Abstract
Translation regulation plays an important role during gametocytogenesis in the malaria parasite, a process that is obligatory for the transmission of the parasite through mosquito vectors. In this study we determined the function of PfPuf2, a member of the Puf family of translational repressors, in gametocytogenesis of Plasmodium falciparum. Tagging of the endogenous PfPuf2 protein with green fluorescent protein showed that PfPuf2 was expressed in both male and female gametocytes, and the protein was localized in the cytoplasm of the parasite. Targeted disruption of the PfPuf2 gene did not affect asexual growth of the parasite, but promoted the formation of gametocytes and differentiation of male gametocytes. Complementation studies were performed to confirm that the resultant phenotypic changes were due to disruption of the PfPuf2 gene. Episomal expression of PfPuf2 under its cognate promoter almost restored the gametocytogenesis rate in a PfPuf2 disruptant to the level of the wild-type parasite. It also partially restored the effect of PfPuf2 disruption on male-female sex ratio. In addition, episomal overexpression of PfPuf2 under its cognate promoter but with a higher concentration of the selection drug or under the constitutive hsp86 promoter in both the PfPuf2-disruptant and wild-type 3D7 lines, further dramatically reduced gametocytogenesis rates and sex ratios. These findings suggest that in this early branch of eukaryotes the function of PfPuf2 is consistent with the ancestral function of suppressing differentiation proposed for Puf-family proteins.
Keywords: Plasmodium, Translation regulation, Puf proteins, RNA-binding protein, Transfection, Genetic complementation
Introduction
Translational control of gene expression plays a critical role during development of eukaryotes (Vardy and Orr-Weaver, 2007). Regulation of spatial localization, stability and translatability of mRNAs is often mediated by cis-acting elements in the 3′ untranslated region (UTR) of the target mRNA (Gray and Wickens, 1998; Kuersten and Goodwin, 2003). Binding of specific regulatory proteins or protein complexes to these elements activates or represses translation of the mRNA. The Puf family RNA-binding proteins (RBPs), named after the two founding members, Drosophila Pumilio protein and Caenorhabditis elegans FBF (fem-3 binding factor), represent a class of translational repressors of specific mRNAs (Wickens et al., 2002). Puf proteins are structurally related and evolutionarily conserved in a wide range of eukaryotes (Spassov and Jurecic, 2003). Although Drosophila has only one Puf protein, many organisms contain multiple Puf members. For example, there are 11 Puf members in the nematode C. elegans, and six in the yeast Saccharomyces cerevisiae. Puf proteins are characterized by the presence of a domain consisting of eight tandem imperfect repeats of ~36 amino acids each (Zamore et al., 1997; Zhang et al., 1997). This repeat region, together with short flanking sequences, constitutes the functional sequence-specific RNA-binding domain, the Puf domain. Crystal structures of human and yeast Puf proteins revealed a modular nature of RNA binding, with each Puf repeat recognizing a single RNA base (Gupta et al., 2008; Miller et al., 2008; Wang et al., 2002). Binding of the mRNAs by Puf, together with other effector proteins, results in translational repression and/or acceleration of decay of target mRNAs (Goldstrohm et al., 2007; Olivas and Parker, 2000; Wharton et al., 1998).
Functional studies in different organisms have revealed diverse roles of Puf proteins in embryonic development, stem-cell maintenance and neurogenesis (Spassov and Jurecic, 2003; Wickens et al., 2002). Although some Puf members in C. elegans act redundantly (Bachorik and Kimble, 2005; Crittenden et al., 2002; Lamont et al., 2004), most of them have distinct functions. In C. elegans, FBF-1 and FBF-2 regulate the sperm-oocyte switch in the hermaphrodite germline (Zhang et al., 1997), and they also regulate the mitosis-meiosis decision in the germline to prevent stem cells from entering meiosis (Crittenden et al., 2002). FBF-1 is also required for regulation of expression of a cGMP-dependent protein kinase in adult olfactory sensory neurons (Kaye et al., 2009). In primary spermatocytes, after initiation of meiosis, PUF-8 is required to maintain meiosis and prevent return to mitosis (Subramaniam and Seydoux, 2003). Several Puf members (PUF-5, PUF-6 and PUF-7) have additional functions in post-mitotic differentiation by regulating maternal mRNAs (Lublin and Evans, 2007). PUF-9 controls the differentiation of epidermal stem cells at the larva-adult transition (Nolde et al., 2007). In S. cerevisiae, Puf3, Puf5, and Puf6 regulate the decay of COX17, HO and ASH1 mRNAs, respectively (Deng et al., 2008; Gu et al., 2004; Olivas and Parker, 2000; Tadauchi et al., 2001). In addition, recent studies described combinatorial actions of several yeast Puf proteins (Hook et al., 2007; Ulbricht and Olivas, 2008). Although Drosophila has only one Pumilio protein, it plays multiple roles in embryonic morphogenesis (Murata and Wharton, 1995), germline development and maintenance (Asaoka-Taguchi et al., 1999; Forbes and Lehmann, 1998; Parisi and Lin, 1999), and dendrite morphogenesis, neuronal excitability and learning (Mee et al., 2004; Menon et al., 2004; Muraro et al., 2008; Schweers et al., 2002; Ye et al., 2004). Based on related functions of Pufs in controlling germline switch in C. elegans, regulating aging and mitochondrial function in yeast, and promoting vegetative growth in the slime mold Dictyostelium (Souza et al., 1999), it has been suggested that the ancestral function of Puf proteins is to promote proliferation and to suppress differentiation (Wickens et al., 2002).
Translational regulation of gene expression in protozoan parasites is not well understood. In malaria parasites of the genus Plasmodium, the life cycle takes place in two hosts. In the blood of a vertebrate host, in response to poorly defined cues, a small proportion of the parasites differentiates into gametocytes, a process termed gametocytogenesis. Transmission to the mosquito vector is accomplished by the sexually dimorphic male and female gametocytes. Molecular investigations into the sexual biology of malaria parasites have led to the identification of a number of gametocyte- and sex-specific molecules and elucidation of their functions during sexual development (reviewed by Alano, 2007; Dixon et al., 2008; Kooij and Matuschewski, 2007; Talman et al., 2004a), but the processes of gametocytogenesis and sex differentiation are still poorly understood. Earlier studies have suggested the existence of genes that are regulated post-transcriptionally. In mature gametocytes, the mRNAs of two transmission-blocking vaccine candidates, P25 and P28, are abundantly present, but the proteins are expressed subsequently in zygotes and ookinetes (Kaslow et al., 1988; Paton et al., 1993). Similarly, comparisons of mRNA expression and proteomic data in the human and rodent parasites revealed a considerable number of genes that are potentially regulated at the translation level (Hall et al., 2005; Le Roch et al., 2004). In the rodent parasite Plasmodium berghei, some of these mRNA species are found to be associated with a DDX6-class RNA helicase, DOZI (development of zygote inhibited), in the female gametocytes (Mair et al., 2006). Deletion of PbDOZI causes a near complete loss of P25 and P28 mRNAs and significant reduction of many other mRNA species in unactivated gametocytes, suggesting a role of DOZI in controlling mRNA stability. Many of these translationally regulated genes contain in the putative 3′ UTRs, a 47-base U-rich motif which is shown to be associated with translation repression of the mRNAs, regardless of its position relative to the coding region (Braks et al., 2008). Interestingly, the U-rich motif also contains a submotif, UUGU, which resembles the UGUR tetranucleotide consensus motif recognized by Puf proteins. However, this 47-base motif has not been identified in P. falciparum. All malaria parasites have two conserved Puf proteins that are differentially expressed in gametocytes (Cui et al., 2002). In P. falciparum, both PfPuf1 (PFE0935c) and PfPuf2 (PFD0825c) are upregulated in gametocytes (Cui et al., 2002; Fan et al., 2004), and microarray analysis detected increasing amounts of PfPuf1 and PfPuf2 mRNAs during gametocyte maturation (Young et al., 2005). Interestingly, the highest level of PfPuf2 expression was in sporozoites (Le Roch et al., 2003). Although PfPuf1 was found to be upregulated only in gametocytes from microarray analysis, a subtraction library of P. berghei sporozoites identified the PfPuf1 orthologue (uis9) as upregulated in salivary gland sporozoites (Matuschewski et al., 2002). These results suggest that both PfPuf proteins may function in several developmental stages. In vitro binding assay and yeast three-hybrid analysis have demonstrated that the PfPuf proteins possess RNA-binding activities toward the Drosophila nanos response elements (Fan et al., 2004), but the cellular targets of these proteins remain to be identified and the functions of Puf proteins in gametocyte development are unknown.
In order to determine the roles of Puf proteins in the development of Plasmodium falciparum, we generated stable lines with PfPuf2 disruption. The effect of PfPuf2 disruption on parasite growth and development was assessed, and further confirmed by genetic complementation and overexpression studies. We show that PfPuf2 plays an important role in repressing gametocytogenesis and male gametocyte differentiation in P. falciparum, consistent with the ancestral role of Puf proteins in suppression of differentiation.
Results
PfPuf2 is expressed in both female and male gametocytes
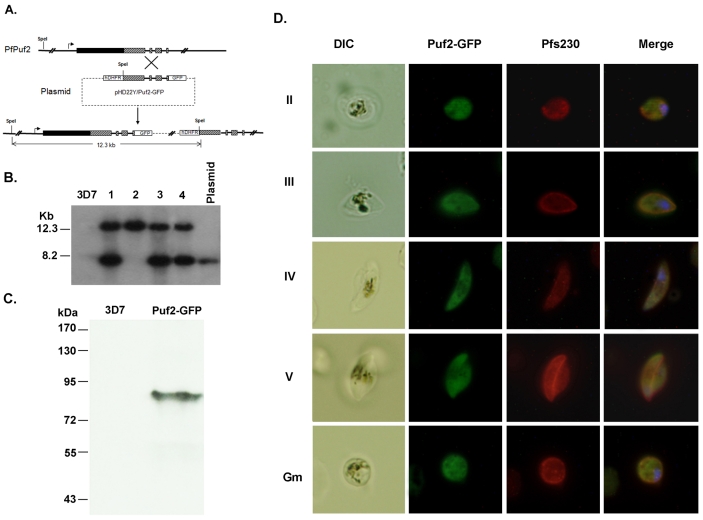
To study stage-specific expression and subcellular localization of the PfPuf2 protein in gametocytes, we transfected the 3D7 parasite clone and tagged the C-terminus of the endogenous PfPuf2 with green fluorescent protein (GFP). Correct integration of the plasmid pHD22Y/Puf2-GFP into the PfPuf2 locus was confirmed by genomic Southern blot (Fig. 1A,B). Among the four clones characterized, clone 2 had a single copy plasmid integrated at the PfPuf2 locus and was selected for protein expression analysis (Fig. 1B). Western blotting with anti-GFP antibodies detected a specific protein band of 90 kDa, consistent with the predicted size of the PfPuf2-GFP fusion protein (Fig. 1C). Compared with 3D7, the GFP-tagged parasite did not show noteworthy differences in asexual growth rates, morphology and gametocyte production (supplementary material Fig. S1). Consistent with its sexual-stage-specific expression, GFP was not observed in asexual stages (data not shown). The GFP-tagged parasites showed GFP signal in all gametocyte stages and also in rounded female gametes (Fig. 1D). GFP was present in all gametocytes that were detected with antibodies for the gametocyte-specific protein Pf230, indicating that PfPuf2 was expressed in both male and female gametocytes (Fig. 1D). As expected for a potential role of PfPuf2 in translational regulation, PfPuf2-GFP was observed in the parasite cytosol with a relatively even distribution (Fig. 1D).
Fig. 1.
GFP-tagging of PfPuf2 and subcellular localization. (A) Predicted integration event of GFP fusion at the endogenous PfPuf2 locus. Top: PfPuf2 locus on chromosome 4. Solid lines indicate introns or intergenic regions, filled boxes represent the exons, and hatched boxes the PfPuf2 region used for homologous recombination in the transfection plasmid. Middle: the plasmid pHD22Y/Puf2-GFP. Bottom: The resultant single-crossover event at the PfPuf2 locus with integration of one copy of the plasmid. The SpeI restriction sites are shown. (B) Confirmation of integration by Southern blot. Genomic DNA from 3D7 and four clones (1-4) of PfPuf2-GFP was digested with SpeI and separated on a 0.6% agarose gel. The blot was probed with the labeled GFP gene. The predicted 12.3 kb DNA band and the 8.2 kb plasmid are marked. (C) Confirmation of PfPuf2-GFP expression by western blotting. Gametocyte lysates from 3D7 control and PfPuf2-GFP clone 2 were separated by 10% SDS-PAGE and probed with anti-GFP antibodies. (D) GFP fluorescence and IFA of gametocytes from the PfPuf2-GFP clone 2. Representative GFP fluorescent images of stage II-IV gametocytes and a female gamete (Gm) showing the expression and localization of PfPuf2-GFP. Gametocytes and gametes were confirmed by IFA with the anti-Pf230 antibodies. Nuclei were counterstained with Hoechst 33342.
Targeted disruption of PfPuf2
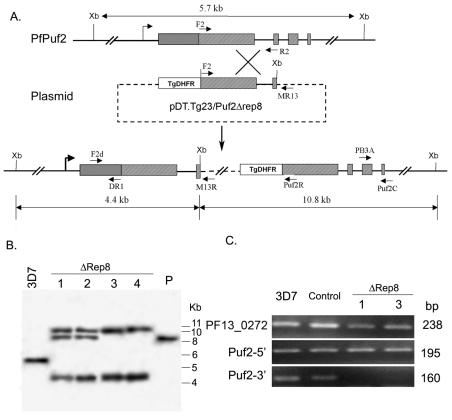
To study the function of PfPuf2, plasmid pDT.Tg23/Puf2Δrep8 was transfected into 3D7 parasites in order to disrupt PfPuf2 after repeat 7 of the RNA-binding domain (Fig. 2A). After transfection, parasites were selected using pyrimethamine, and three alternating cycles of drug-on and drug-off were applied to facilitate the loss of episomal plasmids and enrich parasites with chromosomal integration of the plasmid. Integration events were monitored for each cycle by using integration-specific polymerase chain reaction (PCR). From two independent transfection experiments, 20 single clones were obtained for further analysis. The presence of the plasmid backbone in all selected parasite clones was confirmed by the generation of a 1370 bp PCR product with primers F2 and M13R; the integration of the plasmid at the PfPuf2 locus was verified by PCR with primers F2d and M13R (data not shown). From ten clones selected for PCR analysis, eight were positive for integration-specific PCR at the PfPuf2 locus. To show that these were single clones, PCR was performed with F2d × Puf2R, which produced a 1.5 kb fragment in wild-type (wt) 3D7 and two F2d × M13R-negative clones (suggesting plasmid integration elsewhere), but not in the eight F2d × M13R-positive clones (too large to be amplified; data not shown). Four single clones with PfPuf2 site integration were selected for further analysis by genomic Southern blot (Fig. 2B). As expected from a single-crossover event, a 10.8 kb and a 4.4 kb band were observed in the PfPuf2-disrupted lines, in contrast to the 5.7 kb band in wt 3D7. The presence of an extra 8.5 kb band of the same size as the plasmid construct in clones 1 and 2 indicated that these clones most probably resulted from integration of plasmid concatemers (O'Donnell et al., 2001).
Fig. 2.
Disruption of the PfPuf2 gene. (A) Predicted PfPuf2 disruption from a single-crossover event. The positions and orientations of the primers on chromosome 4 and the plasmid are shown. Restriction enzyme XbaI (Xb) sites and the expected sizes of DNA fragments after XbaI digestion are also shown. Top: PfPuf2 locus on chromosome 4. Solid lines represent introns or intergenic regions, filled boxes the exons, and hatched boxes the RNA-binding domain. Middle: the transfection plasmid pDT.Tg23/Puf2Δrep8 showing the PfPuf2 genomic fragment and the drug selection cassette TgDHFR. Bottom: the predicted single-crossover event at the PfPuf2 locus showing the integration of one copy of the plasmid. The primers F2d and MR13 were used for identification of this integration event. (B) Confirmation of PfPuf2 disruption by Southern blot. Genomic DNA from wt 3D7, four disruption clones (ΔRep8 1-4) and 100 ng of the plasmid (P) were digested with XbaI and separated in a 0.6% agarose gel. The blot was hybridized to 32P-labeled PCR product using primers F2 × R2 (shown in A). (C) Confirmation of PfPuf2 disruption by RT-PCR. The primers for amplifying the 5′ end (F2d and DR1) and the 3′ end (PB3A and Puf2C) of PfPuf2 are shown in A. The constitutively expressed gene PF13_0272 was used as a control. The sizes of the RT-PCR products are shown on the right. Two representative PfPuf2-disruption clones ΔRep8-1 and ΔRep8-3, wt 3D7, and a transfection control (Control) were tested for PfPuf2 expression in mixed gametocytes.
To further confirm disruption of PfPuf2, its expression was analyzed by reverse transcriptase (RT)-PCR (RT-PCR) using RNA from gametocytes (mostly stages IV and V) in two PfPuf2-disrupted clones, wt 3D7, and one control parasite line that had the pDT.Tg23/Puf2ΔRep8 construct integrated elsewhere in the genome. Compared with a housekeeping gene PF13_0272, a similar level of PfPuf2 expression was detected in all clones with primers F2d × DR1, which are upstream of the integration site (Fig. 2A,C). By contrast, the downstream primers PB3A × Puf2C only detected PfPuf2 expression in wt 3D7 and transfection control parasites, demonstrating the deletion of domain downstream of the repeat 7 of the RNA-binding domain in the two PfPuf2 disruptants.
PfPuf2 disruption increases gametocytogenesis and male gametocyte differentiation
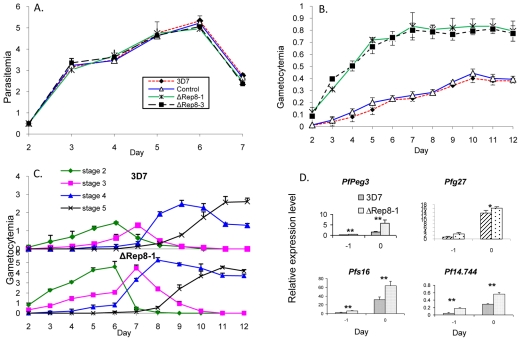
Since the RNA-binding activity of Puf proteins requires all eight repeats and short flanking regions, we determined whether C-terminal truncation of PfPuf2 (deletion of sequence after repeat 7) was associated with any phenotypic changes. Consistent with its gametocyte-specific expression and potential functions in gametocytes, disruption of PfPuf2 did not affect P. falciparum asexual growth (Fig. 3A). Daily parasitemias were not significantly different in the four clones examined (P>0.05, ANOVA). In the PfPuf2 disruptant parasites, gametocytes were observed during routine culture. No morphological abnormalities were detected during examination of the parasites under a light microscope (data not shown). However, gametocytogenesis rates in the PfPuf2-disrupted clones were dramatically different from those in the controls (Fig. 3B). The transfection control did not exhibit significant differences from the wt 3D7 in daily gametocytemias (P>0.05, ANOVA), suggesting that the observed changes in gametocytogenesis in PfPuf2-disrupted clones were not due to integration of the plasmid in the parasite genome. Starting from the same initial asexual parasitemia, the two PfPuf2 disruptants produced significantly higher gametocytemias than the transfection control and wt 3D7 (P<0.01, ANOVA). This increase in gametocyte formation in the PfPuf2 disruptants was especially evident during the early days after gametocyte induction, and such a trend was maintained throughout the entire gametocytogenesis period. On day 12 when the majority of gametocytes were at stages IV and V, gametocytemias in the PfPuf2 disruptants were still twofold higher than in the control and wt 3D7 (Fig. 3B). To determine whether the enhanced gametocyte production in the PfPuf2-disrupted clones was due to accelerated maturation of gametocytes, we followed the dynamics of gametocyte development in two lines. When the developmental stages (II-V) of the daily gametocytemias were determined, the dynamics of corresponding gametocyte stages was similar between the PfPuf2 disruptant and wt 3D7 (Fig. 3C). This result suggested that gametocytes of the PfPuf2 disruptants developed at a similar speed, and disruption of PfPuf2 led to an overall increase in gametocytogenesis. In agreement with this observation, enhanced gametocytogenesis could be detected immediately after induction by molecular analysis, as four genes expressed in early gametocytes (Pfs16, Pfs27, PfPeg3 and Pf14.744) showed significantly higher levels of expression in the PfPuf2 disruptant than in wt 3D7 as analyzed by real-time RT-PCR analysis (Fig. 3D).
Fig. 3.
Asexual growth and gametocytogenesis. Four clones, wt 3D7, transfection control and two PfPuf2 disruptants (ΔRep8-1 and ΔRep8-3) were compared. (A) Asexual growth. Synchronized parasite cultures were initiated at 0.5% parasitemia on day 1. Parasitemia was determined daily from Giemsa-stained thin smears. Each point represents the mean and standard deviation from three experiments. Statistical analysis by two-way ANOVA followed by Tukey's pairwise comparison showed that daily parasitemias were not significantly different among the parasite lines (P>0.05). (B) Daily gametocytemias after induction of gametocytogenesis. Values are means ± standard deviations of triplicate samples. Statistical analysis showed that the gametocytemias were significantly different in the PfPuf2 disruptants and controls (two-way ANOVA and Tukey's t-test; P<0.0001), whereas gametocytemias were not significantly different in the two disruptants or in the two controls (P>0.05). Symbols are the same as in A. (C) Gametocyte development and maturation dynamics. The dynamics of different gametocyte stages (except stage 1) was determined for wt 3D7 (upper panel) and ΔRep8-1 (lower panel) lines. Gametocyte stages were differentiated on the basis of morphology of the gametocytes. Values are the means and standard deviations of stage-specific gametocytemias determined from three replicates. (D) Real-time RT-PCR of representative genes expressed during early gametocyte stages in the PfPuf2 disruptant clone ΔRep8-1 and wt 3D7. The data were log-transformed and the two samples from the same day were compared by t-test. Asterisks indicate significant differences between the two samples: *P=0.05 and **P=0.01.
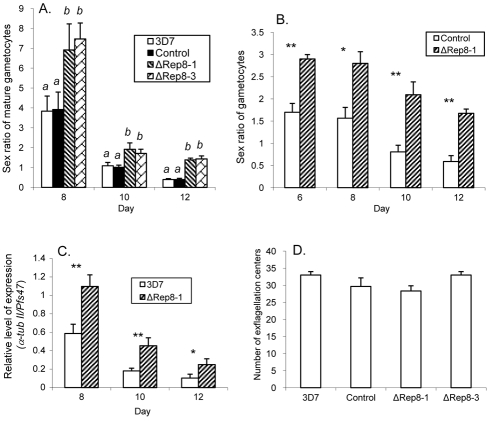
To determine whether PfPuf2 truncation affected sexual differentiation, we counted male and female gametocytes in the four parasite clones based on morphology. In wt 3D7 and control parasites, sex ratios (male:female) of mature gametocytes were male-biased at early times of gametocytogenesis, suggesting that male gametocytes matured earlier than females (Fig. 4A). This male-biased sex ratio was reversed to a female-biased ratio at late stages of gametocytogenesis. In the two PfPuf2-disrupted clones, sex ratios were significantly higher than in the control and wt 3D7 at all time points of the entire gametocytogenesis period. In stark contrast to a female-biased sex ratio on day 12 in wt 3D7 and control parasites, male-biased sex ratios were maintained in PfPuf2-disrupted clones (Fig. 4A). To verify this observation, we determined the sex of all gametocytes (both developing and mature) using an indirect immunofluorescence assay (IFA) with antibodies against the male-specific α-tubulin II and gametocyte-specific Pfs230. PfPuf2 disruption led to significant increases of male gametocytes from day 6 to 12 (t-test, P<0.01; supplementary material Fig. S2A). By comparison, whereas a 2.2-fold increase in female gametocytemia was detected on day 6 in the PfPuf2-disruptant (t-test, P<0.05), there was only a slight, insignificant increase in female gametocytemia on day 12 (t-test, P>0.05; supplementary material Fig. S2B). This resulted in significantly higher gametocyte sex ratios in the PfPuf2 disruptants than in the control parasites (t-test, P<0.05, Fig. 4B). We further compared the relative expression levels of the male-specific α-tubulin II gene and female-specific gene Pfs47 by real-time RT-PCR analysis (Fig. 4C). This experiment again confirmed that PfPuf2 truncation resulted in significantly higher sex ratios (t-test, P<0.05). Altogether, these experiments consistently demonstrated that PfPuf2 truncation led to significantly elevated male:female gametocyte sex ratios. Next, we wanted to determine whether PfPuf2 truncation could influence the exflagellation ability of mature male gametocytes (Fig. 4D). Using 12-day gametocyte cultures, we counted exflagellation centers and normalized the numbers by the mature male gametocytemias in the four parasite clones. No significant differences in exflagellation were observed in the two PfPuf2 disruptants, transfection control and wt 3D7 (ANOVA, P=0.125). This result demonstrated the maturity of the male gametocytes and their competence for exflagellation in the PfPuf2-disrupted clones, even though this result is not necessarily predictive of mosquito infectiveness (Noden et al., 1994).
Fig. 4.
Sex differentiation and male gametocyte exflagellation. (A) Mature male:female ratios in ΔRep8-1, ΔRep8-3, transfection control and wt 3D7 clones on day 8, 10 and 12. The means and standard deviations are shown from three gametocyte induction experiments. Daily sex ratios in the clones were compared by one-way ANOVA with Bonferroni corrections. For each time point, different letters indicate significant differences between the clones (P<0.05). (B) Sex ratios (male:female) of all gametocytes were compared between the ΔRep8-1 clone and transfection control. From day 6 to 12, IFA was performed with anti-Pfs230 antibodies to detect all gametocytes, and anti-α-tubulin II antibodies to detect male gametocytes. Data were analyzed by t-tests with Bonferroni corrections. For each time point, asterisks indicate significant differences between the two parasite clones: *P=0.05, **P=0.01. (C) The ratios of relative mRNA levels, determined by real-time RT-PCR, of the male-specific α-tubulin II gene (α-tub II) and female-specific gene Pfs47. Asterisks indicate significant differences between the two samples: *P=0.05 and **P=0.01 (t-test with Bonferroni corrections). (D) Comparison of male gametocyte exflagellation centers. Exflagellation centers were counted and normalized to mature male gametocytemia of each clone. One-way ANOVA did not detect significant differences among these clones (P>0.05).
Complementation of the PfPuf2 truncation mutation
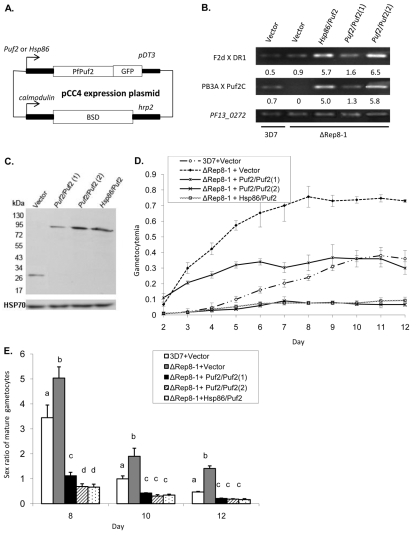
To confirm that PfPuf2 truncation was responsible for the observed changes in gametocytogenesis, we performed complementation studies by episomally expressing PfPuf2. PfPuf2 expression was directed by either its cognate promoter to confer stage-specific expression of PfPuf2 or the hsp86 promoter for a higher level of expression (Fig. 5A). Since the constitutively active hsp86 promoter has a completely different expression profile and may not faithfully reflect the effect of PfPuf2 overexpression on sexual development, we also sought to achieve higher PfPuf2 expression under its cognate promoter by using higher drug selection pressure. Altering drug selection pressure has been shown to modulate the copy number of the concatemeric episomes and thereby regulate the expression level of the transgene (Dzikowski and Deitsch, 2008). All constructs were transfected into the ΔRep8-1 clone, and the pCC4-GFP control vector was also transfected into 3D7 to serve as an additional control. All resistant parasites were established under 2.5 μg/ml of blasticin D (BSD) selection. In order to obtain a higher level of PfPuf2 expression, PfPuf2 expression under its cognate promoter was further selected under 10 μg/ml of BSD. By quantitative PCR it was estimated that the copy number of the pCC4-Puf2/Puf2 plasmid was increased from 2.3 under 2.5 μg/ml of BSD to 12.7 under 10 μg/ml of BSD. RT-PCR analysis of day-10 gametocytes detected expression of PfPuf2 C-terminal fragment in the ΔRep8-1 clone transfected with the PfPuf2 expression constructs (Fig. 5B). In addition, PfPuf2 expression driven by the hsp86 promoter and the PfPuf2 promoter under 10 μg/ml of BSD selection resulted, respectively, in 3.8- and 4.5-fold higher PfPuf2 expression than the PfPuf2 promoter under 2.5 μg/ml of BSD selection (Fig. 5B). Western blots showed that the increase in PfPuf2 mRNA corresponded to a 4.5- and 3.2-fold increase, respectively, in the amount of recombinant PfPuf2-GFP protein (Fig. 5C). Gametocytogenesis was compared among the five parasite lines (Fig. 5D). Whereas the vector controls did not change the gametocytogenesis phenotypes of the ΔRep8-1 or 3D7 clones, episomal expression of PfPuf2 resulted in significantly lower gametocytemias from day 3 than ΔRep8-1 transfected with the control vector (Fig. 5D; t-test, P<0.01). The gametocytemias in ΔRep8-1 transfected with the pCC4-Puf2/Puf2 construct under 2.5 μg/ml of BSD were initially higher than those in the 3D7 control (from day 2 to 6; t-test, P<0.05), but subsequent daily gametocytemias were not significantly different in the two lines (from day 7 to 12; t-test, P>0.05). This suggests that PfPuf2 expression under its own promoter partially restored the gametocytogenesis phenotype. More remarkably, ΔRep8-1 transfected with the pCC4-Hsp86/Puf2 construct or the pCC4-Puf2/Puf2 construct under 10 μg/ml of BSD produced significantly fewer gametocytes than the 3D7 control (t-test, P<0.05), suggesting that higher levels of PfPuf2 expression further repressed gametocytogenesis (Fig. 5D). Analysis of the gametocyte male:female sex ratios found that moderate expression of PfPuf2 (pCC4-Puf2/Puf2 with 2.5 μg/ml of BSD) had significantly lowered the sex ratio of mature gametocytes (one-way ANOVA, P<0.05; Fig. 5E). Moreover, higher levels of PfPuf2 expression under the hsp86 promoter or its cognate promoter with 10 μg/ml of BSD selection led to further significant reductions in sex ratio (one-way ANOVA, P<0.05; Fig. 5E). Consistent with a similar level of PfPuf2 expression resulting from transfection with pCC4-Hsp86/Puf2 and pCC4-Puf2/Puf2 under 10 μg/ml of BSD, these two lines had no significant differences in daily gametocytemia and sex ratio (P>0.05, one-way ANOVA; Fig. 5D,E).
Fig. 5.
Complementation of PfPuf2 disruption. (A) Diagram of the pCC4 expression plasmid used for the episomal expression of PfPuf2-GFP. PfPuf2-GFP was directed by either the PfPuf2 or hsp86 promoter, and GFP expression in the pCC4-GFP control (Vector) was directed by the PfPuf2 promoter. (B) Episomal expression of PfPuf2 in the ΔRep8-1 clone. RT-PCR was performed to detect PfPuf2 expression at N- and C-termini using the primers (F2d × DR1) and primers (PB3A × Puf2C), respectively (same as in Fig. 2A). PF13_0272 was used as an internal control. Parasites were transfected with either pCC4-GFP control vector or PfPuf2 coding sequence driven by the hsp86 promoter (Hsp86/Puf2) or PfPuf2 promoter (Puf2/Puf2). Puf2/Puf2(1) and Puf2/Puf2(2) were selected with 2.5 and 10 μg/ml of BSD, respectively. The numbers below the DNA bands indicate average relative expression levels of PfPuf2 in each parasite line from real-time RT-PCR analysis determined using the ΔCt method with PF13_0272 as the control. (C) Expression of the recombinant PfPuf2-GFP in transfected PfPuf2 disruptant. The GPF-tagged PfPuf2 was detected by western blotting using anti-GFP antibodies (upper panel) and protein loading was monitored with anti-HSP70 antibodies (lower panel). (D) Gametocytogenesis in the parasite lines described in B. Two-way ANOVA showed significant difference between each line (d.f.=3, 88; F=1166; P<0.001) except between Hsp86/Puf2 and Puf2/PfPuf2(2). Bonferroni simultaneous tests indicate that gametocytemias between 3D7+vector and ΔRep8-1+Puf2/Puf2(1) were significantly different from day 2 to 6 (P<0.05), but not significantly different at subsequent time points (P>0.05). (E) Sex ratios (male:female) in the parasite lines described in B. One-way ANOVA and Bonferroni simultaneous pairwise comparisons were performed for each time point. Different letters indicate significant differences between the parasite lines (P<0.05).
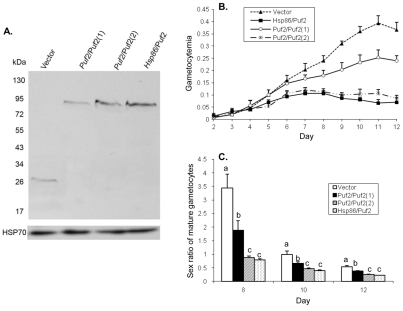
To verify the role of PfPuf2 in repressing gametocytogenesis and male gametocyte differentiation in P. falciparum, we transfected the wt 3D7 parasite with the control and PfPuf2 expression constructs and selected resistant parasites with BSD. Parasites transfected with the constructs pCC4-Hsp86/Puf2 or pCC4-Puf2/Puf2 with 10 μg/ml of BSD selection had a similar level of recombinant PfPuf2 expression, 3.1- or 3.8-fold higher than that with the pCC4-Puf2/Puf2 construct under 2.5 μg/ml of BSD (Fig. 6A). The gametocytogenesis and sex ratio results in the wt 3D7 were similar to those from the PfPuf2 complementation studies in the ΔRep8-1 background. From day 7 after induction for gametocytogenesis, episomal expression of PfPuf2 in wt 3D7 under its cognate promoter and 2.5 μg/ml of BSD selection resulted in significantly lower gametocytemias than transfection with the control vector (t-test, P<0.05; Fig. 6B). Consistently, higher levels of PfPuf2 expression under the hsp86 promoter or PfPuf2 promoter under 10 μg/ml of BSD further repressed gametocytogenesis, resulting in significantly lower daily gametocytemias than those of the control and pCC4-Puf2/Puf2 under 2.5 μg/ml of BSD from day 5 after induction (one-way ANOVA, P<0.05; Fig. 6B). In terms of sex ratio of mature gametocytes, episomal expression of PfPuf2 in the wt 3D7 background also led to significantly lower sex ratios than in wt 3D7 transfected with the control vector (one-way ANOVA, P<0.05; Fig. 6C). Taken together, these results indicate that higher levels of PfPuf2 expression repress gametocytogenesis and male gametocyte differentiation.
Fig. 6.
Overexpression of PfPuf2 in wt 3D7. (A) Expression of PfPuf2-GFP. The wt 3D7 was transfected with the plasmids shown in Fig. 5A. The GFP-tagged PfPuf2 protein expression was determined by western blotting with anti-GFP antibodies (upper panel), and protein loading was monitored with anti-HSP70 antibodies (lower panel). 3D7 parasites were transfected with either pCC4-GFP control vector (Vector) or PfPuf2-GFP driven by the hsp86 (Hsp86/Puf2) or PfPuf2 promoter (Puf2/Puf2). Puf2/Puf2(1) and Puf2/Puf2(2) were selected with 2.5 and 10 μg/ml of BSD, respectively. (B) Gametocytogenesis in the parasite lines described in A. Two-way ANOVA showed significant difference between pCC4-GFP control (Vector) and Puf2/Puf2(1) from day 7 to day 12 (P<0.05). No significant difference was found between Hsp86/Puf2 and Puf2/Puf2(2) at all time points (P>0.05), whereas these two parasite lines showed significant difference in daily gametocytemias from the control (Vector) and Puf2/Puf2(1) lines (P<0.001). (C) Sex ratios (male:female) in the parasite lines described in A. One way ANOVA and Bonferroni simultaneous pairwise comparison were performed for each time point. Different letters indicate significant difference between the parasite lines (P<0.05).
Discussion
Sexual development and transmission through an invertebrate vector are intimately connected in the life cycle of the malaria parasites. During sexual development, malaria parasites have to choose two pathways: one is the commitment to sexual development (transition from asexual replication to gametocytogenesis), and the other is sex differentiation (development into either male or female gametocytes). Although global expression analyses have identified large sets of mRNAs and proteins that are specific to gametocytes (Florens et al., 2002; Lasonder et al., 2002; Young et al., 2005), the mechanism underlying sexual development remains poorly understood. Recent investigations into the mechanism of sexual development have revealed that gametocytes, like Drosophila eggs, also store maternal transcripts for subsequent development, implying the significance of translational regulation (Hall et al., 2005; Le Roch et al., 2004). In P. falciparum, the two differentially expressed Puf family proteins, PfPuf1 and PfPuf2, may serve as potential translational regulators in gametocytes (Cui et al., 2002; Fan et al., 2004). In this study, we performed functional analysis of PfPuf2 and found that genetic disruption of this gene has resulted in enhanced gametocytogenesis and elevated sex ratio, suggesting that PfPuf2 is involved in both pathways. Yet, the loss of capability to infect mosquitoes in control and manipulated lines, possibly as a result of extensive culture, meant that it was not possible to examine the potential effect of PfPuf2 disruption in subsequent development in mosquitoes (data not shown).
The capacity for sexual development is genetically determined in malaria parasites, and there are considerable variations in the rate of commitment to gametocytogenesis among parasite strains, and even between parasite clones derived from the same isolate (Dyer and Day, 2000; Talman et al., 2004a). However, there is overwhelming evidence demonstrating the plasticity of this process as the commitment to gametocytogenesis can be modulated by many environmental factors such as host immunity, host hormones, drug treatment, etc. As developmental decision for gametocytogenesis occurs during the formation of the asexual schizont (Bruce et al., 1990), it is important that regulatory genes are expressed in schizonts that are committed to sexual differentiation (Alano, 2007). Global analyses of gene expression by several groups have revealed relatively small numbers of genes showing evidence of upregulation after induction of sexual development (Eksi et al., 2005; Silvestrini et al., 2005; Young et al., 2005). Some early gametocyte genes are probably involved in host cell remodeling, since they are found to be exported to the parasitophorous vacuole and/or the cytoplasm of erythrocytes (Eksi et al., 2005; Lanfrancotti et al., 2007). Others such as pfs27 and pfgig may play regulatory roles during the commitment to sexual development. Disruption of early gametocyte genes such as pfs16 and pfgig reduced or eliminated gametocyte production (Gardiner et al., 2005; Lobo et al., 1999). An early study suggested an essential role of pfg27 in gametocytogenesis (Kongkasuriyachai et al., 2004), but a recent study found that it was dispensable for gametocyte and gamete production and important for maintaining cell integrity (Oliveri et al., 2009). Our results suggest that PfPuf2 may be a regulator in gametocytogenesis of malaria parasites. Disruption of PfPuf2 resulted in at least a two-fold increase in gametocyte formation, whereas overexpression of PfPuf2 repressed this process. It is noteworthy that in addition to a higher level of expression in later gametocyte stages, PfPuf2 also has low levels of expression in asexual stages (Cui et al., 2002) and a much higher level of expression in sporozoites, suggesting that PfPuf2 may participate in translation control in these stages. Interestingly, the function of PfPuf2 in repressing gametocytogenesis is consistent with the proposed ancestral role of Puf family proteins in repressing differentiation, according to findings in several model organisms (Wickens et al., 2002).
The human malaria parasites have a female-biased sex ratio that is subject to considerable variability and modulations by environmental factors (Paul et al., 2002). Although evolutionary biologists have attempted to explain the female-biased ratio and adaptive changes by the sex allocation theory (Schall, 2009; West et al., 2001), the molecular mechanism governing sex differentiation remains obscure. The deciphering of the sex-specific proteomes of the rodent parasite P. berghei has highlighted the molecular dimorphism between the sexes (Khan et al., 2005). Because the sex of a gametocyte is predetermined in sexually committed schizonts (Silvestrini et al., 2000; Smith et al., 2000), the regulatory mechanism determining sex must act early in the sex differentiation pathway. An early sexual-stage gene PfPeg3 (also known as Pfmdv1) is linked to defects in male gametocyte formation and its disruption caused a significant decrease in the number of mature male gametocytes (Furuya et al., 2005; Vaidya et al., 1995). Its localization to the parasitophorous membrane suggests that it may not play a regulatory role in sex determination (Furuya et al., 2005; Lanfrancotti et al., 2007). In P. falciparum, male-biased gametocyte sex ratios are normally observed at the early stages of gametocyte development, but later change to female-biased sex ratios. As differential deaths of male and female gametocytes are not observed in P. falciparum (Smalley and Sinden, 1977), this temporal change in sex ratio indicates that male gametocytes matured more rapidly. The cumulative effects of differential sex maturation dynamics during the lengthy gametocytogenesis process (~10 days) and cytoadherence of early gametocyte stages in the human host may contribute to the variations in P. falciparum sex ratio observed in peripheral blood of patients (Talman et al., 2004a). Using both genetic disruption and complementation, we have demonstrated in this study that PfPuf2 is involved in sex differentiation. In PfPuf2-disrupted clones, male-biased gametocyte sex ratios were maintained throughout the gametocytogenesis period. We have further shown that overexpression of PfPuf2 further reduced male gametocyte formation, resulting in even lower sex ratios than in the 3D7 control (Figs 5, 6). Given the detection of PfPuf2 expression in both male and female gametocytes (Fig. 1), it is unlikely to be the most upstream regulator of the sex determination process.
Enhanced gametocytogenesis in response to drug treatment, host factors such as anemia, and infections by genetically distinct malaria parasite strains in bird, rodent and human malarias is often associated with altered sex ratios (Paul et al., 2000; Reece et al., 2008; Robert et al., 2003; Sowunmi et al., 2008), suggesting a mechanistic link between the two pathways. Although sexual development and sex determination may respond to different environmental cues (Talman et al., 2004b), our results from PfPuf2 disruption and rescue studies also suggested an intrinsic link of the two pathways. The involvement of RNA-binding proteins in sexual development may be responsible for the phenotypic plasticity of gametocyte development and sex ratio, by providing simple adjustment through controlled expression of the target genes. It is also noteworthy that an early gametocyte gene, Pfs27, which may also play a regulatory role in the sexual pathway, also encodes an RNA-binding protein (Sharma et al., 2003). In yeast, fruit fly and human, global analysis has revealed that Pufs are potentially associated with large sets of mRNAs (Gerber et al., 2004; Gerber et al., 2006; Morris et al., 2008). Therefore, it is highly possible that PfPuf2 regulates different subsets of genes, which are involved in the two pathways of sexual development. Bioinformatic analysis of the P. falciparum genome using conserved binding sites for Puf proteins found 95 genes with potential Puf elements in 3′ regions (Le Roch et al., 2004). In addition, a number of translationally regulated genes have abundant (possibly repressed) mRNAs in gametocytes, coinciding with PfPuf expression. Furthermore, some of them contain consensus Puf motifs (UGUR) in the untranslated regions (Braks et al., 2008) and, therefore, are potentially subject to regulation by PfPufs. The PfPuf2 mutant and tagged lines from this study may facilitate the identification of the target gene repertoire in the parasite genome.
Materials and Methods
DNA constructs
To tag PfPuf2 with GFP at the C-terminus, a PfPuf2 fragment [nucleotides (nt) 967-2021] was amplified from P. falciparum genomic DNA and cloned into pBluescript to fuse with GFP and pDT 3′UTR (Fan et al., 2009). This cassette was subcloned into pHD22Y at BamHI and NotI sites to produce pHD22Y/Puf2-GFP (Fidock and Wellems, 1997). To disrupt PfPuf2, a PfPuf2 fragment of nt 491-1553 was amplified using primers F2 and R2 (supplementary material Table S1) and cloned at the BamHI site of pDT.Tg23 to generate pDT.Tg23/Puf2ΔRep8 (Wu et al., 1996). For complementation, the vector pCC4 (with the replacement of drug selection cassette hdhfr to BSD in pCC1 or pHHT-FCU vector) was modified for episomal expression of the transgenes (Maier et al., 2006). The full-length PfPuf2 coding region was amplified with primers puf2exF1 and puf2exR1 using gametocyte cDNA as the template (supplementary material Table S1) and cloned into pBluescript to fuse with GFP and pDT 3′UTR (Fan et al., 2009). PfPuf2 expression was driven by either the hsp86 promoter or the PfPuf2 promoter (1310 bp upstream of the PfPuf2 translation start codon). The transgene under this PfPuf2 promoter showed similar pattern of expression to that of the endogenous PfPuf2 in asexual stages and gametocytes (data not shown). The PfPuf2 expression cassette was cloned into the pCC4 vector at SpeI and NotI sites to obtain the constructs pCC4-Puf2/Puf2 and pCC4-Hsp86/Puf2 (Fig. 5A). In the control plasmid pCC4-GFP, GFP expression was driven by the PfPuf2 promoter. For parasite transfection, all constructs and control plasmids were purified using a Maxiprep kit (Qiagen).
Parasite culture and transfection
P. falciparum clone 3D7 was maintained as described previously (Trager and Jensen, 1976) and synchronized by sorbitol treatment (Lambros and Vanderberg, 1979). Transfection was done by electroporation of ~100 μg of plasmid DNA into 3-4% of synchronized ring-stage parasites using an established procedure (Fidock and Wellems, 1997). For pDT.Tg23-Puf2Δrep8 and the control plasmid pDT.Tg23, parasites were selected with 100 ng/ml of pyrimethamine for 48 hours and subsequently 20 ng/ml of pyrimethamine for ~3 weeks with weekly replenishment of fresh red blood cells (RBCs) until resistant parasites were seen. Parasites containing integrated forms of the constructs were enriched using three drug on-off cycles with 500 ng/ml of pyrimethamine (Crabb and Cowman, 1996). Drug-resistant parasites were screened by PCR and Southern blot to detect plasmid integration at the PfPuf2 locus. Single clones of parasites with stable integration of the constructs were obtained by limiting dilution (Rosario, 1981). Four clones from two different transfection experiments were randomly selected for the characterization of the integration events and two were used for phenotypic analysis. For the PfPuf2 localization study, pHD22Y/Puf2-GFP was transfected into 3D7 and selected with 2.5 nM WR99210. Drug cycles and cloning of GFP-tagged parasites were performed similarly. To complement the PfPuf2 disruption mutants and overexpress PfPuf2 in the wt 3D7 background, pCC4-Puf2/Puf2, pCC4-Hsp86/Puf2 and pCC4-GFP control vector were transfected into a PfPuf2 disruptant clone (ΔRep8-1) and wt 3D7 parasites and selected with BSD at 2.5 μg/ml until resistant parasites appeared. For transfection with the pCC4-Puf2/Puf2 construct, the BSD concentration was subsequently elevated to 10 μg/ml to increase the plasmid copy number and PfPuf2 expression.
Parasite growth and development
For each parasite clone, the growth experiments were performed in triplicate. To compare asexual stage parasite growth, 1 ml of synchronized ring-stage parasites at a parasitemia of 0.5% and hematocrit of 5% was placed into each well of a 24-well plate. Medium was changed daily for a week. Giemsa-stained thin smears were made to determine the daily parasitemias by counting parasites in at least 3000 red blood cells. Induction of gametocytogenesis was performed as described previously (Fivelman et al., 2007). Briefly, 2% synchronous trophozoites were incubated overnight on a rotation shaker to obtain 8% ring stage parasites. The culture was stressed by replacing only half of the spent medium with fresh medium. On the next day, (designated as day –1) when parasites reached mid-schizont stage, the culture was split to obtain 2% schizonts with the retention of one-third of the spent medium. After being gently shaken overnight, 8% ‘stressed’ ring-stage parasites appeared in the culture on the next day, which was designated as day 0. Subsequently, the medium was changed daily and the culture was maintained for 12 days until gametocytes became mature. Gametocytemia was determined by counting gametocytes in at least 5000 red blood cells in each treatment. Mature male and female gametocytes were differentiated using morphological characteristics of mature gametocytes in Giemsa-stained thin films (Robert et al., 1996; Talman et al., 2004b). The sex ratio of male to female was determined by counting at least 500 gametocytes on each slide. Exflagellation of mature microgametocytes from each clone was examined using a standard procedure (Bhattacharyya and Kumar, 2001). In brief, the gametocytes were pelleted at 37°C at 400 g, washed twice with warm PBS, and resuspended in warm PBS at a final hematocrit of 50%. Twenty microliters of the suspension with 5 μM of xanthurenic acid was dispersed to form an erythrocytic monolayer under the coverslip of a hemocytometer at room temperature. The exflagellation centers were observed under a microscope between 10 and 20 minutes later. Ten random fields were counted at 200× magnification and the numbers of exflagellation centers were normalized after dividing by the mean number of mature male gametocytes of each parasite clone.
Confirmation of chromosomal integration of the plasmids
Genomic DNA was extracted from the parasites using a proteinase K digestion and a phenol-chloroform extraction procedure (Cui et al., 2002). To detect integration of pDT-Tg23/Puf2Δrep8 plasmid at the PfPuf2 locus, genomic DNA from pyrimethamine-resistant parasites was amplified using the primers F2d and M13R (supplementary material Table S1). Another set of primers F2d and Puf2R was used to confirm that the obtained parasites were from single clones without contamination from parasites that had the plasmid integrated elsewhere. For genomic Southern blot, 3 μg of parasite DNA from each clone were digested with SpeI (for GFP-tagging) or XbaI (for PfPuf2 disruption) and separated on a 0.6% agarose gel. DNA was transferred to a nylon membrane, denatured, and UV-crosslinked to the membrane using Stratalinker 2400 (Stratagene). The membrane was prehybridized in 7% SDS, 0.5 M NaH2PO4 (pH 7.2), 2% dextran sulfate for 3 hours at 65°C and then hybridized with the denatured probes for 12 hours at 65°C (Kyes et al., 2000). Two washes were then performed at 60°C for 20 minutes with 2× SSC, 0.5% SDS and once for 50 minutes with 1× SSC, 0.1% SDS. The membrane was exposed to a Kodak X-ray film at −80°C for autoradiography. For Southern blots in the GFP tagging and PfPuf2 disruption experiments, the PCR fragments of GFP and PfPuf2 (F2 × R2) were used a probes, respectively.
RT-PCR
RNA extraction, cDNA synthesis, RT-PCR and real-time RT-PCR were performed as previously described (Miao et al., 2006). Briefly, 2 μg of parasite total RNA were reverse transcribed with Superscript III (Invitrogen, CA) at 50°C for 60 minutes. RT-PCR was performed with primers F2d and DR1 to detect the expression of the 5′ region of PfPuf2, which would generate a 195 bp PCR product for both genomic DNA and cDNA. To verify the lack of expression of PfPuf2 downstream of the integration sites in the disrupted parasite clones, RT-PCR was performed with PB3A and Puf2C, which would produce a 282 bp PCR product from genomic DNA and a 160 bp product from cDNA in the control clones. These primers were also used for real-time RT-PCR analysis of the mRNA levels of PfPuf2 in the complementation lines. The constitutively expressed PF13_0272 gene was used as a control using primers C341F and C341R (supplementary material Table S1). For real-time RT-PCR analysis of genes expressed during early stages of gametocytogenesis, primers (supplementary material Table S1) were designed for Pfs16 (Moelans et al., 1991), Pfs27 (Alano et al., 1991), PfPeg3 (Lanfrancotti et al., 2007) and PF14_744 (Eksi et al., 2005). For sex-specific gene expression, primers were designed for the male-specific α-tubulin II gene and female-specific gene Pfs47 (Rawlings et al., 1992; van Schaijk et al., 2006). The relative expression value of each gene was calculated as described previously using the ΔCt method (Drew and Reece, 2007; Miao et al., 2006). Copy numbers of the episomal plasmids were calculated by quantitative PCR and then comparing the ΔCt of the BSD gene to that of the single-copy merozoite surface protein 1 gene as described previously (Dzikowski and Deitsch, 2008).
Western blotting
To detect Puf2-GFP expression in the GFP-tagged parasite clone and from episomal expression, pellets of mixed gametocytes were resuspended in 1 × protein inhibitor mixture (Sigma), passed through a 1 cc syringe three or four times, and clarified by centrifugation. The lysate was mixed with SDS-polyacrylamide gel electrophoresis (PAGE) loading buffer, incubated at 95°C for 5 minutes, and separated in a 10% SDS-PAGE gel at ~50 μg of proteins per lane. The proteins were transferred to a nitrocellulose membrane and probed with primary anti-GFP antibodies (Roche Diagnostics) at 1:1000 and secondary horseradish peroxidase (HRP)-conjugated antibodies at 1:5000. As a loading control, the membrane was probed with antibodies against the heat shock protein 70 (HSP70). Proteins were visualized using an ECL Kit (Invitrogen). The relative levels of the GFP-tagged PfPuf2 in the overexpression studies were estimated using a densitometer.
Fluorescence microscopy
To determine PfPuf2 expression and subcellular locations during gametocytogenesis, GFP signal in a parasite clone with GFP-tagged PfPuf2 was observed under a fluorescence microscope using a previously described procedure (Tonkin et al., 2006). An immunofluorescence assay (IFA) was performed using antibodies against Pfs230 to label all gametocytes, and parasite nuclei were counterstained with Hoechst 33342. Images were merged using Adobe Photoshop. To determine the total male and female gametocytemias, monoclonal antibody against α-tubulin II was used to detect males, and rabbit antibodies against Pfs230 were used to detect the entire gametocyte population. At least 500 total gametocytes were counted to obtain the sex ratios.
Statistical analysis
The data were analyzed using Minitab 13.0 and SAS 8.0. The data were transformed to satisfy normal distribution when needed. For parasitemias and gametocytemias, data were analyzed by a two-way ANOVA and the interaction term (day by treatment) was included, followed by Tukey's pairwise comparison or Bonferroni simultaneous tests. For comparison of the gametocytogenesis rate between parasites transfected with pCC4-GFP vector and the PfPuf2 expression plasmid (pCC4-Puf2/Puf2 and pCC4-Hsp86/Puf2), Tukey's pairwise comparison and t-test were performed with Bonferroni corrections to ensure that the family error rate was corrected to α=0.0045 for each individual test. For comparison of male:female gametocyte ratios, relative expression levels of different genes by RT-PCR at each time point, and male gametocyte exflagellation center counts, one-way ANOVA and t-test were performed with Bonferroni corrections.
Supplementary Material
Acknowledgments
This work was partially supported by grant R01 AI064553 from National Institute of Allergy and Infectious Diseases, National Institutes of Health. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/7/1039/DC1
References
- Alano P. (2007). Plasmodium falciparum gametocytes: still many secrets of a hidden life. Mol. Microbiol. 66, 291-302 [DOI] [PubMed] [Google Scholar]
- Alano P., Premawansa S., Bruce M. C., Carter R. (1991). A stage specific gene expressed at the onset of gametocytogenesis in Plasmodium falciparum. Mol. Biochem. Parasitol. 46, 81-88 [DOI] [PubMed] [Google Scholar]
- Asaoka-Taguchi M., Yamada M., Nakamura A., Hanyu K., Kobayashi S. (1999). Maternal Pumilio acts together with Nanos in germline development in Drosophila embryos. Nat. Cell Biol. 1, 431-437 [DOI] [PubMed] [Google Scholar]
- Bachorik J. L., Kimble J. (2005). Redundant control of the Caenorhabditis elegans sperm/oocyte switch by PUF-8 and FBF-1, two distinct PUF RNA-binding proteins. Proc. Natl. Acad. Sci. USA 102, 10893-10897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya M. K., Kumar N. (2001). Effect of xanthurenic acid on infectivity of Plasmodium falciparum to Anopheles stephensi. Int. J. Parasitol. 31, 1129-1133 [DOI] [PubMed] [Google Scholar]
- Braks J. A., Mair G. R., Franke-Fayard B., Janse C. J., Waters A. P. (2008). A conserved U-rich RNA region implicated in regulation of translation in Plasmodium female gametocytes. Nucleic Acids Res. 36, 1176-1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M. C., Alano P., Duthie S., Carter R. (1990). Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology 100, 191-200 [DOI] [PubMed] [Google Scholar]
- Crabb B. S., Cowman A. F. (1996). Characterization of promoters and stable transfection by homologous and nonhomologous recombination in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 93, 7289-7294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden S. L., Bernstein D. S., Bachorik J. L., Thompson B. E., Gallegos M., Petcherski A. G., Moulder G., Barstead R., Wickens M., Kimble J. (2002). A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature 417, 660-663 [DOI] [PubMed] [Google Scholar]
- Cui L., Fan Q., Li J. (2002). The malaria parasite Plasmodium falciparum encodes members of the Puf RNA-binding protein family with conserved RNA binding activity. Nucleic Acids Res. 30, 4607-4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Singer R. H., Gu W. (2008). Translation of ASH1 mRNA is repressed by Puf6p-Fun12p/eIF5B interaction and released by CK2 phosphorylation. Genes Dev. 22, 1037-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M. W., Thompson J., Gardiner D. L., Trenholme K. R. (2008). Sex in Plasmodium: a sign of commitment. Trends Parasitol. 24, 168-175 [DOI] [PubMed] [Google Scholar]
- Drew D. R., Reece S. E. (2007). Development of reverse-transcription PCR techniques to analyse the density and sex ratio of gametocytes in genetically diverse Plasmodium chabaudi infections. Mol. Biochem. Parasitol. 156, 199-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer M., Day K. P. (2000). Commitment to gametocytogenesis in Plasmodium falciparum. Parasitol. Today 16, 102-107 [DOI] [PubMed] [Google Scholar]
- Dzikowski R., Deitsch K. W. (2008). Active transcription is required for maintenance of epigenetic memory in the malaria parasite Plasmodium falciparum. J. Mol. Biol. 382, 288-297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksi S., Haile Y., Furuya T., Ma L., Su X., Williamson K. C. (2005). Identification of a subtelomeric gene family expressed during the asexual-sexual stage transition in Plasmodium falciparum. Mol. Biochem. Parasitol. 143, 90-99 [DOI] [PubMed] [Google Scholar]
- Fan Q., Li J., Kariuki M., Cui L. (2004). Characterization of PfPuf2, member of the Puf family RNA-binding proteins from the malaria parasite Plasmodium falciparum. DNA Cell Biol. 23, 753-760 [DOI] [PubMed] [Google Scholar]
- Fan Q., Miao J., Cui L., Cui L. (2009). Characterization of protein arginine methyltransferase I from Plasmodium falciparum. Biochem. J. 421, 107-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidock D. A., Wellems T. E. (1997). Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc. Natl. Acad. Sci. USA 94, 10931-10936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fivelman Q. L., McRobert L., Sharp S., Taylor C. J., Saeed M., Swales C. A., Sutherland C. J., Baker D. A. (2007). Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol. Biochem. Parasitol. 154, 119-123 [DOI] [PubMed] [Google Scholar]
- Florens L., Washburn M. P., Raine J. D., Anthony R. M., Grainger M., Haynes J. D., Moch J. K., Muster N., Sacci J. B., Tabb D. L., et al. (2002). A proteomic view of the Plasmodium falciparum life cycle. Nature 419, 520-526 [DOI] [PubMed] [Google Scholar]
- Forbes A., Lehmann R. (1998). Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development 125, 679-690 [DOI] [PubMed] [Google Scholar]
- Furuya T., Mu J., Hayton K., Liu A., Duan J., Nkrumah L., Joy D. A., Fidock D. A., Fujioka H., Vaidya A. B., et al. (2005). Disruption of a Plasmodium falciparum gene linked to male sexual development causes early arrest in gametocytogenesis. Proc. Natl. Acad. Sci. USA 102, 16813-16818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner D. L., Dixon M. W., Spielmann T., Skinner-Adams T. S., Hawthorne P. L., Ortega M. R., Kemp D. J., Trenholme K. R. (2005). Implication of a Plasmodium falciparum gene in the switch between asexual reproduction and gametocytogenesis. Mol. Biochem. Parasitol. 140, 153-160 [DOI] [PubMed] [Google Scholar]
- Gerber A. P., Herschlag D., Brown P. O. (2004). Extensive association of functionally and cytotopically related mRNAs with Puf family RNA-binding proteins in yeast. PLoS Biol. 2, E79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A. P., Luschnig S., Krasnow M. A., Brown P. O., Herschlag D. (2006). Genome-wide identification of mRNAs associated with the translational regulator PUMILIO in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 103, 4487-4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstrohm A. C., Seay D. J., Hook B. A., Wickens M. (2007). PUF protein-mediated deadenylation is catalyzed by Ccr4p. J. Biol. Chem. 282, 109-114 [DOI] [PubMed] [Google Scholar]
- Gray N. K., Wickens M. (1998). Control of translation initiation in animals. Annu. Rev. Cell Dev. Biol. 14, 399-458 [DOI] [PubMed] [Google Scholar]
- Gu W., Deng Y., Zenklusen D., Singer R. H. (2004). A new yeast PUF family protein, Puf6p, represses ASH1 mRNA translation and is required for its localization. Genes Dev. 18, 1452-1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta Y. K., Nair D. T., Wharton R. P., Aggarwal A. K. (2008). Structures of human Pumilio with noncognate RNAs reveal molecular mechanisms for binding promiscuity. Structure 16, 549-557 [DOI] [PubMed] [Google Scholar]
- Hall N., Karras M., Raine J. D., Carlton J. M., Kooij T. W., Berriman M., Florens L., Janssen C. S., Pain A., Christophides G. K., et al. (2005). A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science 307, 82-86 [DOI] [PubMed] [Google Scholar]
- Hook B. A., Goldstrohm A. C., Seay D. J., Wickens M. (2007). Two yeast PUF proteins negatively regulate a single mRNA. J. Biol. Chem. 282, 15430-15438 [DOI] [PubMed] [Google Scholar]
- Kaslow D. C., Quakyi I. A., Syin C., Raum M. G., Keister D. B., Coligan J. E., McCutchan T. F., Miller L. H. (1988). A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333, 74-76 [DOI] [PubMed] [Google Scholar]
- Kaye J. A., Rose N. C., Goldsworthy B., Goga A., L'Etoile N. D. (2009). A 3′UTR pumilio-binding element directs translational activation in olfactory sensory neurons. Neuron 61, 57-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. M., Franke-Fayard B., Mair G. R., Lasonder E., Janse C. J., Mann M., Waters A. P. (2005). Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell 121, 675-687 [DOI] [PubMed] [Google Scholar]
- Kongkasuriyachai D., Fujioka H., Kumar N. (2004). Functional analysis of Plasmodium falciparum parasitophorous vacuole membrane protein (Pfs16) during gametocytogenesis and gametogenesis by targeted gene disruption. Mol. Biochem. Parasitol. 133, 275-285 [DOI] [PubMed] [Google Scholar]
- Kooij T. W., Matuschewski K. (2007). Triggers and tricks of Plasmodium sexual development. Curr. Opin. Microbiol. 10, 547-553 [DOI] [PubMed] [Google Scholar]
- Kuersten S., Goodwin E. B. (2003). The power of the 3′ UTR: translational control and development. Nat. Rev. Genet. 4, 626-637 [DOI] [PubMed] [Google Scholar]
- Kyes S., Pinches R., Newbold C. (2000). A simple RNA analysis method shows var and rif multigene family expression patterns in Plasmodium falciparum. Mol. Biochem. Parasitol. 105, 311-315 [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. (1979). Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65, 418-420 [PubMed] [Google Scholar]
- Lamont L. B., Crittenden S. L., Bernstein D., Wickens M., Kimble J. (2004). FBF-1 and FBF-2 regulate the size of the mitotic region in the C. elegans germline. Dev. Cell 7, 697-707 [DOI] [PubMed] [Google Scholar]
- Lanfrancotti A., Bertuccini L., Silvestrini F., Alano P. (2007). Plasmodium falciparum: mRNA co-expression and protein co-localisation of two gene products upregulated in early gametocytes. Exp. Parasitol. 116, 497-503 [DOI] [PubMed] [Google Scholar]
- Lasonder E., Ishihama Y., Andersen J. S., Vermunt A. M., Pain A., Sauerwein R. W., Eling W. M., Hall N., Waters A. P., Stunnenberg H. G., et al. (2002). Analysis of the Plasmodium falciparum proteome by high-accuracy mass spectrometry. Nature 419, 537-542 [DOI] [PubMed] [Google Scholar]
- Le Roch K. G., Zhou Y., Blair P. L., Grainger M., Moch J. K., Haynes J. D., de la Vega P., Holder A. A., Batalov S., Carucci D. J., et al. (2003). Discovery of gene function by expression profiling of the malaria parasite life cycle. Science 301, 1503-1508 [DOI] [PubMed] [Google Scholar]
- Le Roch K. G., Johnson J. R., Florens L., Zhou Y., Santrosyan A., Grainger M., Yan S. F., Williamson K. C., Holder A. A., Carucci D. J., et al. (2004). Global analysis of transcript and protein levels across the Plasmodium falciparum life cycle. Genome Res. 14, 2308-2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo C. A., Fujioka H., Aikawa M., Kumar N. (1999). Disruption of the Pfg27 locus by homologous recombination leads to loss of the sexual phenotype in P. falciparum. Mol. Cell 3, 793-798 [DOI] [PubMed] [Google Scholar]
- Lublin A. L., Evans T. C. (2007). The RNA-binding proteins PUF-5, PUF-6, and PUF-7 reveal multiple systems for maternal mRNA regulation during C. elegans oogenesis. Dev. Biol. 303, 635-649 [DOI] [PubMed] [Google Scholar]
- Maier A. G., Braks J. A., Waters A. P., Cowman A. F. (2006). Negative selection using yeast cytosine deaminase/uracil phosphoribosyl transferase in Plasmodium falciparum for targeted gene deletion by double crossover recombination. Mol. Biochem. Parasitol. 150, 118-121 [DOI] [PubMed] [Google Scholar]
- Mair G. R., Braks J. A., Garver L. S., Wiegant J. C., Hall N., Dirks R. W., Khan S. M., Dimopoulos G., Janse C. J., Waters A. P. (2006). Regulation of sexual development of Plasmodium by translational repression. Science 313, 667-669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuschewski K., Ross J., Brown S. M., Kaiser K., Nussenzweig V., Kappe S. H. I. (2002). Infectivity-associated changes in the transcriptional repertoire of the malaria parasite sporozoite stage. J. Biol. Chem. 277, 41948-41953 [DOI] [PubMed] [Google Scholar]
- Mee C. J., Pym E. C., Moffat K. G., Baines R. A. (2004). Regulation of neuronal excitability through pumilio-dependent control of a sodium channel gene. J. Neurosci. 24, 8695-8703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon K. P., Sanyal S., Habara Y., Sanchez R., Wharton R. P., Ramaswami M., Zinn K. (2004). The translational repressor Pumilio regulates presynaptic morphology and controls postsynaptic accumulation of translation factor eIF-4E. Neuron 44, 663-676 [DOI] [PubMed] [Google Scholar]
- Miao J., Fan Q., Cui L., Li J., Li J., Cui L. (2006). The malaria parasite Plasmodium falciparum histones: organization, expression, and acetylation. Gene 369, 53-65 [DOI] [PubMed] [Google Scholar]
- Miller M. T., Higgin J. J., Hall T. M. (2008). Basis of altered RNA-binding specificity by PUF proteins revealed by crystal structures of yeast Puf4p. Nat. Struct. Mol. Biol. 15, 397-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelans I. I., Meis J. F., Kocken C., Konings R. N., Schoenmakers J. G. (1991). A novel protein antigen of the malaria parasite Plasmodium falciparum, located on the surface of gametes and sporozoites. Mol. Biochem. Parasitol. 45, 193-204 [DOI] [PubMed] [Google Scholar]
- Morris A. R., Mukherjee N., Keene J. D. (2008). Ribonomic analysis of human Pum1 reveals cis-trans conservation across species despite evolution of diverse mRNA target sets. Mol. Cell. Biol. 28, 4093-4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraro N. I., Weston A. J., Gerber A. P., Luschnig S., Moffat K. G., Baines R. A. (2008). Pumilio binds para mRNA and requires Nanos and Brat to regulate sodium current in Drosophila motoneurons. J. Neurosci. 28, 2099-2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y., Wharton R. P. (1995). Binding of pumilio to maternal hunchback mRNA is required for posterior patterning in Drosophila embryos. Cell 80, 747-756 [DOI] [PubMed] [Google Scholar]
- Noden B. H., Beadle P. S., Vaughan J. A., Pumpuni C. B., Kent M. D., Beier J. C. (1994). Plasmodium falciparum: the population structure of mature gametocyte cultures has little effect on their innate fertility. Acta Trop. 58, 13-19 [DOI] [PubMed] [Google Scholar]
- Nolde M. J., Saka N., Reinert K. L., Slack F. J. (2007). The Caenorhabditis elegans pumilio homolog, puf-9, is required for the 3′UTR-mediated repression of the let-7 microRNA target gene, hbl-1. Dev. Biol. 305, 551-563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell R. A., Preiser P. R., Williamson D. H., Moore P. W., Cowman A. F., Crabb B. S. (2001). An alteration in concatameric structure is associated with efficient segregation of plasmids in transfected Plasmodium falciparum parasites. Nucleic Acids Res. 29, 716-724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivas W., Parker R. (2000). The Puf3 protein is a transcript-specific regulator of mRNA degradation in yeast. EMBO J. 19, 6602-6611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri A., Camarda G., Bertuccini L., van de Vegte-Bolmer M., Luty A. J., Sauerwein R., Alano P. (2009). The Plasmodium falciparum protein Pfg27 is dispensable for gametocyte and gamete production, but contributes to cell integrity during gametocytogenesis. Mol. Microbiol. 73, 180-193 [DOI] [PubMed] [Google Scholar]
- Parisi M., Lin H. (1999). The Drosophila pumilio gene encodes two functional protein isoforms that play multiple roles in germline development, gonadogenesis, oogenesis and embryogenesis. Genetics 153, 235-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton M. G., Barker G. C., Matsuoka H., Ramesar J., Janse C. J., Waters A. P., Sinden R. E. (1993). Structure and expression of a post-transcriptionally regulated malaria gene encoding a surface protein from the sexual stages of Plasmodium berghei. Mol. Biochem. Parasitol. 59, 263-275 [DOI] [PubMed] [Google Scholar]
- Paul R. E., Coulson T. N., Raibaud A., Brey P. T. (2000). Sex determination in malaria parasites. Science 287, 128-131 [DOI] [PubMed] [Google Scholar]
- Paul R. E., Brey P. T., Robert V. (2002). Plasmodium sex determination and transmission to mosquitoes. Trends Parasitol. 18, 32-38 [DOI] [PubMed] [Google Scholar]
- Rawlings D. J., Fujioka H., Fried M., Keister D. B., Aikawa M., Kaslow D. C. (1992). Alpha-tubulin II is a male-specific protein in Plasmodium falciparum. Mol. Biochem. Parasitol. 56, 239-250 [DOI] [PubMed] [Google Scholar]
- Reece S. E., Drew D. R., Gardner A. (2008). Sex ratio adjustment and kin discrimination in malaria parasites. Nature 453, 609-614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert V., Read A. F., Essong J., Tchuinkam T., Mulder B., Verhave J. P., Carnevale P. (1996). Effect of gametocyte sex ratio on infectivity of Plasmodium falciparum to Anopheles gambiae. Trans. R Soc. Trop. Med. Hyg. 90, 621-624 [DOI] [PubMed] [Google Scholar]
- Robert V., Sokhna C. S., Rogier C., Ariey F., Trape J. F. (2003). Sex ratio of Plasmodium falciparum gametocytes in inhabitants of Dielmo, Senegal. Parasitology 127, 1-8 [DOI] [PubMed] [Google Scholar]
- Rosario V. (1981). Cloning of naturally occurring mixed infections of malaria parasites. Science 212, 1037-1038 [DOI] [PubMed] [Google Scholar]
- Schall J. J. (2009). Do malaria parasites follow the algebra of sex ratio theory? Trends Parasitol. 25, 120-123 [DOI] [PubMed] [Google Scholar]
- Schweers B. A., Walters K. J., Stern M. (2002). The Drosophila melanogaster translational repressor pumilio regulates neuronal excitability. Genetics 161, 1177-1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Sharma I., Kogkasuriyachai D., Kumar N. (2003). Structure of a gametocyte protein essential for sexual development in Plasmodium falciparum. Nat. Struct. Biol. 10, 197-203 [DOI] [PubMed] [Google Scholar]
- Silvestrini F., Alano P., Williams J. L. (2000). Commitment to the production of male and female gametocytes in the human malaria parasite Plasmodium falciparum. Parasitology 121, 465-471 [DOI] [PubMed] [Google Scholar]
- Silvestrini F., Bozdech Z., Lanfrancotti A., Di Giulio E., Bultrini E., Picci L., Derisi J. L., Pizzi E., Alano P. (2005). Genome-wide identification of genes upregulated at the onset of gametocytogenesis in Plasmodium falciparum. Mol. Biochem. Parasitol. 143, 100-110 [DOI] [PubMed] [Google Scholar]
- Smalley M. E., Sinden R. E. (1977). Plasmodium falciparum gametocytes: their longevity and infectivity. Parasitology 74, 1-8 [DOI] [PubMed] [Google Scholar]
- Smith T. G., Lourenco P., Carter R., Walliker D., Ranford-Cartwright L. C. (2000). Commitment to sexual differentiation in the human malaria parasite, Plasmodium falciparum. Parasitology 121, 127-133 [DOI] [PubMed] [Google Scholar]
- Souza G. M., da Silva A. M., Kuspa A. (1999). Starvation promotes Dictyostelium development by relieving PufA inhibition of PKA translation through the YakA kinase pathway. Development 126, 3263-3274 [DOI] [PubMed] [Google Scholar]
- Sowunmi A., Balogun S. T., Gbotosho G. O., Happi C. T. (2008). Plasmodium falciparum gametocyte sex ratios in children with acute, symptomatic, uncomplicated infections treated with amodiaquine. Malar. J. 7, 169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassov D. S., Jurecic R. (2003). The PUF family of RNA-binding proteins: does evolutionarily conserved structure equal conserved function? IUBMB Life 55, 359-366 [DOI] [PubMed] [Google Scholar]
- Subramaniam K., Seydoux G. (2003). Dedifferentiation of primary spermatocytes into germ cell tumors in C. elegans lacking the pumilio-like protein PUF-8. Curr. Biol. 13, 134-139 [DOI] [PubMed] [Google Scholar]
- Tadauchi T., Matsumoto K., Herskowitz I., Irie K. (2001). Post-transcriptional regulation through the HO 3′-UTR by Mpt5, a yeast homolog of Pumilio and FBF. EMBO J. 20, 552-561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talman A. M., Domarle O., McKenzie F. E., Ariey F., Robert V. (2004a). Gametocytogenesis: the puberty of Plasmodium falciparum. Malar. J. 3, 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talman A. M., Paul R. E., Sokhna C. S., Domarle O., Ariey F., Trape J. F., Robert V. (2004b). Influence of chemotherapy on the Plasmodium gametocyte sex ratio of mice and humans. Am. J. Trop. Med. Hyg. 71, 739-744 [PubMed] [Google Scholar]
- Tonkin C. J., Struck N. S., Mullin K. A., Stimmler L. M., McFadden G. I. (2006). Evidence for Golgi-independent transport from the early secretory pathway to the plastid in malaria parasites. Mol. Microbiol. 61, 614-630 [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. (1976). Human malaria parasites in continuous culture. Science 193, 673-675 [DOI] [PubMed] [Google Scholar]
- Ulbricht R. J., Olivas W. M. (2008). Puf1p acts in combination with other yeast Puf proteins to control mRNA stability. RNA 14, 246-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya A. B., Muratova O., Guinet F., Keister D., Wellems T. E., Kaslow D. C. (1995). A genetic locus on Plasmodium falciparum chromosome 12 linked to a defect in mosquito-infectivity and male gametogenesis. Mol. Biochem. Parasitol. 69, 65-71 [DOI] [PubMed] [Google Scholar]
- van Schaijk B. C., van Dijk M. R., van de Vegte-Bolmer M., van Gemert G. J., van Dooren M. W., Eksi S., Roeffen W. F., Janse C. J., Waters A. P., Sauerwein R. W. (2006). Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Mol. Biochem. Parasitol. 149, 216-222 [DOI] [PubMed] [Google Scholar]
- Vardy L., Orr-Weaver T. L. (2007). Regulating translation of maternal messages: multiple repression mechanisms. Trends Cell Biol. 17, 547-554 [DOI] [PubMed] [Google Scholar]
- Wang X., McLachlan J., Zamore P. D., Hall T. M. (2002). Modular recognition of RNA by a human pumilio-homology domain. Cell 110, 501-512 [DOI] [PubMed] [Google Scholar]
- West S. A., Reece S. E., Read A. F. (2001). Evolution of gametocyte sex ratios in malaria and related apicomplexan (protozoan) parasites. Trends Parasitol. 17, 525-531 [DOI] [PubMed] [Google Scholar]
- Wharton R. P., Sonoda J., Lee T., Patterson M., Murata Y. (1998). The Pumilio RNA-binding domain is also a translational regulator. Mol. Cell 1, 863-872 [DOI] [PubMed] [Google Scholar]
- Wickens M., Bernstein D. S., Kimble J., Parker R. (2002). A PUF family portrait: 3′UTR regulation as a way of life. Trends Genet. 18, 150-157 [DOI] [PubMed] [Google Scholar]
- Wu Y., Kirkman L. A., Wellems T. E. (1996). Transformation of Plasmodium falciparum malaria parasites by homologous integration of plasmids that confer resistance to pyrimethamine. Proc. Natl. Acad. Sci. USA 93, 1130-1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B., Petritsch C., Clark I. E., Gavis E. R., Jan L. Y., Jan Y. N. (2004). Nanos and Pumilio are essential for dendrite morphogenesis in Drosophila peripheral neurons. Curr. Biol. 14, 314-321 [DOI] [PubMed] [Google Scholar]
- Young J. A., Fivelman Q. L., Blair P. L., de la Vega P., Le Roch K. G., Zhou Y., Carucci D. J., Baker D. A., Winzeler E. A. (2005). The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol. Biochem. Parasitol. 143, 67-79 [DOI] [PubMed] [Google Scholar]
- Zamore P. D., Williamson J. R., Lehmann R. (1997). The Pumilio protein binds RNA through a conserved domain that defines a new class of RNA-binding proteins. RNA 3, 1421-1433 [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Gallegos M., Puoti A., Durkin E., Fields S., Kimble J., Wickens M. P. (1997). A conserved RNA-binding protein that regulates sexual fates in the C. elegans hermaphrodite germ line. Nature 390, 477-484 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.