Abstract
Background
Preeclampsia (PE) is a prevalent life-threatening hypertensive disorder of pregnancy. The circulating antiangiogenic factor, soluble endoglin (sEng), is elevated in the blood circulation of women with PE and contributes to disease pathology. However, the underlying mechanisms responsible for its induction in PE are unknown.
Methods and Results
Here we discovered that a circulating autoantibody, the angiotensin receptor agonistic autoantibody (AT1-AA), stimulates sang production via AT1 angiogenesis receptor activation in pregnant mice but not non-pregnant mice. Subsequently we demonstrate that the placenta is a major source contributing to sang induction in vivo and AT1-AA injected pregnant mice display the impaired placental angiogenesis. Using drug screening, we identified TNF-α as a circulating factor increased in the serum of autoantibody-injected pregnant mice contributing to AT1-AA-mediated sang induction in human umbilical vascular endothelial cells (HUVECs). Subsequently, among all the drugs screened we found that hemin, an inducer of heme oxygenase-1 (HO-1), functions as a break to control AT1-AA mediated sang induction by suppressing TNF-α signaling in Havocs. Finally, we demonstrated that AT1-AA-mediated decreased angiogenesis seen in human placenta villous explants was attenuated by TNF-α neutralizing antibodies, soluble TNF-α receptors and hemi, an inducer of home oxygenase, by abolishing both sang and sFlt-1 induction.
Conclusions
Our findings demonstrate that AT1-AA-mediated TNF-α induction, by overcoming its negative regulator, HO-1, is a key underlying mechanism responsible for impaired placenta angiogenesis by inducing both sEng and sFlt-1 secretion from human villous explants and provide important new targets for diagnosis and therapeutic intervention in the management of PE.
Keywords: angiogenesis, circulation, endothelium, inflammation, signal transduction
Preeclampsia (PE) is a prevalent life-threatening hypertensive disorder of pregnancy with a highest maternal and fetal morbidity and mortality.1, 2 A growing body of evidence indicates that a circulating maternal autoantibody, the angiotensin II type I receptor agonistic autoantibody (AT1-AA), is a prominent component in the pathogenesis of PE. Numerous early studies demonstrated that AT1-AAs activate AT1 receptors on a variety of cell types and provoke biological responses relevant to the pathophysiology of PE.3–8 Recently we have extended these in vitro studies by showing that key features of PE are generated in pregnant mice injected with either total IgG or affinity purified AT1-AAs from preeclamptic women.9 These studies provided the first direct evidence for the pathogenic nature of AT1-AAs in PE.
Recently, Venkatasha et al showed that a soluble form of endoglin (sEng) is present at significantly elevated levels in the circulation of women with PE compared to women with normotensive pregnancy and that the level of sEng correlated with disease severity.10 Endoglin is a cell-surface co-receptor for transforming growth factors (TGF)-β 1 and TGF-β3 and is mainly expressed on endothelial cells and syncytiotrophoblasts.11–13 The introduction of recombinant adenovirus vectors encoding sEng into pregnant rats resulted in mild hypertension and proteinuria. Notabley, introduction of viral vectors encoding sEng and soluble fms-like tyrosine kinase-1 (sFlt1, a soluble form of VEGF receptor–1) together into pregnant rats resulted in nephrotic-range proteinuria, severe hypertension, and the HELLP syndrome (Hemolysis, Elevated Liver enzymes and Low Platelets), a severe form of preeclampsia. These studies demonstrate that sEng contributing to PE.14 However, factors and signaling pathways responsible for elevated sEng in women with PE were not determined.
Here we show that AT1-AA induces the production of sEng in pregnant mice but not in non-pregnant mice by activation of AT1 receptors and that the placenta is a major source of its induction in vivo. We further provide the compelling mouse and human evidence that AT1-AA leads to impaired placenta angiogenesis via AT1 receptor activation. More importantly, we revealed that AT1-AA-mediated TNF-α induction, by overcoming its negative regulator, heme oxygenase-1 ( HO-1), is a key underlying mechanism responsible for impaired placenta angiogenesis by inducing both sEng and sFlt-1 secretion from human villous placental explants. Overall, our findings are the first to link maternal autoantibodies with increased TNF-α, a prominent inflammatory cytokine, to contribute to impaired placenta angiogenesis by inducing two key antiangiogenic factors, sFlt-1 and sEng. This study provides important new targets for diagnosis and therapeutic intervention in the management of PE.
Methods
For an expanded methods sections, please refer to the online-only Data Supplement.
Patients
Patients who were admitted to Memorial Hermann Hospital were identified by the obstetrical faculty of the University of Texas Medical School at Houston. Detailed patient information has been previously described.6, 9 The research protocol was approved by the Institutional Committee for the Protection of Human Subjects.
Introduction of antibody into mice
All animal studies were reviewed and approved by the Animal Welfare Committee, University of Texas Houston Health Science Center. Forty-eight C57BL/6J pregnant and thirty-two non-pregnant mice (18 to 22 g; Harlan, Indianapolis, Ind.) were used in our study. Experiments were performed in the following groups of mice: pregnant mice (n=32) or non-pregnant mice (n=32) with single (n=16) or double (n=16) injection of IgG purified from normotensive controls (n=8) or preeclamptic patients (n=8). In addition, some of the pregnant mice were coinjected with losartan (0.24 mg, a generous gift from Merck & Co., Inc. Rahway, NJ) (n=8) or a 7-aa peptide (AFHYESQ) (n=8) corresponding to an epitope on the second extracellular loop of the AT1 receptor (1.5 mg) All of the mice were anesthetized with sodium pentobarbital (50 mg/kg ip) and concentrated IgG purified from 200 µl normotenisve controls (n=12) or patients’ serum (n=14) (either combined or single individual) was introduced into pregnant mice on gestation day 13 for single injection and one more injection on gestation 14 for double injection or non-pregnant mice on two consecutive days by orbital sinus injection. Immunoglobulin IgG was prepared as previously describled.5–7, 9 We collected plasma and serum on multiple gestation days for determination of sEng and TNF-α concentration.
Results
IgG from women with PE simulates sEng production in pregnant mice but not non-pregnant mice via AT1 receptor activation
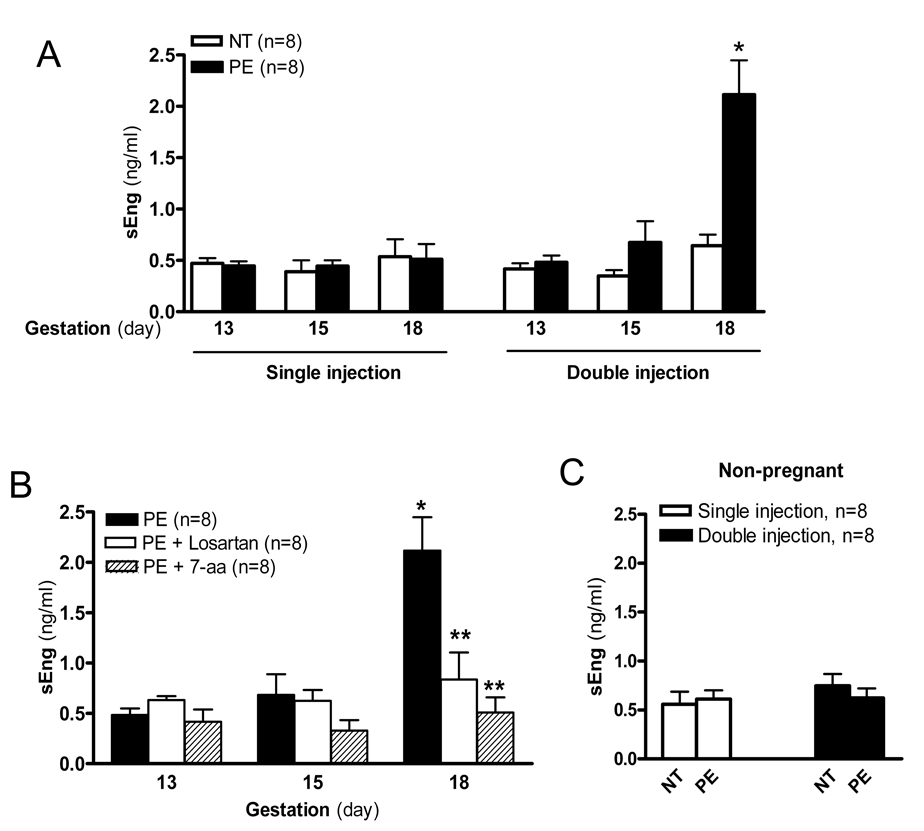
To determine the potential role of pregnancy to the sEng induction by AT1-AA, we injected pregnant or non-pregnant mice with a single dosage (gestation day 13) or a double dosage (gestation days 13 and 14) of IgG from women with PE or women with a normotensive pregnancy. We show that the single injection of IgG from women with PE did not induce a significant increase in sEng production by gestation day 18 in the pregnant mice compared with mice injected with IgG from women with a normotensive pregnancy (Figure 1A). However a four-fold increase in sEng was observed by gestation day 18 in pregnant mice receiving a double injection of IgG from preeclamptic women as compared to mice receiving two injections of IgG from normotensive pregnant women (Figure 1A), which is similar to the increased seen in preeclamptic women.10 Because two autoantibody injections were required to achieve a significant increase in sEng levels in pregnant mice, we chose the double injection protocol for the remainder of the experiments presented here. Antibody mediated induction of sEng production was blocked by co-injection with losartan, an AT1 receptor antagonist, or with a 7-aa epitope peptide that blocks autoantibody-mediated AT1 receptor activation (Figure 1B). These results indicate that IgG from preeclamptic women induced sEng production in pregnant mice via AT1R activation. In contrast to what we observed in pregnant mice, sEng levels in non-pregnant mice were not induced by IgG from women with PE or normotensive pregnant women following single or double injections (Figure 1C). Thus, these findings demonstrate that IgG from women with PE is capable of inducing sEng production via AT1R activation in pregnant mice but not in non-pregnant mice.
Figure 1. IgG from women with PE induces sEng secretion in pregnant but not non-pregnant mice via AT1- receptor activation.
IgG from women with PE or normotensive pregnant women was introduced by intra-orbital injection into pregnant mice or non-pregnant mice for five days with a single injection or double injection. (A) Plasma was collected at different time points as indicated and the concentration of sEng was determined by ELISA. Data are expressed as mean ± SEM. * P < 0.05 versus gestation day 18 pregnant mice injected with normotensive IgG. (B) Co-injection of losartan or the 7-aa epitope peptide inhibited the increase of sEng production by IgG from women with preeclampsia. * P < 0.01 versus gestation day 13 pregnant mice injected with IgG from women with preeclampsia. ** P < 0.05 versus gestation day 18 pregnant mice injected with preeclamptic IgG. (C) No effect on sEng production by IgG from women with preeclampsia in non-pregnant mice. Data are expressed as mean ± SEM. N=8 for each group.
Placenta is a major organ contributing to sEng production in autoantibody-injected pregnant mice
Next, to determine whether the placenta is a major source of sEng production and secretion, we measured Eng mRNA and protein levels in the mouse placenta and kidneys from pregnant mice injected with IgG as described above. We found that total Eng mRNA levels were increased in placenta tissue of mice injected with IgG from women with PE compared to placenta tissue of mice injected with IgG from women with normotensive pregnancies (Figure 2A&B), suggesting that AT1-AA mediated sEng induction is at the mRNA level, a finding consistent with earlier human studies.14 Similarly, we found that the abundance of intact Eng protein and the small amount of sEng remaining in the placentas were also induced in pregnant mice injected with IgG from preeclamptic but not IgG from normotensive pregnant women (Figure 2C–E). Consistently, we found that Eng protein levels were much lower in kidney samples and there was no difference in mice injected with IgG from normotensive pregnant women or those with preeclampsia (Figure 2C–E). Thus, these results provide direct evidence that placenta is a major organ contributing to sEng synthesis and secretion in autoantibody-injected pregnant mice.
Figure 2. Placenta contributes to sEng production in response to IgG from women with PE.
(A) Semi-quantitative RT-PCR was used to quantify endoglin mRNA abundance from mouse placentas. L: losartan, 7-aa, seven amino acid epitope peptide. (B) The ratio of Eng mRNA/β-actin mRNA was obtained by performing densitometric analysis of multiple agarose gels (n=8 mice for each group). * P < 0.05 versus mice injected with IgG from normotensive pregnant women; ** P < 0.05 versus preeclamptic IgG injection. Data are expressed as mean ± SEM. (C) The expression levels of Eng and sEng protein were analyzed by western blot. The ratio of sEng (D) or Eng (E) protein to β-actin is used to represent the sEng and Eng expression levels. N=8 mice for each group. * P < 0.05 versus mice injected with IgG from normotensive pregnant women. Data are expressed as mean ± SEM.
Angiogenesis is impaired in placentas of autoantibody-injected pregnant mice
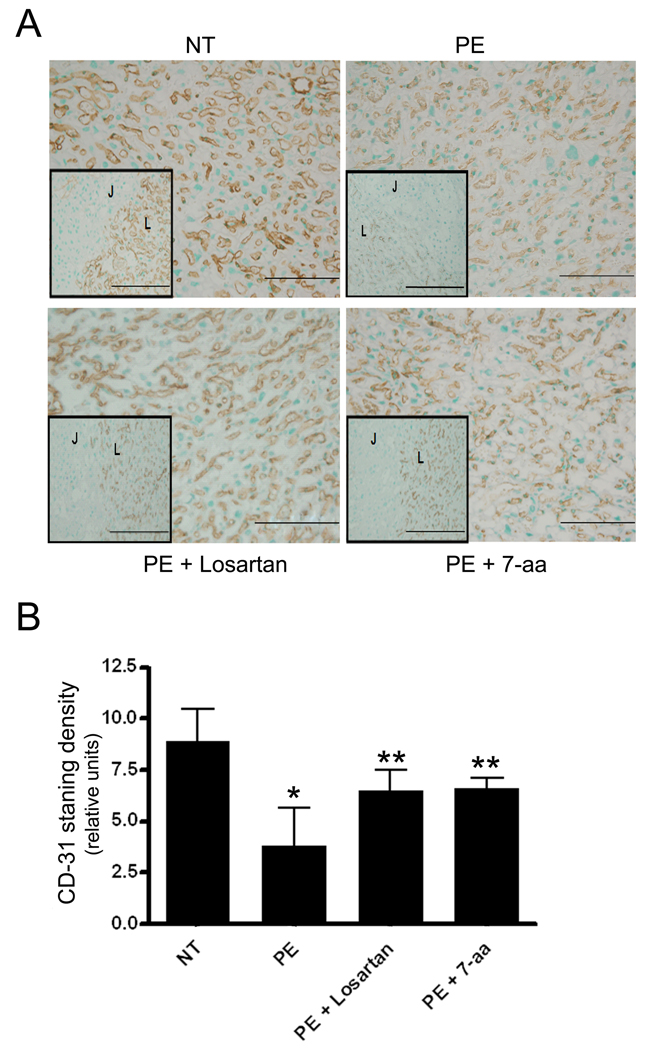
To address whether AT1-AA-induced placental derived antiangiogenic factor lead to impaired placental angiogenesis, we analyzed the vasculature of isolated mouse placentas by immunostaining using antibody recognizing CD31, an endothelial cell specific marker. The results show that CD-31 staining was less prominent in the labyrinth zone of the placentas of mice injected with IgG from women with PE in comparison to those injected with IgG from normotensive pregnant women (Figure 3A). Co-injection of antibody with losartan or the 7-aa epitope peptide reduced this effect (Figure 3A). Quantitative image analysis of CD31 immunostaining demonstrated significantly less immunoreactivity in placenta sections from mice injected with IgG from preeclamptic women compared with those from mice injected with IgG from normotensive pregnant women. Co-injection with losartan or the 7-aa epitope peptide significantly reduced the anti-angiogenic effects of autoantibody-injection (Figure 3B). Taken together, these results indicate that IgG from women with PE is capable of inducing placental-derived antiangiogenic factor production via AT1R activation and thereby contribute to decreased angiogenesis in the placenta of injected mice.
Figure 3. CD-31 immunohistochemical staining of mouse placentas.
(A) CD-31 (PECAM-1) staining of the placentas of mice injected with IgG from women with preeclampsia (PE) and normotensive pregnancy (NT) in absence or presence of Losartan or 7-aa epitope peptide. Inset: Junctional (J) and Labryinth (L) zone border. Four placenta were chosen from each category (n=8 mice). Scale bar: 50µm. (B) Quantification of CD-31 (PECAM-1) staining. Mean scores are represented ± SEM. N=8 mice for each category. * p<0.01versus normotensive IgG injection; **p<0.05 versus preeclamptic IgG injection.
Serum from mice injected with IgG from preeclamptic women stimulates sEng secretion from endothelial cells
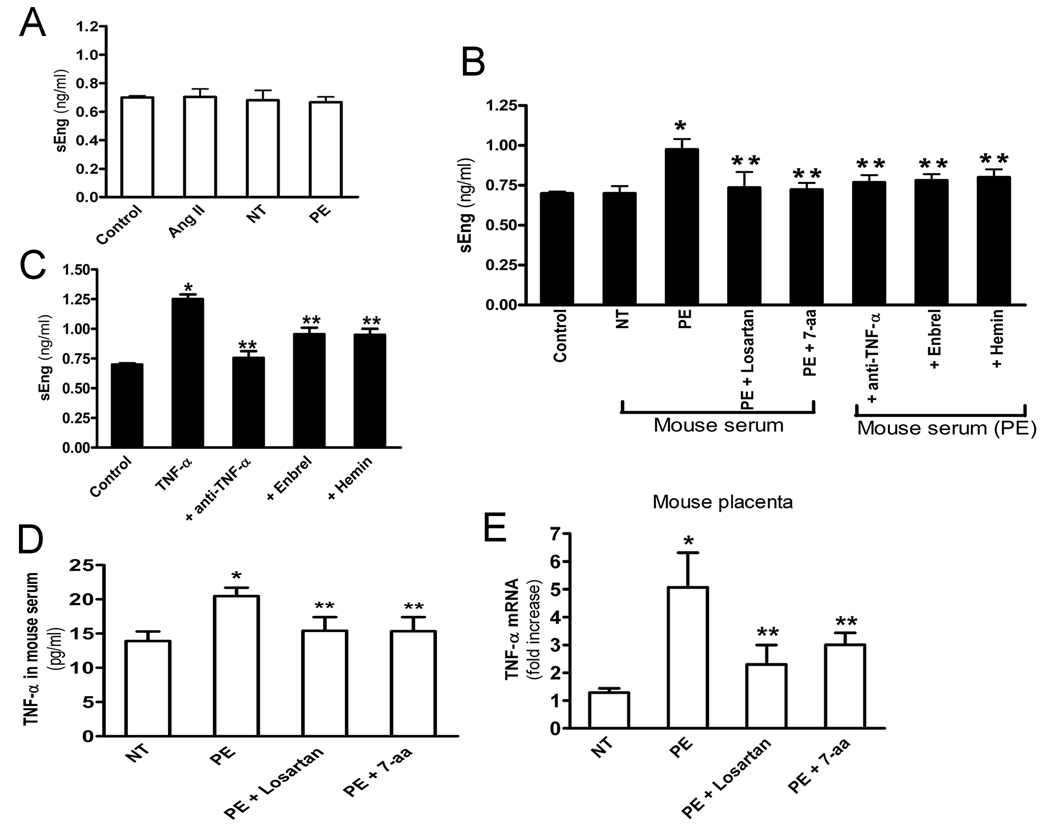
It is difficult to decipher the signaling pathways involved in AT1-AA-mediated sEng induction in intact animals, we therefore chose endothelial cells, a recognized source of sEng production, as a cellular model system. Unexpectedly, we found that neither Ang II nor IgG from preeclamptic and normotensive pregnant women stimulated sEng production by the cultured endothelial cells (Figure 4A). These results suggested that increased sEng in autoantibody-injected pregnant mice did not result from direct AT1 receptor activation. However, the production of sEng secretion was significantly increased by endothelial cells treated with serum of pregnant mice injected with IgG from preeclamptic women compared with that of cells cultured with serum of pregnant mice injected with normotensive IgG (Figure 4B). The increase in sEng production did not occur when using serum from mice that were co-injected with losartan or the 7-aa epitope peptide (Figure 4B). These results imply that serum from pregnant mice injected with IgG from preeclamptic women contain a factor that stimulates human sEng secretion from human endothelial cells. Furthermore, the causative factor present in the serum of antibody injected mice is the result of AT1 receptor activation.
Figure 4. TNF-α is the serum factor in autoantibody-injected pregnant mice that is responsible for induction of sEng production by endothelial cells.
(A) HUVEC cells were treated with Ang II, IgG from preeclamptic or normotensive women and the concentration of sEng in cell culture supernatant was determined. Data are expressed as mean (± SEM) of ≥3 experiments performed in duplicate (n=12–14 patient’s IgG for each group). (B) HUVEC cells treated with serum from control pregnant mice, pregnant mice injected with IgG from normotensive (NT) or preeclamptic women (PE) or coinjected with preeclamptic IgG and losartan (PE+Losartan), 7-aa epitope peptide (PE+7-aa) at day 5 following the initial injections. HUEVC cells also treated with anti-TNF-α, Enbrel or hemin for 30 minitues before adding serum from pregnant mouse injected with IgG from women with preeclampsia (mouse serum (PE)). After 24 hour the sEng concentration in cell culture supernatants were measured. * P < 0.05 versus cells treated with serum from mouse injected with normotensive IgG. ** P < 0.05 versus cells treated with serum from mouse injected with preeclamptic IgG. Data are expressed as mean ± SEM. N =8 mice’s serum for each group. (C) HUVEC cells were treated with TNF-α in the presence of TNF-α neutralizing antibody, Enbrel or hemin for 24 hours. Concentration of sEng in cell culture media was determined by ELISA. * P < 0.01 versus control untreated cells. ** P < 0.05 versus cells treated TNF-α alone. Data are expressed as mean (± SEM) of ≥3 experiments performed in duplicate (n=9 for each group). (D) IgG from normotensive or preeclamptic pregnant women were introduced into pregnant mice at gestation days 13 and 14. Mouse serum was collected at gestation days 18 and levels of TNF-α were measured by ELISA. Data are expressed as mean ± SEM, n=5–8 for each group. * P < 0.05 versus mice treated with normotensive IgG. ** P < 0.05 versus mice treated with preeclamptic IgG alone. (E) Placentas were collected (n=8–10 for each group) at gestation day 18 (5 days following the initial IgG injection). Real-time PCR was performed to quantify the TNF-α mRNA abundance. Data are expressed as mean ± SEM. * P < 0.001 versus normotensive IgG injection. ** P < 0.01 versus preeclamptic IgG injection..
TNF-α is the serum factor in the autoantibody-injected pregnant mice responsible for sEng secretion from endothelial cells
To determine the circulating factors involved in AT1-AA-mediated sEng induction in endothelial cells, we performed a drug screen to identify drugs that blocked the induction of sEng by serum from autoantibody injected mice. Among all the drugs we have tested, we found that Enbrel, a soluble form of the TNF receptor blocks serum-mediated sEng production by human endothelial cells (Figure 4B), indicating that TNF-α is the serum factor responsible for inducing sEng production by endothelial cells. Next, we demonstrated that the ability of sera from autoantibody-injected mice to stimulate sEng production by cultured endothelial cells was also inhibited by anti-TNF, a neutralizing antibody. Finally, we found that TNF-α directly stimulated sEng production by endothelial cells and that the induction was blocked by the presence of anti-TNF-α, a neutralizing antibody, or Enbrel, a soluble form of the TNF receptor that also blocks TNF-α signaling. Taken together, these findings provide strong evidence that TNF-α was the serum factor in the autoantibody-injected pregnant mice responsible for AT1-AA-mediated sEng induction.
TNF-α expression is increased in the blood circulation and placentas of autoantibody injected pregnant mice
The level of TNF-α is elevated in the blood circulation of women with PE15, 16 and it is possible that TNF-α may be induced by AT1-AA. To address this possibility, we measured TNF-α in autoantibody-injected pregnant mice. As shown in Figure 4D the level of TNF-α was elevated in pregnant mice injected with IgG from preeclamptic women compared with that in mice injected with IgG from normotensive pregnant women. The autoantibody-mediated induction of TNF-α was prevented by co-injection with losartan or the 7-aa epitope peptide (Figure 4D) indicating that IgG from preeclamptic women induces TNF-α production in pregnant mice via AT1R activation.
To determine whether TNF-α expression is upregulated in the placentas of antibody-injected mice, the placentas were collected on gestation day 18, five days following the initial IgG injecton and total RNA was isolated and real-time PCR was used to analyze the TNF-α transcript levels. The results (Figure 4E) showed that the level of TNF-α transcripts increased nearly 5-fold in the placentas of mice injected with IgG from preeclamptic women compared with that in the placentas of mice injected with IgG from normotensive pregnant women. The autoantibody-mediated increase in the abundance of placental TNF-α RNA was attenuated by co-injection with losartan or the 7-aa epitope peptide. These data indicate that IgG from women with PE stimulates an increase in the abundance of TNF-α transcripts in the placentas via AT1 receptor activation.
AT1-AA-induced TNF-α overcomes its downstream negative regulator, heme oxygenase-1, to induce sEng secretion by endothelial cells
Among all the drugs we screened, we unexpectedly found that hemin, a well-known inducer of heme oxygenase-1 (HO-1), blocked the ability of TNF-α to stimulate sEng production in human endothelial cells (Figure 4C). These results indicate that HO-1 functions as a break to control TNF-α induced sEng secretion. More importantly, hemin also blocked the ability of sera obtained from pregnant mice injected with preeclamptic IgG to stimulate sEng production by human endothelial cells (Figure 4B). Thus, these findings provide the first evidence that HO-1 functions downstream of AT1-AA-mediated TNF-α signaling to regulate sEng production.
AT1-AA-induced TNF-α production contributes to sEng induction from human placental villous explants
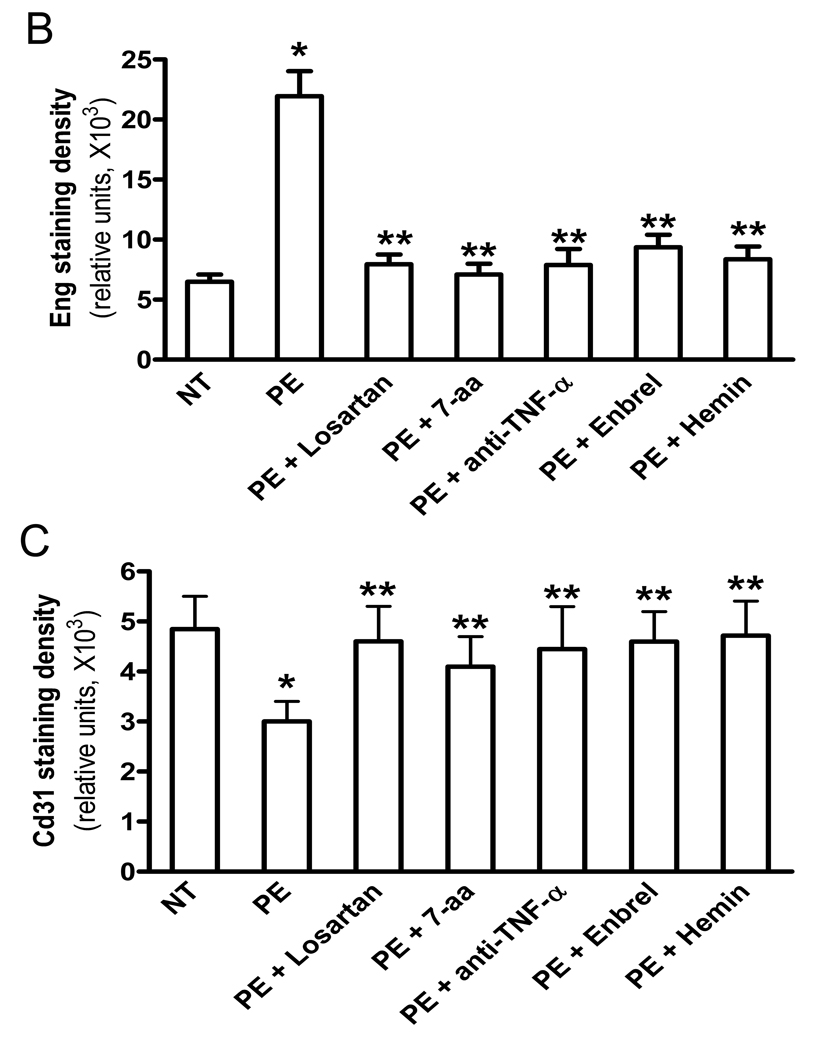
To extend mouse finding to humans and assess the direct pathological role of autoantibody-mediated TNF-α signaling on sEng secretion in humans, we took advantage of human villous explants as an investigative tool. Immunohistostaining showed that Eng is evident in the syncytiotrophoblasts (fetal derived cells) and endothelial cells (maternal origin) (Figure 5A), consistent with results published earlier.14 Quantitative image analysis revealed that the intensity of immunostaining of Eng was higher in the samples treated with IgG from women with PE and that the increase in intensity was prevented by the presence of losartan or the 7-aa epitope peptide indicating that the increase was mediated through AT1 receptor activation (Figure 5A & B). The increase in Eng immunostaining was also prevented by the presence of anti-TNF-α, Enbrel, or hemin, indicating the requirement for TNF-α to overcome the negative regulation by HO-1 (Figure 5A & B). Consistently, ELISA results demonstrated that AT1-AA significantly induced sEng secretion from human villous explants and that the induction was inhibited by compounds that block TNF-α signaling (Figure 5D). Overall, these findings not only reveal the localization of Eng in human placenta, but also provide evidence that the induction of sEng by IgG from women with PE requires downstream TNF-α signaling through processes that are negatively regulated by HO-1.
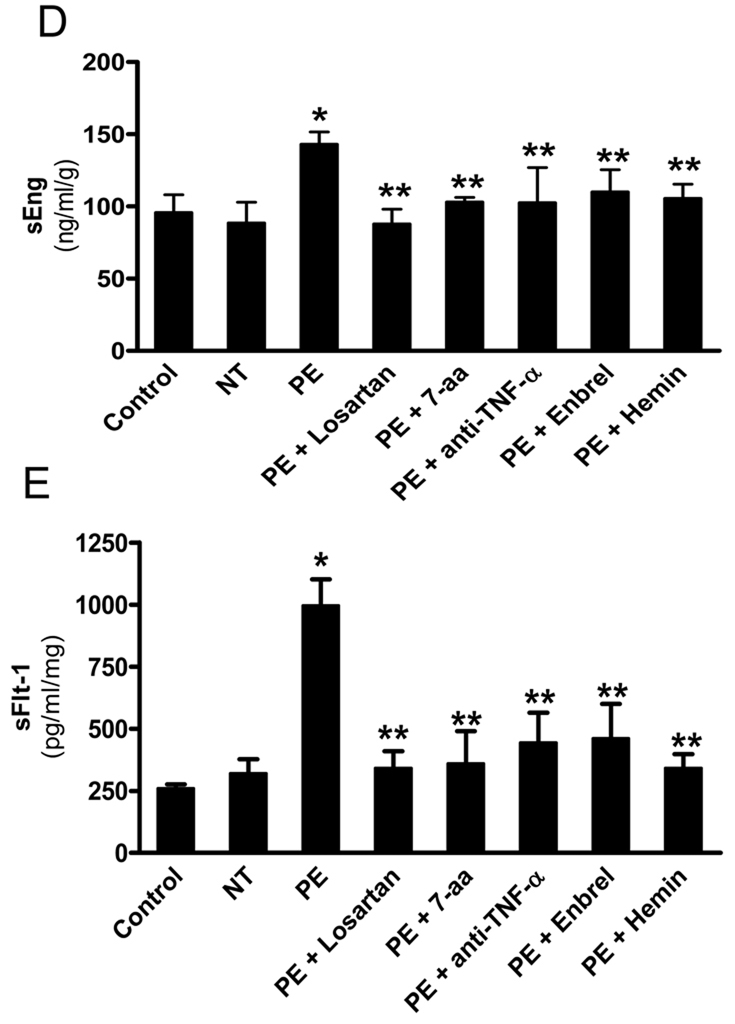
Figure 5. AT1-AA-mediated TNF-α induction contributes to impaired placenta angiogenesis by inducing both sEng and sFlt-1 secretion from human villous placental explants.
Normal human placental villous explants were collected and treated with IgG from women with preeclampsia or normotensive pregnant individuals in the presence or absence of various reagents for 72 hours. (A) At the end of treatment, human placental villous explants were collected, fixed and stained with (H&E), anti-human Eng and anti-human CD31 antibody. Scale bar, 100 µm (H&E and CD31) or 200 µm (Eng). Syncytiotrophoblast (syn) is indicated by arrow head. (B–C) Expression of endoglin (B) and CD31 (D) were quantified using Image-Pro Plus image analysis sosftware. (E–F) Cell culture supernatants were collected for sEng (E) and sFlt-1 (F) measurements by ELISA. Data are expressed as mean ± SEM of ≥4 experiments performed in duplicate (n=12–14 patient’s IgG for each category). * P < 0.001 versus villous explants treated with normotensive IgG. ** P < 0.05 versus villi treated with preeclamptic IgG.
AT1-AA-induced TNF-α signaling plays an important role in dysregulated human placental angiogenesis
Consistent to our mouse finding, we also found that treatment of explants with IgG from women with PE, in contrast to IgG from normotensive pregnant women, resulted in decreased CD31 immunostaining in human villous explants (Figure 5A & 5C). The autoantibody mediated decrease in CD31 immunostaining (i.e., angiogenesis) was attenuated by the presence of losartan or the 7-aa peptide as previously seen in pregnant mice. More importantly, we also found that anti-TNF-α, Enbrel or hemin prevented the reduced angiogenesis caused by AT1-AA in human villous explants (Figure 5A & 5C), indicating that the reduced angiogenesis involved autoantibody induced TNF-α signaling that was suppressed by HO-1. Overall, our studies suggest that autoantibody-induced TNF-α-signaling plays an important role in decreased placental angiogenesis associated with preeclampsia.
TNF-α signaling contributes to AT1-AA-mediated sFlt-1 induction and subsequent impaired placenta angiogenesis
In addition to sEng, sFlt-1 is another key antiangiogenic factor that is elevated in PE. We found that blocking AT1-AA-mediated TNF-α signaling inhibited impaired placenta angiogenesis (Figure 5A and C), suggesting that TNF-α signaling contributes to abnormal placental angiogenesis by inducing not only sEng as we shown in Figure 5D, but also sFlt-1. To test this possibility, we measured sFlt-1 secretion in human placental villous explants following treatment with IgG from women with preeclampsia and normotensive pregnant women. The results (Figure 5E) showed that IgG from women with PE, in contrast to IgG from normotensive pregnant women, stimulated the production of sFlt1. Similar to sEng, the induction was blocked by the presence of losartan or the 7-aa epitope peptide, indicating the requirement for AT1 receptor activation. Autoantibody-induced sFlt1 secretion was also prevented by the presence of human TNF-α neutralizing antibody, Enbrel or hemin (Figure 5E), indicating the contributory role TNF-α signaling in AT1-AA-mediaed sFlt-1 induction in human villous explants. Together, these results significantly strengthen and broaden the pathophysiology role of AT1-AA-mediated TNF-α induction in preeclampsia by linking both sFlt-1 and sEng with impaired angiogenesis in the human placenta.
Discussion
In this study, we have provided both in vivo mouse evidence and in vitro human studies that AT1-AA contributes to impaired placental angiogenesis via AT1 receptor activation. Mechanistically, we have identified for the first time that the induction of sEng and sFlt1 by AT1-AA is mediated through TNF-α signaling that is negatively regulated by HO-1. Overall, both mouse and human studies reported here provide strong evidence that AT1-AA-mediated TNF-α induction is an underlying mechanism for increased secretion of antiangiogenic factors and suggest that these signaling pathways contribute to impaired placenta angiogenesis in PE and novel therapeutic possibilities for the disease (Figure 6).
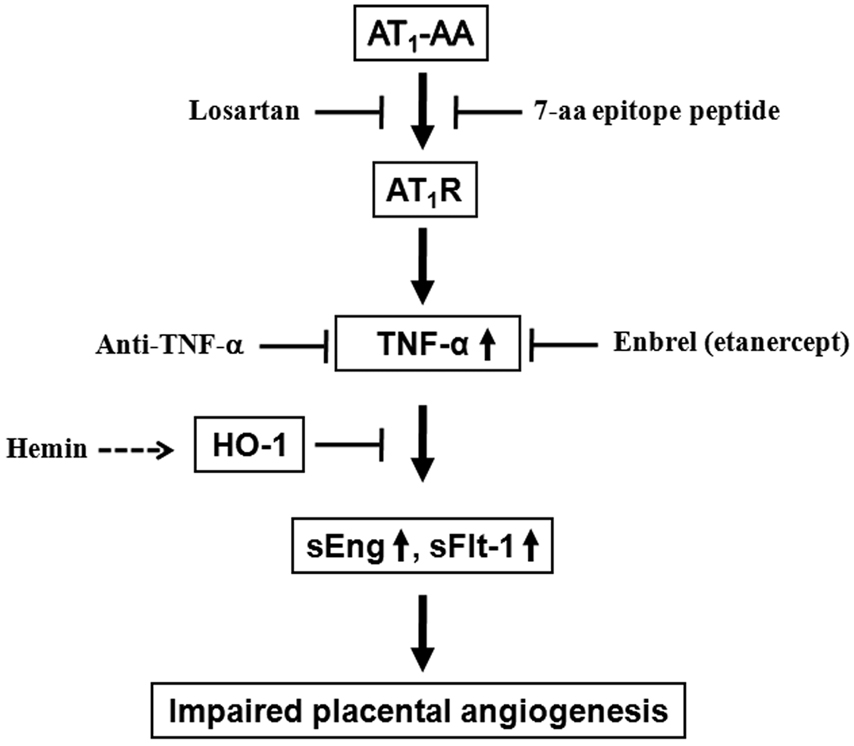
Figure 6. Working model of AT1-AA-mediated TNF-α induction in impaired placental angiogenesis in preeclampsia.
This diagram represents a possible signaling cascade by which the autoantibody-mediated TNF-α induction overcomes HO-1 (heme oxygenase 1), its negative regulator, and contributes to increased sEng and sFlt-1 secretion and subsequent impaired placental angiogenesis, a major feature in preeclampsia. This implies blockade of TNF-α and AT1 receptor or increasing HO-1 signaling may be potential therapeutic strategies in the management of this serious disorder of pregnancy for both mom and babies.
Although the molecular basis for sEng induction in PE is poorly understood , hypoxia-inducible factor-1(HIF-1), TGF-β and TNF-α are reported involved in regulating the endoglin gene expression and the release of sEng from the placenta.17, 18 We have shown here that IgG harbored by women with PE induce TNF-α production via AT1R activation which in turn stimulates sEng production and its release into the maternal circulation from placenta but not kidney. These results indicate that AT1-AA is the causative factor responsible for sEng induction via TNF-α signaling and the placenta is a major organ contributing to increased sEng secretion. Moreover, we provide the compelling evidence that hemin, a well-known inducer of heme oxygenase-1 (HO-1), attenuated AT1-AA-induced impaired human placental angiogenesis by blocking increased secretion of both sFlt1 and sEng from human placenta villous explants. Thus, our studies provide the first evidence that HO-1 is a key intracellular molecule to control AT1-AA-induced dysregualted placental angiogenesis by inhibiting TNF-a-mediated sFlt-1 and sEng induction. HO-1 is an inducible, endoplamic reticulum bound enzyme that catalyzes the nicotinamide adenosine dinucleotide phosphate-cytochrome P450 reductase-dependent oxidation of heme to biliverdin in a 3-step process that liberates carbon monoxide (CO) and Fe2+. Thus, our current findings are strongly supported by the earlier studies showing that HO-1 activity is protective against oxidant injury and inflammatory responses.19 More importantly, it is known that women with PE have significantly decreased CO concentrations in their exhaled breath associated with a decreased expression of HO activity in their placentas.20 Overall, we have provided both mouse and human evidence that AT1-AA-mediated TNF-α induction contributes to sEng and sFlt-1 induction and subsequent impaired placenta angiogenesis by overcoming HO-1 signaling.
TNF-α is a potent pro-inflammatory cytokine found at increased levels in the plasma of preeclamptic women.21–23 Notably, recent studies demonstrate that there are significant increases of soluble TNF-α receptors in the plasma of preeclamtpic patients.24, 25 Although soluble TNF-α receptor is likely to bind with circulating TNF-α and decreases its availability as a ligand, we have provided both human and mouse evidence that AT1-AA-mediated TNF-α induction is responsible for both sEng and sFlt-1 induction and contributes to decreased placental angiogenesis, suggesting that increased TNF-α may contribute to pathogenesis of PE. These implication is strongly supported by recent studies demonstrating that TNFα is an important factor contributing to pathology seen an experimental model of preeclampsia in rats based on reduced uterine perfusion pressure.26, 27 More importantly, chronic perfusion of TNF-α at a similar level as those seen in the plasma of the preeclamptic women into pregnant rats leads to hypertension and proteinuria.26 Although our studies present here and others21, 22, 26 provide a strong evidence of pathoglogical role of TNF-α in PE, the relative importance of increased soluble TNF-α receptors in PE remain to be determined.
While there is general agreement that plasma levels of TNF-α are higher in women with PE 28–30, conflicting reports have appeared regarding the placental contribution to increased levels of TNF-α in women with preeclampsia.16, 31 Two earliest reports28, 30 provided evidence for increased TNF-α in placentas from women with preeclampsia. Two subsequent reports 16, 31 did not find any significant difference in TNF-α levels between placentas from women with preeclampsia compared to placentas from normotensive pregnant women. Because TNF-α is synthesized and released from the placenta the amount of TNF-α remaining in placental tissue may not accurately reflect the amount of TNF-α produced and released by the placenta. The abundance of TNF-α mRNA will more likely reflect the potential for TNF-α protein production. Data presented here show that autoantibody mediated induction of sEng only occurs in pregnant animals and that TNF-α is a critical signaling intermediate. We see a significant increase in TNF-α mRNA in the placentas of pregnant mice injected with IgG from women with preeclampsia. The increase is prevented by co-injection with losartan or the 7-aa epitope peptide, indicating that the increase is due to AT1-AA mediated AT1 receptor activation. Thus, in our AT1-AA induced model of preeclampsia in mice, and the data from human placenta villous explants, suggest that the placenta is a major contributor to the resulting increase in circulating TNF-α.
In conclusion, the work reported here is the first to link AT1-AA with the inflammatory system to regulate placental-derived factors in both human and murine pregnancy and suggest that TNF-α is likely an important mediator of autoantibody-induced pathophysiology associated with PE resulting from increased production of sEng and sFlt1. Therefore the use of TNF-α neutralizing antibodies, soluble forms of TNF-α receptors or inducers of Ho-1 to blunt the effects of elevated TNF-α may be useful in the treatment of PE.
CLINICAL PERSPECTIVE
Preeclampsia (PE) is a life-threatening hypertensive complication of pregnancy that is a leading cause of maternal and neonatal mortality and morbidity in the United States and the world. It is highly prevalent and affects ~7% of first pregnancies. Available strategies used to manage PE are poor and currently limited to the delivery of the baby and placenta, secondary to the lack of fundamental understanding of the etiology and pathophysiology of the disorder. PE accounts for over 80,000 premature births each year in the US (approximately 15% of total premature births) and over $4 billion in medical cost. Recent studies demonstrate that two antiangiogenic factors, sFlt-1 and sEng, are elevated in the sera of preeclamptic women and contribute to pathophysiology of PE. However, the causative factors and molecular mechanisms responsible for their induction remain unknown. In this study, we have identified that a circulating maternal autoantibody, the angiotensin receptor agonistic autoantibody (AT1-AA), recently emerged as a prominent component in the pathogenesis of the disease, stimulates both sFlt-1 and sEng production via AT1 receptor activation and subsequent impaired placental angiogenesis in both pregnant mice and human villous explants. We also discovered that AT1-AA-mediated TNF-α induction, by overcoming its negative regulator, heme oxygenase-1 (HO-1), is a key underlying mechanism responsible for impaired placenta angiogenesis by inducing both sEng and sFlt-1 secretion from human villous explants. Thus, these studies are the first to link AT1-AA with the inflammatory system to regulate placental derived factors and provide important new targets for diagnosis and therapeutic intervention in the management of PE.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by National Institute of Health Grants HL076558, HD34130, grants from the March of Dimes Foundation and the Texas Higher Education Coordinating Board.
Abbreviations
- sFlt1
soluble fms-like tyrosine kinase-1
- Ang II
angiotensin II
- NT
normotensive
- PE
preeclampsia
- TNF-α
tumor necrosis factor-α
- AT1R
AT1 receptor
Footnotes
Disclosures
None.
References
- 1.Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–56. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 2.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science (New York, N.Y. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 3.Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, Juepner A, Gulba DC, Mackman N, Haller H, Luft FC. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101:2382–2387. doi: 10.1161/01.cir.101.20.2382. [DOI] [PubMed] [Google Scholar]
- 4.Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 5.Xia Y, Wen HY, Bobst S, Day MC, Kellems RE. Maternal autoantibodies from Preeclampsia patients Activate Angiotensin Receptors on Human Trophoblast Cells. J. Soc. Gyenocologic Investigation. 2003;10:82–93. doi: 10.1016/s1071-5576(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhou CC, Ahmad S, Mi T, Abbasi S, Xia L, Day MC, Ramin SM, Ahmed A, Kellems RE, Xia Y. Autoantibody from women with preeclampsia induces soluble Fms-like tyrosine kinase-1 production via angiotensin type 1 receptor and calcineurin/nuclear factor of activated T-cells signaling. Hypertension. 2008;51:1010–1019. doi: 10.1161/HYPERTENSIONAHA.107.097790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thway TM, Shlykov SG, Day MC, Sanborn BM, Gilstrap LC, 3rd, Xia Y, Kellems RE. Antibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activation. Circulation. 2004;110:1612–1619. doi: 10.1161/01.CIR.0000142855.68398.3A. [DOI] [PubMed] [Google Scholar]
- 8.Bobst SM, Day MC, Gilstrap LC, 3rd, Xia Y, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human mesangial cells and induce interleukin-6 and plasminogen activator inhibitor-1 secretion. American Journal of Hypertension. 2005;18:330–336. doi: 10.1016/j.amjhyper.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nature medicine. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 11.Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. The Journal of biological chemistry. 1992;267:19027–19030. [PubMed] [Google Scholar]
- 12.Gougos A, St Jacques S, Greaves A, O'Connell PJ, d'Apice AJ, Buhring HJ, Bernabeu C, van Mourik JA, Letarte M. Identification of distinct epitopes of endoglin, an RGD-containing glycoprotein of endothelial cells, leukemic cells, and syncytiotrophoblasts. International immunology. 1992;4:83–92. doi: 10.1093/intimm/4.1.83. [DOI] [PubMed] [Google Scholar]
- 13.St-Jacques S, Forte M, Lye SJ, Letarte M. Localization of endoglin, a transforming growth factor-beta binding protein, and of CD44 and integrins in placenta during the first trimester of pregnancy. Biology of reproduction. 1994;51:405–413. doi: 10.1095/biolreprod51.3.405. [DOI] [PubMed] [Google Scholar]
- 14.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nature medicine. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 15.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–249. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 16.Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. The Journal of clinical endocrinology and metabolism. 2001;86:2505–2512. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Elsner T, Botella LM, Velasco B, Langa C, Bernabeu C. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-beta pathways. The Journal of biological chemistry. 2002;277:43799–43808. doi: 10.1074/jbc.M207160200. [DOI] [PubMed] [Google Scholar]
- 18.Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, Devey LR, Wigmore SJ, Abbas A, Hewett PW, Ahmed A. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed A, Rahman M, Zhang X, Acevedo CH, Nijjar S, Rushton I, Bussolati B, St John J. Induction of placental heme oxygenase-1 is protective against TNFalpha-induced cytotoxicity and promotes vessel relaxation. Molecular medicine (Cambridge, Mass.) 2000;6:391–409. [PMC free article] [PubMed] [Google Scholar]
- 20.Hendler I, Baum M, Kreiser D, Schiff E, Druzin M, Stevenson DK, Seidman DS. End-tidal breath carbon monoxide measurements are lower in pregnant women with uterine contractions. J Perinatol. 2004;24:275–278. doi: 10.1038/sj.jp.7211094. [DOI] [PubMed] [Google Scholar]
- 21.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40:102–111. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 22.Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with preeclampsia. British journal of obstetrics and gynaecology. 1995;102:20–25. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 23.Visser W, Beckmann I, Bremer HA, Lim HL, Wallenburg HC. Bioactive tumour necrosis factor alpha in pre-eclamptic patients with and without the HELLP syndrome. British journal of obstetrics and gynaecology. 1994;101:1081–1082. doi: 10.1111/j.1471-0528.1994.tb13587.x. [DOI] [PubMed] [Google Scholar]
- 24.Sibai B, Romero R, Klebanoff MA, Rice MM, Caritis S, Lindheimer MD, Van Dorsten JP, Landon M, Miodovnik M, Dombrowski M, Meis P. Maternal plasma concentrations of the soluble tumor necrosis factor receptor 2 are increased prior to the diagnosis of preeclampsia. American journal of obstetrics and gynecology. 2009;200:630, e631–e638. doi: 10.1016/j.ajog.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schipper EJ, Bolte AC, Schalkwijk CG, Van Geijn HP, Dekker GA. TNF-receptor levels in preeclampsia--results of a longitudinal study in high-risk women. J Matern Fetal Neonatal Med. 2005;18:283–287. doi: 10.1080/14767050500246466. [DOI] [PubMed] [Google Scholar]
- 26.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005;46:1022–1025. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 27.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008;52:1161–1167. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Walsh SW. TNF alpha concentrations and mRNA expression are increased in preeclamptic placentas. Journal of reproductive immunology. 1996;32:157–169. doi: 10.1016/s0165-0378(96)00998-9. [DOI] [PubMed] [Google Scholar]
- 29.Haider S, Knofler M. Human tumour necrosis factor: physiological and pathological roles in placenta and endometrium. Placenta. 2009;30:111–123. doi: 10.1016/j.placenta.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinehart BK, Terrone DA, Lagoo-Deenadayalan S, Barber WH, Hale EA, Martin JN, Jr, Bennett WA. Expression of the placental cytokines tumor necrosis factor alpha, interleukin 1beta, and interleukin 10 is increased in preeclampsia. American journal of obstetrics and gynecology. 1999;181:915–920. doi: 10.1016/s0002-9378(99)70325-x. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi M, Ueda Y, Yamaguchi T, Sohma R, Shibazaki M, Ohkura T, Inaba N. Tumor necrosis factor-alpha in the placenta is not elevated in pre-eclamptic patients despite its elevation in peripheral blood. Am J Reprod Immunol. 2005;53:113–119. doi: 10.1111/j.1600-0897.2005.00253.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.