Abstract
Following mitosis, specification and migration during embryogenesis, dopamine neurons of the mesencephalon undergo a postnatal naturally occurring cell death event that determines their final adult number, and a period of axonal growth that determines pattern and extent of target contacts. While a number of neurotrophic factors have been suggested to regulate these developmental events, little is known, especially in vivo, of the cell signaling pathways that mediate these effects. We have examined the possible role of Akt/Protein Kinase B by transduction of these neurons in vivo with adeno-associated viral vectors to express either a constitutively active or a dominant negative form of Akt/protein kinase B. We find that Akt regulates multiple features of the postnatal development of these neurons, including the magnitude of the apoptotic developmental cell death event, neuron size, and the extent of target innervation of the striatum. Given the diversity and magnitude of its effects, the regulation of the development of these neurons by Akt may have implications for the many psychiatric and neurologic diseases in which these neurons may play a role.
Keywords: apoptosis, mTor, neurotrophic, Parkinson’s disease, sprouting
Dopamine neurons of the mesencephalon play an important role in the neurobiology of many prevalent and highly disabling neurologic and psychiatric diseases. Dysfunction of these neurons has been implicated in schizophrenia (Jarskog et al. 2007), addictive behaviors (Kauer and Malenka 2007) and satiety disorders (Morton et al. 2006), and they are especially vulnerable to degeneration in a number of adult-onset neurodegenerative diseases, most notably Parkinson’s disease, but also progressive supranuclear palsy and other tauopathies (Lee et al. 2001). An understanding of the neurobiology of these neurons will therefore have important implications for the pathogenesis and treatment of these conditions. Important progress has been made in our understanding of the development of these neurons, including the prenatal processes of specification and differentiation (Prakash and Wurst 2006; Smidt and Burbach 2007) and postnatal processes of determination of neuron number, target contact, and afferent contact. We have shown that like most developing neural systems, dopamine neurons undergo a naturally occurring cell death event (Janec and Burke 1993; Oo and Burke 1997); it is biphasic, with an initial major phase just after birth, and a second minor phase at postnatal day 14. Much has been learned about the trophic factors that regulate this postnatal cell death event, with the principal evidence suggesting roles for glial cell line-derived neurotrophic factor (GDNF) as a target-derived factor (Burke 2004) and brain-derived neurotrophic factor (BDNF) serving in either an afferent (Alonso-Vanegas et al. 1999) or autocrine role.
However, little is known about the cell signaling pathways within mesencephalic dopamine neurons that mediate the trophic responses that suppress developmental apoptosis, or induce axon sprouting, particularly in the in vivo context. One candidate pathway for a cell signaling role in these trophic responses is that of phosphatidylinositol-3 kinase (PI3K) and Akt/protein kinase B activation. In studies of neural cells in tissue culture, PI3K/Akt signaling has been implicated in the survival effects of nerve growth factor, platelet-derived growth factor and insulin-like growth factor (Yao and Cooper 1995; Dudek et al. 1997). A number of trophic effects of GDNF have likewise been attributed to PI3K/Akt activation, including cell survival (Soler et al. 1999; Besset et al. 2000; Mograbi et al. 2001), neurite differentiation (Pong et al. 1998; Encinas et al. 2001) and neuroprotection (Ugarte et al. 2003). We have therefore sought to investigate the role of Akt signaling in the development of dopamine neurons of the substantia nigra (SN) in vivo by the use of constitutively active and dominant negative forms of Akt, delivered by adeno-associated virus 1 (AAV1) viral vector transfer.
Materials and methods
Generation of recombinant adeno-associated virus
A plasmid encoding a 5′-src myristoylation signal in frame with mouse Akt1 was kindly provided by Dr Thomas Franke (Franke et al. 1995; Ahmed et al. 1997). The myristoylated-Akt1 (Myr-Akt) sequence was modified to incorporate a FLAG-encoding sequence at the 3′-end and inserted into an AAV1 packaging construct as previously described (Olson et al. 2006; Ries et al. 2006). The genomic titer was 1.6 × 1012 viral genomes/mL. A plasmid encoding the pleckstrin homology (PH) domain of mouse Akt1 (dominant negative Akt) (DN-Akt) was also kindly provided by Dr Thomas Franke (Songyang et al. 1997), and modified to incorporate a FLAG-encoding sequence at the 3′-end. The genomic titer was 1.8 × 1012 viral genomes/mL. Enhanced green fluorescent protein was subcloned into the same viral backbone and viral stocks were used at a titer of 2.1–9.1 × 1012.
Intranigral injection of postnatal rats with AAV
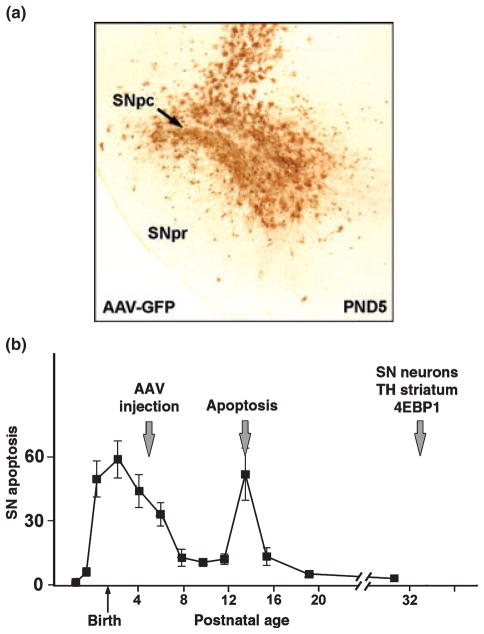
Timed, multiple pregnancy Sprague-Dawley female rats were obtained from Charles River Laboratories (Wilmington, MA, USA) 1 week before delivery. The day of delivery was defined as PND1 (Janec and Burke 1993; Oo and Burke 1997). Intranigral injection of AAV was performed on PND5 or 6. Pups were anesthetized by hypothermia. Animals were then positioned in a stereotaxic apparatus equipped with a hypothermic miniaturized stereotaxic adapter for neonatal rats (Stoelting Co., Wood Dale, IL, USA.). The needle of a Hamilton microliter syringe was lowered to: AP: −0.32 LAT: +0.15 DV: −0.40 (cm) (relative to bregma). Viral vector solution was infused at 0.1 μL/min over 20 min for a total volume of 2.0 μL. The needle was held in place for 5 min and then slowly withdrawn. This procedure consistently transduced the majority of neurons of the SNpc (Fig. 1a). For both AAV Myr-Akt and AAV DN-Akt, neurons were transduced exclusively. Following AAV injection, pups were killed either at PND14 for the quantification of apoptosis in SNpc or at PND33 for morphologic assessment of SNpc neurons or striatal dopaminergic innervation (Fig. 1b).
Fig. 1.
Assessment of the effect of Akt on development of the SNpc. (a) Transduction of SNpc neurons in postnatal rat with AAV GFP at PND5. A PND5 rat was injected dorsal to the SNpc with 2.0 μL of AAV GFP (2.1 × 10E12 vg/mL), Killed at 7 days post-injection, and the brain was then processed for immunoperoxidase staining of GFP. Successful transduction of the majority of SNpc neurons is observed. (b) Timeline for the assessment of Akt in relation to the time course of naturally occurring cell death in dopamine neurons of the SNpc in rat (Oo and Burke 1997). Naturally occurring cell death in SNpc is biphasic, with the major phase occurring at the time of birth, and a second, minor phase occurring at PND14. Due to the time required for protein expression to be achieved by the vector, AAV injection at PND5 will affect predominantly the second phase. Rats were assessed for levels of apoptosis at PND14, and for SN neuron number, striatal TH staining, and phospho-4E-BP1 immunostaining at PND33.
Immunohistochemistry for tyrosine hydroxylase
Pups were anesthetized by halothane inhalation and then perfused intracardially with 0.9% saline for 5 min by gravity, followed by 4% paraformaldehyde (PF) in 0.1 M phosphate buffer (PB) for 10 min at 4°C. The brain was removed and the SN was blocked. The SN was post-fixed in 4% PF in 0.1 M PB for 1 week at 4°C and then placed in 20% sucrose in 0.1 M PB for 24 h prior to sectioning. Each SN was rapidly frozen by immersion in isopentane on dry ice and then sectioned in a cryostat at 30 μm. A complete set of serial sections was obtained, and every fourth section was processed free-floating. After washes with phosphate-buffered saline (PBS), sections were incubated in primary antibody (MAB5280; Chemicon, Temecula, CA, USA) mouse monoclonal anti-TH at 1: 40 in PBS/10% normal horse serum for 24 h at 4°C. Sections were then washed with PBS and incubated with biotinylated horse anti-mouse IgG (Vector Laboratories, Burlingame, CA, USA) at 1: 50 in PBS/10% normal horse serum at 4°C. Sections were washed in PBS at room temperature and then incubated with avidin-biotinylated-horseradish peroxidase complexes (ABC) (Vector Laboratories) at 1: 600 for 1 h at 20–25°C. Following washes in PBS, sections were incubated in a solution of diaminobenzidine (50 mg in 100 mL Tris-buffer, pH 7.6) containing glucose oxidase, ammonium chloride and D(+) glucose to generate H2O2. Sections were then mounted on glass slides, left to dry overnight at 20–25°C and thionin counterstained. Striatal sections were cut at 30 μm from Paxinos–Watson (Paxinos and Watson 1982) planes 8.7–10.2. Sections were rinsed in PBS and then treated with PBS/bovine serum albumin and PBS/bovine serum albumin/0.1% Triton. Sections were then incubated with a rabbit anti-TH polyclonal antibody (Calbiochem, San Diego, CA, USA) for 48 h at 4°C, treated with biotinylated Protein A and ABC as described and incubated with diaminobenzidine and H2O2.
Immunofluorescence double-labeling for FLAG and phosphorylated 4E-BP1
Akt signaling regulates a wide array of cellular targets (Manning and Cantley 2007). However, the cellular pathway downstream of Akt that has been most closely associated with regulation of cell growth is that of mTor kinase and its downstream targets (Manning and Cantley 2007). In relation to responses especially relevant to neural development, such as regulation of neuron size and axon growth, a specific mTor complex, the rapamycin-sensitive mTor complex 1 (mTORC1) appears to be particularly important (Swiech et al. 2008). This complex, in turn, has two principal substrates that regulate protein translation, S6K and eukaryotic initiation factor 4E-binding protein (4E-BP1) (Wullschleger et al. 2006; Manning and Cantley 2007). In the context of axon growth and guidance, the phosphorylation of 4E-BP1 has been used as a valid reporter of mTORC1 activity (Campbell and Holt 2001; Li et al. 2008).
For Phospho-4E-BP1 (p-4E-BP1) and FLAG double staining, postnatal rats were anesthetized by hypothermia with supplemental oxygen and perfused with 0.2 mM Na3VO4 in 4%PF/0.1 M PB by pump at 5 mL/min for 10 min. The brains were post-fixed and cryoprotected. SN sections were cut at 30 μm and processed free-floating with anti-phospho-4E-BP1 (1: 200, Cell Signaling, Beverly, MA, USA) and anti-FLAG (1: 1000; Sigma).
Determination of SN neuron numbers by stereologic analysis
For the experiments performed with AAV DN-Akt, stereologic analysis was performed on TH-immunostained sections under blinded conditions on coded slides. For each animal, the SN on both sides of the brain was analyzed. For each section, the entire SN was identified as the region of interest. Using StereoInvestigator software (MicroBright Field, Inc, Williston, VT, USA), a fractionator probe was established for each section. The number of TH-positive neurons in each counting frame was then determined by focusing down through the section, using 100× objective under oil, as required by the optical disector method. Our criterion for counting an individual TH-positive neuron was the presence of its nucleus either within the counting frame, or touching the right or top frame lines (green), but not touching the left or bottom lines (red). The total number of TH-positive neurons for each side of the SN was then determined by the StereoInvestigator program.
For the experiments performed with AAV Myr-Akt, stereologic analysis was performed on Nissl-stained sections for the reason that we had previously shown that Myr-Akt augments TH expression and artifactually increases total counts of neurons in SNpc (Ries et al. 2006). In addition, the stereologic analysis was performed only on sections anterior to the first appearance of the medial terminal nucleus, because in posterior sections, adjacent to the site of the AAV injection, the SN was distorted by the formation of vascular malformations induced by Myr-Akt (Fig. 2).
Fig. 2.
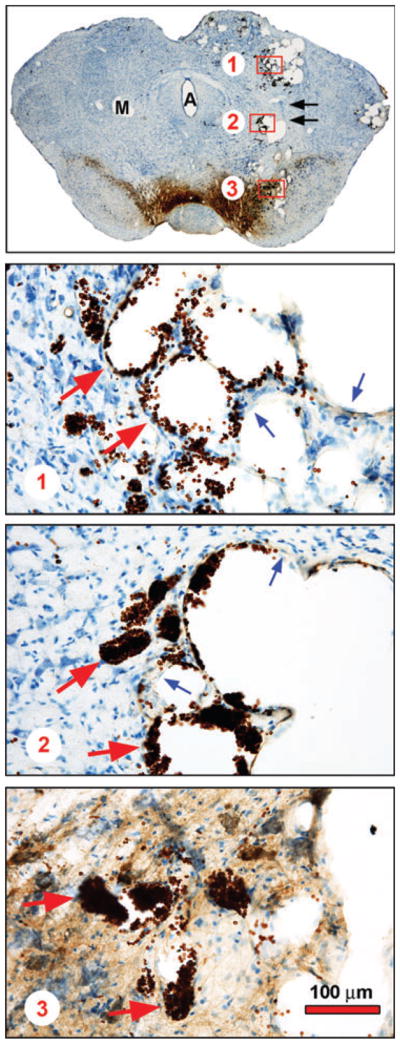
Induction of vascular malformations by AAV Myr-Akt. Injection of AAV Myr-Akt induced vascular malformations within the midbrain in all nine rat pups injected at PND 5 and examined morphologically at PND33. A representative coronal section is shown at low power in the top panel. The uninjected side has been marked (M) on the left. For orientation, the Aqueduct of Sylvius (A) is in the center of the section. Along the path of the needle used to deliver viral vector to the SN (black arrows), multiple, round unstained spaces are observed. Three of them are delineated by red rectangles and shown at higher power in the lower panels. In each of these panels, these clear spaces are demonstrated to be the intravascular compartment of enlarged cerebral vessels cut in cross-section. In each panel, endothelial cells making up the walls of these structures are shown by blue arrows; collections of red blood cells, stained brown due to the presence of endogenous peroxidase, are shown by red arrows.
The size of the cell and nucleus of TH or Nissl-stained neurons in the SN was also determined by use of the StereoInvestigator program.
Qualitative morphologic analysis of apoptosis in SN
In the TH immunoperoxidase-stained sections, apoptosis was identified at the light microscope level by performing a thionin counterstain and visualizing intranuclear chromatin clumps at 600× as one or more intensely basophilic, homogeneously stained, round and distinctly bounded structures. We have previously shown that, for naturally occurring cell death in dopamine neurons and for induction of this death event, apoptotic profiles so identified are confirmed to be apoptotic by electron microscopy (Macaya et al. 1994; Jackson-Lewis et al. 2000; El-Khodor and Burke 2002), TUNEL labeling (Macaya et al. 1994; Jackson-Lewis et al. 2000; El-Khodor and Burke 2002), and by immunostaining for activated caspase-3 and its cleavage products (Jeon et al. 1999; El-Khodor and Burke 2002) and for activated caspase-9 (Ganguly et al. 2004). Apoptotic profiles in each SN were quantified by scanning the SN at 600× in its entirety on 2–4 sections in each of three planes through the SN corresponding to Paxinos-Watson planes 4.2, 3.7 and 3.2 (Paxinos and Watson 1982). The total number of profiles for each plane was averaged, and the sum of these averages provided a measure of the magnitude of apoptosis in SN for that brain (Oo et al. 2003). All quantitative morphologic assessments were performed on coded slides, blind to experimental condition.
Results
Constitutively active Akt (Myr-Akt) increases neuron number and size in the SNpc
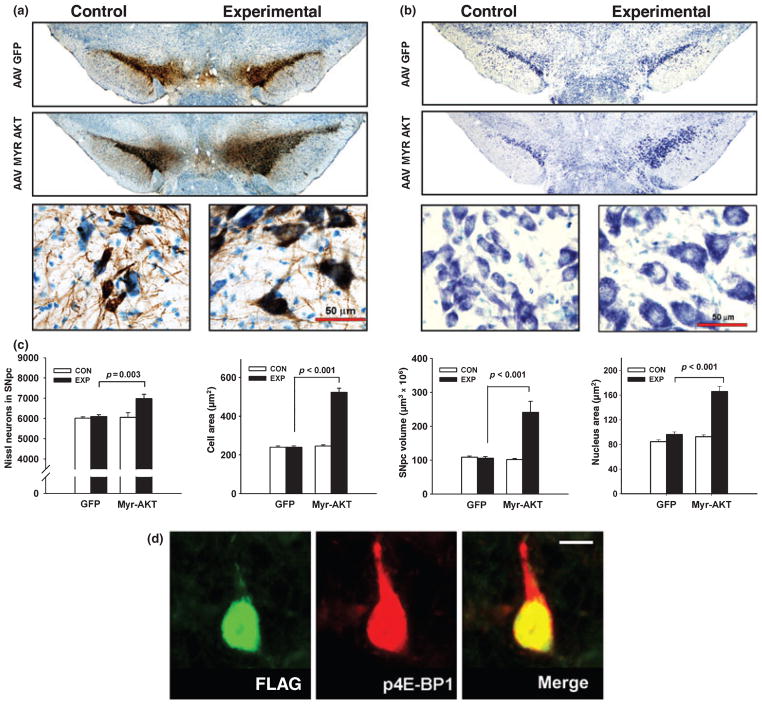
Following AAV transduction of neurons of the SN with Myr-Akt on PND5, there was a striking increase in the regional size of the SNpc at PND33 (120%, p < 0.001) (Fig. 3a–c) at which time the naturally occurring cell death event has ceased (Fig. 1b). This effect was due to an increase in both the number and size of neurons in the SNpc. The increase in the number of Nissl-stained neurons in the SNpc was relatively modest (15%) (Fig. 3c) but significant (p = 0.003). The cellular basis of the increased regional size of the SNpc was primarily that of a comparable increase in the size of individual SNpc neurons. The effect was quantified as a 118% (p < 0.001) increase in the mean cross-sectional area of individual SNpc Nissl-stained neurons (Fig. 3b and c). There was also a 173% increase in the size of the nuclei of neurons in the SNpc (p < 0.001, ANOVA) (Fig. 3b and c).
Fig. 3.
Transduction of SNpc neurons during postnatal development with Myr-Akt results in an increase in their size and number. (a) Immunoperoxidase labeling of tyrosine hydroxylase (TH) within the SNpc at 28 days post-injection (PND33) on one side of the brain of either AAV GFP or AAV Myr-Akt on PND5. The low power photomicrographs in the top panel show, at a regional level, that there is no difference between the injected side (Experimental) and the uninjected side (Control) in the AAV GFP-treated animals, whereas the Experimental side of the AAV Myr-Akt-injected animals demonstrates a markedly increased extent of TH-immunostaining in the SNpc. The higher power micrographs shown in the lower panels demonstrate at a cellular level that this increased extent of TH staining is due primarily to a marked increase in the size of SNpc TH-positive neurons. (b) Nissl stain of the ventral mesencephalon at 28 days post-injection (PND33) of either AAV GFP or AAV Myr-Akt on PND5. The low power photomicrographs in the top panel show that the apparent increase in the size and number of dopamine neurons in the SNpc is observed independently of the expression of TH. An increase in the size of Nissl-stained neurons is observed at the cellular level in the lower panels. (c) Stereologic counts of Nissl-stained neurons in the SNpc demonstrate a 15% increase in neuron number on PND33 (AAV GFP, N = 6 rats; AAV Myr-Akt, N = 5 rats). Injection of AAV Myr-Akt also results in a 118% increase in the cross-sectional area of Nissl-stained neurons (N = 45 neurons, each experimental condition), and a 120% increase in the volume of the SN. Additionally, Myr-Akt induced a 173% increase in the cross-sectional area of nuclei of neurons in the SNpc (AAV GFP, Control (CON) (uninjected side) 84.5 ± 2.9 μm2, Experimental (EXP) (injected side) 96.0 ± 4.3 μm2; AAV Myr-Akt, Control (CON) (uninjected side) 92.6 ± 2.9 μm2, Experimental (EXP) (injected side) 166.3 ± 8.3 μm2; N = 50 nuclei for each condition; p < 0.001, one-way ANOVA). (d) Double immunofluorescence labeling reveals phosphorylation of 4E-BP1 in an SNpc neuron transduced with Myr-Akt, identified by the presence of the FLAG epitope. Scale bar = 10 μm.
To determine whether transduction of SNpc neurons with Myr-Akt has an effect on mTORC1 signaling, we examined the phosphorylation status of 4E-BP1. In studies to characterize the relationship between the presence of p-4E-BP1 staining and transduction with AAV Myr-Akt, we examined 50 p-4E-BP1-positive profiles in adult mice and determined that all were FLAG-positive; i.e., p-4E-BP1 positive immunostaining was observed exclusively in transduced neurons (data not shown). Identical results were observed in postnatal rats: both FLAG and p-4E-BP1 immunoreactivity were observed exclusively in the SNpc injected with AAV Myr-Akt, as was double-labeling for FLAG/p-4E-BP1, and p-4E-BP1 positivity was observed exclusively in FLAG-positive (i.e., transduced) neurons. An example of immunofluorescent staining to demonstrate double-labeling for FLAG/p-4E-BP1 is shown (Fig. 3d).
A dominant negative form of Akt (DN-Akt) decreases neuron number and size in the SNpc
In view of these effects of an exogenous, constitutively active mutant form of Akt on the development of SNpc neurons, we sought next to determine whether endogenous Akt plays a regulatory role. To address this question, we transduced these neurons with a dominant negative form of Akt, consisting of the N-terminal PH domain alone. Specific suppression of endogenous Akt signaling by this construct has previously been demonstrated in the in vitro context (Dudek et al. 1997; Songyang et al. 1997; Wang et al. 2003). When examined 4 weeks after AAV transduction (at PND33), dopamine neurons of the SNpc were reduced in both number and size (Fig. 4). Based on stereologic counts, the number of TH-positive SN neurons was decreased by 16% (p < 0.02) (Fig. 4B). We have previously shown that Akt can regulate the expression of TH in dopamine neurons (Ries et al. 2006). We therefore considered the possibility that this decrease in dopamine neuron number may be due to diminished immunodetection of TH, rather than an actual decrease in neuron number. To address this concern, we quantified the magnitude of naturally occurring cell death directly by determining the number of apoptotic profiles in the SNpc at PND14 (Fig. 1b), following injection of AAV DN-Akt on PND6 (Fig. 1b). This analysis showed a significant induction of apoptosis by DN-Akt (Fig. 4c). We therefore conclude that the diminished mature number of dopamine neurons in animals given DN-Akt is due, at least in part, to an augmentation of the naturally occurring cell death event.
Fig. 4.
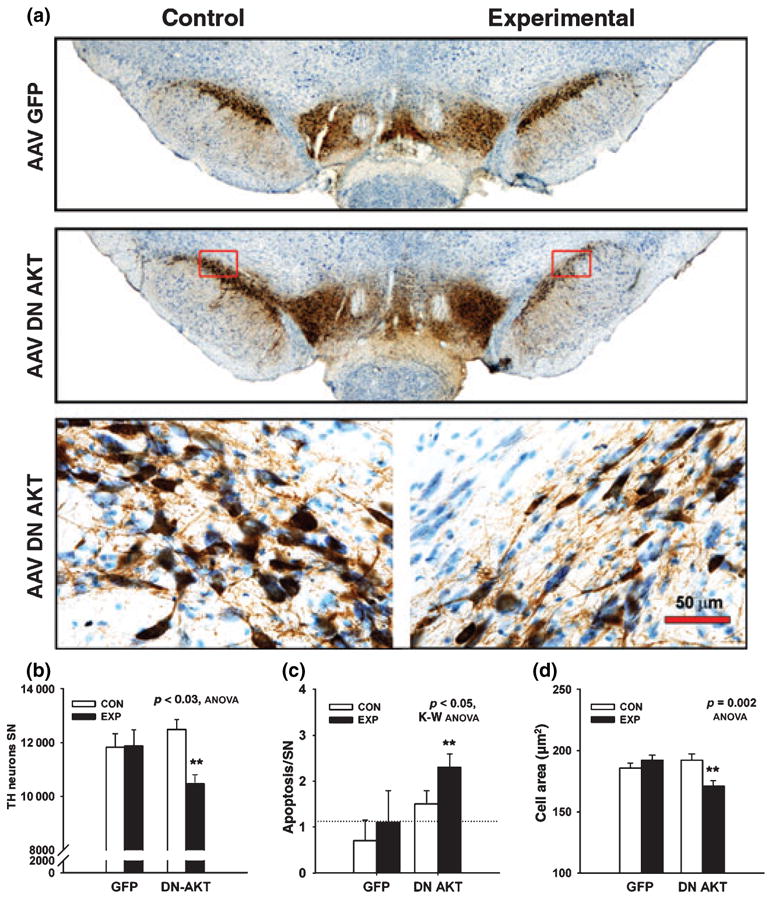
Transduction of SNpc neurons during postnatal development with DN-Akt results in a decrease in their size and number. (a) Immunoperoxidase labeling of TH within the SNpc at 28 days post-injection (PND33) of either AAV GFP or AAV DN-Akt on PND5. The low power photomicrographs in the top panel show, at a regional level, that there is no difference between the injected side (Experimental) and the un-injected side (Control) in the AAV GFP-treated animals. The Experimental side of the AAV DN-Akt-injected animals demonstrates a decreased intensity of TH-immunostaining in the SNpc. The regions delineated by red rectangles are shown at higher power in the lower panels. At a cellular level, the decreased intensity of TH staining is due to decreases in both the size and number of SNpc TH-positive neurons. (b) Stereologic counts of TH-stained neurons in the SNpc. Injection of AAV DN-Akt on PND5 induces a 16% decrease in neuron number on PND33 (AAV GFP, N = 5 rats; AAV DN-Akt, N = 6 rats). (C) Injection of AAV DN-Akt on PND5 induces an increase in the magnitude of apoptotic cell death at PND14 by two-fold, from a count of 1.1 ± 0.7 (SEM) per sampled SN sections to 2.3 ± 0.3 (p < 0.05). (d) AAV DN-Akt induces an 11% decrease in the cross-sectional area of Nissl-stained neurons (N = 50 neurons, each experimental condition).
In addition to this regulation of naturally occurring cell death, endogenous Akt also regulates dopamine neuron size during development. Transduction with DN-Akt resulted in a small (11%), but significant decrease in cross-sectional area (Fig. 4d).
Akt regulates the extent of dopaminergic innervation of the striatum
Among its cellular effects in neurons, Akt not only suppresses apoptosis (Brunet et al. 2001; Downward 2004) but also regulates axon growth and sprouting (Markus et al. 2002; Kwon et al. 2006). We have previously demonstrated that a constitutively active form of Akt is capable of inducing sprouting of adult mesencephalic dopamine neurons in vivo (Ries et al. 2006). We therefore investigated a possible role for Akt in regulating the extent of axonal growth of nigrostriatal dopaminergic neurons during development. Transduction of these neurons with Myr-Akt on PND5 resulted in a 48% increase in the density of TH-positive immunostaining within the striatum at PND33 (Fig. 5b and d). Examination of individual TH-positive fibers revealed increased tortuosity and numbers in regions where they are ordinarily sparse, such that individual morphology is discernable (Fig. 5B). These observations suggest that the increase in density of striatal TH immunostaining is likely to be due, at least in part, to sprouting, rather than to increased expression of TH alone.
Fig. 5.
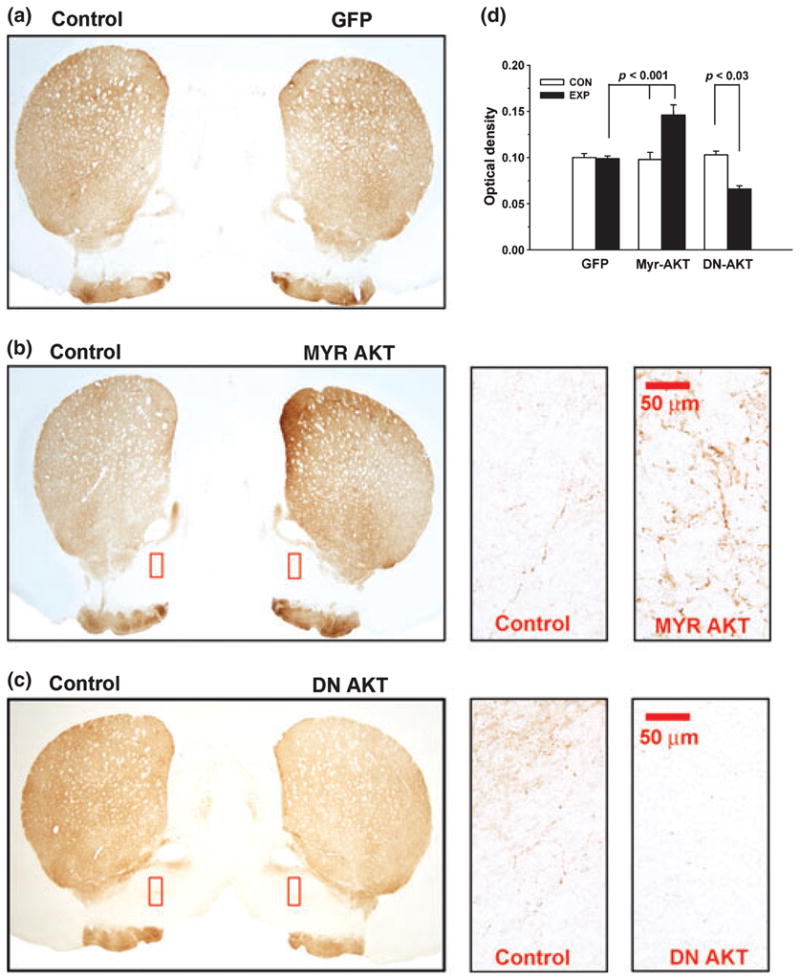
Transduction of SNpc neurons during postnatal development with Myr-Akt or DN-Akt has reciprocal effects on striatal dopaminergic innervation. (a) Immunoperoxidase labeling of TH within the striatum at 28 days post-injection (PND33) with AAV GFP on PND5. The low power photomicrograph shows that there is no difference between the injected side (GFP) and the uninjected side (CONTROL) in the AAV GFP-treated animals. (b) Labeling of TH within the striatum at 28 days post-injection (PND33) with AAV Myr-Akt on PND5. The low power photomicrograph shows that there is an increased intensity of TH immunostaining on the injected side (MYR AKT) in comparison to the uninjected side (CONTROL). The regions delineated by red rectangles are shown at higher power in the right-hand panels. At a cellular level, the increased intensity of TH staining is due to increases in both the caliber and number of SNpc TH-positive fibers following AAV Myr-Akt. (c) Labeling of TH within the striatum at 28 days post-injection (PND33) with AAV DN-Akt on PND5. The low power photomicrograph shows that there is a decreased intensity of TH immunostaining on the injected side (DN AKT) in comparison to the uninjected side (CONTROL). The regions delineated by red rectangles are shown at higher power in the right-hand panels. At a cellular level, the decreased intensity of TH staining is due to a decrease in the number of SNpc TH-positive fibers following AAV DN-Akt. (d) Quantitative analysis of optical densities (ODs) over the striatum in postnatal rats at 28 days following SN injection of AAV GFP (N = 7 rats), AAV Myr-Akt (N = 9 rats) or AAV DN-Akt (N = 6 rats).
To determine whether endogenous Akt may play a role in regulating axonal development, we examined striatal TH immunostaining following transduction of SN neurons with DN-Akt. This dominant negative construct induced a 36% decrease in whole striatal TH staining density in comparison to the contralateral, non-injected side, and GFP-injected controls (Fig. 5c and d). The decrease in TH immunostaining was uniform throughout the striatum; it was not restricted to any subregion, nor was it predominant in either the striosome or matrix compartments (Gerfen et al. 1987). This decrease in the density of striatal TH staining at the regional level corresponded with a decrease in the number of individual TH-positive fibers (Fig. 5c).
Discussion
Following embryonic mitosis, specification and migration, dopamine neurons of the ventral mesencephalon undergo a naturally occurring cell death event that determines their final adult number (Janec and Burke 1993; Oo and Burke 1997). In both rats and mice (Jackson-Lewis et al. 2000), this event begins just before birth and ends by PND28. It is biphasic, with an initial major peak just after birth, and a second minor peak at PND14. In both species, the morphology of cell death is exclusively apoptotic. As envisioned by classic neurotrophic theory (Oppenheim 1991), this cell death event is regulated by target contact with the striatum. There is evidence that GDNF may serve as a striatal target-derived neurotrophic factor regulating the first phase of naturally occurring cell death (Burke 2004). There is additional evidence that BDNF may also regulate postnatal cell death in these neurons, but it appears to be provided in anterograde fashion by afferent projections (Alonso-Vanegas et al. 1999), rather than retrograde from the striatal target.
The possibility that PI3K/Akt signaling could serve in vivo to mediate development trophic effects of either GDNF or BDNF in dopamine neurons is supported by many observations made in tissue culture (Soler et al. 1999; Mograbi et al. 2001; Bogush et al. 2007). In addition, we have previously shown that mRNA for all three isoforms of Akt is expressed during development in the SNpc, and that Akt protein can be identified within dopamine neurons (Ries et al. 2006). Using a viral vector approach, we herein find evidence that Akt plays a cell autonomous role in mesencephalic dopamine neurons to regulate three aspects of their development: the magnitude of the naturally occurring cell death event, neuron size and axon growth.
To examine the role of Akt in regulating developmental apoptosis, we used a strategy based on viral vector-mediated delivery of a dominant negative form for the reason that three Akt isoforms are expressed in the SNpc (Ries et al. 2006), making it difficult to achieve a complete abrogation of Akt signaling by genetic approaches. We used a well-characterized construct comprised of the PH domain of Akt (Dudek et al. 1997; Songyang et al. 1997; Wang et al. 2003). The rationale behind the dominant negative effect of this construct is that Akt depends on its PH domain to interact with phosphoinositides at the cell membrane and become activated by kinases (Hanada et al. 2004). The PH domain construct competes with endogenous Akt for this localization and thereby blocks its activation. In keeping with a role for endogenous Akt in the regulation of developmental apoptosis in SNpc neurons, we have found that transduction on PND5-6 with AAV DN-Akt results in a two-fold increase in the number of apoptotic profiles during the second phase of developmental cell death on PND14.
Since dopamine neurons are post-mitotic in the postnatal period (Burke 2003), it would be predicted that an increase in developmental cell death by DN-Akt would result in a decrease in the final adult number of these neurons. We find that this is the case; transduction at PND5-6 resulted in a 16% decrease in the number of SNpc dopamine neurons on PND33. In view of this modest effect, a question might be raised as to the importance of Akt in regulating developmental apoptosis in these neurons. However, there are two reasons why this effect is likely to be a substantial underestimate. First, AAV transduction is not achieved in all PND5-6 SNpc neurons. Second, and more importantly, transduction was achieved late in the first phase of developmental cell death (Fig. 1b), after much apoptosis has already occurred.
A concern might also be raised that the PH domain alone construct could affect the function of other PH domain-containing proteins. To address the issue of specificity, we performed the complimentary experiment of transducing these neurons with a constitutively active form of Akt, Myr-Akt. If endogenous Akt regulates the naturally occurring cell death event, then it would be predicted that transduction with this form should augment the number of SNpc dopamine neurons surviving the developmental cell death event. Transduction with AAV Myr-Akt on PND5-6 results in a 15% increase in the number of Nissl-stained neurons in the SNpc at PND33. Again, this modest effect is likely to be a substantial underestimate, based on the considerations raised above for AAV DN-Akt. We therefore conclude that Akt signaling endogenous to dopamine neurons of the SNpc regulates the naturally occurring cell death event that occurs postnatally, and thereby regulates the final adult number of these neurons.
The most pronounced developmental effect that we observed following transduction of neurons of the SNpc with Myr-Akt was a striking increase in their individual cell size. The magnitude of this effect (over 100% increase) in postnatal rats was substantially greater than that previously observed in adult mice (about 50%) (Ries et al. 2006), using the same AAV Myr-Akt vector at a similar titer. This observation suggests, not surprisingly, that developing neurons of the SNpc are more responsive to Akt than mature neurons to regulation of cell size. To explore the role of endogenous Akt in regulating cell size, we examined the effect of DN-Akt. Transduction with this construct induced a small (11%), but significant, decrease in size, suggesting that endogenous Akt plays a role. The effect of dominant negative inhibition of endogenous Akt is small in comparison with the marked converse effect of constitutively active Myr-Akt possibly because endogenous Akt plays only a partial role in regulation of cell size, or other signaling pathways are able to compensate. Alternatively, for cell size, there may be a lower limit on the range of size subject to Akt regulation.
These observations that Akt plays a role in the regulation of SNpc neuron cell size are in keeping with prior observations made in mice with deletion of the tumor suppressor phosphatase and tensin homologue (PTEN) gene in select neurons postnatally (Backman et al. 2001; Kwon et al. 2001). PTEN negatively regulates Akt activation by dephosphorylating phosphatidylinositol-3,4,5-triphosphate, thereby inhibiting interaction between the PH domain and the inner plasma membrane. In mice with postnatal PTEN deletion in cerebellar and dentate gyrus neurons, there is a resulting increase in their size, up to 150%. Our observations in SNpc neurons are similar to those of Shioi et al. in cardiac myocytes (Shioi et al. 2002), who noted that transgenic expression of either constitutively active or dominant negative forms resulted in an increase, or decrease, respectively, of cell size. Furthermore, they demonstrated that the effect of the constitutively active form to increase size was dependent on mTor signaling downstream to Akt. Our finding that 4E-BP1, an mTor substrate, is phosphorylated in SNpc neurons transduced with Myr-Akt suggests that it may play a role in SNpc neurons as well.
In addition to regulating developmental apoptosis and cell size of mesencephalic dopamine neurons, Akt also regulated their axon growth. This effect was observed at the regional level as an increase in the density of dopaminergic axon and terminal TH-immunostaining in the striatum following transduction with Myr-Akt, and a decrease following DN-Akt. At the regional level of analysis, it is not possible to distinguish between an increased number of axons due to sprouting and an increased density of immunostaining due to increased expression of TH. However, at the fiber level of analysis, morphologic evidence of sprouting was observed as an increased tortuosity and number of fibers, suggesting that the increased density of striatal staining was due, at least in part, to sprouting. Like the effect on neuron size, the effect on striatal TH staining density was greater in the postnatal setting, with 48% increase, than in adults, with 26% increase (Ries et al. 2006). These possible age differences seem unlikely to be due instead to a species difference, because in both this study in postnatal rats and the earlier study in adult mice, we used a mutant form of mouse Akt1, which would be expected to be more effective in mouse. We have previously shown that Akt-induced sprouting is functionally significant; it results in an increase in dopamine release, and behavioral response to amphetamine (Ries et al. 2006).
In conclusion, these studies provide evidence that Akt plays a role in the regulation of apoptosis, cell size, and axon growth during development in dopamine neurons of the SNpc. Akt may therefore mediate effects of GDNF, BDNF or other neurotrophic factors on the development of these neurons. We have previously shown that in the SN, unlike striatum and cortex, Akt mRNA expression remains highly expressed after development, raising the possibility that it plays a role in the adult maintenance of these neurons. In this regard, it is of interest to note that GDNF has been recently shown to be essential for the maintenance of the viability of SNpc dopamine neurons in adulthood (Pascual et al. 2008). Based on the studies herein, that have identified a developmental role of Akt in maintaining the viability and growth of these neurons, it will be of interest to examine its role in adulthood.
Acknowledgments
This work was supported by NS26836, NS38370, and the Parkinson’s Disease Foundation.
Abbreviations used
- 4E-BP1
eukaryotic initiation factor 4E-binding protein
- AAV
adeno-associated virus
- ABC
avidin-biotinylated-horse-radish peroxidase complexes
- BDNF
brain-derived neurotrophic factor
- BSA
bovine serum albumen
- DN
dominant negative
- GDNF
glial cell line-derived neurotrophic factor
- mTORC1
mammalian target of rapamycin complex 1
- p-4E-BP1
phospho-4E-BP1
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- PF
paraformaldehyde
- PH
pleckstrin homology
- PI3K
phosphatidylinositol-3 kinase
- PND
postnatal day
- PTEN
phosphatase and tensin homologue
- SN
substantia nigra
- SNpc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
References
- Ahmed NN, Grimes HL, Bellacosa A, Chan TO, Tsichlis PN. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Vanegas MA, Fawcett JP, Causing CG, Miller FD, Sadikot AF. Characterization of dopaminergic midbrain neurons in a DBH:BDNF transgenic mouse. J Comp Neurol. 1999;413:449–462. doi: 10.1002/(sici)1096-9861(19991025)413:3<449::aid-cne7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Backman SA, Stambolic V, Suzuki A, et al. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet. 2001;29:396–403. doi: 10.1038/ng782. [DOI] [PubMed] [Google Scholar]
- Besset V, Scott RP, Ibanez CF. Signaling complexes and protein–protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase. J Biol Chem. 2000;275:39159–39166. doi: 10.1074/jbc.M006908200. [DOI] [PubMed] [Google Scholar]
- Bogush A, Pedrini S, Pelta-Heller J, Chan T, Yang Q, Mao Z, Sluzas E, Gieringer T, Ehrlich ME. AKT and CDK5/p35 mediate brain-derived neurotrophic factor induction of DARPP-32 in medium size spiny neurons in vitro. J Biol Chem. 2007;282:7352–7359. doi: 10.1074/jbc.M606508200. [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Burke RE. Postnatal Developmental Programmed Cell Death in Dopamine Neurons. Ann NY Acad Sci. 2003;991:69–79. doi: 10.1111/j.1749-6632.2003.tb07464.x. [DOI] [PubMed] [Google Scholar]
- Burke RE. Ontogenic cell death in the nigrostriatal system. Cell Tissue Res. 2004;318:63–72. doi: 10.1007/s00441-004-0908-4. [DOI] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- El-Khodor BF, Burke RE. Medial forebrain bundle axotomy during development induces apoptosis in dopamine neurons of the substantia nigra and activation of caspases in their degenerating axons. J Comp Neurol. 2002;452:65–79. doi: 10.1002/cne.10367. [DOI] [PubMed] [Google Scholar]
- Encinas M, Tansey MG, Tsui-Pierchala BA, Comella JX, Milbrandt J, Johnson EM., Jr c-Src is required for glial cell line-derived neurotrophic factor (GDNF) family ligand-mediated neuronal survival via a phosphatidylinositol-3 kinase (PI-3K)-dependent pathway. J Neurosci. 2001;21:1464–1472. doi: 10.1523/JNEUROSCI.21-05-01464.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Ganguly A, Oo TF, Rzhetskaya M, Pratt R, Yarygina O, Momoi T, Kholodilov N, Burke RE. CEP11004, a novel inhibitor of the mixed lineage kinases, suppresses apoptotic death in dopamine neurons of the substantia nigra induced by 6-hydroxydopamine. J Neurochem. 2004;88:469–480. doi: 10.1046/j.1471-4159.2003.02176.x. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Herkenham M, Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987;7:3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT–a major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Vila M, Djaldetti R, Guegan C, Liberatore G, Liu J, O’Malley KL, Burke RE, Przedborski S. Developmental cell death in dopaminergic neurons of the substantia nigra of mice. J Comp Neurol. 2000;424:476–488. doi: 10.1002/1096-9861(20000828)424:3<476::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Janec E, Burke RE. Naturally occurring cell death during postnatal development of the substantia nigra of the rat. Mol Cell Neurosci. 1993;4:30–35. doi: 10.1006/mcne.1993.1004. [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Miyamoto S, Lieberman JA. Schizophrenia: new pathological insights and therapies. Annu Rev Med. 2007;58:49–61. doi: 10.1146/annurev.med.58.060904.084114. [DOI] [PubMed] [Google Scholar]
- Jeon BS, Kholodilov NG, Oo TF, Kim S, Tomaselli KJ, Srinivasan A, Stefanis L, Burke RE. Activation of caspase-3 in developmental models of programmed cell death in neurons of the substantia nigra. J Neurochem. 1999;73:322–333. doi: 10.1046/j.1471-4159.1999.0730322.x. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, Eberhart CG, Burger PC, Baker SJ. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet. 2001;29:404–411. doi: 10.1038/ng781. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Li YH, Werner H, Puschel AW. Rheb and mTOR regulate neuronal polarity through Rap1B. J Biol Chem. 2008;283:33784–33792. doi: 10.1074/jbc.M802431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaya A, Munell F, Gubits RM, Burke RE. Apoptosis in substantia nigra following developmental striatal excitotoxic injury. Proc Natl Acad Sci USA. 1994;91:8117–8121. doi: 10.1073/pnas.91.17.8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus A, Zhong J, Snider WD. Raf and akt mediate distinct aspects of sensory axon growth. Neuron. 2002;35:65–76. doi: 10.1016/s0896-6273(02)00752-3. [DOI] [PubMed] [Google Scholar]
- Mograbi B, Bocciardi R, Bourget I, Busca R, Rochet N, Farahi-Far D, Juhel T, Rossi B. Glial cell line-derived neurotrophic factor-stimulated phosphatidylinositol 3-kinase and Akt activities exert opposing effects on the ERK pathway: importance for the rescue of neuroectodermic cells. J Biol Chem. 2001;276:45307–45319. doi: 10.1074/jbc.M101220200. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science. 2006;311:1017–1020. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- Oo TF, Burke RE. The time course of developmental cell death in phenotypically defined dopaminergic neurons of the substantia nigra. Dev Brain Res. 1997;98:191–196. doi: 10.1016/s0165-3806(96)00173-3. [DOI] [PubMed] [Google Scholar]
- Oo TF, Kholodilov N, Burke RE. Regulation of natural cell death in dopaminergic neurons of the substantia nigra by striatal GDNF in vivo. J Neurosci. 2003;23:5141–5148. doi: 10.1523/JNEUROSCI.23-12-05141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW. Cell death during development of the nervous system. Ann Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1982. [Google Scholar]
- Pong K, Xu RY, Baron WF, Louis JC, Beck KD. Inhibition of phosphatidylinositol 3-kinase activity blocks cellular differentiation mediated by glial cell line-derived neurotrophic factor in dopaminergic neurons. J Neurochem. 1998;71:1912–1919. doi: 10.1046/j.1471-4159.1998.71051912.x. [DOI] [PubMed] [Google Scholar]
- Prakash N, Wurst W. Genetic networks controlling the development of midbrain dopaminergic neurons. J Physiol. 2006;575:403–410. doi: 10.1113/jphysiol.2006.113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries V, Henchcliffe C, Kareva T, Rzhetskaya M, Bland RJ, During MJ, Kholodilov N, Burke RE. Oncoprotein Akt/PKB: Trophic effects in murine models of Parkinson’s Disease. Proc Natl Acad Sci USA. 2006;103:18757–18762. doi: 10.1073/pnas.0606401103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidt MP, Burbach JP. How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci. 2007;8:21–32. doi: 10.1038/nrn2039. [DOI] [PubMed] [Google Scholar]
- Soler RM, Dolcet X, Encinas M, Egea J, Bayascas JR, Comella JX. Receptors of the glial cell line-derived neurotrophic factor family of neurotrophic factors signal cell survival through the phosphatidylinositol 3-kinase pathway in spinal cord moto-neurons. J Neurosci. 1999;19:9160–9169. doi: 10.1523/JNEUROSCI.19-21-09160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Baltimore D, Cantley LC, Kaplan DR, Franke TF. Interleukin 3-dependent survival by the Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:11345–11350. doi: 10.1073/pnas.94.21.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiech L, Perycz M, Malik A, Jaworski J. Role of mTOR in physiology and pathology of the nervous system. Biochim Biophys Acta. 2008;1784:116–132. doi: 10.1016/j.bbapap.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Ugarte SD, Lin E, Klann E, Zigmond MJ, Perez RG. Effects of GDNF on 6-OHDA-induced death in a dopaminergic cell line: modulation by inhibitors of PI3 kinase and MEK. J Neurosci Res. 2003;73:105–112. doi: 10.1002/jnr.10632. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT. Control of synaptic strength, a novel function of Akt. Neuron. 2003;38:915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]