Abstract
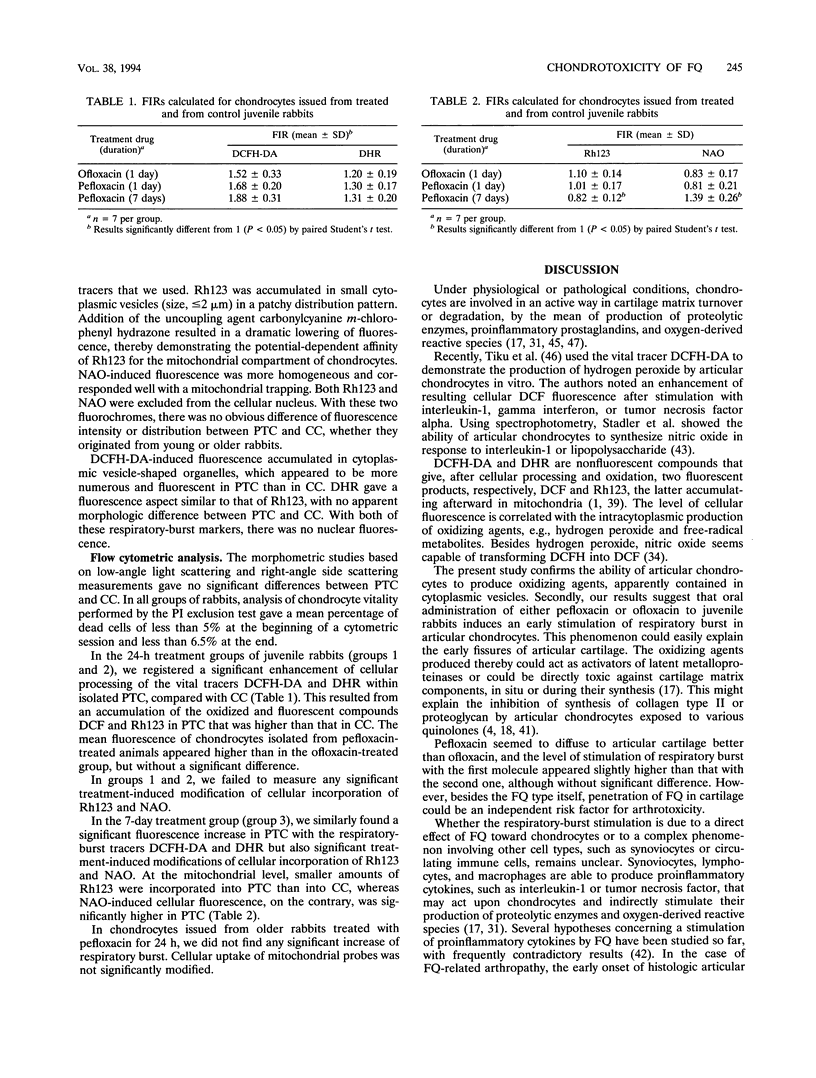
To better understand quinolone-related arthropathy, we conceived an experimental ex vivo model using cell cultures of articular chondrocytes issued from pretreated New Zealand White rabbits (NZW). Juvenile (4- to 5-week-old) NZW were orally dosed with ofloxacin or pefloxacin (300 mg/kg of body weight for 1 day) or with pefloxacin (300 mg/kg for 7 days). Adult (5-month-old) NZW were treated with pefloxacin (300 mg/kg for 1 day). Chondrocytes were enzymatically recovered from cartilage and were analyzed by cytofluorometry using 2',7'-dichlorofluorescein diacetate (DCFH-DA) and dihydrorhodamine 123 (DHR), reflecting cellular respiratory-burst activity, and rhodamine 123 (Rh123) and 10-N-nonyl-acridine orange (NAO), specific for the mitochondrial activity and mass, respectively. A significant increase in the respiratory burst was detected by DCFH-DA and DHR in all treated groups of young animals, compared with untreated control groups. No significant increase of respiratory burst was noted in older treated rabbits. The 7-day treatment resulted in a decrease in mitochondrial uptake of Rh123 and an increase in NAO uptake. Fluoroquinolone arthrotoxicity seems to involve in its early phase the respiratory burst of immature articular chondrocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass D. A., Parce J. W., Dechatelet L. R., Szejda P., Seeds M. C., Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983 Apr;130(4):1910–1917. [PubMed] [Google Scholar]
- Benel L., Ronot X., Kornprobst M., Adolphe M., Mounolou J. C. Mitochondrial uptake of rhodamine 123 by rabbit articular chondrocytes. Cytometry. 1986 May;7(3):281–285. doi: 10.1002/cyto.990070309. [DOI] [PubMed] [Google Scholar]
- Benel L., Ronot X., Mounolou J. C., Gaudemer F., Adolphe M. Compared flow cytometric analysis of mitochondria using 10-n-nonyl acridine orange and rhodamine 123. Basic Appl Histochem. 1989;33(2):71–80. [PubMed] [Google Scholar]
- Burkhardt J. E., Hill M. A., Lamar C. H., Smith G. N., Jr, Carlton W. W. Effects of difloxacin on the metabolism of glycosaminoglycans and collagen in organ cultures of articular cartilage. Fundam Appl Toxicol. 1993 Feb;20(2):257–263. doi: 10.1006/faat.1993.1034. [DOI] [PubMed] [Google Scholar]
- Burkhardt J. E., Hill M. A., Turek J. J., Carlton W. W. Ultrastructural changes in articular cartilages of immature beagle dogs dosed with difloxacin, a fluoroquinolone. Vet Pathol. 1992 May;29(3):230–238. doi: 10.1177/030098589202900307. [DOI] [PubMed] [Google Scholar]
- Castora F. J., Simpson M. V. Search for a DNA gyrase in mammalian mitochondria. J Biol Chem. 1979 Nov 25;254(22):11193–11195. [PubMed] [Google Scholar]
- Castora F. J., Vissering F. F., Simpson M. V. The effect of bacterial DNA gyrase inhibitors on DNA synthesis in mammalian mitochondria. Biochim Biophys Acta. 1983 Sep 9;740(4):417–427. doi: 10.1016/0167-4781(83)90090-8. [DOI] [PubMed] [Google Scholar]
- Champagne A. M., Benel L., Ronot X., Mignotte F., Adolphe M., Mounolou J. C. Rhodamine 123 uptake and mitochondrial DNA content in rabbit articular chondrocytes evolve differently upon transfer from cartilage to culture conditions. Exp Cell Res. 1987 Aug;171(2):404–410. doi: 10.1016/0014-4827(87)90172-8. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Summerhayes I. C., Johnson L. V., Walsh M. L., Bernal S. D., Lampidis T. J. Probing mitochondria in living cells with rhodamine 123. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):141–155. doi: 10.1101/sqb.1982.046.01.018. [DOI] [PubMed] [Google Scholar]
- Chevais M., Reinert P., Rondeau M. C., Tobelem R., Albengres E., Riant P., Tillement J. P. Critical risk/benefit analysis of pefloxacine use in children under 15 years--the problem of arthralgias. Int J Clin Pharmacol Ther Toxicol. 1987 Jun;25(6):306–309. [PubMed] [Google Scholar]
- Fairfield F. R., Bauer W. R., Simpson M. V. Mitochondria contain a distinct DNA topoisomerase. J Biol Chem. 1979 Oct 10;254(19):9352–9354. [PubMed] [Google Scholar]
- Gallagher M., Weinberg R., Simpson M. V. Effect of the bacterial DNA gyrase inhibitors, novobiocin, nalidixic acid, and oxolinic acid, on oxidative phosphorylation. J Biol Chem. 1986 Jul 5;261(19):8604–8607. [PubMed] [Google Scholar]
- Gough A. W., Kasali O. B., Sigler R. E., Baragi V. Quinolone arthropathy--acute toxicity to immature articular cartilage. Toxicol Pathol. 1992;20(3 Pt 1):436–450. doi: 10.1177/019262339202000313. [DOI] [PubMed] [Google Scholar]
- Green W. T., Jr Behavior of articular chondrocytes in cell culture. Clin Orthop Relat Res. 1971 Mar-Apr;75:248–260. doi: 10.1097/00003086-197103000-00030. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A. Oxygen radicals, inflammation, and arthritis: pathophysiological considerations and implications for treatment. Semin Arthritis Rheum. 1991 Feb;20(4):219–240. doi: 10.1016/0049-0172(91)90018-u. [DOI] [PubMed] [Google Scholar]
- Hildebrand H., Kempka G., Schlüter G., Schmidt M. Chondrotoxicity of quinolones in vivo and in vitro. Arch Toxicol. 1993;67(6):411–415. doi: 10.1007/BF01977402. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. The fluoroquinolones: pharmacology, clinical uses, and toxicities in humans. Antimicrob Agents Chemother. 1985 Nov;28(5):716–721. doi: 10.1128/aac.28.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Bockus B. J., Chen L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol. 1981 Mar;88(3):526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajcovic J., Ebringer L., Polónyi J. Quinolones and coumarins eliminate chloroplasts from Euglena gracilis. Antimicrob Agents Chemother. 1989 Nov;33(11):1883–1889. doi: 10.1128/aac.33.11.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maftah A., Petit J. M., Julien R. Specific interaction of the new fluorescent dye 10-N-nonyl acridine orange with inner mitochondrial membrane. A lipid-mediated inhibition of oxidative phosphorylation. FEBS Lett. 1990 Jan 29;260(2):236–240. doi: 10.1016/0014-5793(90)80112-v. [DOI] [PubMed] [Google Scholar]
- Maftah A., Petit J. M., Ratinaud M. H., Julien R. 10-N nonyl-acridine orange: a fluorescent probe which stains mitochondria independently of their energetic state. Biochem Biophys Res Commun. 1989 Oct 16;164(1):185–190. doi: 10.1016/0006-291x(89)91700-2. [DOI] [PubMed] [Google Scholar]
- Maggiolo F., Caprioli S., Suter F. Risk/benefit analysis of quinolone use in children: the effect on diarthrodial joints. J Antimicrob Chemother. 1990 Oct;26(4):469–471. doi: 10.1093/jac/26.4.469. [DOI] [PubMed] [Google Scholar]
- Modica-Napolitano J. S., Aprille J. R. Basis for the selective cytotoxicity of rhodamine 123. Cancer Res. 1987 Aug 15;47(16):4361–4365. [PubMed] [Google Scholar]
- Montay G., Tassel J. P. Improved high-performance liquid chromatographic determination of pefloxacin and its metabolite norfloxacin in human plasma and tissue. J Chromatogr. 1985 Apr 12;339(1):214–218. doi: 10.1016/s0378-4347(00)84647-2. [DOI] [PubMed] [Google Scholar]
- Obernauerová M., Subík J., Ebringer L. Ofloxacin induces cytoplasmic respiration-deficient mutants in yeast Saccharomyces cerevisiae. Curr Genet. 1992 May;21(6):443–446. doi: 10.1007/BF00351653. [DOI] [PubMed] [Google Scholar]
- Paraidathathu T., de Groot H., Kehrer J. P. Production of reactive oxygen by mitochondria from normoxic and hypoxic rat heart tissue. Free Radic Biol Med. 1992 Oct;13(4):289–297. doi: 10.1016/0891-5849(92)90176-h. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Roughley P. J., DiBattista J. A., McCollum R., Martel-Pelletier J. Are cytokines involved in osteoarthritic pathophysiology? Semin Arthritis Rheum. 1991 Jun;20(6 Suppl 2):12–25. doi: 10.1016/0049-0172(91)90024-t. [DOI] [PubMed] [Google Scholar]
- Petit J. M., Maftah A., Ratinaud M. H., Julien R. 10N-nonyl acridine orange interacts with cardiolipin and allows the quantification of this phospholipid in isolated mitochondria. Eur J Biochem. 1992 Oct 1;209(1):267–273. doi: 10.1111/j.1432-1033.1992.tb17285.x. [DOI] [PubMed] [Google Scholar]
- Polónyi J., Ebringer L., Krajcovic J., Kapeller K. Injured mitochondria in cells of Euglena gracilis after DNA gyrase inhibitors treatment. Z Mikrosk Anat Forsch. 1990;104(1):61–78. [PubMed] [Google Scholar]
- Rao K. M., Padmanabhan J., Kilby D. L., Cohen H. J., Currie M. S., Weinberg J. B. Flow cytometric analysis of nitric oxide production in human neutrophils using dichlorofluorescein diacetate in the presence of a calmodulin inhibitor. J Leukoc Biol. 1992 May;51(5):496–500. doi: 10.1002/jlb.51.5.496. [DOI] [PubMed] [Google Scholar]
- Rathakrishnan C., Tiku K., Raghavan A., Tiku M. L. Release of oxygen radicals by articular chondrocytes: a study of luminol-dependent chemiluminescence and hydrogen peroxide secretion. J Bone Miner Res. 1992 Oct;7(10):1139–1148. doi: 10.1002/jbmr.5650071005. [DOI] [PubMed] [Google Scholar]
- Ribard P., Kahn M. F. Rheumatological side-effects of quinolones. Baillieres Clin Rheumatol. 1991 Apr;5(1):175–191. doi: 10.1016/s0950-3579(05)80301-2. [DOI] [PubMed] [Google Scholar]
- Riesbeck K., Bredberg A., Forsgren A. Ciprofloxacin does not inhibit mitochondrial functions but other antibiotics do. Antimicrob Agents Chemother. 1990 Jan;34(1):167–169. doi: 10.1128/aac.34.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronot X., Benel L., Adolphe M., Mounolou J. C. Mitochondrial analysis in living cells: the use of rhodamine 123 and flow cytometry. Biol Cell. 1986;57(1):1–7. doi: 10.1111/j.1768-322x.1986.tb00458.x. [DOI] [PubMed] [Google Scholar]
- Rothe G., Oser A., Valet G. Dihydrorhodamine 123: a new flow cytometric indicator for respiratory burst activity in neutrophil granulocytes. Naturwissenschaften. 1988 Jul;75(7):354–355. doi: 10.1007/BF00368326. [DOI] [PubMed] [Google Scholar]
- Rothe G., Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2',7'-dichlorofluorescin. J Leukoc Biol. 1990 May;47(5):440–448. [PubMed] [Google Scholar]
- Shalit I. Immunological aspects of new quinolones. Eur J Clin Microbiol Infect Dis. 1991 Apr;10(4):262–266. doi: 10.1007/BF01966999. [DOI] [PubMed] [Google Scholar]
- Stadler J., Stefanovic-Racic M., Billiar T. R., Curran R. D., McIntyre L. A., Georgescu H. I., Simmons R. L., Evans C. H. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J Immunol. 1991 Dec 1;147(11):3915–3920. [PubMed] [Google Scholar]
- Stahlmann R., Merker H. J., Hinz N., Chahoud I., Webb J., Heger W., Neubert D. Ofloxacin in juvenile non-human primates and rats. Arthropathia and drug plasma concentrations. Arch Toxicol. 1990;64(3):193–204. doi: 10.1007/BF02010725. [DOI] [PubMed] [Google Scholar]
- Stefanovic-Racic M., Stadler J., Evans C. H. Nitric oxide and arthritis. Arthritis Rheum. 1993 Aug;36(8):1036–1044. doi: 10.1002/art.1780360803. [DOI] [PubMed] [Google Scholar]
- Tiku M. L., Liesch J. B., Robertson F. M. Production of hydrogen peroxide by rabbit articular chondrocytes. Enhancement by cytokines. J Immunol. 1990 Jul 15;145(2):690–696. [PubMed] [Google Scholar]



