on E-cadherin and α-SMA expression. Expression of these proteins was normalized to β-actin content in cells treated as described in panel A. Data are expressed as mean ± SEM; n = 3 per treatment; *P < 0.01 versus empty vector; †P < 0.05 versus TGF-β1-treated empty-vector cells.
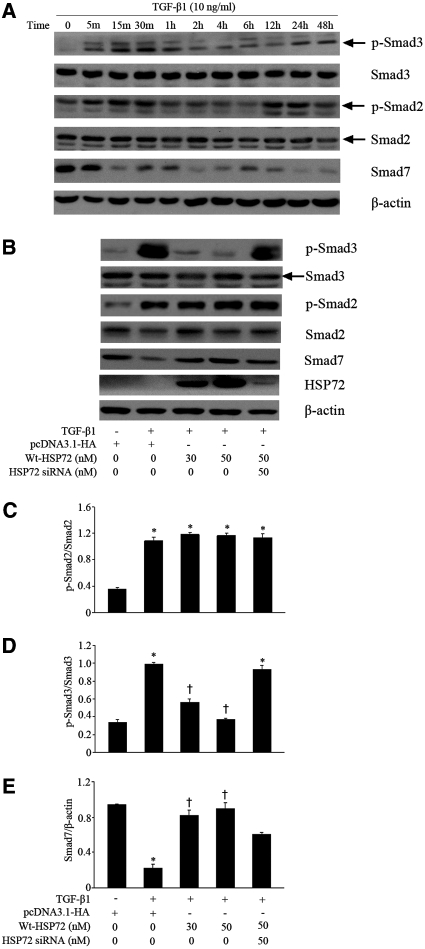
HSP72 Inhibited Activation of the TGF-β1/Smads Pathway
To elucidate the mechanism by which HSP72 inhibits TGF-β1-induced EMT, the role of HSP72 in regulating of Smad2/Smad3 phosphorylation and Smad7 expression was investigated in NRK-52E cells. Exposure of cells to TGF-β1 resulted in phosphorylation of Smad3, which was detected as early as 5 minutes, peaked at 30 minutes, slightly decreased by 1 hour, and then increased again between 24 and 48 hours. Similar results were detected for Smad2 phosphorylation after TGF-β1 exposure. Smad7 protein content was decreased at 15 minutes after TGF-β1 treatment and remained at the low levels thereafter (Figure 3A).
Figure 3.
HSP72 suppresses activation of the TGF-β/Smads pathway. (A) Serum-deprived NRK-52E cells were treated with 10 ng/ml of TGF-β1 for the indicated time period. Cell lysates were probed with antibodies against p-Smad3, Smad3, p-Smad2, Smad2, Smad7 or β-actin. (B) NRK-52E cells were transfected with pcDNA3.1-HA-Wt-HSP72 (30 and 50 nM) or specific HSP72 siRNA were stimulated with 10 ng/ml of TGF-β1 for 30 minutes. (C through E) Smad protein content evaluated by Western blotting with densitometric analysis of the effect of HSP72 expression on p-Smad3, p-Smad2 and Smad7 content normalized with Smad2, Smad3, or β-actin content in TGF-β1-treated cells. Data are expressed as mean ± SEM; n = 3 per treatment; *P < 0.01 versus negative control; †P < 0.05 versus TGF-β1-treated cells without HSP72 overexpression or HSP72 siRNA.
Because phosphorylated Smad3 content peaked at 30 min, this time point was used in subsequent experiments. Compared with empty vector, HSP72 overexpression inhibited Smad3 phosphorylation and simultaneously increased Smad7 content in a dose-dependent manner (Figure 3B). Treatment with HSP72-specific siRNA eliminated these responses. However, HSP72 expression did not block TGF-β1-induced Smad2 phosphorylation (Figure 3, B and C). Quantitative analysis revealed that transfection of HSP72 at a dose of 50 nM reduced p-Smad3 levels by 63% (Figure 3D) and increased Smad7 content by 75% (Figure 3E). Without TGF-β1 exposure, neither HSP72 overexpression nor HSP72 siRNA treatment changed the steady-state content of Smad2, Smad3, or Smad7 and did not affect the phosphorylation of Smad2/3 in NRK-52E cells (data not shown). These results demonstrate that HSP72 inhibits Smad3 but not Smad2 phosphorylation and reverses TGF-β1-induced depression of Smad7 content.
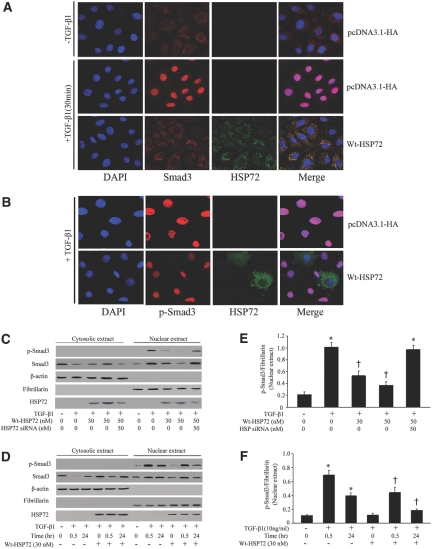
HSP72 Attenuated TGF-β1-induced p-Smad3 Nuclear Translocation
Because phosphorylation of Smad3 and its subsequent nuclear translocation are critical steps in TGF-β signaling,22 the effect of HSP72 on p-Smad3 nuclear translocation and accumulation was examined. In the absence of TGF-β1 exposure, Smad3 was distributed primarily in the cytoplasm and weakly in the nucleus (Figure 4A). Upon TGF-β1 stimulation, most Smad3 accumulated in the nucleus. Overexpression of HSP72 decreased the content of the nuclear Smad3. Colocalization of Smad3 and HSP72 indicates their physical interaction. The primary distribution and nuclear translocation of p-Smad3 under the above conditions are virtually identical to those observed for Smad3 (Figure 4B). To confirm those observations in intact cells, nuclei were isolated after TGF-β1 exposure. Consistent with immunofluorescence results, Smad3 was predominantly cytoplasmic under baseline conditions. TGF-β1 treatment resulted in Smad3 and p-Smad3 nuclear translocation and accumulation as well as a corresponding decrease in their cytoplasmic content. Transient transfection of Wt-HSP72 increased steady-state HSP72 content in the cytoplasm and nucleus and also attenuated TGF-β1-stimulated nuclear p-Smad3 accumulation in dose- and time-dependent manners (Figure 4C through 4F). Quantitative analysis revealed that 50 nM HSP72 inhibited TGF-β1-induced nuclear accumulation of p-Smad3 by 54% (Figure 4E). This HSP72-mediated effect persisted for at least 24 hours (Figure 4F). In contrast, knockdown of HSP72 by siRNA blocked the inhibitory effect of Wt-HSP72 on TGF-β1-induced p-Smad3 nuclear translocation. HSP72 overexpression did not affect p-Smad2 nuclear translocation (data not shown). Therefore, inhibition of EMT by HSP72 is associated with suppression of Smad3 activation and nuclear accumulation.
Figure 4.
HSP72 blocks p-Smad3 nuclear translocation and accumulation in a time- and dose-dependent manner. (A) NRK-52E cells were treated with 10 ng/ml of TGF-β1 for 30 minutes. Representative confocal microscopic images showed the cellular localization of Smad3 (red) and HSP72 (green) by indirect immunofluorescence staining in cells. Original magnification × 400. (B) The cellular localization of p-Smad3 was examined by confocal microscopic images of p-Smad3 (red), HSP72 (green), and nuclear staining (blue) in empty-vector control and HSP72 overexpressing cells after 10 ng/ml of TGF-β1 exposure for 24 hours. Original magnification × 400. (C) NRK-52E cells were treated with wild-type HSP72 (30 and 50 nM) or HSP72 siRNA after 10 ng/ml of TGF-β1 exposure for 30 minutes. Empty vector served as negative control. Smad3 and p-Smad3 nuclear translocation and accumulation were assessed by Western blot analysis. (D) Wild-type HSP72 or HSP72 siRNA were individually expressed in NRK-52E cells before treatment with 10 ng/ml of TGF-β1 for different time periods before the assessment of p-Smad3 nuclear translocation and accumulation. Empty vector served as negative control. Smad3 and p-Smad3 nuclear translocation and accumulation were assessed by Western blot analysis. (E, F) Quantitative determination of the relative abundance of p-Smad3 nuclear accumulation among different groups. Data are expressed as mean ± SEM of three experiments. *P < 0.01 versus negative control; †P < 0.05 versus TGF-β1-treated cells without HSP72 overexpression or HSP72 siRNA.
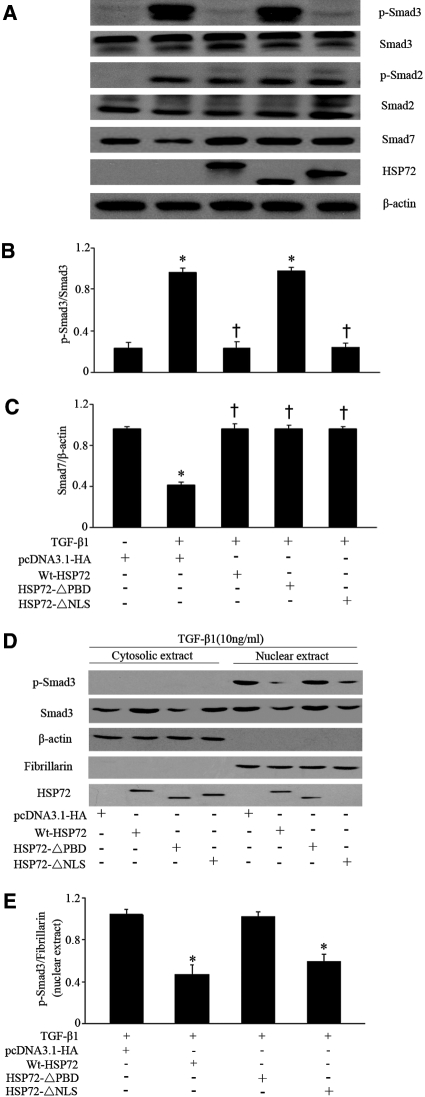
Prevention of TGF-β1-Induced Smad3 Phosphorylation and Nuclear Translocation by HSP72 Required Its Intact Chaperone Function
Because the PBD of HSP72 is required to inhibit EMT (Figure 2), the role of the PBD on Smad activation and expression was examined. Wt-HSP72 and both HSP72 mutants inhibited TGF-β1-induced Smad7 downregulation (Figure 5A), although neither Wt-HSP72 nor its mutants altered Smad7 steady-state content at baseline (data not shown). Only Wt-HSP72 and HSP72-ΔNLS, but not HSP72-ΔPBD, inhibited Smad3 phosphorylation after TGF-β1 exposure (Figure 5, A and B). These data suggest that the PBD, but not the NLS, is required for HSP72 to inhibit Smad3 phosphorylation. However, this domain is dispensable for upregulating Smad7 expression.
Figure 5.
PBD is required for HSP72 to prevent Smad3 activation. (A) NRK-52E cells were transiently transfected with plasmids encoding Wt-HSP72, HSP72-ΔPBD, or HSP72-ΔNLS followed by incubation with 10 ng/ml of TGF-β1 for 30 minutes. Smad protein levels were examined by Western blotting. (B, C) p-Smad3 and Smad7 contents were quantitatively analyzed using a densitometer. Values are mean ± SEM; n = 3 per treatment. *P < 0.05 versus negative control; †P < 0.05 versus TGF-β1-treated cells without HSP72 overexpression. (D) p-Smad3 nuclear translocation and accumulation were assessed by Western blot analysis after overexpression of Wt-HSP72, HSP72-ΔPBD, and HSP72-ΔNLS, respectively. Empty vector served as negative control. (E) Quantitative determination of the relative abundance of p-Smad3 nuclear accumulation among different groups. Data are expressed as mean ± SEM of three experiments. *P < 0.01 versus empty-vector control.
Because HSP72 localization to the nucleus is dependent on its NLS, HSP72-ΔNLS did not accumulate in the nucleus (Figure 5D). Quantitative analysis showed that Wt-HSP72 and HSP72-ΔNLS, but not HSP72-ΔPBD, significantly blocked TGF-β1-induced nuclear translocation of p-Smad3 (Figure 5, D and E). Therefore, these results show that HSP72 likely suppresses p-Smad3-mediated gene transcription primarily by preventing p-Smad3 nuclear translocation. In addition, the HSP72 PBD is critical for inhibiting Smad3 activation and subsequent nuclear translocation.
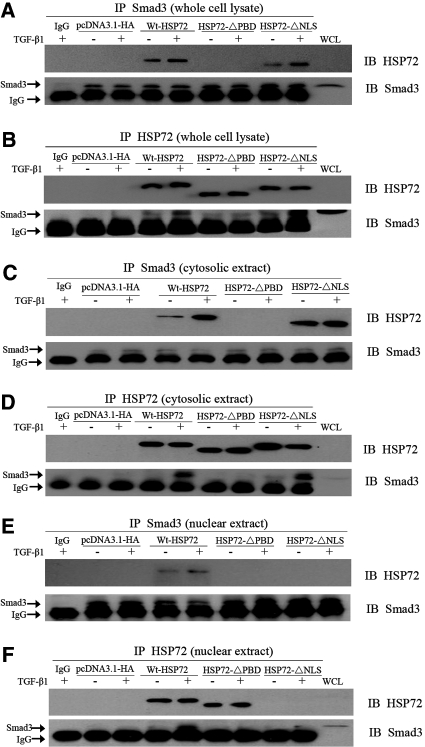
The PBD of HSP72-Mediated Smad3 Interaction
The PBD of HSP72 mediates its interaction with many client proteins and could be responsible for binding Smad3. To test this hypothesis, coimmunoprecipitation assays were performed. Compared with empty vector, overexpression of Wt-HSP72 or HSP72-ΔNLS significantly increased the interaction between HSP72 and Smad3 under all experimental conditions (Figure 6, A and B). This interaction was no longer detected in cells that expressed HSP72-ΔPBD. The amount of immunoprecipitable Smad3 (Figure 6A, lower panel) or HSP72 (Figure 6B, upper panel) was identical in cells expressing various forms of HSP72, excluding the possibility that differing amounts HSP72 or Smad3 in the immunoprecipitates were responsible for these results. Thus, these results indicate that the functional PBD is required for HSP72 interaction with Smad3.
Figure 6.
HSP72 interacts with Smad3. (A) Smad3 was immunoprecipitated from whole NRK-52E cell lysates using a rabbit polyclonal anti-Smad3 antibody. After separation by 7% SDS-PAGE, HSP72 content was assessed in cells that overexpressed either wild-type or mutant HSP72 using an anti-HSP72 antibody (upper panel). Immunoblot analysis (IB) was used to localize Smad3 in cell lysates (WCL, right-hand lane, lower panel). IgG was used as negative IP control (first lane). Results are representative of at least three separate experiments. (B) Whole NRK-52E cell lysates were immunoprecipitated with antibody directed against HSP72. After SDS-PAGE, the immunoprecipitates were probed with antibodies directed against Smad3 and HSP72. (C, D) HSP72 and Smad3 in cytosolic extracts were immunoprecipitated with antibodies against either Smad3 or HSP72. (E, F) Nuclear extracts were isolated and Smad3 or HSP72 were immunoprecipitated using the indicated antibodies. Immunoprecipitates were probed with antibodies directed against Smad3 or HSP72.
To further determine the location of the interaction of these two proteins, coimmunoprecipitation of HSP72 and Smad3 was also performed in cytosolic and nuclear extracts harvested from NRK-52E cells. Although interaction of HSP72 and Smad3 was detected in the cytoplasm (Figure 6, C and D) and nucleus (Figure 6, E and F) of cells expressing Wt-HSP72 and was also detected in the cytosol of HSP72-ΔNLS-expressing cells, there was no measurable interaction in the cytoplasm or nucleus of cells expressing HSP72-ΔPBD. This absence of interaction between HSP72-ΔPBD and Smad3 in the nuclear extract is expected. Taken together, these data indicate that the HSP72 PBD mediates interaction with Smad3 and is responsible for disrupting Smad3 activation, nuclear translocation, and subsequent suppression of EMT.
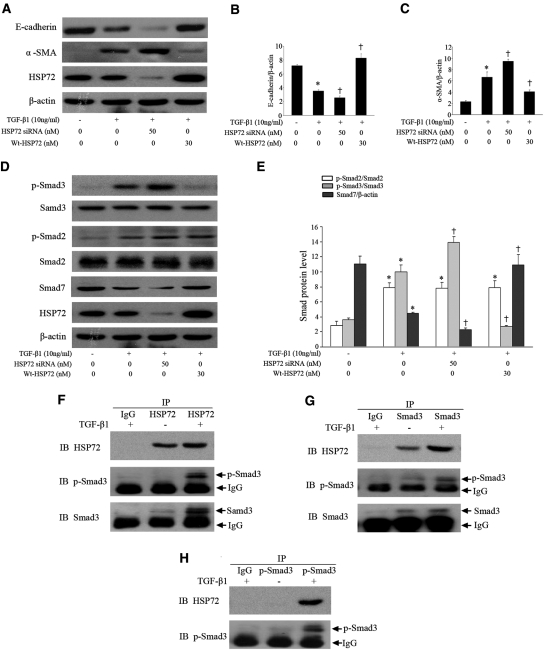
Endogenous HSP72 Suppressed EMT through Blockage of Smad Signaling
To investigate whether endogenous HSP72 affects Smad signaling and EMT, we knocked down endogenous HSP72 using siRNA or increased HSP72 content in HK-2 cells. Compared with HSP72 siRNA treatment, the presence of moderate endogenous HSP72 inhibited TGF-β1-induced EMT (Figure 7A) as evidenced by increasing E-cadherin by 27% and decreasing α-SMA expression by 30% (Figure 7, B and C). Endogenous HSP72 blocked Smad3 phosphorylation by 39% and increased Smad7 levels by 48% (Figure 7, C and D). Induction of exogenous Wt-HSP72 demonstrated the significant additional inhibitory effects on TGF-β1-induced Smad signaling and EMT. Endogenous HSP72 interacted with Smad3/p-Smad3 after TGF-β1 stimulation (Figures 7F through 7H). Of note, HSP72 overexpression and HSP72 siRNA treatment per se did not affect steady-state levels of E-cadherin, α-SMA, and Smads and phosphorylation of Smad2/3 in HK-2 cells without TGF-β1 exposure (data not shown). Collectively, these results are consistent with our observations in NRK-52E cells that HSP72 attenuates TGF-β1-induced EMT by blocking Smad3 phosphorylation.
Figure 7.
Endogenous HSP72 suppresses Smad signaling and EMT. (A) HK-2 cells transfected with pcDNA3.1-HA-Wt-HSP72 or specific HSP72 siRNA were exposed to 10 ng/ml of TGF-β1 for 48 hours. Western blotting was carried out as described in Figure 2A. (B, C) Graphic representation of densitometric quantification of the protein bands in panel A with E-cadherin or α-SMA normalized to the corresponding β-actin levels. Data are expressed as mean ± SEM; n = 3 per treatment; *P < 0.01 versus negative control; †P < 0.05 versus TGF-β1-treated cells alone. (D) Cells were treated and described as above. Expression of Smad2, Smad3, and Smad7 and phosphorylation of Smad2 and Smad3 were assayed by Western blot analyses. (E) Protein levels were quantified with scanning densitometry and normalized with Smad2, Smad3, or β-actin. Data are expressed as mean ± SEM of three independent experiments; *P < 0.01 versus negative control; †P < 0.05 versus TGF-β1-treated cells alone. (F through H) Coimmunoprecipitation of endogenous HSP72 and Smad3 or p-Smad3. Levels of HSP72, Smad3, and p-Smad3 in the IP products were analyzed by Western blotting.
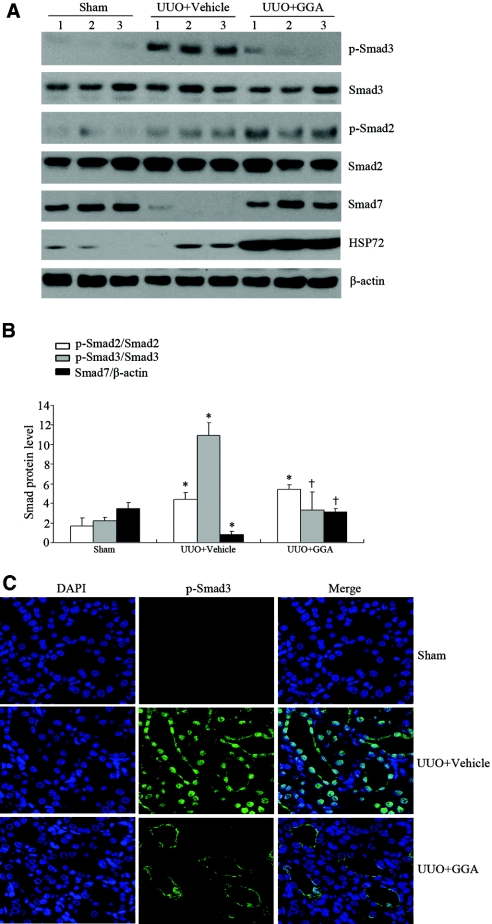
HSP72 Blocks Smad Signaling in the Obstructed Kidney Induced by Unilateral Ureteral Obstruction
Our previous studies demonstrated that selective overexpression of HSP72 by geranylgeranylacetone (GGA) prevented renal interstitial fibrosis in a rat unilateral ureteral obstruction (UUO) model.10 To evaluate whether HSP72 plays a role in the regulation of Smad signaling in vivo, we examined Smad proteins at 7 days after UUO. UUO induced Smad2 and Smad3 phosphorylation and reduced Smad7 content compared with sham controls. Induction of HSP72 expression by GGA significantly inhibited Smad3, but not Smad2, phosphorylation and increased Smad7 content (Figure 8, A and B). Similarly, immunofluorescence staining revealed that overexpressed HSP72 inhibits Smad3 activation in renal tubule cells (Figure 8C). Therefore, these results suggest that HSP72 prevents renal fibrosis in vivo by inhibiting Smad signaling.
Figure 8.
HSP72 inhibits Smad signaling in vivo. (A) Western blot analysis of Smad2, p-Smad2, Smad3, p-Smad3, and Smad7 protein levels from sham, vehicle, and GGA-treated rat kidneys after UUO. Numbers (1, 2, and 3) denote each individual animal within a given group. (B) Densitometric quantification of the corresponding bands was performed using an image analyzer. The data are presented after normalization to Smad2, Smad3, or β-actin expression and expressed as mean ± SEM. *P < 0.05 versus sham; †P < 0.05 versus vehicle. (C) Representative micrographs of p-Smad3 immunofluorescence in different experimental groups as indicated. Original magnification × 400.
Discussion
The study presented here was undertaken to address whether HSP72 suppresses TGF-β1 mediated EMT via its chaperone activity and, if so, to elucidate the underlying mechanisms. By transiently expressing Wt-HSP72, mutants of HSP72, or HSP72 siRNA in NRK-52 cells, we demonstrated that Wt-HSP72 and HSP72-ΔNLS, but not HSP72-ΔPBD suppresses TGF-β1-triggered EMT, suggesting that the PBD of HSP72 is essential for inhibiting EMT in renal tubular epithelial cells. The PBD is also required for HSP72-Smad3 interaction and subsequent inhibition of TGF-β1-induced Smad3 activation. Furthermore, endogenous HSP72 suppressed Smad3 phosphorylation in cultured HK-2 cells and renal tubule cells of rat UUO kidneys. These findings provide significant insight into the mechanism by which HSP72 inhibits TGF-β1-mediated EMT and emphasize the importance of the PBD in mediating this cytoprotective effect.
HSP72 is an abundant, inducible molecular chaperone. Previous studies on the protective effects of HSP72 against various insults have focused mainly on acute injury models and emphasized that HSP72 possesses potent antiapoptotic property in vivo and in vitro.23,24 However, the potential role of HSP72 in regulating the chronic tissue damage is less well studied. Recently, HSP72 was shown to be upregulated in some chronic diseases.25–27 Our recent study, as well as reports by others, showed that HSP72 suppresses EMT and fibrosis,10,28–30 critical processes that promote chronic renal injury.
Although EMT occurs under several pathologic conditions and is regulated by multiple pathways, TGF-β-mediated activation of Smad signaling plays a critical role in EMT.31 Specifically, TGF-β stimulation promotes Smad2 and Smad3 phosphorylation, Smad complex formation, and nuclear accumulation that ultimately result in the induction of a profibrotic gene expression program. Smad7 negatively regulates the TGF-β signaling pathway.5 Therefore, disruption of Smad2/Smad3 activation/translocation, and/or upregulation of Smad7 would be expected to inhibit TGF-β signaling and subsequent EMT.
Given the fact that HSP72 antagonizes EMT,10,28 we examined the effect of HSP72 on TGF-β1-induced Smad2/3 activation and nuclear translocation and Smad7 expression. Our results showed that specific HSP72 domain(s) suppress EMT and the activation of Smad signaling. In fact, the PBD of HSP72 is required for inhibition of TGF-β1-induced Smad3 activation and nuclear translocation. In contrast, the NLS of HSP72 is dispensable for the anti-EMT effect because HSP72 lacking this domain is capable of inhibiting Smad3 activation, p-Smad3 nuclear translocation, and EMT. Despite HSP72 interaction with Smad3 and p-Smad3, it is not entirely clear whether HSP72 interferes with p-Smad3 nuclear migration in a manner that is independent of its effect on Smad3 phosphorylation. It is conceivable that HSP72 at least in part inhibits TGF-β1-induced p-Smad3 nuclear translocation and accumulation through interfering with its phosphorylation. The effects of HSP72 on EMT appear to rely on directly inhibiting Smad3 activation and its downstream events, rather than on upstream Smad7 induction, because HSP72-ΔPBD failed to suppress TGF-β1-triggered EMT despite increased expression of Smad7. Taken together with prior studies,23,24 these results emphasize the diverse, domain-specific effects of HSP72 in mediating cytoprotection in the TGF-β1-induced EMT signaling pathway.
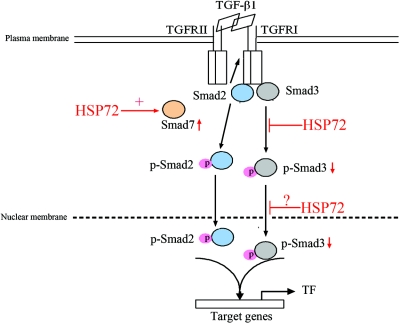
HSP72 has been shown to bind to various kinases and signaling molecules and thus may have effects on several aspects of cell regulation.24,32–34 In the study presented here, Wt-HSP72 and HSP72-ΔNLS in inhibiting TGF-β1-induced EMT provide important clues how HSP72 interacts with the TGF-β signaling pathways. Our immunoprecipation experiments confirm a regulatory role of HSP72 in Smad signal events. Wt-HSP72 interacted with Smad3 in the cytoplasm and the nucleus. However, the HSP72-ΔNLS maintained a high affinity for Smad3 in the cytoplasm but not in the nucleus, implying that the NLS is critical for mediating the nuclear interaction between HSP72 and Smad3. Enhanced HSP72 expression prevents TGF-β1-triggered p-Smad3 nuclear accumulation, most likely by binding and sequestering it in the cytoplasm. Consistent with this hypothesis, we demonstrated that the interaction between wild type or HSP72-ΔNLS and Smad3 was greater after TGF-β1 exposure. Deletion of HSP72 PBD completely disrupted HSP72-Smad3 interaction in the cytoplasm and the nucleus. However, the Smad3 domain that mediates interaction with HSP72 is presently unknown. Together, these observations suggest that HSP72 limits nuclear p-Smad3 accumulation by reducing Smad3 activation and sequestering p-Smad3 in the cytoplasmic and nuclear compartments, as depicted in Figure 9.
Figure 9.
Simplified schematic shows TGF-β signal transduction and postulated mechanisms of inhibition by HSP72. Overexpression of HSP72 inhibits EMT by decreasing Smad3 phosphorylation, its subsequent nuclear translocation, and maybe in a lesser degree by increasing Smad7 protein expression.
Endogenous HSP72 physically interacts with Smad3 and p-Smad3 after TGF-β1 stimulation. Knockdown of endogenous HSP72 increased TGF-β1-induced Smad3 phosphorylation and reduced Smad7. A reversal outcome was achieved by reintroduction of exogenous HSP72. Similarly, specific overexpressed HSP72 by GGA significantly inhibited Smad3 phosphorylation and increased Smad7 content in the obstructed kidneys caused by UUO. This further supports the notion that the HSP72 protective effect is specific for Smad signaling in vitro and in vivo.
In summary, our data demonstrate for the first time that HSP72 plays a critical role in protecting the renal epithelial cell from EMT, and this cytoprotective effect is dependent on the HSP72 PBD. The regulatory effects of HSP72 on Smad proteins are isoform-specific and involve reciprocal changes in Smad3 and Smad7. In view of its ability to interfere with multiple checkpoints in the TGF-β signaling pathway, manipulation of HSP72 may be useful for preventing the onset and/or progression of renal fibrosis.
Concise Methods
Materials
Reagents were purchased from the following vendors: human recombinant TGF-β1 was from R&D Systems (Minneapolis, MN); anti-phospho-Smad3 (p-Smad3, Ser423/425), anti-phospho-Smad2 (p-Smad2, Ser465/467), anti-Smad2, anti-Smad3, and anti-Smad7 from Cell Signaling Technology (Beverly, MA); anti-E-cadherin antibody and anti-HA tag antibody from BD (Biosciences Pharmingen, San Jose, CA); anti-α-SMA was from DAKO (Cupertino, CA); anti-HSP72 from Stressgen Biotechnologies (Victoria, British Columbia, Canada); and anti-fibrillarin from Abcam (Cambridge, MA); anti-β-actin from Boster (Wuhan, China). Horseradish peroxidase (HRP)-conjugated anti-mouse IgG, HRP-conjugated anti-rabbit IgG, Alexa Fluro 546-conjugated anti-rabbit IgG, and Alexa Fluro 488-conjugated anti-mouse IgG were purchased from Cell Signal Technology (Beverly, MA); pcDNA3.1-HA mammalian expression vector was from Invitrogen Life Technologies (Paisley, United Kingdom). Scrambled and HSP72-specific siRNA products were from Shanghai GenePharma Co., Ltd (Shanghai, China), and Lipofectamine 2000 transfection reagent was from Invitrogen Life Technologies (Paisley, United Kingdom). Restriction enzymes were purchased from TaKaRa Biotechnology Co., Ltd (Dalian, China). Protein A/G agarose and NE-PER nuclear and cytoplasmic extraction reagents were from Pierce Biotechnology (Rockford, IL). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Culture
A normal rat kidney proximal tubular epithelial cell line (NRK-52E) and an immortalized proximal tubule epithelial cell line from normal adult human kidney (HK-2) were purchased from American Type Culture Collection (Rockville, MD). Cells were cultured at 37°C in a 5% carbon dioxide atmosphere in DME medium mixed 1:1 (vol:vol) with F12 medium (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% FBS. Cells were grown to approximately 70% to 80% confluence and subjected to serum-deprivation for 24 hours before experimental manipulation.
Plasmid Constructs, siRNA Inhibition, and Transfection
A full-length human HSP72 gene from Genebank (NM005345) was generated by PCR and subcloned into the pcDNA3.1-HA mammalian expression vector (Wt-HSP72) at the restriction enzyme sites of EcoR1/XohI. Mutants of the HSP72 lacking either PBD 383 to 542 (HSP72-ΔPBD) or NLS 246 to 273 (HSP72-ΔNLS) were constructed by insertion in the pcDNA3.1-HA vector. The identity of all HSP72 constructs was confirmed by sequencing before use. pcDNA3.1-HA empty vector was used as control. HSP72 siRNA knockdown was performed by transient transfection with validated HSP72 siRNA (sense, CCGUGCCCGCCUACUUCAATT; antisense, UUGAAGUAGGCGGGCACGGTG). Scrambled RNAi was used as control. For determination of the efficiency of HSP72 knockdown, Western analysis for HSP72 was performed. Several concentrations of HSP72 siRNA (20 to 100 nM) were tested to determine the optimal knockdown conditions as described previously.10 Lipofectamine 2000 and either siRNA or plasmids were separately diluted in serum-free medium and incubated at room temperature for 5 minutes, then mixed and incubated at room temperature for a further 20 minutes. Aliquots of the transfection mixture were added to cell culture dishes. Medium was replaced with fresh DME/F12 medium containing 10% FBS and cultured for another 24 hours after 4-hour transfection. The cells were then subjected to serum-deprivation for 24 hours before treated with or without TGF-β1.
Preparation of Cell Fraction
Harvested cells were resuspended in cell lysis buffer and then sonicated followed by centrifugation at 10,000 × g for 5 minutes at 4°C. The supernatant was designated as the whole cell lysate. At the indicated time after TGF-β1 treatment, stepwise separation and preparation of cytoplasmic and nuclear extracts from cultured cells were performed by using a commercial kit following the manufacturer's instructions. In brief, cytoplasmic extraction reagents I and II were added to a cell pellet to disrupt cell membranes, releasing cytoplasmic contents. The integrity of the nuclei was verified by examination with light microscopy. After recovering the intact nuclei from the cytoplasmic extract by centrifugation, the nuclei were lysed with a nuclear extraction reagent to yield the nuclear extract. Immunoblot analysis was used to assess the adequacy of nuclear purification by measuring fibrillarin (a nuclear protein) and β-actin (cytoplasm protein) content. The protein content of the cytoplasmic and nuclear extracts was determined using the Bradford protein assay.
Kidney cortex and harvested cultured cells were performed as previously described.10
Western Blot Analysis
Protein was isolated from cultured cell extracts or kidney cortex and homogenized in lysis buffer (150 mM NaCl, 10 mM Tris-HCl, 5 mM EDTA, 1 mM EGTA, and 1% Triton X-100) containing a protease inhibitor cocktail. The supernatants of cell or tissue lysates were extracted after centrifugation at 10,000 rpm for 15 minutes at 4°C. The protein concentration was measured with the Bradford protein assay (Bio-Rad, Hercules, CA). Equal amounts of protein from lysates were loaded and separated by 10% SDS-PAGE and transferred onto nitrocellulose membrane. The blots were probed overnight at 4°C with primary antibodies against E-cadherin, α-SMA, HSP72, Smad2, Smad3, Smad7, p-Smad2, p-Smad3, fibrillarin or β-actin, respectively. After incubation with the appropriate HRP-conjugated secondary antibodies, HRP activity was visualized by an enhanced chemiluminescence system (Kodak Medical X-Ray Processor, Rochester, NY). Densitometric analysis was performed as described previously.10
Coimmunoprecipitation Analysis
To assess HSP72-Smad3/p-Smad3 interaction in the whole cell or nuclear lysates, aliquots of samples were subjected to immunoprecipitation (IP). Samples were dissolved in IP buffer (0.5 to 1 mg of protein/ml). Lysates were precleared with nonimmune serum (10 μl/mg protein) obtained from the same host species as the primary antibody and 20 μl of protein A or G agarose-sepharose bead slurry (1.5 g protein A or G agarose-sepharose beads in 30 ml of 50 mM Tris-HCl, pH 7.5) at 4°C for 1 hour followed by centrifugation at maximal speed for 5 minutes. The cell lysates were then incubated overnight at 4°C with a polyclonal rabbit antibody directed against Smad3/p-Smad3 or HSP72 or rabbit preimmune serum. After incubation with 50 μl of protein A or G agarose-sepharose beads for an additional 2 hours, the immunocomplexes were collected by centrifugation at 2400 × g for 60 seconds, washed 3 times with the IP buffer, suspended in 25 μl 2× SDS sample buffer (Bio-Rad, Richmond, CA), and heated at 95°C. Proteins were evaluated by Western blot analysis as described above.
Immunofluorescence Stainings
Indirect immunofluorescence staining was performed using an established procedure.10 The paraffin-embedded kidney sections (4 μm) were deparaffinized and rehydrated. Sections were incubated with polyclonal rabbit anti-phospho-smad3 (1:100) antibody at 4°C overnight followed by Alexa Fluro 546-conjugated anti-rabbit IgG (1:1000). Cells were cultured on glass cover slips and fixed in methanol for 10 minutes at −20°C. Fixed cells were washed with PBS, permeabilized in 0.1% Triton X-100 for 10 minutes at room temperature, and incubated in blocking buffer (5% BSA in PBS) for 1 hour at room temperature. To detect HSP72, Smad3, and p-Smad3, cells were incubated with monoclonal mouse anti-HSP72 (1:100), polyclonal rabbit anti-Smad3 (1:50), and polyclonal rabbit anti-phospho-smad3 (1:50) at 4°C overnight followed by Alexa Fluro 488-conjugated anti-mouse IgG (1:1000) or Alexa Fluro 546-conjugated anti-rabbit IgG (1:1000) antibody, respectively. To identify nuclei, cells were counterstained with the fluorescent dye Hoechst 33258 for 3 minutes. In all cases, antibody controls were evaluated to ensure that the results were not a consequence of crossreactivity or nonspecific binding of the secondary antibodies. The positive stainings were measured using a laser scanning confocal microscope (Zeiss LSM 510 META, Carl Zeiss, Germany).
Animal Model
Experiments were performed with male Sprague-Dawley rats (200 to 250 g) obtained from the Sun Yat-Sen University Animal Center (Guangzhou, China). UUO was performed using an established procedure and rats received daily oral administration with 400 mg/kg GGA to optimally induce HSP72 overexpression as described previously.10 Groups of mice (n = 5) were sacrificed at 7 days after UUO and both kidneys were harvested and then subjected to the studies described above. The Animal Care and Use Committee of the Sun Yat-Sen University approved all experimental protocols.
Statistical Analysis
The data are expressed as mean ± SEM. Analysis was performed with standard statistical software (SPSS for Windows, version 11.0). Comparison among groups was made with one-way ANOVA followed by the Student-Newman-Keuls test. P < 0.05 was considered statistically significant.
Disclosures
None.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (30671055) and the Ministry of Education of the People's Republic of China (107087) to Dr. Haiping Mao. Part of these studies was presented as an abstract at the 40th Annual Meeting of the American Society of Nephrology; November 4 through 9, 2008, in Philadelphia, Pennsylvania. Y.Z. and H.M. contributed equally to this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1. Massague J, Blain SW, Lo RS: TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 103: 295– 309, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Blobe GC, Schiemann WP, Lodish HF: Role of transforming growth factor beta in human disease. N Engl J Med 342: 1350– 1358, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Macias-Silva M, Abdollah S, Hoodless PA, Pirone R, Attisano L, Wrana JL: MADR2 is a substrate of the TGFbeta receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell 87: 1215– 1224, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Chen HB, Rud JG, Lin K, Xu L: Nuclear targeting of transforming growth factor-beta-activated Smad complexes. J Biol Chem 280: 21329– 21336, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Ross S, Hill CS: How the Smads regulate transcription. Int J Biochem Cell Biol 40: 383– 408, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Hill CS: Nucleocytoplasmic shuttling of Smad proteins. Cell Res 19: 36– 46, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Zeisberg M, Kalluri R: The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med 82: 175– 181, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Hartl FU: Molecular chaperones in cellular protein folding. Nature 381: 571– 579, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Bukau B, Horwich AL: The Hsp70 and Hsp60 chaperone machines. Cell 92: 351– 366, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Mao H, Li Z, Zhou Y, Li Z, Zhuang S, An X, Zhang B, Chen W, Nie J, Wang Z, Borkan SC, Wang Y, Yu X: HSP72 attenuates renal tubular cell apoptosis and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 295: F202– F214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruchalski K, Mao H, Li Z, Wang Z, Gillers S, Wang Y, Mosser DD, Gabai V, Schwartz JH, Borkan SC: Distinct hsp70 domains mediate apoptosis-inducing factor release and nuclear accumulation. J Biol Chem 281: 7873– 7880, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Knowlton AA: Mutation of amino acids 246–251 alters nuclear accumulation of human heat shock protein (HSP) 72 with stress, but does not reduce viability. J Mol Cell Cardiol 31: 523– 532, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Knowlton AA: Mutation of amino acids 566–572 (KKKVLDK) inhibits nuclear accumulation of heat shock protein 72 after heat shock. J Mol Cell Cardiol 33: 49– 55, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Chow AM, Steel R, Anderson RL: Hsp72 chaperone function is dispensable for protection against stress-induced apoptosis. Cell Stress Chaperones 14: 253– 263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B: The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol 20: 7146– 7159, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stege GJ, Li L, Kampinga HH, Konings AW, Li GC: Importance of the ATP-binding domain and nucleolar localization domain of HSP72 in the protection of nuclear proteins against heat-induced aggregation. Exp Cell Res 214: 279– 284, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Tang D, Kang R, Xiao W, Jiang L, Liu M, Shi Y, Wang K, Wang H, Xiao X: Nuclear heat shock protein 72 as a negative regulator of oxidative stress (hydrogen peroxide)-induced HMGB1 cytoplasmic translocation and release. J Immunol 178: 7376– 7384, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Voss MR, Gupta S, Stice JP, Baumgarten G, Lu L, Tristan JM, Knowlton AA: Effect of mutation of amino acids 246–251 (KRKHKK) in HSP72 on protein synthesis and recovery from hypoxic injury. Am J Physiol Heart Circ Physiol 289: H2519– H2525, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Shi Y, Thomas JO: The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol Cell Biol 12: 2186– 2192, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beckmann RP, Mizzen LE, Welch WJ: Interaction of Hsp 70 with newly synthesized proteins: Implications for protein folding and assembly. Science 248: 850– 854, 1990 [DOI] [PubMed] [Google Scholar]
- 21. Healy DA, Daly PJ, Docherty NG, Murphy M, Fitzpatrick JM, Watson RW: Heat shock-induced protection of renal proximal tubular epithelial cells from cold storage and rewarming injury. J Am Soc Nephrol 17: 805– 812, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Margarit SM, Sondermann H, Hall BE, Nagar B, Hoelz A, Pirruccello M, Bar-Sagi D, Kuriyan J: Structural evidence for feedback activation by Ras.GTP of the Ras-specific nucleotide exchange factor SOS. Cell 112: 685– 695, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C: Heat shock proteins: Essential proteins for apoptosis regulation. J Cell Mol Med 12: 743– 761, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beck FX, Neuhofer W, Muller E: Molecular chaperones in the kidney: Distribution, putative roles, and regulation. Am J Physiol Renal Physiol 279: F203– F215, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA: HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A 105: 1739– 1744, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ogawa F, Shimizu K, Hara T, Muroi E, Hasegawa M, Takehara K, Sato S: Serum levels of heat shock protein 70, a biomarker of cellular stress, are elevated in patients with systemic sclerosis: Association with fibrosis and vascular damage. Clin Exp Rheumatol 26: 659– 662, 2008 [PubMed] [Google Scholar]
- 27. Yaglom JA, Gabai VL, Sherman MY: High levels of heat shock protein Hsp72 in cancer cells suppress default senescence pathways. Cancer Res 67: 2373– 2381, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Bidmon B, Endemann M, Arbeiter K, Ruffingshofer D, Regele H, Herkner K, Eickelberg O, Aufricht C: Overexpression of HSP-72 confers cytoprotection in experimental peritoneal dialysis. Kidney Int 66: 2300– 2307, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Wakisaka O, Takahashi N, Shinohara T, Ooie T, Nakagawa M, Yonemochi H, Hara M, Shimada T, Saikawa T, Yoshimatsu H: Hyperthermia treatment prevents angiotensin II-mediated atrial fibrosis and fibrillation via induction of heat-shock protein 72. J Mol Cell Cardiol 43: 616– 626, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Hagiwara S, Iwasaka H, Matsumoto S, Noguchi T, Yoshioka H: Association between heat stress protein 70 induction and decreased pulmonary fibrosis in an animal model of acute lung injury. Lung 185: 287– 293, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Xu J, Lamouille S, Derynck R: TGF-beta-induced epithelial to mesenchymal transition. Cell Res 19: 156– 172, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kee HJ, Eom GH, Joung H, Shin S, Kim JR, Cho YK, Choe N, Sim BW, Jo D, Jeong MH, Kim KK, Seo JS, Kook H: Activation of histone deacetylase 2 by inducible heat shock protein 70 in cardiac hypertrophy. Circ Res 103: 1259– 1269, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Cizkova D, Rosocha J, Vanicky I, Radonak J, Galik J, Cizek M: Induction of mesenchymal stem cells leads to HSP72 synthesis and higher resistance to oxidative stress. Neurochem Res 31: 1011– 1020, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Johnson JD, Fleshner M: Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol 79: 425– 434, 2006 [DOI] [PubMed] [Google Scholar]