Abstract
Mutation of the mouse laminin α5 gene results in a variety of developmental defects, including defects in kidney structure and function. Whereas the total absence of laminin α5 results in breakdown of the glomerular basement membrane (GBM) and failed glomerular vascularization, a hypomorphic Lama5 mutation (the Lama5neo allele) results in proteinuria, hematuria, polycystic kidney disease (PKD), and death 3 to 4 weeks after birth. Here, we examined the role of podocyte-derived laminin α5 via podocyte-specific inactivation of Lama5 and podocyte-specific rescue of the Lama5neo mutation. Podocyte-specific inactivation of Lama5 resulted in varying degrees of proteinuria and rates of progression to nephrotic syndrome. The GBM of proteinuric mice appeared thickened and “moth-eaten,” and podocyte foot processes became effaced. Podocyte-specific restoration of laminin α5 production using two distinct strategies in Lama5neo/neo mice resulted in the resolution of proteinuria, hematuria, and PKD. These results suggest that the development of normal GBM structure and function requires podocyte-derived laminin α5 during and after glomerulogenesis and present a unique mechanism for the pathogenesis of PKD in these mice.
The glomerular capillary wall consists of podocytes with interdigitated foot processes bridged by slit diaphragms, fenestrated endothelial cells, and the intervening glomerular basement membrane (GBM)1 that these two cell types together produce.2 A defect in or injury to any one of these three components can cause pathologic leak of albumin and other plasma proteins into the urine. This and a wealth of other evidence suggest that the three layers interact to establish and maintain the glomerular filtration barrier to plasma macromolecules.
Most research into the mechanisms of glomerular disease is currently focused on the structure of the podocyte slit diaphragm and its interaction with the actin cytoskeleton (reviewed in reference 3). Yet the podocyte's intimate association with the GBM via specific receptors, coupled with the fact that mutations in four genes encoding GBM components cause glomerular disease in humans and mice,4–7 make the GBM an attractive—if not necessary—target for research aimed at providing a complete understanding of the glomerular filtration barrier. Indeed, the GBM's prominence in the filtration barrier was hypothesized several decades ago,8 and recent studies have provided additional support.9–11
Like all basement membranes, the GBM is comprised of four major extracellular matrix proteins: laminin, collagen IV, nidogen, and sulfated proteoglycans. Laminins are obligate α-β-γ heterotrimers12 that self-polymerize in the extracellular matrix to provide the basis for basement membrane formation via interactions with cellular receptors.13 The major laminin in mature GBM is laminin-521 (α5β2γ1), but during glomerulogenesis there are developmental transitions in which laminin-111 (α1β1γ1) is replaced by laminin-511 (α5β1γ1), which is then joined and eventually replaced by laminin-521.14–16
Here we focus on the laminin α5 chain. Laminin α5 is widely expressed,17 and Lama5−/− mice die at late fetal stages with multiple developmental defects.18 Kidneys are small and occasionally absent in Lama5−/− embryos, and in those mutant kidneys that do form, the GBM breaks down during glomerulogenesis, which prevents glomerular vascularization and filtration.19 Studies of chimeric laminin chains in transgenic mice revealed that the α5 COOH-terminal laminin globular domain is required for proper adhesion of mesangial cells to the GBM20 and for a proper filtration barrier.21 In addition, mice with a hypomorphic Lama5 mutation (Lama5neo) that reduces α5 expression because of neo insertion causes proteinuria, hematuria, and a form of polycystic kidney disease (PKD).22
Here we manipulated Lama5 expression in vivo specifically in podocytes in three different ways to investigate the function of laminin α5 in the GBM. (1) We used a podocyte-specific Cre mouse to mutate Lama5 during glomerulogenesis, resulting in a new model of nephrotic syndrome. (2) We generated novel transgenic mice expressing FLP recombinase specifically in podocytes, which rescues the Lama5neo mutation via FLPase-mediated removal of neo. This prevented proteinuria, hematuria, and, unexpectedly, cystogenesis. (3) We used a novel tetracycline-resistance operon (tetO)-regulated human laminin α5 transgene and a podocyte-specific reverse tetracycline transactivator (rtTA)23 to express human laminin α5 in podocytes during and after glomerulogenesis in Lama5neo/neo mice. This resulted in deposition of human α5 in the GBM, which ameliorated proteinuria, hematuria, and, again unexpectedly, the PKD. Together, these results implicate podocyte-derived laminin α5 as a crucial component of the glomerular filtration barrier, and they show that in mice a disease with features of PKD can be causally related to a GBM defect.
Results
Podocyte-Specific Inactivation of Lama5 by Cre Recombinase
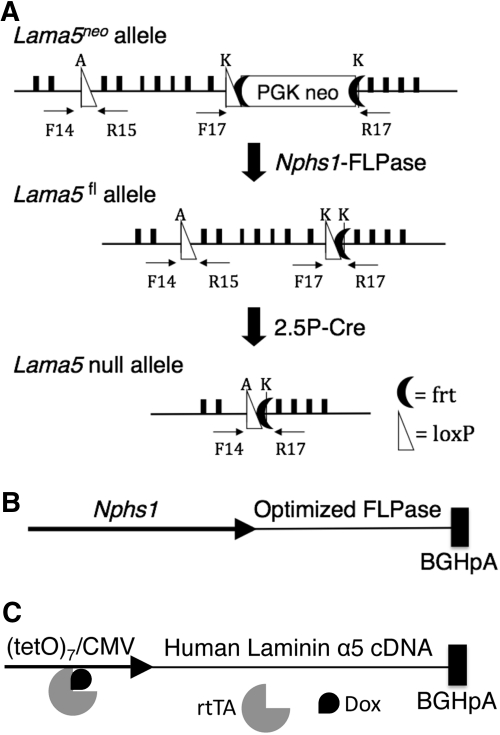
Lama5 was mutated during glomerulogenesis specifically in podocytes by crossing the podocyte-specific 2.5P-Cre transgene24 onto the conditionally mutant Lama5fl/fl (Figure 1A) and Lama5fl/− genetic backgrounds.25 PCR analysis of genomic DNA showed that excision of the seven floxed Lama5 coding exons by Cre recombinase (Figure 1A) occurred in kidney but not in other tissues (Figure 2A and A′), consistent with the known specificity of the 2.5P-Cre transgene.24 Lama5fl/fl;2.5P-Cre and Lama5fl/-;2.5P-Cre mice were viable and most presented with mild proteinuria early in life that eventually progressed to the nephrotic range (Figure 3, A and B). As the affected mice aged, they became edematous, hypoalbuminemic, hypercholesterolemic, hematuric, and their kidneys yellowed (data not shown). Light microscopy revealed protein casts in tubules and tubulointerstitial nephritis, but glomerular lesions, including glomerulosclerosis and mesangial matrix expansion, were not obvious until later stages of disease (Figures 3C through 3F and data not shown). Ultrastructural analysis revealed few abnormalities at early stages (approximately 3 weeks) despite mild proteinuria (data not shown). At later stages of disease the GBM was thickened, had a moth-eaten appearance, and there were irregular contours with frequent subepithelial outpocketings and extensive foot process effacement (Figures 3G through 3J and data not shown).
Figure 1.
Schematic diagrams of Lama5 alleles and new transgenes used in these studies. (A) The hypomorphic Lama5neo allele is converted to the functional conditional Lama5fl allele by FLPase-mediated removal of the FRT-flanked PGK-neo cassette. A Lama5 null allele is generated by the activity of Cre recombinase, which removes the seven exons between the loxP sites. The primers used for PCR of genomic DNA are indicated. (B) The Nphs1-FLPo transgene contains the mouse nephrin (Nphs1) promoter driving FLPo and a bovine growth hormone polyA signal sequence. (C) The doxycycline-inducible human laminin α5 transgene (tetO-LA5) contains seven copies of tetO with a CMV minimal promoter driving the human LAMA5 cDNA. The binding of doxycycline (Dox) to the rtTA promotes recruitment to and activation of the promoter.
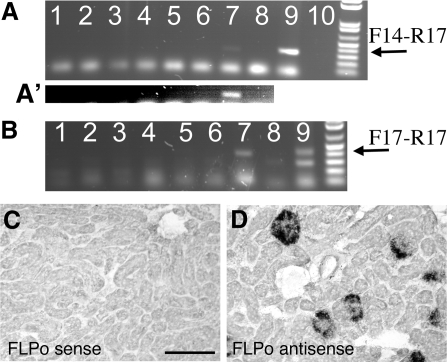
Figure 2.
Expression of Cre or FLPo alters Lama5 alleles. (A) PCR analysis of DNA extracted from multiple tissues of a Lama5fl/+;2.5P-Cre mouse only generates the appropriate product (arrow) in kidney. Lane 1, lung; 2, liver; 3, spleen; 4, intestine; 5, cerebrum; 6, cerebellum; 7, kidney; 8, no DNA; 9, positive control tail; 10, blank. (A′) The region of interest in lanes 1 to 8 was brightened to better reveal the kidney-specific product. (B) PCR analysis of DNA extracted from multiple tissues of a Lama5neo/neo;Nphs1-FLPo mouse only generates the appropriate product (arrow) in kidney. Lanes are as in panel A; the positive control here is from a Lama5fl/+ mouse. (C, D) In situ hybridization with a (C) sense or (D) antisense FLPo riboprobe on kidney sections from a Nphs1-FLPo transgenic mouse shows expression confined to podocytes.
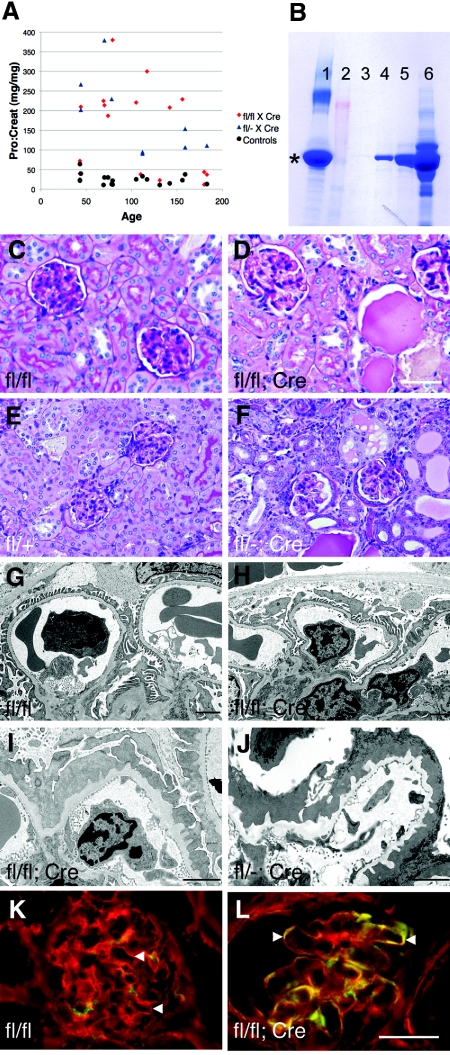
Figure 3.
Podocyte-specific mutation of Lama5 causes nephrotic syndrome. (A) Graph showing protein:creatinine ratios of mutant and control mice plotted against age. Note that all Lama5fl/−;2.5P-Cre mice became overtly proteinuric. (B) SDS-PAGE analysis of 1 μl of urine shows that albumin is the major protein. Lane 1, 10 μg BSA; 2, markers; 3, Lama5fl/fl, postnatal day (P) 131; 4 and 5, Lama5 fl/fl;2.5P-Cre, P131; 6, Lama5fl/fl;2.5P-Cre, P69. * denotes albumin. The three P131 mice were littermates. (C through F) Periodic acid–Schiff staining of kidney sections from mice of the indicated genotypes at (C, D) P215 and (E, F) P78. (G through J) Ultrastructural analysis of the glomerular capillary wall of mice of the indicated genotypes at (G through I) P215 and (J) P78. Panels H and I are from the same mouse, which was already albuminuric at P131 (lane 5 in panel B), demonstrating significant variation in the ultrastructural changes. (K, L) Immunofluorescence analysis of laminin α5 (red) and laminin α1 (green) deposition in the GBM (arrowheads) of P156 mice of the indicated genotypes. Note the lack of green in (K) the control GBM but frequent green and yellow staining in (L) the mutant. Bar in D: 40 μm for C and D, 53 μm for E and F; bars in G through J: 2 μm; bar in L, 33 μm for K and L.
The rate of disease progression was highly variable, because some Lama5fl/fl;2.5P-Cre mice lived only a few weeks, whereas others showed little sign of disease even at 8 months of age (Figure 3A and data not shown). This variability may have stemmed from the mixed genetic background; from heterogeneity in the onset, level, or extent of Cre expression; or from a combination of these variables. The notion that Cre might be limiting in some cases is supported by the fact that all of the Lama5fl/−;2.5P-Cre mice, which only required one rather than two floxed Lama5 alleles to be recombined by Cre to generate Lama5−/− podocytes, exhibited nephrotic levels of proteinuria (Figure 3A).
Immunofluorescence analysis of laminin α5 deposition in the GBM of proteinuric animals at an early stage showed similar levels in controls and mutants, but at later stages laminin α5 levels were reduced compared with control (data not shown). The residual laminin α5 in the GBM of mutants was likely synthesized by glomerular endothelial cells, which do not express the 2.5P-Cre transgene, and some may have been secreted by podocytes during and after glomerulogenesis before they became depleted of Lama5 mRNA. Laminins α1 and α2 could be detected ectopically in the mature mutant GBM (Figure 3, K and L, and data not shown), presumably as attempted compensation for the reduced α5, but they were not sufficient to prevent proteinuria. This is consistent with our previous studies showing that replacing endogenous laminin α5 with a modified version carrying COOH-terminal globular domain segments of laminin α1 also resulted in proteinuria.21 These studies show that podocyte-derived laminin α5 is required to maintain the structural integrity of the GBM and the glomerular filtration barrier.
Podocyte-Specific Rescue of the Lama5neo Insertional Mutation
In the process of creating the conditional Lama5fl allele, we generated mice carrying a FLP recognition target (FRT) site-flanked neo insertion in intron 21. This genetic alteration leads to proteinuria, hematuria, PKD, and death at 3 to 4 weeks of age in Lama5neo/neo homozygotes. Molecular analyses revealed that the insertion causes aberrant Lama5 RNA splicing and reduced laminin α5 protein deposition.22 We concluded from these data that reduced laminin α5 expression in glomerular cells caused GBM defects and impaired permselectivity, whereas reduced expression in tubular cells caused cystogenesis because of aberrant cell/matrix interactions. Alternatively, because the loss of laminin α5 from tubular basement membranes (TBMs) appeared more dramatic than the loss from the GBM (Figure 4F and reference 22), the possibility existed that the proteinuria stemmed in part from tubular cell dysfunction, consistent with concepts proposed by Comper and colleagues.26,27 To distinguish the pathologic effects of laminin α5 reduction in the GBM from those stemming from reduction in TBMs, we devised two different strategies to specifically rescue the Lama5neo insertion mutation in podocytes.
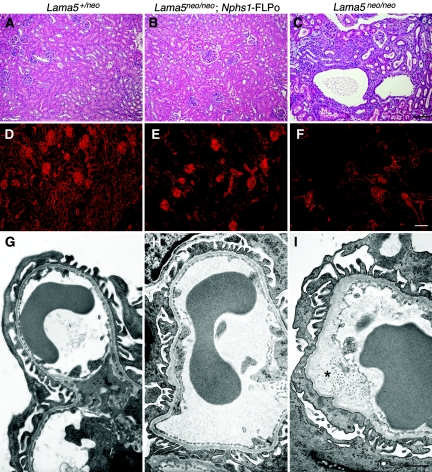
Figure 4.
Podocyte-specific expression of FLP recombinase rescues renal defects in Lama5neo/neo mice. (A through C) Hemotoxylin and eosin staining of P21 kidney sections of the indicated genotypes reveals overall renal architecture. Note the lack of any histopathology in (B) the Lama5neo/neo kidney expressing FLPo in podocytes and (C) the presence of cysts and tubulointerstitial disease in the nonrescued mutant littermate. (D through F) Immunofluorescence analysis of laminin α5 deposition in kidney sections from the same mice. Note the reduced TBM signals in panels E and F as compared with panel D. (G through I) Ultrastructural analysis of glomerular capillary loops from the same mice. GBM thickening (*) and adjacent foot process effacement is evident in (I) the mutant but not in (G) the control or in (H) the rescued mutant. Bar in C, 100 μm for A through C; bar in F, 80 μm for D through F; bar in I, 1.1 μm for G through I.
Strategy 1: Podocyte-Specific Removal of the neo Insertion
To remove the pathogenic neo insertion specifically in podocytes, we generated transgenic mice designed to express a codon-optimized FLP recombinase (FLPo)28 under the control of the 4.2-kb mouse nephrin (Nphs1) promoter29 (Figure 1B). FLP recombinase activity should excise the FRT site-flanked neo insertion during glomerulogenesis and convert the hypomorphic Lama5neo allele to the fully functional Lama5fl allele (Figure 1A).
We generated eighteen Nphs1-FLPo transgenic founders and bred seven different integrants onto the Lama5+/neo background. PCR using Lama5 primers flanking the 2-kb neo insertion was used to look for evidence of excision of the neo in DNA extracted from kidney cortex without excision in other tissues. Successful removal of neo was observed for three of the lines (Figure 2B and data not shown), and in situ hybridization with a FLP riboprobe demonstrated podocyte-specific expression of FLPo in all three (Figure 2, C and D, and data not shown). Each of these three transgenes was then bred onto the Lama5neo/neo background.
Surprisingly, all three rescued not only proteinuria and hematuria but also prevented the development of cysts that appeared in Lama5neo/neo littermates lacking Nphs1-FLPo (Figures 4A through 4C and data not shown). The resulting Lama5neo/neo;Nphs1-FLPo mice were viable and fertile, with no signs of proteinuria or kidney disease even at several months of age. Immunohistochemical analysis of laminin α5 deposition showed normal levels in the Lama5neo/neo;Nphs1-FLPo GBM and, in one line (line 5), the expected low level in TBMs (Figures 4D through 4F). However, in the other two lines (lines 1 and 3), which showed higher podocyte FLPo expression, the TBM levels of laminin α5 appeared normal (data not shown). This suggests that the FLPase was expressed in some tubular epithelial cells despite the absence of in situ signals in tubules (Figure 2C). Alternatively, FLPase may have been secreted by podocytes and taken up by tubular cells, but there is no definitive evidence for this.
Consistent with the lack of proteinuria, ultrastructural analysis of the glomerular capillary wall revealed no defects in Lama5neo/neo;Nphs1-FLPo kidneys, in contrast to the GBM thickening and occasional splitting observed in Lama5neo/neo glomeruli (Figures 4G through 4I). From these studies of Lama5neo/neo;Nphs1-FLPo mice we conclude that restoration of Lama5 expression to normal levels specifically in podocytes during glomerulogenesis is sufficient to rescue GBM architecture and the integrity of the glomerular filtration barrier.
Strategy 2: Podocyte-Specific Expression of a Laminin α5 Transgene
To express a full-length laminin α5 cDNA specifically in podocytes during and after glomerulogenesis using a doxycycline-inducible system, we produced a new transgenic mouse line carrying the human LAMA5 cDNA under the control of the tetO-cytomegalovirus (CMV) regulatory element (tetO-LA5; Figure 1C). tetO-LA5 mice were crossed to 2.5P-rtTA mice expressing rtTA under control of the human podocin (NPHS2) promoter.23 Next, we mated both transgenes onto the Lama5neo/neo background to generate Lama5neo/neo;2.5P-rtTA;tetO-LA5 mice. Doxycycline was administered when pregnancy became obvious (usually embryonic day 12 to 15) and continuously thereafter such that human laminin α5 synthesis should occur in podocytes from when they first begin to differentiate.
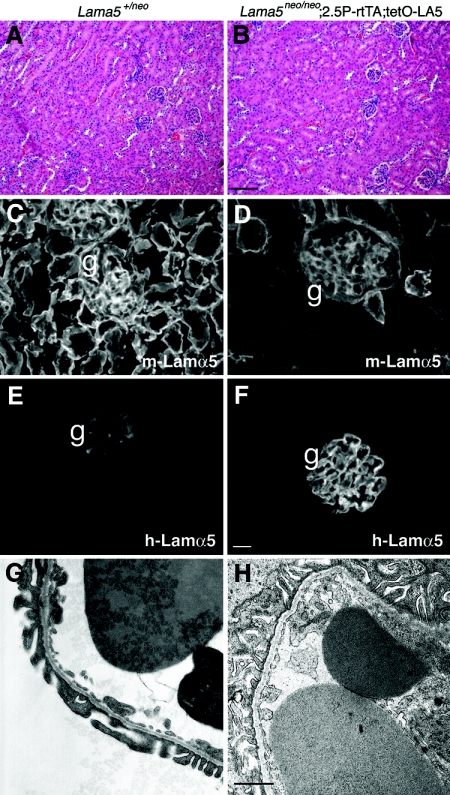
Similar to the results presented above, the Lama5neo/neo;2.5P-rtTA;tetO-LA5 mice treated with doxycycline did not develop proteinuria or hematuria. They were fertile and developed no signs of kidney disease as adults, at least to 6 months of age. Light microscopy revealed normal glomeruli, no cysts, and normal renal architecture (Figure 5, A and B). Immunohistochemical analysis revealed an apparent reduction of mouse laminin α5 in the GBM and the expected low levels in the TBMs (Figure 5, C and D). Human laminin α5 deposition was restricted to the GBM and was doxycycline-dependent (Figure 5, E and F, and data not shown). Electron microscopic analysis revealed normal architecture of the glomerular capillary wall in the rescued mutant (Figure 5, G and H), consistent with the lack of proteinuria. Thus, human laminin α5 can compensate for the reduced levels of mouse α5 in the mutant GBM, allowing for the establishment and maintenance of an intact glomerular filtration barrier.
Figure 5.
Podocyte-specific expression of human laminin α5 induced by doxycycline rescues renal defects in Lama5neo/neo mice. (A, B) Hemotoxylin and eosin staining of P42 kidney sections of the indicated genotypes reveals normal renal architecture. (C, D) Immunofluorescence analysis of mouse laminin α5 deposition shows reduced levels in the rescued mutant GBM (g) and in most TBMs (D) as compared with control (C). (E, F) Immunostaining for human laminin α5 shows deposition in the GBM (g) but not in TBMs of the rescued mutant (F) but only background fluorescence in the control glomerulus, perhaps because of low-level accumulation of mouse IgG1 in the mesangium (E). (G, H) Ultrastructural analysis of a glomerular capillary loop from the same mice reveals normal GBM and foot process architecture. Bar in B, 100 μm for A and B; bar in F, 20 μm for C through F; bar in H, 1.1 μm for G and H.
Discussion
Together with our prior results, the data presented here show that expression of laminin α5 by podocytes and its secretion into the GBM is essential for establishing and maintaining the glomerular filtration barrier. We previously showed that with no laminin α5 the GBM breaks down19; with only endothelial laminin α5, the GBM is intact, but podocytes do not form normal foot processes30; and with reduced laminin α5 throughout the kidney because of a neo insertion in Lama5, there is proteinuria, hematuria, and development of multiple cysts.22 Here we demonstrated that knockout of Lama5 in podocytes during glomerulogenesis causes a variable, delayed onset nephrotic syndrome. This variability likely stems from the interplay of variabilities in the spatial and temporal expression of Cre from the podocin promoter, in the level of Cre expression, in the efficiency of recombination, and the potentially long half-lives of laminin α5 mRNA and protein. Despite the variability, we conclude that ongoing expression of laminin α5 in podocytes is a necessity.
To further explore the role of podocyte-derived laminin α5 in the filtration barrier, we used two different podocyte-specific strategies to attempt to rescue the glomerular defects in mice carrying the neo insertion. Removing the pathogenic neo insertion with FLP recombinase or expressing a human laminin α5 transgene prevented proteinuria and hematuria and restored the architecture of the glomerular capillary wall to normal. In both cases, cystogenesis in the tubules was also prevented, despite the glomerulus-specific nature of the rescues. This suggests that under the right circumstances a glomerular defect can cause cystic kidney disease. Although an attractive explanation for this finding is the notion that cysts begin in the glomeruli because of filtration defects and then propagate down the tubule, glomerular cysts were rarely, if ever, observed.
Regarding the etiology of cyst development in this model, we had initially hypothesized that the reduced laminin α5 in TBMs of Lama5neo/neo mice led to impaired cell-matrix interactions and subsequent cystogenesis,22 despite the fact that there are no reports of laminin α5 alterations in PKD. Furthermore, a recent report showed that laminin α5 in the hair follicle basement membrane is important for cilia structure and function.31 However, here a direct effect of laminin α5 reduction on tubular epithelial cell behavior seems not to be the complete story; neither mode of glomerular rescue should affect the TBMs, yet cystogenesis was inhibited. There are no precedents for congenital nephrotic syndrome and/or hematuria causing early cystogenesis in humans or in mice.
In human autosomal dominant PKD, cysts form in only a small percentage of tubules despite the equal genetic susceptibility of all nephrons, giving rise to the theory that a “second hit” is necessary for cystic transformation. What might be unique about the Lama5neo/neo mice is the combination of GBM and TBM defects. Perhaps a factor that is filtered at higher than normal levels in all proteinuric conditions mildly injures tubular epithelial cells, but in Lama5neo/neo mice these cells are more sensitive to injury because of impaired cell-matrix interactions (the second hit), resulting in cystic transformation. We are testing this hypothesis by attempting to selectively rescue the TBMs without affecting the abnormal GBM.
Alternatively, it is possible that despite the selective nature of the podocyte rescue, a small amount of laminin α5 may have found its way from glomeruli to TBM at sufficient levels to prevent cystogenesis. This would be difficult to demonstrate in the podocyte-specific FLP recombinase rescue because mouse laminin α5 is already weakly detectable in the TBMs of Lama5neo/neo mice.22 However, the restriction of human laminin α5 deposition to the GBM in the doxycycline-inducible system, with complete absence from the TBMs, makes this mechanism for tubular rescue unlikely.
It is interesting that despite the widespread expression of Lama5 during development and the many defects observed in its absence,32 the GBM manifests the most sensitivity to the reduced levels in Lama5neo/neo mice. The death of these mice at 3 to 4 weeks of age from renal failure may have masked abnormalities in other tissues, such as the lung.25 Yet restoration of laminin α5 expression only to podocytes led to viable, fertile mice, suggesting that only the glomerular filter is defective. It is notable that some of these rescued mice were smaller than their control littermates, perhaps because of placental insufficiency, as observed in Lama5−/− mice.18
Finally, as part of these studies we generated a new tool for manipulation of podocyte gene expression that should be useful for the nephrology community. Expression of FLPo recombinase driven by the nephrin promoter was demonstrated to be podocyte-specific in the kidney. Combined with available Cre transgenic mice and inducible systems, the design of increasingly complex genetic experiments now becomes feasible.
Concise Methods
Genetically Altered Mice
All animal experiments conformed to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Lama5−,18 Lama5fl,25 Lama5neo,22 2.5P-Cre,24 and 2.5P-rtTA23 mice have been described previously and were maintained on mixed, primarily B6/CBA/129 genetic backgrounds. Nphs1-FLPo transgenic mice were generated by microinjection of the isolated transgene (Figure 1B) into the pronuclei of B6CBAF2/J single-celled embryos. The 4.2-kb mouse nephrin promoter,29 a gift from Susan Quaggin (Samuel Lunenfeld Research Institute, Toronto, Canada), was placed upstream of FLPo recombinase with a 3′ bovine growth hormone polyadenylylation signal sequence28 (Addgene Inc., Cambridge, MA). Transgenic mice were identified by PCR using the FLPo primers 5′-CCACCTTCATGAGCTACAACACCATC-3′ and 5′-ACTTGTTCTGCACCAGCTTGAAGCTC-3′.
tetO-LA5 transgenic mice were generated by microinjection of the isolated transgene (Figure 1C) into the pronuclei of C57BL/6NTac single-celled embryos. The full-length human laminin α5 cDNA with a bovine growth hormone polyadenylylation signal sequence (a gift from Kiyotoshi Sekiguchi, Osaka University) was placed under the control of the (tetO)7-CMV regulatory element (a gift from Jeffrey Whitsett, University of Cincinnati). tetO-LA5 transgenic mice were identified by PCR using human LAMA5 primers 5′-TGCATCGAGATGGACACG-3′ and 5′-GCTTCAGGAAGAAGAGCAC-3′. To induce expression of tetO-LA5 in podocytes in animals also carrying the 2.5P-rtTA transgene, mice were treated with 1 mg/ml doxycycline in drinking water containing 5% sucrose. Of fifteen founders produced, the offspring of two expressed the transgene in the desired fashion, and one of these lines was used herein.
PCR Analysis of Genomic DNA
Crude DNA extracts from multiple tissues were obtained as described.33 To assay for mutation of the Lama5fl allele by Cre-mediated excision of the floxed exons, primers F14 (5′-ACCATGGGTATCCCGACTGTCACG-3′ and R17 (5′-GTTGAAGCCAAAGCGTACAGCG-3′) were used. These typically lie 2.5 kb apart, a segment normally too large to amplify, but after excision by Cre this segment shrinks to approximately 330 bp (Figures 1A and 2A). To assay for excision of the neo insertion from the Lama5neo allele by FLP recombinase, primers F17 (5′-GTGCCGCCCTAACACCCAAGG-3′) and R17, which flank the neo, were used. Once the approximately 2-kb neo gene is removed, these primers amplify a 425-bp product, which represents the functional Lama5fl allele; a 300-bp product representing the wild-type Lama5+ allele was also amplified (Figures 1A and 2B).
Renal Chemistry Assays
Analysis of blood and urine was performed on a Roche Cobas Mira Plus Analyzer. In some cases, 1 μl of urine was analyzed on SDS-PAGE gels stained with Coomassie brilliant blue.
Antibodies and Histology
Rabbit anti-mouse laminin α5,14 mouse anti-human laminin α5 clone 4C734 (Chemicon, Temecula, CA), rat anti-mouse laminin α1 clone 8B3,35 rat anti-human laminin α2 clone 4H8-2 (Alexis Biochemicals/Enzo Life Sciences, Plymouth Meeting, PA), and fluorescence secondary antibodies (Molecular Probes/Invitrogen, Carlsbad, CA) were used to immunostain frozen sections of unfixed kidneys as described.36 For light and electron microscopy, tissues were fixed and processed for paraffin and plastic sectioning by standard methods. Sections were stained and viewed as described previously.36 In situ hybridizations were performed as described.37
Disclosures
None.
Acknowledgments
We thank Jeanette Cunningham, Gloriosa Go, Jennifer Richardson, the Mouse Genetics Core, and the Mouse Core in the Pulmonary and Critical Care Division for valuable technical assistance; Marcus Moeller (RWTH University of Aachen, Aachen, Germany) and Larry Holzman (University of Pennsylvania, Philadelphia) for 2.5P-Cre mice; Jeffrey Kopp (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD) for 2.5P-rtTA mice; and Dale Abrahamson (Kansas University Medical Center, Kansas City, KS) for the 8B3 antibody. J.H.M. thanks Gerd Walz for an enlightening discussion. This research was supported by NIH grants R21DK074613, R01DK064687, R01GM060432, and R01DK078314 to J.H.M. and grant P01HL029594 to R.M.S. S.G. was supported by NIH training grant T32DK007126. T.L.A.-K. was supported by a Francis Family Foundation fellowship. Electron microscopy, production of Nphs1-FLPo transgenic mice, and renal chemistry assays were supported in part by the Washington University George M. O'Brien Center for Kidney Disease Research (P30DK079333) and the Research Center for Auditory and Visual Studies (P30DC004665).
This work was presented at the 2009 Annual Meeting of the American Society of Nephrology, San Diego, CA, October 29 through November 1.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Revisiting Basement Membrane Pathology in Renal Cystic Disease,” on pages 548–549.
References
- 1. Miner JH: Building the glomerulus: A matricentric view. J Am Soc Nephrol 16: 857– 861, 2005 [DOI] [PubMed] [Google Scholar]
- 2. St. John PL, Abrahamson DR: Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int 60: 1037– 1046, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428– 437, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Hasselbacher K, Wiggins RC, Matejas V, Hinkes BG, Mucha B, Hoskins BE, Ozaltin F, Nurnberg G, Becker C, Hangan D, Pohl M, Kuwertz-Broking E, Griebel M, Schumacher V, Royer-Pokora B, Bakkaloglu A, Nurnberg P, Zenker M, Hildebrandt F: Recessive missense mutations in LAMB2 expand the clinical spectrum of LAMB2-associated disorders. Kidney Int 70: 1008– 1012, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Hudson BG: The molecular basis of Goodpasture and Alport syndromes: Beacons for the discovery of the collagen IV family. J Am Soc Nephrol 15: 2514– 2527, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Miner JH, Go G, Cunningham J, Patton BL, Jarad G: Transgenic isolation of skeletal muscle and kidney defects in laminin beta2 mutant mice: Implications for Pierson syndrome. Development 133: 967– 975, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wuhl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dotsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A: Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet 13: 2625– 2632, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Farquhar MG: Editorial: The primary glomerular filtration barrier—Basement membrane or epithelial slits? Kidney Int 8: 197– 211, 1975 [DOI] [PubMed] [Google Scholar]
- 9. Farquhar MG: The glomerular basement membrane: Not gone, just forgotten. J Clin Invest 116: 2090– 2093, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jarad G, Cunningham J, Shaw AS, Miner JH: Proteinuria precedes podocyte abnormalities in Lamb2−/− mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest 116: 2272– 2279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abrahamson DR, Isom K, Roach E, Stroganova L, Zelenchuk A, Miner JH, St John PL: Laminin compensation in collagen alpha3(IV) knockout (Alport) glomeruli contributes to permeability defects. J Am Soc Nephrol 18: 2465– 2472, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD: A simplified laminin nomenclature. Matrix Biol 24: 326– 332, 2005 [DOI] [PubMed] [Google Scholar]
- 13. McKee KK, Harrison D, Capizzi S, Yurchenco PD: Role of laminin terminal globular domains in basement membrane assembly. J Biol Chem 282: 21437– 21447, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR: The laminin alpha chains: Expression, developmental transitions, and chromosomal locations of alpha1–5, identification of heterotrimeric laminins 8–11, and cloning of a novel alpha3 isoform. J Cell Biol 137: 685– 701, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miner JH, Sanes JR: Collagen IV α3, α4, and α5 chains in rodent basal laminae: Sequence, distribution, association with laminins, and developmental switches. J Cell Biol 127: 879– 891, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miner JH: Developmental biology of glomerular basement membrane components. Curr Opin Nephrol Hypertens 7: 13– 19, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Miner JH, Lewis RM, Sanes JR: Molecular cloning of a novel laminin chain, α5, and widespread expression in adult mouse tissues. J Biol Chem 270: 28523– 28526, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Miner JH, Cunningham J, Sanes JR: Roles for laminin in embryogenesis: Exencephaly, syndactyly, and placentopathy in mice lacking the laminin α5 chain. J Cell Biol 143: 1713– 1723, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miner JH, Li C: Defective glomerulogenesis in the absence of laminin α5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev Biol 217: 278– 289, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Kikkawa Y, Virtanen I, Miner JH: Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin alpha5 in the glomerular basement membrane. J Cell Biol 161: 187– 196, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kikkawa Y, Miner JH: Molecular dissection of laminin alpha 5 in vivo reveals separable domain-specific roles in embryonic development and kidney function. Dev Biol 296: 265– 277, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Shannon MB, Patton BL, Harvey SJ, Miner JH: A hypomorphic mutation in the mouse Laminin alpha5 gene (Lama5) causes polycystic kidney disease. J Am Soc Nephrol 17: 1913– 1922, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shigehara T, Zaragoza C, Kitiyakara C, Takahashi H, Lu H, Moeller M, Holzman LB, Kopp JB: Inducible podocyte-specific gene expression in transgenic mice. J Am Soc Nephrol 14: 1998– 2003, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of Cre recombinase in transgenic mice. Genesis 35: 39– 42, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Nguyen NM, Kelley DG, Schlueter JA, Meyer MJ, Senior RM, Miner JH: Epithelial laminin alpha5 is necessary for distal epithelial cell maturation, VEGF production, and alveolization in the developing murine lung. Dev Biol 282: 111– 125, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D: Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol 20: 489– 494, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD: The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: Retrieval is disrupted in nephrotic states. Kidney Int 71: 504– 513, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Raymond CS, Soriano P: High-efficiency FLP and PhiC31 site-specific recombination in mammalian cells. PLoS ONE 2: e162, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eremina V, Wong MA, Cui S, Schwartz L, Quaggin SE: Glomerular-specific gene excision in vivo. J Am Soc Nephrol 13: 788– 793, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Abrahamson DR, St John PL, Isom K, Robert B, Miner JH: Partial rescue of glomerular laminin alpha5 mutations by wild-type endothelia produce hybrid glomeruli. J Am Soc Nephrol 18: 2285– 2293, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Gao J, Derouen MC, Chen CH, Nguyen M, Nguyen NT, Ido H, Harada K, Sekiguchi K, Morgan BA, Miner JH, Oro AE, Marinkovich MP: Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev 22: 2111– 2124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miner JH, Yurchenco PD: Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol 20: 255– 284, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML: Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 29: 52, 54, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Tiger C-F, Champliaud M-F, Pedrosa-Domellof F, Thornell L-E, Ekblom P, Gullberg D: Presence of laminin α5 chain and lack of laminin α1 chain during human muscle development and in muscular dystrophies. J Biol Chem 272: 28590– 28595, 1997 [DOI] [PubMed] [Google Scholar]
- 35. St. John PL, Wang R, Yin Y, Miner JH, Robert B, Abrahamson DR: Glomerular laminin isoform transitions: Errors in metanephric culture are corrected by grafting. Am J Physiol Renal Physiol 280: F695– F705, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Harvey SJ, Jarad G, Cunningham J, Rops AL, van der Vlag J, Berden JH, Moeller MJ, Holzman LB, Burgess RW, Miner JH: Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Pathol 171: 139– 152, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miner JH, Li C, Mudd JL, Go G, Sutherland AE: Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development 131: 2247– 2256, 2004 [DOI] [PubMed] [Google Scholar]