Abstract
Hepatocyte growth factor (HGF) is found in tumor microenvironments, and interaction with its tyrosine kinase receptor Met triggers cell invasion and metastasis. It was previously shown that acidic extracellular pH stimulated peripheral lysosome trafficking, resulting in increased cathepsin B secretion and tumor cell invasion, which was dependent upon sodium-proton exchanger (NHE) activity. We now demonstrate that HGF induced the trafficking of lysosomes to the cell periphery, independent of HGF-induced epithelial-mesenchymal transition. HGF-induced anterograde lysosome trafficking depended upon the PI3K pathway, microtubules and RhoA, resulting in increased cathepsin B secretion and invasion by the cells. HGF-induced NHE activity via increased net acid production, and inhibition of NHE activity with 5-(N-ethyl-N-isopropyl)-amiloride (EIPA), or a combination of the NHE1-specific drug cariporide and the NHE3-specific drug s3226 prevented HGF-induced anterograde trafficking and induced retrograde trafficking in HGF-overexpressing cells. EIPA treatment reduced cathepsin B secretion and HGF-induced invasion by the tumor cells. Lysosomes were located more peripherally in Rab7-shRNA-expressing cells and these cells were more invasive than control cells. Overexpression of the Rab7 effector protein, RILP, resulted in a juxtanuclear location of lysosomes and reduced HGF-induced invasion. Together, these results suggest that the location of lysosomes is an inherently important aspect of invasion by tumor cells.
Keywords: Lysosome trafficking, HGF, EIPA, Rab7, RILP, Cell invasion, Cathepsin secretion, Glycolysis, NHE1, ORP1L
Introduction
The development of metastatic foci, resulting from an invasive primary tumor, is the leading cause of cancer-associated deaths. The hepatocyte growth factor (HGF)-Met signaling complex is known to activate a number of signaling pathways that contribute to the invasion and metastasis of a primary tumor (Birchmeier et al., 2003); Met is a tyrosine kinase receptor and HGF is its only known ligand. HGF-Met signaling induces an epithelial-mesenchymal transition (EMT) where epithelial cells lose cell-cell adhesions and take on a motile, mesenchymal phenotype, which is thought to enhance migration and invasion through the basement membrane (Acloque et al., 2008; Benvenuti and Comoglio, 2007; Levayer and Lecuit, 2008; Yilmaz and Christofori, 2009). Cell invasion is considered an important step for eventual distant metastatic foci formation (Tsuji et al., 2009; Yilmaz and Christofori, 2009). Prolonged Met signaling most probably constitutes the tumor-promoting properties of HGF, which include, in addition to invasion, cellular proliferation, survival, actin remodeling and motility (Birchmeier et al., 2003; Gentile et al., 2008). Met overexpression and activating mutations are strongly associated with aggressive cancers, including carcinomas of breast, prostate, lung, brain, gastric and pancreatic origin (Agarwal et al., 2009; Sawada et al., 2007; Taniguchi et al., 1998).
Increased levels of HGF are common in tumor microenvironments and reactive stroma, and prolonged Met signaling induced by this growth factor promotes tumor progression (Birchmeier et al., 2003; Matsumoto and Nakamura, 2006). HGF can be produced by both cancer-associated fibroblasts and tumor cells in a paracrine or autocrine manner, respectively. Moreover, many different cell types found in and around primary tumors can influence the behavior of the tumor via the secretion of cytokines, chemokines and growth factors (review by Joyce and Pollard, 2009; Mantovani et al., 2006; Singh et al., 2007). In addition to HGF, other factors of the tumor microenvironment such as acidic extracellular pH (pHe), hypoxia, and serum deprivation also play significant regulatory roles (Boccaccio and Comoglio, 2006; Pennacchietti et al., 2003). How these factors and the Met signaling pathway influence primary tumors to regulate gene expression, angiogenesis, proteinase secretion, and cell motility and invasion are major areas of ongoing research.
Tumor cells demonstrate enhanced metabolism because of increased glycolytic rates, which affects cytoplasmic pH (pHi) as a result. Sodium-proton exchangers (NHEs), sodium-dependent and -independent HCO3 −/Cl− exchangers, and H+/lactate co-transporters are engaged as the main mediators of tumor cell pHi homeostasis. pHi plays a major role in many cellular and tumorigenic processes, including controlling cell volume, cell cycle, membrane potential, oncogenesis and malignant transformation (Grinstein et al., 1989; Harguindey et al., 2005; Putney et al., 2002). Moreover, the activity of NHEs, especially NHE1, are regulated by acidic pHi and oncogenic transformation, causing protons to be expelled outside the cell, thus creating an acidic pHe (Kaplan and Boron, 1994; Reshkin et al., 2000). Acidic pHe and NHE1 activity have both been shown to enhance the invasive capability of tumor cells through gene expression changes and regulation of the actin cytoskeleton (Martinez-Zaguilan et al., 1996; Moellering et al., 2008; Paradiso et al., 2004). In addition, HGF (Kaneko et al., 1995) and other growth factors (Grinstein et al., 1989; Strazzabosco et al., 1995) have been reported to activate NHEs, suggesting that multiple mechanisms may regulate NHE activity.
Previous studies have shown that acidic pHe can induce the trafficking of cathepsin-rich lysosomes to the cell periphery (Glunde et al., 2003; Rozhin et al., 1994), a process that is dependent on NHE activity (Steffan et al., 2009), and this may play a role in ECM proteolysis and cell invasion and metastasis (Koblinski et al., 2000; Nomura and Katunuma, 2005). Since HGF has been shown to activate NHEs (Kaneko et al., 1995), we wanted to determine if this growth factor also induced the peripheral trafficking of lysosomes and cathepsin B secretion. In this report, we demonstrate that HGF stimulates the trafficking of lysosomes to the cell periphery, resulting in the secretion of lysosomal proteinases and increased invasion by the cells. Moreover, we report that HGF stimulates proton production, which we propose induces the activation of sodium-proton exchangers, responsible for the anterograde trafficking of lysosomes. Finally, we demonstrate that by altering the spatial distribution of lysosomes, we can control the invasiveness of tumor cells, suggesting that the location of lysosomes in cells may be a key event in invasion by tumor cells.
Results
HGF induces cell scattering and lysosome trafficking in DU145 prostate cancer cells
In a previous study, we showed that the DU145 androgen-independent prostate cancer cell line scattered in response to HGF (Coleman et al., 2009), and that this cell line was an excellent model for acidic pHe-induced lysosome anterograde trafficking (Steffan et al., 2009). Therefore, to determine if HGF affects the intracellular distribution of lysosomes, DU145 cells were treated with HGF for 16 hours before lysosome-associated membrane protein-1 (LAMP-1)-positive vesicles were visualized by immunofluorescence (IF) microscopy. Fig. 1A,B illustrates the ability of HGF to induce cell scattering and the trafficking of LAMP-1-positive vesicles to the cell periphery. Using previously described software to measure and quantify the distance of lysosomes from the cell nucleus (Glunde et al., 2003; Steffan et al., 2009), we confirmed that HGF quantitatively induces the movement of LAMP-1-positive vesicles to the periphery, compared with untreated cells (Fig. 1C). LAMP-1-positive vesicles in control cells were located primarily juxtanuclear, at an average distance of 3.1 μm from the cell nucleus, but after HGF treatment, LAMP-1-positive vesicles were found to be located an average distance of 6.3 μm from the cell nucleus, indicating the vesicles had undergone anterograde trafficking toward the cell periphery.
Fig. 1.
Overnight treatment with HGF induces cell scattering and the trafficking of lysosomes to the cell periphery. (A,B) Representative merged IF images depicting the trafficking of lysosomes (red) before and after overnight HGF treatment; shown also is actin (green) and nuclei (blue; top row). (C) Quantification of lysosomal distribution, shown as mean distance from individual cell nuclei. Error bars represent the s.e.m. of 30 cells from at least three independent experiments; *denotes statistical significance (P<0.0001) versus the control (Cntl). (D-I) Cathepsin B (green; D,G) and LAMP-1 (red; E,H) colocalize (F and I) in the presence (G-I) or absence (D-F) of HGF. Arrows indicate the same vesicles in each image. Scale bars: 10 μm.
To determine if these LAMP-1-positive vesicles had the properties of lysosomes, cells were co-stained to detect cathepsin B and LAMP-1 after overnight incubation with HGF. Colocalization of these two lysosomal markers is shown in Fig. 1D-I. In addition, supplementary material Fig. S1 illustrates that lysosomes appeared to be the only organelle undergoing HGF-induced anterograde trafficking, as early endosomes (using EEA1 as a marker), recycling endosomes (transferrin receptor), late endosomes [(mannose-6-phosphate receptor (M6PR) and lysobisphosphatidic acid (LBPA)], and the trans-Golgi (GM130) did not undergo peripheral trafficking in response to HGF. Therefore, the LAMP-1-positive vesicles undergoing HGF-induced trafficking in Fig. 1 are most probably lysosomes.
The PI3K pathway is required for HGF-induced lysosome trafficking
The addition of HGF to cell culture media induces the phosphorylation of the Met receptor and the activation (phosphorylation) of a number of downstream signaling pathways including the PI3K-Akt, MAPK (Erk1/2), and JNK pathways that is sustained for up to 4 hours before attenuating (Fig. 2A). To determine the pathway(s) required for HGF-induced anterograde lysosome trafficking, signaling pathway-specific pharmacological inhibitors were employed. Fig. 2B,C demonstrate that inhibition of the PI3K pathway with 10 μM LY294002 prevented HGF-induced anterograde lysosome trafficking; whereas concentrations of LY294002 below 5 μM did not prevent HGF-induced anterograde lysosome trafficking (results not shown). However, inhibition of the MAPK, p38-MAPK, and JNK pathways with 10 μM U0126, SB203580 or SP600125, respectively, did not prevent HGF-induced anterograde lysosome trafficking (supplementary material Fig. S2), suggesting that the PI3K pathway plays the major role in HGF-induced lysosome trafficking. Control experiments demonstrated that each drug inhibited its target kinase at the concentrations used (Steffan and Cardelli, 2010). Furthermore, treatment with LY294002 and U0126 prevented HGF-induced cell scattering (Fig. 2B, supplementary material Fig. S2). Since lysosomes still underwent peripheral trafficking in the presence of U0126, these data suggest that HGF-induced cell scattering (EMT) is not necessary for HGF-induced lysosome trafficking.
Fig. 2.
The PI3K pathway is required for HGF-induced lysosome trafficking. (A) Western blot analysis of DU145 cells indicates that HGF rapidly induced a sustained phosphorylation of Met (Tyr1234/1235), Akt (Ser483), Erk (The202/Tyr204) and Jnk (The183/Tyr185); actin is shown as a protein load control. (B) Cells were pretreated with LY294002 for 30 minutes before the addition of HGF overnight and the location of lysosomes (red) were observed using IF microscopy; shown also are actin (green) and nuclei (blue). (C) Quantification of lysosomal distribution is shown as mean distance from individual cell nuclei. Error bars represent s.e.m. of 30 cells from at least three independent experiments; *statistical significance (P<0.0001) versus control (Cntl). Scale bars: 10 μm.
Microtubules, but not actin filaments, are required for HGF-induced lysosome trafficking
Endocytic organelles traffic throughout cells along both microtubules and actin filaments (Cordonnier et al., 2001; Swanson et al., 1992). Microtubules are commonly used for rapid, long-distance lysosome movement; whereas the use of actin filaments usually results in slower movement over short distances (Matteoni and Kreis, 1987). To determine the role of microtubules in HGF-induced lysosome trafficking, DU145 cells were treated with nocodazole, or left untreated, for 2 hours prior to treatment with HGF. Nocodazole effectively disrupted microtubules (results not shown) as previously published (Steffan and Cardelli, 2010). IF microscopy indicated that lysosomes were unable to undergo HGF-induced anterograde movement when microtubules were disrupted (supplementary material Fig. S3E,F). In addition, we found that HGF treatment caused an increase of microtubules extending into cell surface protrusions, often coinciding with the location of lysosomes (supplementary material Fig. S3A-D). To evaluate the role of the actin cytoskeleton in HGF-induced lysosome trafficking, cells were pretreated with cytochalasin D for 30 minutes before HGF treatment. Disruption of the actin cytoskeleton did not prevent HGF-induced lysosome trafficking (supplementary material Fig. S4). In fact, it is interesting to note that disruption of the actin cytoskeleton itself allows the trafficking of lysosomes to the cell periphery, suggesting that the actin cytoskeleton, particularly cortical actin, may be an important negative regulator of anterograde lysosome trafficking, acting as a barrier which lysosomes are unable to navigate through under normal conditions.
RhoA is required for HGF-induced lysosome trafficking
The Rho subfamily of GTPases and their downstream effector proteins are known to play a role in lysosome trafficking along microtubules, and we have previously implicated RhoA as the principle Rho GTPase facilitating acidic pHe-induced lysosome trafficking (Steffan et al., 2009). Therefore, to determine if RhoA also facilitates HGF-induced lysosome trafficking, GFP-tagged dominant-negative (DN) RhoA was transiently transfected into DU145 cells. As demonstrated in supplementary material Fig. S5, expression of DN-RhoA-GFP prevented HGF-induced lysosome trafficking, suggesting that RhoA also facilitates HGF-induced lysosome trafficking.
HGF increases proton production, inducing acidification of the extracellular medium
Growth factors, including HGF, EGF and TNF-α, have been shown to increase glucose utilization in a variety of cells (Bertola et al., 2007; Boussouar and Benahmed, 1999; Hsu and Sabatini, 2008; Perdomo et al., 2008; Véga et al., 2002). Furthermore, Kaplan and colleagues (Kaplan et al., 2000) have shown that HGF also enhances glycolysis and oxidative phosphorylation in murine mammary cells. This study also found that, although there was a significant increase in energy metabolism, there was no apparent intracellular acidification, suggesting that proton exchangers may be activated to achieve pHi homeostasis. Thus, we predicted that HGF-induced increases in energy metabolism (leading to an increase in proton production) would cause an increased acidic pHe. DU145 cells were treated with or without HGF overnight, and the net acid production was determined via the change in pHe. Fig. 3A demonstrates that HGF significantly increased net acid production. In addition, using the pH-sensitive dye BCECF-AM, we found a slight but insignificant increase in pHi, but did find that the pH of the cell culture medium (pHe) did become more acidic (Fig. 3B,C). Therefore, these data suggest that HGF increases energy metabolism, leading to an increase in cytoplasmic protons, which are extruded from the cells to maintain pHi homeostasis, causing acidification of the pHe. Consequently, the activity of proton exchangers, such as NHE1, are implicated in HGF-induced lysosome trafficking.
Fig. 3.
Effect of HGF on pHi and pHe. Treatment with HGF overnight (A) increases net acid production, causing acidification of the extracellular pH (C), but not the intracellular pH (B). This suggests the activity of sodium-proton exchangers in mediating intracellular pH homeostasis in the presence of HGF. See Materials and Methods for more information.
HGF-induced lysosome trafficking requires NHE-mediated sodium-proton exchange activity
Our previous study implicated NHE activity in acidic pHe-induced lysosome trafficking (Steffan et al., 2009). Since HGF stimulates proton production, leading to an acidic pHe (Fig. 3) and NHE activation (Kaneko et al., 1995), we hypothesized that NHE inhibitors may block HGF-induced lysosome trafficking. DU145 cells were treated with 25 μM EIPA [5-(N-ethyl-N-isopropyl)-amiloride; a relatively non-isoform-specific NHE inhibitor with a preference for NHE1] ± HGF for 16 hours. IF microscopy revealed that treatment with EIPA did not block cell scattering, but prevented HGF-induced anterograde lysosome movement (Fig. 4A). EIPA was also able to prevent HGF-induced anterograde lysosome trafficking in the PC3 prostate cancer cell line (supplementary material Fig. S6). Additionally, we examined the H1993 lung cancer cell line, in which Met is overexpressed due to MET gene amplification, causing constitutively active Met signaling (Milligan et al., 2009). We observed that lysosomes in H1993 cells were more peripherally localized under neutral pH culture conditions compared with DU145 and PC3 cells. Intriguingly, EIPA mediated the retrograde movement of lysosomes to a juxtanuclear position, demonstrating that EIPA not only prevented HGF-induced lysosome trafficking, but also reversed the location of lysosomes after they were dispersed (supplementary material Fig. S6).
Fig. 4.
EIPA, a broad inhibitor of sodium-proton exchangers, prevents and reverses HGF-induced lysosome trafficking, cathepsin B secretion, and invasion. (A) DU145 cells were pretreated with EIPA for 30 minutes before the addition of HGF overnight, followed by IF microscopy to visualize lysosomes (red); also shown are actin (green) and nuclei (blue). (B) IF images of a DU145 HGF-overexpressing cell line after two days in culture. Lysosomes have undergone anterograde trafficking in these cells, which did not occur in vector control cells, and EIPA was able to reverse this effect and induce lysosomes to traffic toward the cell nucleus. (C) Quantification of lysosomal distribution is shown as mean distance from individual cell nuclei. Xs indicate the addition of HGF and/or EIPA; O/E, overexpressing cells. Error bars represent s.e.m. of 30 cells from at least three independent experiments. (D) DU145 cells were treated for 24 hours with EIPA and/or HGF and the amount of cathepsin B secreted into the culture medium was determined. (E) DU145 cells were seeded onto Matrigel-coated transwell chambers and allowed to invade for 24 hours in the presence of EIPA and/or HGF. See Materials and Methods for more information. *Statistical significance (P<0.0001) versus –HGF control; **statistical significance (P<0.001) versus vector control. Scale bars: 10 μm.
The studies described thus far have shown that acute treatment of prostate tumor cells with HGF stimulated anterograde lysosome movement; however, there is no evidence that this relocation can be maintained over a longer time period. Therefore, we generated a DU145 cell line that overexpressed HGF (confluent cultures contained 2-3 ng/ml HGF); these cells remained scattered during culturing (results not shown). Fig. 4B illustrates that lysosomes had undergone anterograde movement towards the plasma membrane in HGF overexpressing cells that was comparable with HGF-treated cells (Fig. 4A). A vector control cell line did not scatter, nor did lysosomes undergo anterograde movement, under similar culture conditions (Fig. 4B). Furthermore, EIPA reversed anterograde lysosome trafficking in the HGF-overexpressing cell line, suggesting the importance of NHEs in initiating and maintaining HGF-induced lysosome trafficking. Quantification of the lysosome-nucleus distance is shown in Fig. 4C.
EIPA prevents HGF-induced cathepsin B secretion and invasion by tumor cells
It has been proposed that increased cathepsin B and/or cathepsin D secretion promotes localized ECM proteolysis and cell invasion (Colella and Casey, 2003; Colella et al., 2004; Tu et al., 2008). To determine if there was a physiological consequence to HGF-induced lysosome trafficking, secreted cathepsin B was measured in the medium after 24 hours. HGF treatment induced a twofold increase in the secretion of cathepsin B compared with non-HGF-treated cells, and EIPA treatment prevented this increase (Fig. 4D). Cell invasion (transwell) assays were also performed to determine the effects of HGF ± EIPA on invasion by tumor cells. Fig. 4E indicates that, similar to what was observed for cathepsin B secretion, HGF-induced invasion by tumor cells was prevented by EIPA. Therefore, we note a correlation between, (1) the ability of lysosomes to traffic to the cell periphery, (2) secreted cathepsin B and (3) increased invasion by tumor cells.
NHE1 and NHE3 participate in lysosome trafficking, cathepsin B secretion and invasion by tumor cells
Since EIPA has been shown to inhibit multiple NHE isoforms (Masereel et al., 2003), we employed more specific inhibitors of NHE1 and NHE3 (cariporide and s3226, respectfully) to determine if these exchangers played a role in HGF-induced lysosome redistribution. DU145 cells were pretreated with 10 μM cariporide and/or s3226 for 30 minutes prior to HGF treatment overnight. Treatment with cariporide or s3226 alone had a small effect (cariporide treatment was statistically significant) on HGF-induced lysosome redistribution; however, treatment with both NHE inhibitors more robustly (P<0.001) prevented HGF-induced lysosome trafficking (Fig. 5), suggesting that NHE1 and NHE3 both play a role in HGF-induced lysosome trafficking. Moreover, since EIPA prevented lysosome trafficking to a greater degree than the combination of cariporide and s3226, we predict that other EIPA-sensitive NHE isoforms must also play a role. In fact, DU145 tumor cells transcribe mRNA for eight different NHE isoforms (Steffan et al., 2009).
Fig. 5.
NHE1 and NHE3 are both involved in HGF-induced lysosome trafficking. (A) DU145 cells were pretreated for 30 minutes with 10 μM cariporide and/or 10 μM s3226 before the addition of HGF overnight, followed by IF microscopy to visualize the distribution of lysosomes (red); also shown are actin (green) and nuclei (blue). Cariporide and s3226 work additively to prevent HGF-induced lysosome trafficking. (B) Quantification of lysosomal distribution is shown as mean distance from individual cell nuclei. Error bars represent s.e.m. of 30 cells from at least three independent experiments; *Statistical significance (P<0.0001) versus control; **statistical significance (P<0.001) versus HGF; $statistical significance (P<0.01) versus HGF. Scale bars: 10 μm.
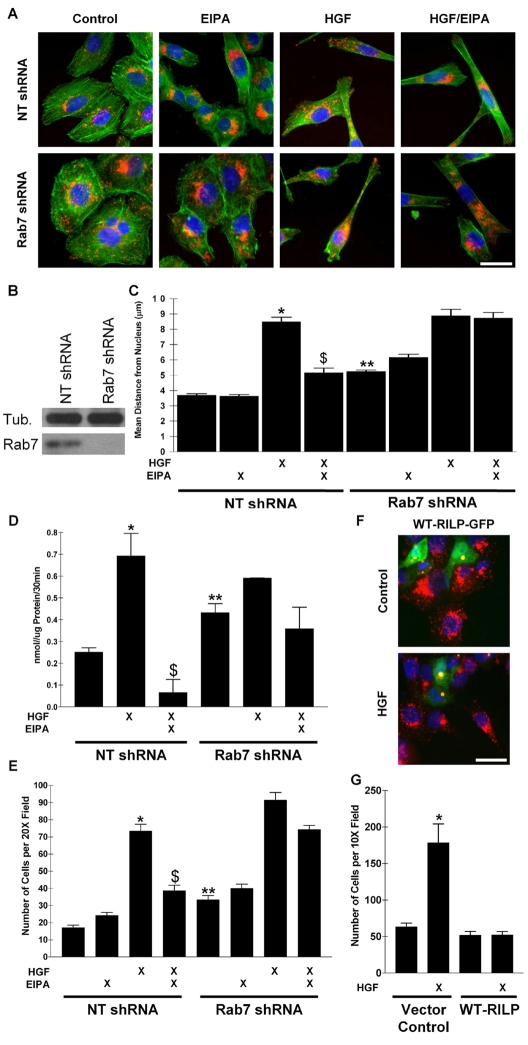
Rab7 is required for EIPA-mediated prevention of HGF-induced lysosome trafficking, cathepsin B secretion, and invasion by tumor cells
Rab7 and its effector RILP (Rab7-interacting lysosomal protein) are known to play a role in the retrograde trafficking of late endosomes and lysosomes (Jordens et al., 2001; Steffan and Cardelli, 2010). To determine if Rab7 was involved in the EIPA-based inhibition of HGF-induced lysosome trafficking, we utilized lentiviral vectors to express shRNA to Rab7 in DU145 cells. A cell line demonstrating greater than 90% Rab7 knockdown efficiency (Fig. 6B) was used for the following experiments. EIPA was unable to prevent HGF-induced lysosome trafficking in the Rab7-shRNA-expressing cells (Fig. 6A,C), and Rab7 downregulation resulted in lysosomes being more peripherally distributed under normal culture conditions than in a mismatched (non-target; NT) shRNA-expressing cell line as previously shown (Steffan and Cardelli, 2010). In addition, expression of DN-RILP-GFP resulted in a more peripheral distribution of lysosomes, similar to the silencing of Rab7, and EIPA was unable to induce retrograde lysosome trafficking when DN-RILP-GFP was expressed (supplementary material Fig. S7). Thus, we conclude that Rab7 and RILP play key roles in maintaining lysosomes in the perinuclear area and EIPA requires functional Rab7 and RILP to induce retrograde trafficking.
Fig. 6.
Rab7 is required for EIPA-mediated prevention of HGF-induced lysosome redistribution, cathepsin B secretion and invasion. (A) Representative merged IF images depicting the effects on the distribution of lysosomes (red); also shown are actin (green) and nuclei (blue) in vector control and Rab7-shRNA-expressing cells. EIPA requires Rab7 to prevent HGF-induced lysosome trafficking. HGF was added overnight after a 30 minute pretreatment with EIPA. (B) Western blot analysis indicates efficient Rab7 knockdown in one of the cell pools expressing Rab7 shRNA. Tubulin is shown as a protein load control. (C) Quantification of lysosomal distribution is shown as mean distance from individual cell nuclei. Error bars represent s.e.m. of 30 cells from at least three independent experiments or s.e.m. from three independent assays. (D) Rab7-shRNA-expressing cells secrete more cathepsin B than non-target (NT)-shRNA-expressing cells and EIPA no longer reduces secretion in Rab7-shRNA-expressing cells. See Materials and Methods for secretion assay details. (E) Invasion assay indicates that Rab7-shRNA-expressing cells are more invasive and EIPA does not prevent HGF-induced invasion when Rab7 is downregulated. See Materials and Methods for more assay details. (F) IF microscopy shows that lysosomes in cells expressing WT-RILP-GFP are clustered near the cell nucleus. Lysosomes are shown in red and transfected cells in green. Yellow color represents WT-RILP-GFP and lysosome colocalization. (G) Invasion assays indicate that RILP overexpression prevents HGF-induced invasion. *Statistical significance (P<0.0001) versus NT cntl; **statistical significance (P<0.001) versus NT cntl; $statistical significance (P<0.0001) versus NT HGF treated. Scale bars: 10 μm.
Since EIPA prevented cathepsin B secretion in WT cells, we quantified the effect that downregulation of Rab7 had on HGF-induced cathepsin B secretion. Fig. 6D illustrates that EIPA prevented HGF-induced cathepsin B secretion in the NT-shRNA-expressing cell line in a similar manner to the parental DU145 cell line, but EIPA was not able to completely prevent HGF-induced cathepsin B secretion in the Rab7-shRNA-expressing cells. Furthermore, similar to the lysosome distribution noted above, Rab7-shRNA-expressing cells secreted more cathepsin B under normal culture conditions compared with the NT-shRNA-expressing cell line. We observed a similar trend regarding invasion; Rab7-shRNA-expressing cells were inherently more invasive, and EIPA was unable to prevent HGF-induced invasion compared with the NT-shRNA-expressing cell line (Fig. 6E). These data suggest that Rab7 activity was required for EIPA-mediated prevention of HGF-induced lysosome trafficking, cathepsin B secretion, and invasion by tumor cells.
Lysosome distribution may control invasion by tumor cells
The results presented above suggest that the location of lysosomes may be important in regulating invasion by tumor cells. As a further test of this idea, we overexpressed RILP in DU145 cells. Transient overexpression of WT-RILP-GFP has been shown to induce the clustering of lysosomes near the cell nucleus (Cantalupo et al., 2001; Jordens et al., 2001; Steffan and Cardelli, 2010), as was the case in our hands (Fig. 6F). Moreover, HGF was unable to induce lysosome anterograde trafficking when WT-RILP-GFP was overexpressed (Fig. 6F). Finally, we engineered DU145 cells to stably express WT-RILP and compared their ability to invade relative to vector control cells. We observed that HGF-induced invasion was largely prevented in cells overexpressing WT-RILP (Fig. 6F). Thus, this provides further evidence that the spatial location of lysosomes may be a major determinant of invasion by tumor cells.
Redistribution of cellular cholesterol may be responsible for EIPA-induced retrograde lysosome trafficking
ORP1L is a cholesterol sensing protein, which has been shown to sense increased cholesterol levels and induce retrograde late endosome trafficking by controlling the Rab7-RILP-p150Glued complex (Johansson et al., 2005; Johansson et al., 2007; Rocha et al., 2009). To determine if EIPA increased levels of cholesterol or caused a shift in the distribution of intracellular cholesterol, DU145 cells were treated with 25 μM EIPA ± HGF overnight. Fixed cells were stained with filipin to visualize cholesterol and/or LAMP-1 to visualize lysosomes. HGF had no effect of cholesterol distribution; however, EIPA caused a shift of cellular cholesterol from a mainly membrane distribution to a vesicular and/or punctate distribution (Fig. 7A). In addition, cells co-stained with filipin and LAMP-1 demonstrate co-localization when treated with EIPA (Fig. 7B) with many of the vesicular structures appearing enlarged and near the cell nucleus (see Discussion). These data suggest that EIPA may cause an increase of cholesterol in lysosomal membranes and this may activate ORP1L to induce lysosome retrograde trafficking.
Fig. 7.
EIPA increases vesicular-associated cholesterol, which colocalizes with LAMP-1. (A) Filipin staining demonstrates that EIPA, but not HGF induces an increase in vesicular cholesterol as demonstrated by punctate staining. (B) IF microscopy shows that cholesterol-rich vesicles (flipin staining; green) colocalize with LAMP-1 (red). EIPA induces these cholesterol- and LAMP-1-positive vesicles to enlarge and accumulate in the perinuclear region. Scale bars: 10 μm (A); 5 μm (B).
Discussion
Factors in the tumor microenvironment, such as acidic pHe and growth factors, can regulate tumor growth and invasion. HGF is a potent mitogen and pro-invasive growth factor that plays a major role in tumor progression. We report here a novel role for the HGF-Met signaling pathway. This pathway, when activated, induces the peripheral trafficking of lysosomes, resulting in an increase in cathepsin B secretion and invasion. HGF-induced lysosome trafficking required the PI3K pathway, microtubules, RhoA and at least two NHE isoforms. HGF did not increase the invasion by tumor cells or affect lysosome redistribution in cells overexpressing the Rab7 effector, RILP, where lysosomes remained close to the nucleus. Furthermore, Rab7-shRNA-expressing (Rab7-KD) DU145 cells were inherently more invasive, lysosomes were more peripheral, and inhibitors of NHE family members did not block invasion or prevent or reverse lysosome redistribution in these cells. These data suggest that HGF controls lysosomal distribution in tumor cells and that lysosome distribution plays a major role in regulating invasion by tumor cells.
Inhibitors of the PI3K pathway prevented HGF-induced cell scattering and lysosome redistribution, consistent with cell scattering itself inducing lysosome movement. However, MAPK inhibitors blocked HGF-induced scattering but did not block lysosome redistribution, suggesting the two events are mutually exclusive. The JNK and p38 MAPK pathways did not contribute to lysosome movement.
Treatment of DU145 tumor cells with HGF induced a net acid production and acidification of the pHe without an increase in acidification of the pHi (Fig. 3), an indirect indicator of proton exchange activity, perhaps due to an HGF-induced increase in glycolysis. It is interesting to note that in our previous study, demonstrating acidic pHe-induced lysosome movement, only a 2-hour treatment of cells was required to induce maximum anterograde lysosome redistribution. However, even though HGF rapidly induced the phosphorylation of Akt and Erk1/2 (downstream of PI3K and MAPK, respectively), HGF-induced lysosome trafficking required overnight treatment with this growth factor. This supports the idea that protons must accumulate in the culture medium and/or inside cells, thus activating NHEs. Thus, one possible interpretation of this data, is that HGF- and acidic pHe-induced lysosome trafficking act through a similar mechanism – namely the activation of NHEs.
Consistent with our previous study, we determined that NHE1 and NHE3 mediated, in part, HGF-induced lysosome trafficking, since EIPA, or the combination of cariporide and s3226 (Fig. 5) prevented or attenuated HGF-induced lysosome trafficking, respectively. Since the non-isoform-specific NHE inhibitor EIPA was more effective at preventing HGF-induced lysosome trafficking, we conclude that other NHE isoforms, in addition to NHE1 and NHE3, may also mediate the trafficking of lysosomes. In fact, DU145 cells have been shown to contain mRNA for NHE1-3 and NHE5-9 (Steffan et al., 2009). A future goal will be to determine the individual and cooperative roles of these additional NHE isoforms in regulating HGF-induced lysosome trafficking. Our results demonstrated that EIPA not only blocked acute HGF-mediated lysosome movement, but also reversed the chronic redistribution observed in cells overexpressing HGF or in cells where the Met receptor is greatly overexpressed and constitutively active. This suggests that NHEs are required not only to initiate lysosome movement, but also to maintain the redistribution of these organelles. This may have significant clinical impact as lysosomes may already be nearer the cell periphery in cancer patients upon presentation.
EIPA-modulated retrograde movement of lysosomes depended on the activity of Rab7, a GTPase known to traffic late endosomes and lysosomes towards the nucleus (Johansson et al., 2007) and the Rab7 effector RILP, which is similar to the mechanism of Troglitazone-induced retrograde lysosome trafficking (Steffan and Cardelli, 2010). In fact, lysosomes in Rab7-KD- and DN-RILP-expressing cells were more peripherally located than in vector control cells. Also, HGF-induced invasion by Rab7-KD cells was not blocked by EIPA, in contrast to control cells. Finally, Fig. 6 demonstrates that Rab7-KD cells were more invasive and secreted more cathepsin B than control cells in the absence of HGF. We conclude that a more peripheral cellular location of lysosomes may be important in regulating invasion, and that EIPA blocks invasion by stimulating retrograde lysosome transport or preventing anterograde movement.
In support of this idea, overexpression of WT-RILP induced lysosome aggregation near the nucleus and these cells were much less invasive in the presence or absence of HGF. Although RILP overexpression was thought only to induce nuclear aggregation of late endosomes and/or lysosomes (Cantalupo et al., 2001), it has also been shown that cells overexpressing RILP have delayed degradation of internalized EGFR, suggesting that receptor signaling would be sustained (Progida et al., 2007; Wang and Hong, 2006). However, if this were the case in our system, we would expect that the addition of HGF to the RILP-expressing cells should enhance invasion, as Met degradation would be delayed, allowing for sustained endosomal Met signaling.
We have previously reported that Troglitazone-induced retrograde lysosome trafficking was independent of ORP1L, a cholesterol sensor which mediates retrograde trafficking in high cholesterol environments (Steffan and Cardelli, 2010). However, as reported here, EIPA-induced retrograde lysosome trafficking may be dependent on ORP1L as EIPA increases vesicle-associated cholesterol which colocalizes with LAMP-1 (Fig. 7B). In fact, perinuclear located LAMP-1-positive vesicles were enlarged in cells treated with EIPA as previously described (Fretz et al., 2006), which we suggest may be due to the increase in lysosomal membrane cholesterol. Thus, it appears that although these two compounds (Troglitazone and EIPA) inhibit NHEs, induce retrograde lysosome trafficking, and prevent cathepsin B secretion and invasion, they may act by different mechanisms. Future studies will be required to create ORP1L knockdown cells to fully examine the role of ORP1L and cholesterol in EIPA-induced retrograde lysosome trafficking.
Our conclusion that lysosome distribution in cells is important in regulating invasion is supported by earlier studies. The Sloane group found that altered lysosomal locations correlated with increased grades of invasive brain cancer (Rempel et al., 1994), and later showed that cathepsin B released from these more peripheral lysosomes played a role in invasion (Koblinski et al., 2000; Rozhin et al., 1994). Most recently, Tu et al. (Tu et al., 2008) demonstrated that cathepsin B delivered to podosomes by lysosomes in NIH3T3 cells was involved in matrix degradation. These studies are important, but essentially correlative with regard to location of lysosomes in the cell and involvement in invasion by tumor cells The research described here more clearly demonstrates, by altering expression of proteins only thought regulate lysosome trafficking, that lysosome location is directly involved in invasion by tumor cells. Essentially, Rab7-KD cells have peripheral lysosomes and are more invasive, while RILP-overexpressing cells have lysosomes aggregating in a juxtanuclear position and they are less invasive.
Our data supports a model in which HGF-induced proton production, linked with NHE activity, drives peripheral lysosome movement, cathepsin B secretion and increased invasion. However, it is possible that Met and NHE interact together as a complex and that activation of Met, directly or indirectly activates the signal transduction activity of NHE members, independent of proton production. There is no evidence, to our knowledge, of a direct interaction between Met and NHEs; however, there is evidence that Met may induce NHE activity through a, yet to be defined, tyrosine kinase-calcium/calmodulin-dependent mechanism (Kaneko et al., 1995). In addition, it is possible that CD44, the cell-surface receptor for hyaluronan, is involved, as both Met and NHEs have been shown to interact with CD44 (Bourguignon et al., 2004; Orian-Rousseau et al., 2002; Singleton et al., 2007). It would be interesting to determine if Met, CD44 (specifically the v6 splice variant), and NHEs interact and if the calcium/calmodulin pathway is involved in lysosome trafficking. Consistent with this, CD44 has been shown to activate RhoA signaling (reviewed by Bourguignon, 2008), suggesting that CD44 may play a role in lysosome trafficking. Therefore, future studies may find additional complex interactions that are responsible for not only lysosome trafficking, but also EMT, because CD44 and NHEs are known to interact with the actin cytoskeleton through the ezrin/radixin/moesin family of proteins (Orian-Rousseau et al., 2007; Stock and Schwab, 2006).
In conclusion, we report that HGF-induced peripheral lysosome trafficking, cathepsin B secretion, and cell invasion depend on NHE activity. In addition, we present data suggesting that, by controlling the spatial distribution of lysosomes by downregulating Rab7 expression or by RILP overexpression, the invasiveness of tumor cells is modulated. Although the mechanism by which the NHEs facilitate lysosome trafficking remains unknown, it will be informative to determine the signaling pathway(s) connecting NHE activity and Met activity.
Materials and Methods
Cell culture
The human prostate cancer cell lines DU-145 and PC3, and the lung adenocarcinoma H1993, were purchased from ATCC and maintained in RPMI-1640 (Mediatech) with 10% FBS (Gemini Bio-Products) and 1% penicillin-streptomycin (Mediatech). Cells were maintained in a 37°C incubator with 5% CO2 and were sub-cultured upon attaining >75% confluence.
Reagents and antibodies
LY294002, U0126, SP600126, SB203580 and Nocodazole were purchased from Calbiochem and were all used at 10 μM. Phalloidin (1:100) was purchased from Molecular Probes. α-Tubulin antibody (1:1000) was purchased from Lab Vision. Cathepsin B and D antibodies (1:100) were purchased from Calbiochem. LAMP-1 antibody (H4A3; 1:50), was purchased from the Developmental Studies Hybridoma Bank at the University of Iowa. Secondary antibodies, Texas-Red or FITC, donkey anti-mouse or anti-rabbit (1:100), were purchased from Jackson ImmunoResearch Laboratories. pErk1/2, pAKT, pMet and pJNK antibodies (Cell Signaling) were used at 1:1000. HGF (Calbiochem) was used at 33 ng/ml. EIPA [5-(N-ethyl-N-isopropyl)-amiloride], cytochalasin D, and filipin (Sigma) were used at 25 μM, 1 μM and 0.01 mg/ml, respectively. Anti-actin antibody was used at 1:2500 and purchased from Sigma. EEA1 (1:100), GM130 (1:100) and transferrin receptor (1:50) antibodies were purchased from BD. M6PR antibody was purchased from Abnova. LBPA antiserum was a kind gift from Jean Gruenberg.
Cathepsin B secretion assay
Assay was performed as previously described (Steffan et al., 2009). HGF was added to cultures for 24 hours at 33 ng/ml.
Immunofluorescence staining and microscopy
IF microscopy was performed as previously described (Steffan et al., 2009).
Western blot analysis
Western blot analysis was performed as previously described (Steffan et al., 2009).
Lentiviral delivery of short-hairpin RNA
Short hairpin RNAs (shRNA) directed towards Rab7 were delivered into DU145 prostate cancer cells using Mission Lentiviral Transduction Particles (Sigma) according to manufacturer's protocol and as previously described (Steffan et al., 2009).
Transfection
GFP-expressing WT-RILP and DN-WILP expression plasmids were obtained from Rene Harrison (University of Toronto-Scarborough) and DN-RhoA plasmids were obtained from Addgene. Transfection experiments were carried out using Lipofectamine transfection reagent (Invitrogen) according to manufacturer's protocol for 24 hours prior to treatment with HGF.
Creation of stable cells line expressing RILP
A DU145 cells line stably expressing WT-RILP was previously described (Steffan and Cardelli, 2010)
Invasion assay
Invasion assay were performed as previously described (Steffan and Cardelli, 2010). HGF in serum-free medium was added to both the top and bottom chambers at a concentration of 33 ng/ml.
Net acid production and intracellular pH measurement
The amount of acid produced by cells with and without HGF was measured by determining the pH change in the cell culture medium. The medium of cells ± HGF was changed and the pH was measured (time zero). After 18 hours, the pH was measured again. Cultures were kept at 37°C and 5% CO2 throughout the experiment. Cells were then lysed and protein concentrations measured using the BCA protein assay (Pierce) using known BSA concentrations for a standard curve. The change in pHe represents net acid production, as pHi was found unchanged. pHi was measured using the pH-sensitive fluorescent dye, (2,7)-biscarboxyethyl-5(6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM; Molecular Probes) at 37°C. Details have been previously described (Steffan et al., 2009; Turturro et al., 2004).
Filipin staining
Cells were stained with filipin as previously described (Steffan and Cardelli, 2010). Cells were treated with EIPA and/or HGF overnight. In colocalization experiments, cholesterol was stained first, followed by permeabilization and staining for LAMP-1. Identical exposure and capture settings were used for each experimental condition.
Statistical analysis
GraphPad software, Prism 3.0 was utilized to perform all statistics. One or two-tailed Mann-Whitney t-tests were performed to indicate statistical significance. All graphs show the standard error of the mean (s.e.m.).
Supplementary Material
Acknowledgments
The authors would like to acknowledge Rebecca Bigelow, David Coleman and Stephanie Wang for their critical reading of the manuscript. A special thanks to Meiyappan Solaiyappan (Johns Hopkins) for the lysosome quantification software, Jean Gruenberg (University of Geneva) for the LBPA antiserum, and Rene Harrison (University of Toronto-Scarborough) for the RILP constructs. This work was supported by grant no. NIH R01 CA104242-01. The authors declare no conflicts of interest. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/7/1151/DC1
References
- Acloque H., Thiery J., Nieto M. (2008). The physiology and pathology of the EMT. EMBO J. 9, 22-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S., Zerillo C., Kolmakova J., Christensen J. G., Harris L. N., Rimm D. L., DiGiovanna M. P., Stern D. F. (2009). Association of constitutively activated hepatocyte growth factor receptor (Met) with resistance to a dual EGFR//Her2 inhibitor in non-small-cell lung cancer cells. Br. J. Cancer 100, 941-949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenuti S., Comoglio P. M. (2007). The MET receptor tyrosine kinase in invasion and metastasis. J. Cell. Physiol. 213, 316-325 [DOI] [PubMed] [Google Scholar]
- Bertola A., Bonnafous S., Cormont M., Anty R., Tanti J.-F., Tran A., Le Marchand-Brustel Y., Gual P. (2007). Hepatocyte growth factor induces glucose uptake in 3T3-L1 adipocytes through a Gab1/phosphatidylinositol 3-kinase/Glut4 pathway. J. Biol. Chem. 282, 10325-10332 [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Birchmeier W., Gherardi E., Vande Woude G. F. (2003). Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 4, 915-925 [DOI] [PubMed] [Google Scholar]
- Boccaccio C., Comoglio P. M. (2006). Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat. Rev. Cancer 6, 637-645 [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y. (2008). Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin. Cancer Biol. 18, 251-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L., Singleton P., Diedrich F., Stern R., Gilad E. (2004). CD44 interaction with Na+/H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breat tumor cell invasion. J. Biol. Chem. 279, 26991-27007 [DOI] [PubMed] [Google Scholar]
- Boussouar F., Benahmed M. (1999). Epidermal growth factor regulates glucose metabolism through lactate dehydrogenase a messenger ribonucleic acid expression in cultured porcine Sertoli cells. Biol. Repro. 61, 1139-1145 [DOI] [PubMed] [Google Scholar]
- Cantalupo G., Alifano P., Roberti V., Bruni C. B., Bucci C. (2001). Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 20, 683-693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella R., Casey S. F. (2003). Decreased activity of cathepsins L + B and decreased invasive ability of PC3 prostate cancer cells. Biotech. Histochem. 78, 101-108 [DOI] [PubMed] [Google Scholar]
- Colella R., Jackson T., Goodwyn E. (2004). Matrigel invasion by the prostate cancer cell lines, PC3 and DU145, and cathepsin L+B activity. Biotech. Histochem. 79, 121-127 [DOI] [PubMed] [Google Scholar]
- Coleman D. T., Bigelow R., Cardelli J. A. (2009). Inhibition of fatty acid synthase by luteolin post-transcriptionally down-regulates c-Met expression independent of proteosomal/lysosomal degradation. Mol. Cancer Ther. 8, 214-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier M.-N., Dauzonne D., Louvard D., Coudrier E. (2001). Actin filaments and myosin I alpha cooperate with microtubules for the movement of lysosomes. Mol. Biol. Cell 12, 4013-4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fretz M., Jin J., Conibere R., Penning N., Al-Taei S., Storm G., Futaki S., Takeuchi T., Nakase I., Jones A. (2006). Effects of Na+/H+ exchanger inhibitors on subcellular localisation of endocytic organelles and intracellular dynamics of protein trandsuction domains HIV-TAT peptide and octaarginine. J. Control. Release 116, 247-254 [DOI] [PubMed] [Google Scholar]
- Gentile A., Trusolino L., Comoglio P. M. (2008). The Met tyrosine kinase receptor in development and cancer. Cancer Met. Rev. 27, 85-94 [DOI] [PubMed] [Google Scholar]
- Glunde K., Guggino S. E., Solaiyappan M., Pathak A. P., Ichikawa Y., Bhujwalla Z. M. (2003). Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia 5, 533-545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Rotin D., Mason M. J. (1989). Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochem. Biophys. Acta 988, 73-97 [DOI] [PubMed] [Google Scholar]
- Harguindey S., Orive G., Luis Pedraz J., Paradiso A., Reshkin S. J. (2005). The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin - one single nature. Biochem. Biophys. Acta 1756, 1-24 [DOI] [PubMed] [Google Scholar]
- Hsu P., Sabatini D. M. (2008). Cancer cell metabolism: Warburg and beyond. Cell 134, 703-707 [DOI] [PubMed] [Google Scholar]
- Johansson M., Lehto M., Tanhuanpaa K., Cover T. L., Olkkonen V. M. (2005). The oxysterol-binding protein homologue ORP1L interacts with Rab7 and alters functional properties of late endocytic compartments. Mol. Biol. Cell 16, 5480-5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M., Rocha N., Zwart W., Jordens I., Janssen L., Kuijl C., Olkkonen V. M., Neefjes J. (2007). Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor {beta}lll spectrin. J. Cell Biol. 176, 459-471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordens I., Fernandez-Borja M., Marsman M., Dusseljee S., Janssen L., Calafat J., Janssen H., Wubbolts R., Neefjes J. (2001). The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 11, 1680-1685 [DOI] [PubMed] [Google Scholar]
- Joyce J. A., Pollard J. W. (2009). Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Hayashi N., Tanaka Y., Horimoto M., Ito T., Sasaki Y., Fusamoto H., Kamada T. (1995). Activation of Na+/H+ exchanger by hepatocyte growth factor in hepatocytes. Hepatology 22, 629-636 [PubMed] [Google Scholar]
- Kaplan D., Boron W. (1994). Long-term expression of c-H-ras stimulates Na-H and Na(+)-dependent Cl− HCO3 exchange in NIH-3T3 fibroblasts. J. Biol. Chem. 269, 4116-4124 [PubMed] [Google Scholar]
- Kaplan O., Firon M., Vivi A., Navon G., Tsarfaty I. (2000). HGF/SF activates glycolysis and oxidative phosphorylation in DA3 murine mammary cancer cells. Neoplasia 2, 365-377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblinski J. E., Ahram M., Sloane B. F. (2000). Unraveling the role of proteases in cancer. Clin. Chim. Acta 291, 113-135 [DOI] [PubMed] [Google Scholar]
- Levayer R., Lecuit T. (2008). Breaking down EMT. Nat. Cell Biol. 10, 757-759 [DOI] [PubMed] [Google Scholar]
- Mantovani A., Schioppa T., Porta C., Allavena P., Sica A. (2006). Role of tumor-associated macrophages in tumor progression and invasion. Cancer Met. Rev. 25, 315-322 [DOI] [PubMed] [Google Scholar]
- Martinez-Zaguilan R., Seftor E. A., Seftor R. E. B., Chu Y., Gillies R. J., Hendrix M. J. C. (1996). Acidic pH enhances the invasive behavior of human melanoma cells. Clin. Exp. Metastasis 14, 176-186 [DOI] [PubMed] [Google Scholar]
- Masereel B., Pochet L., Laeckmann D. (2003). An overview of inhibitors of Na+/H+ exchanger. Eur. J. Med. Chem. 38, 547-554 [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Nakamura T. (2006). Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int. J. Cancer 119, 477-483 [DOI] [PubMed] [Google Scholar]
- Matteoni R., Kreis T. E. (1987). Translocation and clustering of endosomes and lysosomes depends on microtubules. J. Cell Biol. 105, 1253-1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan S. A., Burke P., Coleman D. T., Bigelow R. L., Steffan J. J., Carroll J. L., Williams B. J., Cardelli J. A. (2009). The green tea polyphenol EGCG potentiates the antiproliferative activity of c-Met and epidermal growth factor receptor inhibitors in non-small cell lung cancer cells. Clin. Cancer Res. 15, 4885-4894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R., Black K., Krishnamurty C., Baggett B. K., Stafford P., Rain M., Gatenby R. A., Gillies R. J. (2008). Acid treatment of melanoma cells selects for invasive phenotypes. Clin. Exp. Metastasis 25, 411-425 [DOI] [PubMed] [Google Scholar]
- Nomura T., Katunuma N. (2005). Involvement of cathepsins in the invasion, metastasis and proliferation of cancer cells. J. Med. Invest. 52, 1-9 [DOI] [PubMed] [Google Scholar]
- Orian-Rousseau V., Chen L., Sleeman J. P., Herrlich P., Ponta H. (2002). CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 16, 3074-3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orian-Rousseau V., Morrison H., Matzke A., Kastilan T., Pace G., Herrlich P., Ponta H. (2007). Hepatocyte growth factor-induced Ras activation requires ERM proteins linked to both CD44v6 and F-actin. Mol. Biol. Cell 18, 76-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso A., Cardone R. A., Bellizzi A., Bagorda A., Guerra L., Tommasino M., Casavola V., Reshkin S. J. (2004). The Na+-H+ exhanger-1 induces cytoskeletal changes involving reciprocal RhoA and Rac1 signaling, resulting in motility and invasion in MDA-MB-435 cells. Breast Cancer Res. 6, R616-R628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchietti S., Michieli P., Galluzzo M., Mazzone M., Giordano S., Comoglio P. M. (2003). Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 3, 347-361 [DOI] [PubMed] [Google Scholar]
- Perdomo G., Martinez-Brocca M. A., Bhatt B. A., Brown N. F., O'Doherty R. M., Garcia-Ocana A. (2008). Hepatocyte growth factor is a novel stimulator of glucose uptake and metabolism in skeletal muscle cells. J. Biol. Chem. 283, 13700-13706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Progida C., Malerod L., Stuffers S., Brech A., Bucci C., Stenmark H. (2007). RILP is required for the proper morphology and function of late endosomes. J. Cell Sci. 120, 3729-3737 [DOI] [PubMed] [Google Scholar]
- Putney L. K., Denker S. P., Barber D. L. (2002). The changing face of the Na+/H+ exchanger, NHE1: structure, regulation, and cellular actions. Annu. Rev. Pharmacol. Toxicol. 42, 527-552 [DOI] [PubMed] [Google Scholar]
- Rempel S. A., Rosenblum M. L., Mikkelsen T., Yan P.-S., Ellis K. D., Golembieski W. A., Sameni M., Rozhin J., Ziegler G., Sloane B. F. (1994). Cathepsin B expression and localization in glioma progression and invasion. Cancer Res. 54, 6027-6031 [PubMed] [Google Scholar]
- Reshkin S. J., Bellizzi A., Caldeira S., Albarani V., Malanchi I., Poignee M., Alunni-Fabbroni M., Casavola V., Tommasino M. (2000). Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 14, 2185-2197 [DOI] [PubMed] [Google Scholar]
- Rocha N., Kuijl C., van der Kant R., Janssen L., Houben D., Janssen H., Zwart W., Neefjes J. (2009). Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150Glued and late endosome positioning. J. Cell Biol. 185, 1209-1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhin J., Sameni M., Ziegler G. H., Sloane B. F. (1994). Pericellular pH affects distribution and secretion of cathepsin b in malignant cells. Cancer Res. 54, 6517-6525 [PubMed] [Google Scholar]
- Sawada K., Radjabi A. R., Shinomiya N., Kistner E., Kenny H., Becker A. R., Turkyilmaz M. A., Salgia R., Yamada S. D., Vande Woude G. F., et al. (2007). c-Met overexpression is a prognostic factor in ovarian cancer and an effective target for inhibition of peritoneal dissemination and invasion. Cancer Res. 67, 1670-1679 [DOI] [PubMed] [Google Scholar]
- Singh S., Sadanandam A., Singh R. (2007). Chemokines in tumor angiogenesis and metastasis. Cancer Met. Rev. 26, 453-467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton P. A., Salgia R., Moreno-Vinasco L., Moitra J., Sammani S., Mirzapoiazova T., Garcia J. G. N. (2007). CD44 regulates hepatocyte growth factor-mediated vascular integrity. J. Biol. Chem. 282, 30643-30657 [DOI] [PubMed] [Google Scholar]
- Steffan J. J., Cardelli J. A. (2010). Thiazolidinediones induce Rab7-RILP-MAPK-dependent juxtanuclear lysosome aggregation and reduce tumor cell invasion. Traffic 11, 274-286 [DOI] [PubMed] [Google Scholar]
- Steffan J. J., Snider J. L., Skalli O., Welbourne T., Cardelli J. A. (2009). Na+/H+ exchangers and RhoA regulate acidic extracellular pH-induced lysosome trafficking in prostate cancer cells. Traffic 10, 737-753 [DOI] [PubMed] [Google Scholar]
- Stock C., Schwab A. (2006). Role of the Na/H exchanger NHE1 in cell migration. Acta Physiol. 187, 149-157 [DOI] [PubMed] [Google Scholar]
- Strazzabosco M., Poci C., Spirli C., Zsembery A., Granato A., Massimino M. L., Crepaldi G. (1995). Intracellular pH regulation in Hep G2 cells: effects of epidermal growth factor, transforming growth factor-alpha, and insulinlike growth factor-II on Na+/H+ exchange activity. Hepatology 22, 588-597 [PubMed] [Google Scholar]
- Swanson J., Locke A., Ansel P., Hollenbeck P. (1992). Radial movement of lysosomes along microtubules in permeabilized macrophages. J. Cell Sci. 103, 201-209 [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Yonemura Y., Nojima N., Hirono Y., Fushida S., Fujimura T., Miwa K., Endo Y., Yamamoto H., Watanabe H. (1998). The relation between the growth patterns of gastric carcinoma and the expression of hepatocyte growth factor receptor (c-met), autocrine motility factor receptor, and urokinase-type plasminogen activator receptor. Cancer 82, 2112-2122 [PubMed] [Google Scholar]
- Tsuji T., Ibaragi S., Hu G. (2009). Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 69, 7135-7139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C., Ortega-Cava C. F., Chen G., Fernandes N. D., Cavallo-Medved D., Sloane B. F., Band V., Band H. (2008). Lysosomal cathepsin B participates in the podosome-mediated extracellular matrix degradation and invasion via secreted lysosomes in v-Src fibroblasts. Cancer Res. 68, 9147-9156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turturro F., Friday E., Fowler R., Surie D., Welbourne T. (2004). Troglitazone acts on cellular pH and DNA synthesis through a peroxisome proliferator-activated receptor {gamma}-independent mechanism in breast cancer-derived cell lines. Clin. Cancer Res. 10, 7022-7030 [DOI] [PubMed] [Google Scholar]
- Véga C., Pellerin L., Dantzer R., Magistretti P. J. (2002). Long-term modulation of glucose utilization by IL-1alpha and TNF-alpha in astrocytes: Na+ pump activity as a potential target via distinct signaling mechanisms. Glia 39, 10-18 [DOI] [PubMed] [Google Scholar]
- Wang T., Hong W. (2006). RILP interacts with VPS22 and VPS36 of ESCRT-II and regulates their membrane recruitment. Biochem. Biophys. Res. Commun. 350, 413-423 [DOI] [PubMed] [Google Scholar]
- Yilmaz M., Christofori G. (2009). EMT, the cytoskeleton, and cancer cell invasion. Cancer Met. Rev. 28, 15-33 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.