Abstract
Preeclampsia, a syndrome affecting 5% of pregnancies characterized by hypertension and proteinuria, is a leading cause of maternal and fetal morbidity and mortality. The condition is often accompanied by the presence of a circulating maternal autoantibody, the angiotensin II type I receptor agonistic autoantibody (AT1-AA). However, the prevalence of AT1-AA in preeclampsia remains unknown and the correlation of AT1-AA titers to the severity of the disease remains undetermined. We used a sensitive and high throughput luciferase bioassay to detect AT1-AA levels in the serum of 30 normal, 37 preeclamptic (10 mild and 27 severe) and 23 gestational hypertensive (GH) individuals. Here we report that AT1-AA is highly prevalent in preeclampsia (~95%). Next, by comparing the levels of AT1-AA among women with mild and severe preeclampsia, we found that the titer of AT1-AA is proportional to the severity of the disease. Intriguingly, among severe preeclamptic patients, we discovered that the titer of AT1-AA is significantly correlated with the clinical features of preeclampsia: systolic blood pressure (r=0.56), proteinuria (r=0.70) and sFlt-1 level (r=0.71), respectively. Notably, only AT1-AA but not sFlt-1 levels are elevated in GH patients. These data serve as compelling clinical evidence that AT1-AA is highly prevalent in preeclampsia and its titer is strongly correlated to the severity of the disease.
Keywords: preeclampsia, gestational hypertension, angiotensin receptor autoantibodies, sFlt-1, proteinuria
INTRODUCTION
Preeclampsia is a serious hypertensive disorder of pregnancy that affects approximately 5% of pregnancies and remains a leading cause of maternal and neonatal mortality and morbidity in the United States and the world.1–3 The disease is multifactorial and includes such clinical features as high blood pressure, proteinuria, inflammation, endothelial dysfunction, vasoconstriction and placental abnormalities.4–7 The clinical symptoms in the advanced stages of preeclampsia, include cerebral hemorrhage, renal failure and the HELLP syndrome. In serious cases termination of pregnancy is the only available option to prevent further deterioration of the fetus and mother. Despite being a leading cause of maternal death and a major contributor to maternal and perinatal morbidity, the triggering factors and underlying mechanisms responsible for the pathogenesis of preeclampsia remain elusive.
Numerous studies have shown that women with preeclampsia possess angiotensin receptor agonistic autoantibodies (AT1-AAs) that bind to and activate the AT1 angiotensin receptor in multiple cellular systems and provoke biological responses that are relevant to the pathophysiology of preeclampsia.8–13 For example, AT1-AAs increase the contraction rate of rat cardiomyocytes, elevate levels of the anti-angiogenic factor soluble fms-like tyrosine kinase-1 (sFlt-1) leading to decreased angiogenesis in endothelial cells, increased plasminogen activator inhibitor-1 (PAI-1) production resulting in decreased trophoblast invasion and increased NADPH oxidase production in trophoblast cells resulting in oxidative stress.14–17 However, these studies were restricted to the use of in vitro cultured cell systems and therefore did not directly address the relevance of AT1-AAs to hypertension and proteinuria, the defining features of preeclampsia. However, recent experiments have demonstrated that the injection of pregnant mice with AT1-AAs recapitulates the key features of preeclampsia, including hypertension, proteinuria, renal and placental morphologic changes and an increase in the concentration of anti-angiogenic factor sFlt-1.18 Thus, these in vivo studies provide the first direct evidence for a pathophysiological role of AT1-AA in preeclampsia and suggest that these autoantibodies contribute to the pathogenesis of preeclampsia. However, the prevalence of AT1-AA in preeclampsia remains unknown and the correlation of AT1-AA to the severity of the disease remains undetermined due to the lack of a sensitive and convenient assay to accurately measure AT1-AA in human sera.
In this study, because of our newly developed sensitive and high throughput luciferase bioassay, we were able to address two important clinical questions: 1) What percentage of women with preeclampsia contain AT1-AA, and, 2) Does the titer of AT1-AA correlate to the severity of disease? Using this bioassay, we have provided the first compelling patient evidence that AT1-AA is highly prevalent in preeclampsia and its titer strongly correlates to the severity of the disease. These findings add support to the novel concept that preeclampsia is an autoimmune disease associated with AT1-AA.13 We believe these initial clinical studies coupled with our bioassay have provided a strong foundation for us to perform a large scale clinical studies in the future.
METHODS
Materials
Tissue culture medium (RPMI 1640), fetal bovine serum (FBS), and antibiotics such as penicillin-streptomycin (100×), and geneticin (G418, 50 mg/ml) were purchased from Invitrogen Life Technologies (Carlsbad, CA). Human Angiotensin II was obtained from Sigma (St. Louis, MO). Losartan (COZAAR) was a gift from Merck Research Laboratory (Rahway, NJ). The seven amino acid peptide (7aaAFHYESQ), is an epitope sequence present on the second extracellular loop of the AT1 receptor that is recognized by AT1-AA. These peptides were synthesized by the Protein Chemistry Core Laboratory, Baylor College of Medicine (Houston, TX). Protein G Sepharose 4 Fast Flow, used for IgG isolation was purchased from Amersham Pharmacia Biotech (Piscataway, NJ). PathDetect NFAT cis-Reporting system and synthetic Renilla luciferase reporter vector were purchased from Stratagene (La Jolla, CA) and PromegaCorp. (Madison, WI) respectively.
Patients
Patients who were admitted to Memorial Hermann Hospital were identified by the obstetrics faculty of the University of Texas Medical School at Houston. Twenty seven patients were diagnosed with severe preeclampsia based on the definition set by the National High Blood Pressure Education Program Working Group report.19 The criteria include the presence of high blood pressure of ≥ 160/110 mmHg and urinary protein of 300 mg in a 24 hr period or a dipstick value of 1+ or greater. These women had no previous history of hypertension. Other criteria included the presence of persistent headache, visual disturbances, epigastric pain, or the HELLP syndrome in women with blood pressure of ≥ 140/90 mmHg. For patients with mild preeclampsia the blood pressure criteria were ≥140/90 mmHg and urinary protein of 300 mg/24 hr or a dipstick value of 1+ or greater. Patients with a blood pressure of ≥140/90 mmHg appearing after 20 wks gestation and having less than 300 mg urinary protein per 24 hr period were classified as having gestational hypertension. Blood samples collected from the patients were were allowed to clot and then centrifuged at 20,000 ×g for 20 min and the serum samples were stored at −80° C. Patients were generally approached for the study during the prepartum or early intrapartum period. Patient enrollment occurred from May 2007 to April 2009. The research protocol, including the consent form, was approved by the institutional Committee for the Protection of Human Subjects. The general clinical features of the patients involved in the study are shown in Table 1.
Table 1.
Clinical features of patients from various groups in the present study.
| Patient Group | NT | Gestational Hypertension |
Mild PE | Severe PE |
|---|---|---|---|---|
| Age | 28±2 | 28±2 | 25±2 | 28±2 |
| Systolic BP (mm Hg) | 120±2 | 153±4 | 145±2 | 168±3 |
| Diastolic BP (mm Hg) | 73±2 | 88±3 | 91±4 | 98±2 |
| Urin. Protein(mg/24 hrs) | 25±12 | 71±20 | 363±37 | 1201±250 |
| Ser. Creatinine(mg/dl) | 0.66±0.05 | 0.63±0.02 | 0.7±0.02 | 0.69±0.02 |
| sFlt-1 (ng/ml) | 5±1 | 7±1 | 11±2 | 20±2 |
| Weeks Gest. Age (WGA) | 38±0.5 | 36±1 | 35±1 | 32±1* |
For early onset preeclampsia (delivery < 32 weeks), WGA = 26±2, n=10
For late preeclampasia, (delivery at > 32 weeks), WGA = 36±0.5, n=17
Cell Culture
Chinese hamster ovary cells stably transfected with rat angiotensin II receptor type 1A (CHO.AT1A) were kindly provided by Dr. Terry S. Elton (Ohio State University, Coulmbus, OH). Cells were maintained at 37 °C and 5% CO2 and cultured in RPMI 1640 medium containing 5 % FBS, 1 % antibiotics, 8.75 g/liter L-proline and 100 µg/ml gentamycin. The CHO.AT1.luc cells were isolated by introducing the 4 × NFAT luciferase construct bearing a hygromycin phosphotransferase gene. Stable transformants were isolated in the cell culture media described above including hygromycin (100µg/ml).
Preparation of the immunoglobulin G fraction
The IgG fraction was isolated by the batch purification method using Protein Sepharose G 4 Fast flow as described previously.17 The purity of the isolated IgGs was ascertained using gel electrophoresis. The presence of two bands at ~ 50 KDa and ~25KDa indicated the presence of the heavy and light chains of the IgG.
Transient Transfection assay
CHO.AT1A cells were plated at a density of 1 × 105 cells in 24- well plates for 2 hr. Cells were transfected using 500 ng of the NFAT-luciferase reporter construct containing 4 copies of the NFAT binding element (PathDetect NFAT cis-Reporting system), 20 ng of phRTK, a synthetic Renilla luciferase reporter construct (for internal control) and 5 µl of Lipofectamine Reagent (Invitrogen Life Technologies, Carlsbad, CA) for 5hr. The cells were serum starved for 24 hr and treated with Ang II overnight where indicated. Similar experiments were carried out using the 2x-EGR-luciferase reporter construct. The treated cells were lysed in 100 µl of passive lysis buffer (Promega Inc.) at room temperature for 45 min. Luciferase activity (measured in relative light units) was measured using 10 µl of lysate with Dual Luciferase system (Promega Inc).
Luciferase Activity
CHO.AT1A (1×105 cells) containing stably integrated copies of a minigene encoding the rat AT1 receptor and a 4xNFAT-driven luciferase construct were plated on 24-well plates overnight. The next day cells were changed to serum-free medium and treated with IgG (1:10 dilution) for 24 hours. Luciferase activity in cell lysates was measured using a luciferase assay kit (Promega). To test the reproducibility of our bioassay, we carried out the assay mulitple times with different IgG isolations obtained from the same patient and also carried out the assay with the same IgG sample multiple times. We obtained very reproducible activation levels with the IgGs obtained from normotensive pregnant women and women with severe preeclampsia. In general we observed no more than a ± 10% variation when assaying multiple IgG samples from the same patient.
sFlt-1 determination
Commercially available ELISA kits (R&D Systems, CA) was used according to the manufacturer’s recommendations to determine the maternal serum sFlt-1 concentrations.
Data calculation
All data were calculated as a percent change (increase/decrease) of Luciferase activity measured in terms of Relative Light Units (RLU) as determined by monolight luminometer (Pharmingen) of (over) basal. The average luciferase activity (RLU) obtained for basal was 250±50.
Statistical Analysis
Results are expressed as mean ± SEM. All of the data were subjected to statistical analyses using GraphPad Prism 5 (San Diego, CA). One-way ANOVA and Student t tests were performed to determine the significance of differences between different groups. Data were also subjected to correlation analysis using the same software to determine Spearman ‘r’ values. Statistical significance was set at P<0.05.
RESULTS
Construction of a cell line that reports the activation of AT1 receptors as increased luciferase activity
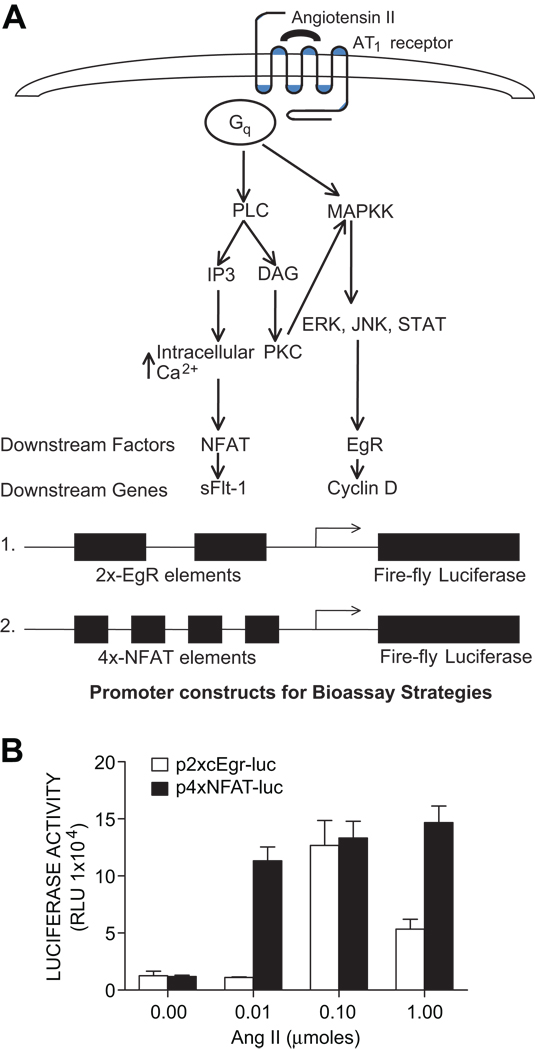
In view of known signaling events downstream of AT1 receptor activation (Fig. 1A) we chose two luciferase reporter constructs for potential use in monitoring AT1 receptor activation. One reporter construct, termed 2X-Egr-luciferase, contains two copies of a consensus Egr (early growth response factor) response element followed by a cytomegalovirus (CMV) promoterdriven firefly luciferase reporter gene. The other construct, termed 4X-NFAT-luciferase, is a CMV promoter-driven luciferase reporter plasmid under the control of four nuclear factor of activated T cells (NFAT) cis-regulatory elements. These DNA constructs were transiently transfected into CHO.AT1 cells that were incubated with a range of Ang II concentrations (10–1000 nM). After 24 hr the cells were lysed and luciferase activity determined in cell extracts. The results (Fig. 1B) show a dose-dependent increase in luciferase activity with both luciferase reporter genes following treatment with Ang II. However, the NFAT-luciferase construct was maximally activated over a broader range of Ang II concentrations and for this reason it was chosen for use in subsequent experiments.
Figure 1. Signaling pathways downstream of AT1 receptor activation.
A. Various downstream molecules involved in the AT1 receptor signaling, PLC: Phospholipase C, MAPKK: Mitogen activated protein kinase kinase, IP3: Inositol (1,4,5) P3, DAG: Diacyl glycerol, NFAT: Nuclear Factor of Activated T cells, EgR: immediate early growth response factor. Reporter constructs used to detect AT1 receptor activation. B. Construction of a cell line that reports the activation of AT1 receptors with increased luciferase activity Ang II regulation of 2XcEgR and 4XNFAT luciferase reporter constructs in AT1 receptor expressing CHO cells. CHO.AT1A cells were plated in either 12-wells or 24-wells plates and transiently transfected with 2XcEgR and 4XNFAT reporter constructs along with synthetic Renilla luciferase reporter (internal control) using Fugene 6 transfection reagent (Roche diagnostics, IN) for 6 hrs. Cells were serum starved for 24 hrs, treated with different concentrations of Ang II O/N, harvested and measured for relative luciferase activityusing a luminometer. Graph points denote the Relative Light Units (RLU).
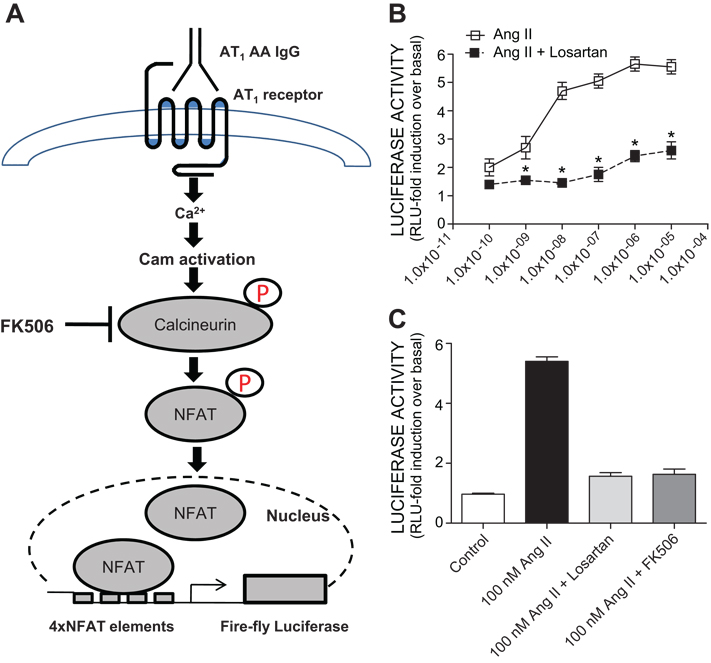
To convert the CHO.AT1 cell line to one that easily reports the activation of AT1 receptors we stably introduced 4X-NFAT-luciferase expression plasmids using co-transfection with a selectable marker. A schematic illustration of the use of the genetically engineered cells to detect AT1 receptor activation by measuring luciferase activity is shown in Fig. 2A. Stable transformants (termed CHO.AT1.luc) were isolated, expanded and tested for the ability to synthesize increased amounts of luciferase in response to increasing concentrations of Ang II. The results (Fig. 2B) show that luciferase activity increased over a concentration range of 0.1 nM to 10 µM, reaching a maximum of approximately 5-fold over the basal (non-treated cells) at 100 nM. The increased luciferase synthesis was completely blocked by the presence of 1 µM Losartan, an AT1 receptor specific antagonist, and by FK506, an inhibitor of Ca2+/calmodulin-dependent phosphatase 2C (calcineurin) (Fig. 2C). These results show that the Ang II-induced stimulation of luciferase activity in CHO.AT1.luc cells was mediated through AT1 receptor activation and downstream signaling through the calcineurin/NFAT pathway.
Figure 2. Construction of a cell line that reports the activation of AT1 receptors with increased luciferase activity.
A. Schematic illustration of genetically engineered cell line, CHO.AT1.luc (4XNFAT-luciferase) used in our bioassay to detect AT1 receptor activation by measurement of luciferase activity. B. Concentration response curve of Angiotensin II with the CHO.AT1A cells. Ang II showed a concentration dependent luciferase activation which was blocked significantly by Losartan (1 µM), an AT1 receptor antagonist. C. Angiotensin II induced luciferase activation is mediated via AT1 receptor activation and calcineurin/NFAT signaling as evidenced by the attenuation of the Ang II mediated luciferase activation in the presence of Ang II type 1 receptor antagonist, Losartan and Ca2+/calmodulin-dependent phosphatase 2B (calcineurin), inhibitor, FK506.
The use of CHO.AT1.luc cells to measure AT1-AA activity
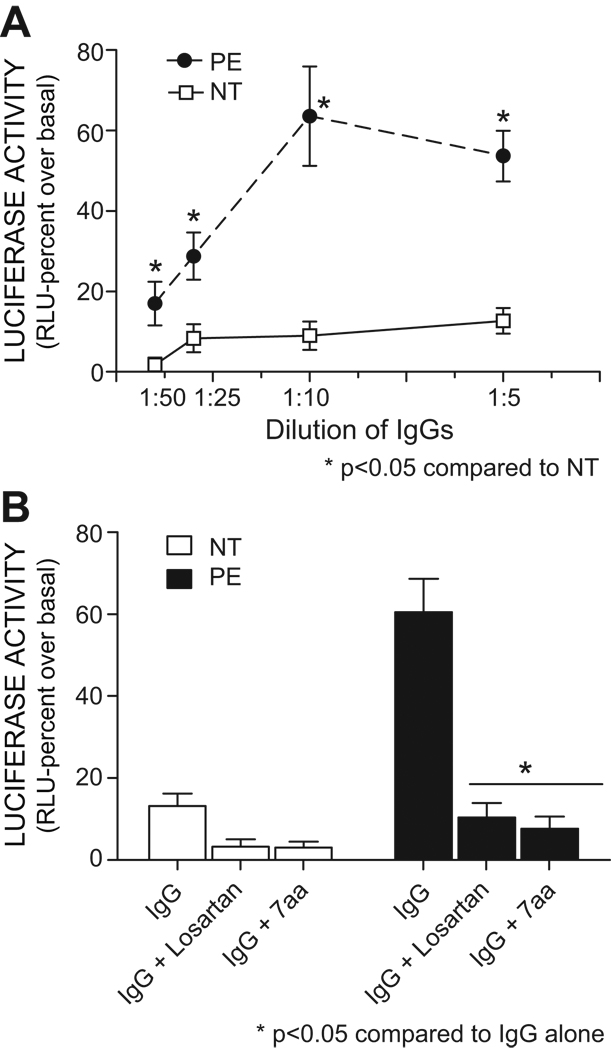
To determine whether autoantibodies from women with preeclampsia are able to activate AT1 receptors on CHO.AT1.luc cells and stimulate luciferase activity, we treated these cells with a ten-fold concentration range (1:50 to 1:5) of IgG from women with severe preeclampsia (PE) and from normotensive pregnant women. After 24 hr cells were lysed and extracts assayed for luciferase activity. The results (Fig. 3A) show a concentration dependent increase in luciferase activity when using IgG from women with preeclampsia that was much greater that that observed with IgG from normotensive pregnant women. Maximal stimulation was achieved at a 1:10 antibody dilution where the luciferase activity expressed as a percent increase over basal was found to be 9±3 for normotensive vs 64±13 for the severe preeclampsia samples. The antibody-mediated stimulation of luciferase activity was blocked by the presence of Losartan. These results indicate that increased luciferase activity resulted from antibody-mediated AT1 receptor activation. Overall, the results indicate that the synthesis of luciferase by CHO.AT1.luc cells served as a bioassay to detect AT1-AAs present in the IgG of women with severe preeclampsia.
Figure 3. Measurement of AT1-AA activity by a luciferase assay in CHO.AT1.Luc cells.
A. Dose dependent response profile of IgGs (AT1-AAs) isolated from the sera of women with preeclampsia (PE) and women with normotensive (NT) pregnancies. n= 6–10 for each group. B. IgGs (AT1-AAs) induced increase in lucifease activation is significantly blocked by AT1 receptors antagonist, Losartan and 7AA (seven amino acid peptide corresponding to this common epitope which blocks the binding of AT1-AA to the AT1 receptor), n=8 for NT and PE.
A characteristic and defining feature of AT1-AAs is the interaction with a seven amino acid (7aa) peptide epitope present on the second extracellular loop of the AT1 receptor. The presence of the 7aa epitope peptide in the culture media prevents the binding of AT1-AAs to AT1 receptors. As a test for the specificity of the NFAT-luciferase bioassay we added the 7aa epitope peptide (0.25 µg/ml) to CHO.AT1.luc cells prior to the addition of IgG from women with severe preeclampsia or normotensive pregnant women. The presence of the 7 aa epitope peptide completely blocked the antibody mediated induction of luciferase activity, including the relatively small increase in luciferase activity observed for cells treated with IgG from normotensive pregnant women (Fig. 3B). These results suggest that the antibody mediated induction of luciferase activity is mediated through interaction with the common peptide epitope associated with the second extracellular loop of the AT1 receptor. Overall these results indicate that increased luciferase activity observed in IgG treated CHO.AT1.luc cells is a measure of AT1-AA-mediated AT1 receptor activation.
Prevalence and abundance of AT1-AA in women with hypertensive disorders of pregnancy
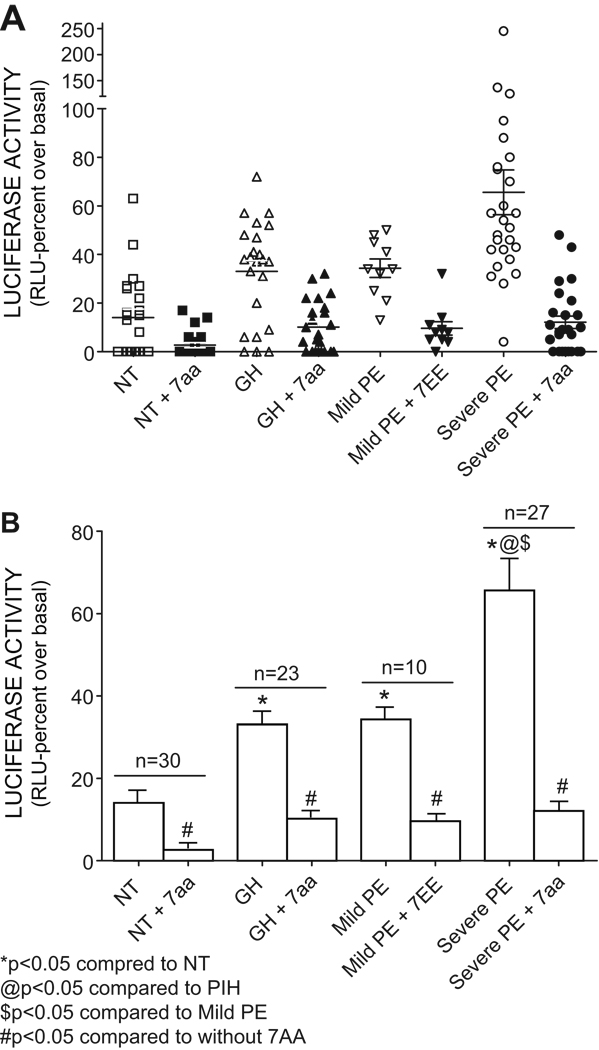
The CHO.AT1.luc cells were used to determine the prevalence and abundance of AT1-AA in normotensive pregnant women, women with gestational hypertension and women with preeclampsia (mild and severe). Luciferase activity (in relative light units, RLU) was expressed as a percent increase over basal activity. The results (Fig. 4A) show that the highest stimulation and the broadest range of activities was achieved with IgG isolated from women with severe preeclampsia. The broad range of NFAT-luciferase activation observed for this group was also associated with a broad spectrum of clinical features of preeclampsia. It is of note that 10 patients in this category fell into the severe early onset category of preeclampsia with delivery before 32 weeks. The level of AT1-AA was not different between the early and late onset cases of severe preeclampsia. The stimulation of luciferase activity by IgG from the severe preeclampsia group was inhibited by the 7aa epitope peptide indicating that it resulted from AT1-AA mediated AT1 receptor activation. In this group 25 of the 26 samples tested showed significant stimulation of luciferase activity with an average stimulation of 66±9 % that was nearly fivefold greater than that observed with IgG from normotensive pregnant women.
Figure 4. Prevalence and abundance of AT1-AA in women with hypertensive disorders of pregnancy.
A. Activation levels of IgGs (AT1-AAs), expressed as luciferase activity, obtained from individual serum samples from various groups of patients. The AT1-AAs induced luciferase activation is significantly blocked in the presence of 7AA that blocks blocks the binding of AT1-AA to the AT1 receptor. Data is calculated according to the percent change (increase) in luciferase synthesis, determined as Relative Light Units (RLU) compared to the basal (no treatment). n=30 for NT, 23 for gestational hypertension (GH), 10 for Mild PE and 27 for Severe PE. B. Average (Mean±SEM) activation of luciferase activity induced by the IgGs (AT1-AAs) as determined by the luciferase activity, from various group of patients. The luciferase activation is significantly blocked by the 7aa that blocks the binding of AT1-AA to the AT1 receptor, thereby also establishing the increase in luciferase synthesis is indeed caused by the AT1-AAs * P <0.05, significantly different compared to NT, @ P < 0.05 significantly different compared to GH, $ P <0.05 significantly different compared to Mild PE, # P < 0.05 significantly different compared to the absence of 7aa. Data analyzed by student’s t-test.
A significant increase in luciferase activity was also observed with IgG from women with mild preeclampsia, although not as high as the severe preeclampsia group. The range of activities was more narrow for the mild preeclampsia group than that that observed for the severe preeclampsia group. All ten of the mild preeclampsia samples showed significant stimulation, with an average value of 35±4 % (Fig. 4B). The stimulation of luciferase activity was blocked by the 7aa epitope peptide indicating that this was due to AT1-AA mediated AT1 receptor activation.
IgG isolated from the normotensive pregnant women showed the lowest range of activity analyzed with an average stimulation of only 14±3 % over basal, a value that was significantly less than that of all other groups. It is noteworthy that approximately half of the normotensive samples had no detectable activity in this assay. The low level of activity displayed by most of the normotensive pregnancy samples was inhibited by the presence of the 7aa epitope peptide, indicating that the low level of activity observed in these samples was likely the result of low titers of AT1-AA.
We draw several conclusions from the data presented in Figure 4. 1) Greater than 95% of women with preeclampsia (10/10 of those with mild preeclampsia and 26/27 with severe preeclampsia) harbor AT1-AAs. 2) Normotensive pregnant women harbor low or undetectable levels of AT1-AA and the overall average antibody levels in these women are approximately 5 fold less than that in women with severe preeclampsia. 3) The average level of AT1-AA activity for each of the three groups of women examined shows a relationship to the clinical severity of the disease.
AT1-AA activity significantly correlates to blood pressure, proteinuria and sFlt-1 in severe preeclampsia
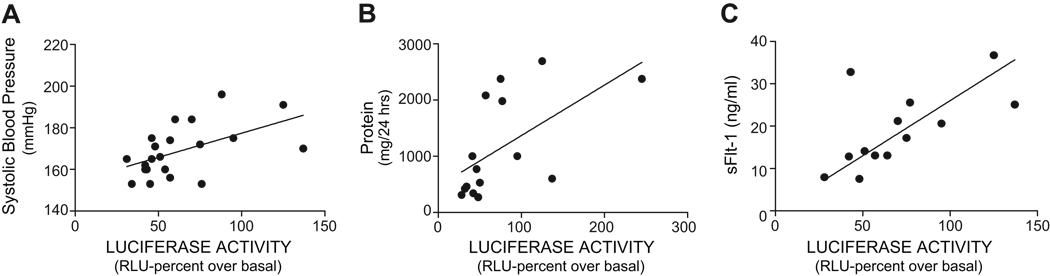
The wide distribution of AT1-AA levels among the women with severe preeclampsia (Fig. 4) was associated with a wide range in the severity of clinical features of the disease. For this reason this group of patients provided a favorable opportunity to examine the relationships between the AT1-AA activity, blood pressure, urinary protein and sFlt-1 levels. This was accomplished by plotting AT1-AA against blood pressure, proteinuria and sFlt-1 for individual patients in the severe preeclampsia group. The results (Fig. 5A) show that the concentration of AT1-AAs in the serum of these women shows a strong positive correlation with systolic blood pressure (r=0.56, n=21, p<0.05). The correlation analysis between the AT1-AA concentration and urinary protein is illustrated in Fig 5B (r=0.70, n=15, p<0.05) and in Fig 5C we show the positive correlation between the plasma sFlt-1 levels with the concentration of AT1-AAs in women with severe preeclampsia (r= 0.71, n=16, p<0.05). Thus, among women with severe preeclampsia there is a strong positive correlation between the abundance of AT1-AA and blood pressure, urinary protein and sFlt-1 levels.
Figure 5. Positive correlation between AT1-AA, blood pressure, urinary protein and sFlt-11 in serve preeclampsia.
A Correlation analysis between AT1-AA activity and systolic blood pressure (r=0.51; P<0.05). B The positive correlation betweeen AT1-AA activity and urinary protein (r=−, P<0.05). C. The relation between AT1-AA and serum sFlt-1 (r=0.71, p<0.05)
AT1-AA is significantly increased in women with gestational hypertension
We also examined IgG from women with gestational hypertension for the presence of AT1-AA. These women are characterized by hypertension appearing after 20 weeks gestation and the absence of proteinuria. The results (Fig. 4A) show that IgG obtained from women with gestational hypertension showed an average stimulation of 33±4% (Fig. 4B) in the NFAT-luciferase bioassay. The activation of luciferase was inhibited by the presence of the 7aa epitope peptide, indicating that the luciferase activation resulted from AT1-AA-mediated AT1 receptor activation. The AT1-AA activity levels obtained with the IgG from the gestational hypertension and mild preeclampsia groups were quite similar (Fig. 4) and the degree of blockage obtained with the 7aa epitope peptide was also similar (10±2 vs 10±3, p<0.05 compared to respective activation without 7aa). These findings indicate that the abundance of AT1-AA is similar in the two hypertensive groups (gestational hypertension and mild preeclampsia) and likely contributes to hypertension by mimicking the vasoconstrictive actions of Ang II.
sFlt-1 levels are not significantly elevated in gestational hypertension
We also measured sFlt-1 concentrations in patients with mild preeclampsia, gestational hypertension and normotensive pregnant women. To our surprise, we found that sFlt-1 levels were not significantly elevated in the blood circulation of patients with gestational hypertension compared to those of normotensive controls. However, sFlt-1 levels were significantly increased in both mild and severe preeclamptic patients as summarized in Table 1. Thus, patients with gestational hypertension show a discordance between AT1-AA and sFlt-1 levels.
DISCUSSION
In this study, our newly developed sensitive and high throughput luciferase bioassay allowed us to address two important questions: 1) What percentage of women with preeclampsia have AT1-AA? 2) Does the titer of AT1-AA correlate to the severity of the disease? Our results show that 1) Greater than 95% of women with preeclampsia harbor significantly elevated levels of AT1-AAs; 2) The level of AT1-AA activity increases with the severity of the disease; 3) There is a strong correlation of AT1-AA activity to hypertension, proteinuria and sFlt-1 in severe preeclampsia; 4) Elevated levels of AT1-AA are present in women with gestational hypertension, lacking proteinuria. 5) Normotensive pregnant women harbor low or undetectable levels of AT1-AAs and the average antibody level in these women is approximately 5-fold less than that in women with severe preeclampsia In summary, our findings show that AT1-AA is highly prevalent in preeclampsia and that its titer strongly correlates to the severity of the disease.
We have recently extended multiple in vitro findings to in vivo studies showing that the introduction of AT1-AA from preeclamptic patients into pregnant mice results in key features of preeclampsia. These findings provide support for the hypothesis that AT1-AAs contribute to pathophysiology in preeclampsia.18 However, the prevalence of AT1-AAs in preeclampsia is largely undetermined due to lack of a sensitive bioassay to accurately measure autoantibody activity. In this study, because of successful establishment of a sensitive and convenient bioassay to quantify AT1-AA activity in patients, we are able to provide the first compelling evidence that AT1-AA is present in nearly all women diagnosed with preeclampsia (both mild and severe). These studies complement our recent animal studies showing that AT1-AA cause features of preeclampsia when injected into pregnant mice. More importantly, we also discovered that AT1-AA activity is significantly higher in patients with severe preeclampsia compared to those with mild preeclampsia. Notably, we found that there is a significant correlation of the titer of AT1-AA to hypertension, proteinuria and sFlt-1 levels in patients with severe preeclampsia. The significant correlation of AT1-AA activity with severity of the disease in humans5, 20, 21 is in good agreement with our mouse studies showing that AT1-AA induces preeclamptic-like features in a dosage-dependent way in pregnant mice.18 In addition, the correlation of AT1-AA to sFlt-1 levels seen in severe preeclampsia is also consistent with earlier reports that link sFlt-1 production with AT1 receptor activation.14, 22 Thus, the results of both human and animal studies show that the levels of AT1-AA increase with the severity of the disease.
In contrast to high prevalence of AT1-AA in preeclampsia, we found that normotenive patients were characterized by low to non-detectable levels of AT1-AA. The average AT1-AA activity in normotensive pregnant women was much lower than that of women with mild preeclampsia and severe preeclampsia. However, the low levels of AT1-AA activity in these samples presumably represent a low titer of AT1-AA because the activity was blocked by either losartan or the 7aa epitope peptide. These findings imply that among normotensive pregnant individuals, the titer of AT1-AA is not high enough or has not been present for sufficient duration to cause the clinical features seen in preeclampsia. Notably, two normotensive individuals contain a relatively high AT1-AA activity, similar to the average observed in patients with preeclampsia. One possible explanation is that AT1-AA may not have been present long enough to cause symptoms. Thus, it will be critical to perform a prospective clinical study to determine when AT1-AA occurs in both normal and preeclamptic patients.
Among 23 patients with gestational hypertension, we found that the average concentration of AT1-AA was significantly elevated and similar to that of mild preeclampsia. AT1-AA is likely to be a causative factor for the hypertension in women with gestational hypertension because AT1-AAs, as AT1 receptor agonists, are functional mimics of Ang II, a well-known hypertensive agent. However, it is puzzling that these patients only display hypertension and not proteinuira since AT1-AA is capable of inducing both hypertension and proteinuria in pregnant mice.18 The answer came unexpectedly when we found that sFlt-1 levels were not significantly elevated in patients with gestational hypertension compared to those of the normotensive pregnant controls. However, sFlt-1 was significantly elevated in women with mild preeclampsia and even higher in the severe preeclamptic group. Our findings are in agreement with those of others who have reported a strong correlation between preeclampsia and elevated sFlt-1 levels and a positive correlation between sFlt-1 levels and disease severity.5, 6, 20, 21, 23 The lack of proteinuria in the gestational hypertension group may be due to a low level of sFlt-1, a factor believed to contribute to proteinuria in preeclampsia. 5, 6, 20, 21 Because gestational hypertension may be a precursor to preeclampsia it is possible that the increase in AT1-AAs we observed for the gestational hypertension group had not been present long enough at sufficiently elevated concentrations to induce levels of sFlt-1 adequate to contribute to proteinuria. Thus, it is possible that in cases where a discordance between AT1-AA levels and sFlt-1 has been noted 21, 24 this may be due to an insufficient concentration or an inadequate time for the autoantibody to induce sFlt-1 levels. This possibility will be addressed in future experiments.
In summary, we have provided initial patient studies showing that AT1-AA is highly prevalent in preeclampsia and its titer increases with disease severity. This study adds additional support to the novel hypothesis that preeclampsia is an autoimmune disease in which AT1-AAs contribute to the pathophysiology of the disease.13
Clinical Perspective
Considerable evidence indicates that a circulating maternal autoantibody, AT1-AA is associated with preeclampsia and contributes to the pathogenesis of the disease. Here we report the use of a convenient and sensitive bioassay to show that these autoantibodies are present in nearly all women diagnosed with preeclampsia and that the titer of the autoantibodies increases with the severity of the disease. Overall, our experimental evidence supports the novel concept that preeclampsia is an autoimmune disease in which disease symptoms result from autoantibody-induced AT1 receptor activation. Our findings have significant prognostic, diagnostic and therapeutic implications with regard to the medical management of this devastating disease for both mom and fetus.
Acknowledgments
Source of Funding:
The present work was supported from funds by U.S. National Institutes of Health grants (HL076558 to YX and HD34130 to REK) and March of Dimes Grant 6-FY06-323 and the Texas Higher Education Coordinating Board (THECB).
Footnotes
Disclosures:
None
REFERENCES
- 1.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 2.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 3.Lindheimer MD, Umans JG. Explaining and predicting preeclampsia. N Engl J M. 2006;355:1056–1058. doi: 10.1056/NEJMe068161. [DOI] [PubMed] [Google Scholar]
- 4.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 5.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res. 2005;57:1R–7R. doi: 10.1203/01.PDR.0000159567.85157.B7. [DOI] [PubMed] [Google Scholar]
- 7.Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiol. 2009;24:147–158. doi: 10.1152/physiol.00043.2008. [DOI] [PubMed] [Google Scholar]
- 8.Xia Y, Wen HY, Bobst S, Day MC, Kellems RE. Maternal autoantibodies from Preeclampsia patients Activate Angiotensin Receptors on Human Trophoblast Cells. J Soc Gyen Invest. 2003;10:82–93. doi: 10.1016/s1071-5576(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 9.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dechend R, Llinas M, Caluwaerts S, Herse F, Lamarca B, Mueller DN, Luft FC, Pijnenborg R, Wallukat G, Granger JP. Agonistic autoantibodies to the AT1 receptor in rat models of preeclampsia: induced by chronic reduction in uterine perfusion pressure (RUPP) and low dose TNF-α infusion. Hypertens in pregnancy. 2006;25(70) (abstract) [Google Scholar]
- 11.Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Brasen JH, Fiebeler A, Schneider W, Caluwaerts S, Vercruysse L, Pijnenborg R, Luft FC, Muller DN. Agonistic autoantibodies to the AT1 receptor in a transgenic rat model of preeclampsia. Hypertension. 2005;45:742–746. doi: 10.1161/01.HYP.0000154785.50570.63. [DOI] [PubMed] [Google Scholar]
- 12.Herse F, Dechend R, Harsem NK, Wallukat G, Janke J, Qadri F, Hering L, Muller DN, Luft FC, Staff AC. Dysregulation of the circulating and tissue-based renin-angiotensin system in preeclampsia. Hypertension. 2007;49:604–611. doi: 10.1161/01.HYP.0000257797.49289.71. [DOI] [PubMed] [Google Scholar]
- 13.Xia Y, Kellems RE. Is preeclampsia an autoimmune disease? Clin Immunol. 2009;133:1–12. doi: 10.1016/j.clim.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou CC, Ahmad S, Mi T, Abbasi S, Xia L, Day MC, Ramin SM, Ahmed A, Kellems RE, Xia Y. Autoantibody From Women With Preeclampsia Induces Soluble Fms-Like Tyrosine Kinase-1 Production via Angiotensin Type 1 Receptor and Calcineurin/Nuclear Factor of Activated T-Cells Signaling. Hypertension. 2008;51:1010–1019. doi: 10.1161/HYPERTENSIONAHA.107.097790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, Juepner A, Gulba DC, Mackman N, Haller H, Luft FC. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101:2382–2387. doi: 10.1161/01.cir.101.20.2382. [DOI] [PubMed] [Google Scholar]
- 16.Bobst SM, Day MC, Gilstrap LC, 3rd, Xia Y, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human mesangial cells and induce interleukin-6 and plasminogen activator inhibitor-1 secretion. Am J Hypertens. 2005;18:330–336. doi: 10.1016/j.amjhyper.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Walther T, Wallukat G, Jank A, Bartel S, Schultheiss HP, Faber R, Stepan H. Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension. 2005;46:1275–1279. doi: 10.1161/01.HYP.0000190040.66563.04. [DOI] [PubMed] [Google Scholar]
- 18.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 20.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J M. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 21.Herse F, Verlohren S, Wenzel K, Pape J, Muller DN, Modrow S, Wallukat G, Luft FC, Redman CWG, Dechend R. Prevalence of agonistic autoantibodies against the Angiotensin II type 1 receptor and soluble fms-like tyrosine kinase 1 in a gestational age-matched case study. Hypertension. 2009;53:393–398. doi: 10.1161/HYPERTENSIONAHA.108.124115. [DOI] [PubMed] [Google Scholar]
- 22.Zhou CC, Ahmad S, Mi T, Xia L, Abbasi S, Hewett PW, Sun C, Ahmed A, Kellems RE, Xia Y. Angiotensin II induces soluble fms-Like tyrosine kinase-1 release via calcineurin signaling pathway in pregnancy. Circ Res. 2007;100:88–95. doi: 10.1161/01.RES.0000254703.11154.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 24.Stepan H, Faber R, Wessel N, Wallukat G, Schultheiss HP, Walther T. Relation between circulating Angiotensin II type 1 receptor agonistic antibodies and soluble fms-like tyrosinase kinase 1 in the pathogenesis of preeclampsia. J Clin Endocrinol Metabol. 2006;91:2424–2427. doi: 10.1210/jc.2005-2698. [DOI] [PubMed] [Google Scholar]