Abstract
Background
Perturbed sleep might contribute to cardiovascular disease by accelerating atherosclerosis. Sleep is poor in Alzheimer caregivers who are also a group at increased cardiovascular risk. Objective: To test the hypothesis that impaired sleep relates to elevated levels of biomarkers of atherosclerosis in community-dwelling elderly and that this association would possibly be stronger in caregivers than in non-caregiving controls.
Methods
We studied 97 Alzheimer caregivers and 48 non-caregiving controls (mean age 71 ± 8 years, 72% women) who underwent wrist actigraphy at their homes. Measures of objective sleep were averaged across 3 consecutive nights. The Pittsburgh Sleep Quality Index was administered by an interviewer to rate subjective sleep quality. Morning fasting blood samples were collected to determine measures of inflammation, coagulation and endothelial dysfunction.
Results
There were independent associations between decreased subjective sleep quality and increased levels of fibrin D-dimer (p = 0.022, ΔR2 = 0.029) and von Willebrand factor antigen (p = 0.029, ΔR2 = 0.034) in all participants. Percent sleep (p = 0.025) and subjective sleep quality (p = 0.017) were lower in caregivers than in controls. In caregivers, the correlation between decreased percent sleep and elevated levels of interleukin-6 (p = 0.042, ΔR2 = 0.039) and C-reactive protein (p < 0.10, ΔR2 = 0.027) was significantly stronger than in controls.
Conclusion
Perceived impairment in sleep related to increased coagulation activity and endothelial dysfunction in all participants, whereas objectively impaired sleep related to inflammation activity in caregivers. The findings provide one explanation for the increased cardiovascular risk in elderly poor sleepers and dementia caregivers in particular.
Key Words: Cardiovascular disease, Coagulation, Inflammation, Psychological stress, Sleep
Introduction
Sleep disorders as diverse as obstructive sleep apnea, sleep curtailment and insomnia have previously been related to increased risk of cardiovascular disease (CVD) [1,2,3]. Interestingly enough, this research suggests that it is not only the objectively assessed sleep disturbance which impacts cardiovascular health, but also an individual's perception of his or her impaired sleep. The pathophysiology underlying the link between poor sleep and CVD risk is only beginning to emerge [2]. In sleep apnea, repetitive hypoxia, fragmented sleep and concomitant sympathetic arousal, among other cardiovascular perturbations, might elicit low-grade systemic inflammation, coagulation activation and endothelial dysfunction, all of which contribute to atherosclerosis and its thrombotic complications [4,5,6]. Similar mechanisms might be involved in CVD related to sleep curtailment and complaints, but research on this issue is scant [2,3].
Chronic sleep deprivation activates inflammatory processes in healthy adults as evidenced by increased circulating levels of C-reactive protein (CRP) [7] and interleukin (IL)-6 [8]. These inflammatory markers have prospectively been associated with increased cardiovascular risk, particularly incident myocardial infarction (MI) [9]. Independent of sleep apnea, the total arousal index correlates with plasma levels of the von Willebrand factor (VWF) antigen in middle-aged subjects [10]. VWF is a marker of endothelial cell activation and damage [11], previously shown to predict incident coronary artery disease [12].
In elderly subjects, sleep efficiency assessed by polysomnography was inversely correlated with D-dimer levels [13]. In caregivers of patients with Alzheimer's disease, stage 2 sleep [13]and wake after sleep onset (WASO) [14]were positively associated with D-dimer. An elevated D-dimer level indicates coagulation activation and is a prospective risk factor of incident and recurrent coronary events [15]. Therefore, the observation of increased D-dimer levels in dementia caregivers related to sleep is of potential clinical importance. Alzheimer caregivers run an increased risk of developing CVD [16,17] and, relative to their non-caregiving counterparts, also experience poorer sleep both objectively and subjectively [18,19]. A multitude of factors may predispose and contribute to impaired sleep in caregivers. These include age-related changes in sleep architecture and circadian rhythm, night-time awakenings related to nocturnal disruption by the care recipient, physical activity restriction, and the development of poor sleep hygiene practices [20].
We investigated the relationship between subjective and objective sleep assessed in the home environment and plasma concentration of atherosclerosis biomarkers IL-6, CRP, D-dimer and VWF in elderly Alzheimer caregivers and non-caregiving controls. We expanded on our previous investigations on an association between polysomnographic measures of disrupted sleep and biomarkers of atherosclerosis [13,14]. The novel aspect of the present study was the examination of objective sleep, as assessed by actigraphy over 3 nights, and subjective sleep quality in relation to markers of inflammation, coagulation and endothelial dysfunction in Alzheimer caregivers and non-caregiving elderly controls. Our primary hypothesis was that poor sleep relates to elevated levels of IL-6, CRP, D-dimer and VWF in all participants. Since we anticipated relatively poorer sleep in caregivers, our second hypothesis was that the relationship between relatively poorer sleep and higher levels of IL-6, CRP, D-dimer and VWF (all of which indicate an increased atherosclerotic risk) would be stronger in caregivers than in controls.
Methods
Study Participants
As part of a larger longitudinal study on effects of dementia caregiving stress on health, cross-sectional data were collected in 145 elderly subjects (97 caregivers and 48 non-caregiving controls). Inclusion criteria included being ≥55 years old and not taking anticoagulant and β-blocker medication. Caregivers were spouses of patients with Alzheimer's disease who lived with and provided informal in-home care for the patient. Controls were community-dwelling elderly not providing care to a household member, but also living with their spouse. In order to yield a representative sample of non-caregivers, controls were recruited in the same proportion of gender and were not excluded for life stress.
Participants were recruited from the University of California San Diego (UCSD) Alzheimer Disease Research Centers, through community support groups and from physician referrals. Controls were also recruited through senior communities and events. All participants gave their informed signed consent, which was approved by the UCSD Institutional Review Board.
Demographic and Medical Data
A research nurse conducted a structured medical history at the participants’ homes. For the present analysis, we specifically considered health characteristics which might affect circulating levels of studied biomarkers. Body mass index (BMI) was calculated as the ratio between weight in kilograms and height in square meters. Participants were asked whether a physician had ever told them that they had hypertension, hypercholesterolemia, type II diabetes or MI/stroke (grouped together as CVD) with answers coded as yes/no. Information about current cardiovascular medications (yes/no) was obtained in terms of regular use of aspirin, statins and angiotensin-converting enzyme (ACE) inhibitors. Smoking status was categorized into current smokers vs. former/never smokers. Alcohol intake was quantified in terms of how many days subjects consumed an alcoholic beverage in an average week during the previous 6 months with answers categorized into ≤1 day per week vs. ≥2 days per week of alcohol consumption. Physical exercise was quantified in terms of the number of days subjects exercised in an average week during the last 6 months with answers categorized into ≤1 day per week vs. ≥2 days per week of exercise.
Psychometric Assessment
Participants completed self-rated psychometric questionnaires on life stress and psychological distress during the nurse's home visit.
Life Stress
The Role Overload Scale was completed as a measure of life stress with respect to the extent to which caregivers and controls felt overwhelmed by their daily responsibilities [21]. The scale has 4 items rated between 1 (not at all) and 4 (completely) points, e.g. ‘you work hard (as a caregiver), but never seem to make any progress’. The sections of the items in parentheses specific to caregivers were omitted in the items presented to non-caregiving controls. We categorized total scores into tertiles (4–6 points, 38%; 7–9 points, 30%; 10–16 points, 32%).
Psychological Distress
The 53-item Brief Symptom Inventory (BSI) was applied to assess psychological distress over the past 6 months [22]. Response choices range from 0 (not at all) to 4 (extremely). Average responses to different subscales were used to calculate an overall score of global psychological distress (i.e. Global Severity Index) ranging from 0 to 4.
Sleep Assessment
Sleep Quality
Perceived characteristics of sleep quality were assessed with the interviewer-administered Pittsburg Sleep Quality Index (PSQI) [23]. The PSQI comprises 19 items, which can be grouped along 7 component scores, namely subjective sleep quality, sleep duration, sleep latency, sleep disturbances, sleep efficacy, use of sleep medication and daytime dysfunction. The 7 component scores are summed up to yield a global PSQI score of subjective sleep quality (range 0–21 points) to be used in our analysis. Higher scores on the PSQI indicate poorer sleep quality.
Objective Measures of Sleep
Objective characteristics of sleep were assessed with actigraphy. Actigraphy has been compared against polysomnography (the gold standard for assessing sleep) and has been shown to be reliable and valid [24]. Actigraphy has also been validated in older adults and has been particularly recommended for use in this population [25,26]. The SleepWatch-O actigraph (Ambulatory Monitoring Inc., Ardsley, N.Y., USA) was worn on the non-dominant wrist for 3 consecutive 24-hour periods (i.e. 72 h). The device detects movement via a piezoelectric bimorph-ceramic cantilever that generates a voltage each time the actigraph is moved. Voltages are gathered continuously and summarized over 1-min intervals. We report data based on the digital integration mode (PIM or proportional integration mode) corresponding most closely to polysomnography in the elderly. ActionW-2 software (Ambulatory Monitoring Inc.) was used to analyze actigraphy data. Participants also completed a sleep diary on which they were asked to record bed time and wake time as well as intervals during which the actigraph was removed for particular activities like showering or bathing. This information was utilized in editing the actigraph records as previously described [27].
Sleep variables used in the present analysis were selected a priori and averaged across 3 nights. These variables included: (1) total sleep time (the minutes per night spent sleeping while in bed); (2) percent sleep (the percent of minutes scored as sleep); (3) WASO (the time awake in minutes after sleep onset during the in-bed interval); (4) number of awakenings (bouts) after sleep onset; (5) duration of awakenings (mean duration of awakenings in minutes occurring after sleep onset). Because of partial redundancy among these sleep variables, we used total sleep time and WASO to merely describe sleep, but not for statistical analysis.
Analysis of Biomarkers
Subjects were studied between 9 a.m. and 11 a.m. At the participants’ home, venous fasting blood was drawn through a 22-gauge venous forearm catheter after a 20-min rest. Blood for the D-dimer and VWF antigen assays was dispensed into polypropylene tubes containing 3.8% sodium citrate (9:1, v/v) and spun at 2,000 g for 10 min at room temperature. Blood for the IL-6 and CRP assays was dispensed in ETDA tubes and spun at 3,000 g for 10 min at 4–8°C. Plasma aliquots were transported to the core lab and stored at −80°C in plastic tubes until analyzed. Plasma D-dimer and VWF antigen levels were measured by enzyme-linked immunosorbent assays (Asserachrom®, Stago, Asnières, France). Plasma levels of IL-6 were measured by a high-sensitive immunoassay kit (Quantikine; R&D Systems, Minneapolis, Minn., USA). Plasma CRP levels were determined by the High Sensitive CRP Reagent Set (DiaSorin, Stillwater, Minn., USA) using the Roche Cobas Mira Plus analyzer. Intra- and interassay coefficients of variation were <10% for all assays. Because of occasional assay problems, data were missing for IL-6 in 5 caregivers and 1 control, for CRP in 9 caregivers and 1 control, for D-dimer in 4 caregivers and 1 control, and for VWF in 3 caregivers.
Data Analysis
Data analysis was conducted with SPSS 15.0 (SPSS Inc., Chicago, Ill., USA). The significance level was set at p < 0.05 (2-tailed). Borderline significance (p < 0.10) is also presented to show trends that may help to illustrate relationships. To obtain a normal distribution, all sleep data were subject to Blom normal score transformation. BMI and all biomarker values were logarithmically transformed. After transformation, one D-dimer and one VWF value were >3 SDs above the sample mean and were consequently dropped, leaving 139, 135, 139 and 141 subjects, respectively, for the IL-6, CRP, D-dimer and VWF analysis. For clarity, we provide the original values in the text and tables.
Student's t test and Fisher's exact test were used, respectively, to calculate differences in continuous and categorical variables between groups. Bivariate associations between 2 variables were estimated using Pearson's correlation analysis. Multivariate linear regression analysis was employed, using forced entry, to identify which sleep characteristics were significantly associated with biomarkers independent of covariates. To prevent overfitted models, we restricted covariates to variables, which differed between caregivers and controls (i.e. age, exercise and role overload, but not the Global Severity Index because of redundancy with role overload), and to those showing bivariate correlations with biomarkers at p < 0.10. We first conducted regression analyses on the entire sample and, in a second step, separately on caregivers and controls, using Fisher Z-transformation to test whether correlations strengths between sleep variables and biomarkers would significantly differ between caregivers and controls [28].
Results
Health Characteristics
Table 1 shows that caregivers were older and exercised less often than controls with no significant differences in other demographic variables. Caregivers endorsed significantly more psychological distress and role overload relative to controls. Caregivers had higher D-dimer levels and, with borderline significance, higher IL-6 than controls.
Table 1.
Participant characteristics (n = 145)
| Caregivers (n = 97) | Controls (n = 48) | p | |
|---|---|---|---|
| Female, % | 71 | 73 | 1.000 |
| Age, years | 72.4 ± 8.7 | 67.9 ± 7.0 | 0.001 |
| BMI | 25.4 ± 4.4 | 25.8 ± 4.9 | 0.620 |
| CVD, %1 | 11 | 4 | 0.221 |
| Hypertension, % | 32 | 21 | 0.176 |
| Hypercholesterolemia, % | 32 | 35 | 0.710 |
| Type II diabetes, % | 4 | 6 | 0.685 |
| Aspirin use, % | 28 | 19 | 0.308 |
| Statin use, % | 18 | 19 | 1.000 |
| ACE inhibitor use, % | 21 | 12 | 0.260 |
| Current smoker, % | 7 | 4 | 0.718 |
| <2 alcohol days per week, % | 53 | 46 | 0.483 |
| <2 exercise days per week, % | 49 | 29 | 0.022 |
| Role overload, score | 9.19 ± 3.18 | 6.23 ± 2.09 | <0.001 |
| Psychological distress, score2 | 0.46 ± 0.42 | 0.30 ± 0.24 | 0.014 |
| IL-6, pg/ml | 1.40 ± 1.51 | 1.04 ± 0.97 | 0.071 |
| CRP, mg/l | 3.54 ± 6.53 | 2.39 ± 3.00 | 0.657 |
| D-dimer, ng/ml | 710 ± 491 | 465 ± 199 | <0.001 |
| VWF antigen, IU/ml | 160 ± 79 | 189 ± 137 | 0.298 |
| Perceived sleep quality, score3 | 7.8 ± 4.1 | 6.1 ± 3.5 | 0.017 |
| Total sleep time, min | 415 ± 77 | 413 ± 82 | 0.895 |
| Percent sleep, % | 88.9 ± 10.0 | 91.7 ± 10.3 | 0.025 |
| WASO, min | 51 ± 44 | 35 ± 36 | 0.010 |
| Awakenings, n | 8.0 ± 5.2 | 7.6 ± 4.6 | 0.870 |
| Mean duration of awakenings min | 6.5 ± 4.1 | 4.9 ± 5.1 | <0.001 |
Values are given as means ± SD or percentages. Comparison between groups done by Student's t test or Fisher's exact test.
ACE = Angiotensin-converting enzyme; PAI-1 = type 1 plasminogen activator inhibitor; t-PA = tissue-type plasminogen activator.
Combination of previous MI/stroke.
Measured by the Global Severity Index.
Measured by the PSQI.
Caregivers reported worse sleep quality and had lower percent sleep than controls. While the number of awakenings did not differ between groups, caregivers had significantly longer awakenings, corresponding to their increased WASO.
Bivariate Associations between Health Characteristics and Biomarkers
Table 2 shows the bivariate correlation coefficients for the associations between health characteristics and biomarkers. Women had higher CRP than men (3.49 ± 6.19 mg/l vs. 2.27 ± 3.54 mg/l, p = 0.032). Age was positively correlated with IL-6 and D-dimer (p < 0.001). BMI was positively correlated with IL-6 (p = 0.004), CRP (p < 0.001) and, with borderline significance, VWF (p = 0.076). Compared to former/never smokers, current smokers had higher CRP (5.51 ± 5.36 mg/l vs. 2.97 ± 5.57 mg/l, p = 0.042) and, with borderline significance, also higher IL-6 (1.88 ± 1.37 pg/ml vs. 1.24 ± 1.35 pg/ml, p = 0.088). Subjects with a positive history for hypertension and hypercholesterolemia, respectively, had higher D-dimer levels (787 ± 545 ng/ml vs. 560 ± 356 ng/ml, p = 0.014) and VWF (198 ± 128 IU/ml vs. 156 ± 86 IU/ml, p = 0.033) than subjects with a negative history.
Table 2.
Bivariate correlations with biomarkers
| IL-6 (n = 139) | CRP (n = 135) | D-dimer (n = 139) | VWF (n = 141) | |
|---|---|---|---|---|
| Gender | −0.06 | 0.19∗ | −0.12 | 0.06 |
| Age | 0.33∗∗∗ | −0.01 | 0.46∗∗∗ | 0.01 |
| BMI | 0.24∗∗ | 0.36∗∗∗ | 0.10 | 0.15+ |
| CVD | 0.11 | 0.03 | 0.11 | 0.02 |
| Hypertension | 0.13 | −0.03 | 0.23∗∗ | 0.04 |
| Hypercholesterolemia | 0.06 | −0.10 | 0.09 | 0.18∗ |
| Diabetes | −0.02 | 0.03 | <–0.01 | −0.09 |
| Aspirin use | 0.11 | −0.01 | 0.01 | −0.11 |
| Statin use | 0.03 | −0.13 | 0.02 | 0.08 |
| ACE inhibitor use | 0.15+ | −0.09 | 0.08 | 0.01 |
| Current smoker | 0.15+ | 0.18∗ | 0.06 | 0.02 |
| Alcohol (≥2 alcohol days/week) | −0.10 | −0.09 | −0.14 | −0.09 |
| Exercise (≥2 exercise days/week) | <–0.01 | −0.02 | 0.07 | 0.04 |
| Psychological distress | −0.02 | 0.05 | 0.02 | 0.01 |
| Role overload | −0.05 | 0.11 | 0.07 | 0.01 |
p < 0.10,
p < 0.05,
p < 0.01,
p < 0.001. ACE = Angiotensin-converting enzyme.
Correlations among Sleep Measures
In all participants, there was no correlation between PSQI scores and objective measures of percent sleep (p = 0.16), the number of awakenings (p = 0.07) or duration of awakenings (p = 0.21). The lack of association was seen both in caregivers and controls. Within the objective measures of sleep, percent sleep was inversely correlated with the number (r = −0.75, p < 0.001) and duration of awakenings (r = −0.65, p < 0.001). The number and mean duration of awakenings were not associated with each other (p = 0.14). Similar correlations were observed among sleep variables in caregivers and controls separately.
Correlations among Biomarkers
In the entire sample, IL-6 correlated with CRP (r = 0.37, p < 0.001), D-dimer (r = 0.28, p = 0.001) and VWF (r = 0.017, p = 0.049). D-dimer and VWF were also correlated with each other (r = 0.28, p = 0.001) and CRP showed borderline significance for a correlation with VWF (r = 0.15, p = 0.089). Similar correlations were observed in each group separately.
Correlations among Sleep Measures and Biomarkers
Subjective Sleep Quality and Biomarkers
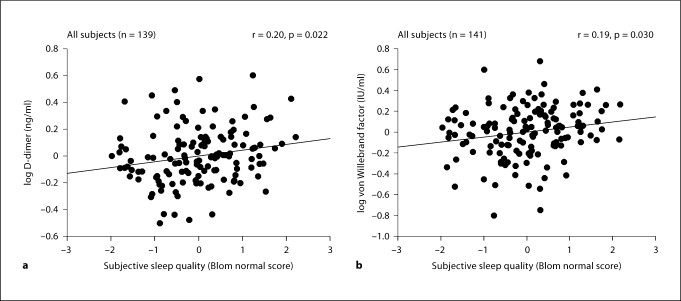
PSQI scores were inversely correlated with D-dimer and VWF in all participants, such that worse subjective sleep quality was associated both with higher levels of D-dimer (p = 0.009) and VWF (p = 0.025; table 3). These correlations maintained significance after taking into account covariates (D-dimer: p = 0.022, ΔR2 = 0.029; VWF: p = 0.029, ΔR2 = 0.034; fig. 1. a, b). When individual groups were analyzed, there were similar significant correlations between sleep quality and VWF in caregivers, but not in controls, and between sleep quality and D-dimer in controls, but not in caregivers. Correlation coefficients did not significantly differ between groups.
Table 3.
Crude and adjusted correlations between subjective sleep quality and biomarkers
| PSQI |
||||
|---|---|---|---|---|
| all | caregivers | controls | p | |
| IL-61 | 0.05 | 0.05 | −0.05 | 0.602 |
| 0.04 | 0.05 | −0.07 | 0.511 | |
| CRP2 | −0.02 | −0.03 | −0.03 | 0.979 |
| −0.09 | −0.07 | −0.09 | 0.909 | |
| D-dimer3 | 0.22∗∗ | 0.12 | 0.36∗ | 0.167 |
| 0.20∗ | 0.08 | 0.33∗ | 0.157 | |
| VWF4 | 0.19∗ | 0.29∗∗ | 0.06 | 0.176 |
| 0.19∗ | 0.27∗ | 0.06 | 0.220 | |
All analyses were adjusted for age, exercise and role overload. The columns show the bivariate (upper row) and adjusted (lower row) correlation coefficients with significance level for all participants, caregivers and controls, and the p value for the comparison of the strength of the correlation coefficients between caregivers and controls.
p < 0.05,
p < 0.01.
Additionally adjusted for BMI, angiotensin-converting enzyme inhibitor use and smoking.
Additionally adjusted for gender, BMI and smoking.
Additionally adjusted for hypertension.
Additionally adjusted for BMI and hypercholesterolemia.
Fig. 1.
The panels depict the partial regression plots with fit line for the relationship between subjective sleep quality (PSQI) and D-dimer (a) and VWF (b) in all participants. All analyses were controlled for age, BMI and role overload. In addition, D-dimer values were adjusted for hypertension, and VWF values were adjusted for BMI and hypercholesterolemia.
Objective Sleep and Biomarkers
For all participants, there were significant inverse correlations between percent sleep and IL-6 (p = 0.004) and D-dimer (p = 0.032), as well as positive correlations between mean duration of awakenings and IL-6 (p = 0.002) and D-dimer (p = 0.004), and between the number of awakenings and VWF (p = 0.026). After adjusting for covariates, these associations were no longer significant. The positive association between the number of awakenings and VWF (p = 0.026) became of borderline significance when taking covariates into account (p = 0.053, ΔR2 = 0.026).
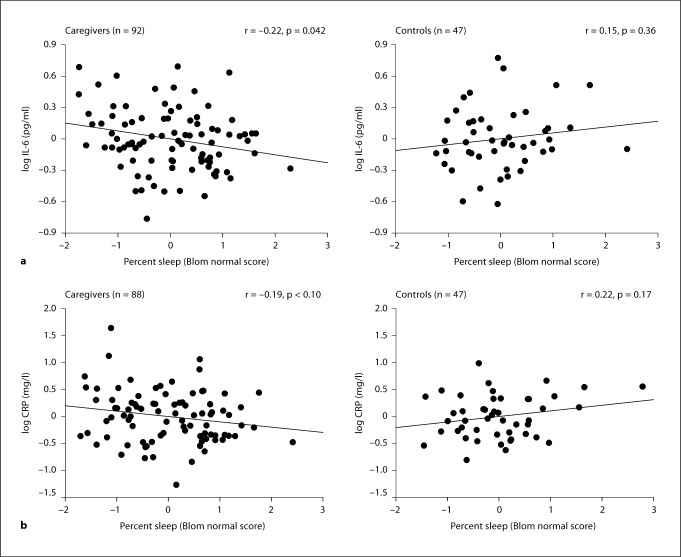
When caregiver and control groups were compared, there were significant correlations between some objective measures of sleep and biomarkers in caregivers, but not in controls. Specifically in caregivers, percent sleep showed an inverse correlation with IL-6 (p = 0.006) and, with borderline significance, CRP (p = 0.098). The significance of these correlations was maintained after controlling for covariates (IL-6: p = 0.042, ΔR2 = 0.039; CRP: p = 0.095, ΔR2 = 0.027) and were also significantly different from those in controls (fig. 2. a, b).
Fig. 2.
The panels depict the partial regression plots with fit line for the relationship between percent sleep and IL-6 (a) and CRP (b) in caregivers and controls. The strength of the partial correlations was significantly different between caregivers and controls for IL-6 (p = 0.043) and CRP (p = 0.028). All analyses were controlled for age, BMI, exercise, smoking and role overload. In addition, IL-6 and CRP values were adjusted for angiotensin converting enzyme inhibitor use and gender, respectively.
Moreover, caregivers showed a positive correlation between the number of awakenings and VWF (p = 0.012), maintaining significance after adjusting for covariates (p = 0.013, ΔR2 = 0.065); however, this correlation did not significantly differ between caregivers and controls. In caregivers, the mean duration of awakenings correlated positively with IL-6 (p = 0.014) and D-dimer (p = 0.007), whereby both associations lost significance when taking covariates into account. Moreover, the bivariate correlation between the mean duration of awakenings and D-dimer was significantly different in caregivers from the one in controls, with this difference losing its significance after adjusting for covariates. Although not significant within each group, the multivariate correlations between the higher number of awakenings and elevated IL-6 and CRP in caregivers showed borderline significance for being different from controls.
Discussion
In the entire sample of our community-dwelling elderly participants, we found an inverse relationship between impaired subjective sleep quality and elevated D-dimer and VWF concentration independent of covariates. These findings provide one possible explanation for the previously observed association between sleep complaints and the risk for developing incident fatal and non-fatal coronary artery disease. Difficulties falling asleep, maintaining sleep and waking up unrefreshed have predicted MI and coronary death in various populations [29,30,31,32]. In particular, there was a gradual relationship between the risk of incident MI and more frequent experience of troubles falling asleep, waking too early and restless sleep in participants aged 65 years or over [33]. In our study, across all participants, several significant bivariate correlations between impaired sleep and elevated biomarkers of increased atherosclerotic risk were accounted for by covariates. Borderline significance was observed for the positive association between the number of awakenings and VWF.
Confirming our assumption, both objective measures of sleep and subjective sleep quality were worse in caregivers than in controls. As opposed to control subjects, caregivers showed several significant associations between objectively assessed poor sleep and biomarkers. The inverse relationships between percent sleep and IL-6 and the positive association between the number of awakenings and VWF withstood adjustment for covariates. However, nonsignificant associations observed in caregivers might partly relate to decreased statistical power since the associations between lowered percent sleep and elevated IL-6 and CRP alone were significantly different in caregivers compared to controls in multivariate analysis. Additionally, the relationship between more awakenings and elevated levels of IL-6 and CRP in caregivers showed borderline significance for being different from the associations found in controls.
Autonomic dysfunction appears to be a key mechanism in the link between poor cardiovascular health and sleep complaints and objectively perturbed sleep [2,3]. Specific to our findings, VWF was shown to be responsive to epinephrine infusion [34], and morning D-dimer levels were shown to be associated with overnight catecholamine excretion [35]. Catecholamines have also been shown to increase IL-6 gene expression in different cells [36,37], systemic IL-6 [37,38] and CRP [39]. In addition, in a comparably smaller sample of caregivers than investigated in the present study, we found that elevated plasma norepinephrine levels accounted for a significant proportion of the positive relationship between WASO and D-dimer levels [14].
Our results must be interpreted within the limitations of the study design and population. Moreover, our findings are preliminary in several respects and their clinical relevance needs to be demonstrated. The individual variance of biomarkers explained by poor sleep was rather small. One reason for this might be that we sampled biomarkers at only one point in time. A single point assessment of biomarker levels does not account for the intra-individual variation over time and it captures only partially the functioning of complex biologic systems like coagulation, inflammation and the endothelium [40]. On the other hand, we collected blood during a relatively narrow time window of 9:00–11:00 a.m., which would have reasonably restricted any diurnal variation in IL-6, CRP, D-dimer and VWF [41,42]. We performed multiple comparisons, but decided not to adjust the significance level because we were testing one a priori hypothesis in several different ways (i.e. poor sleep would be associated with elevated levels of biomarkers of increased atherosclerotic risk) [43]. Some findings only showed a trend towards significance or lost significance in analyses adjusting for covariates. With a larger sample size, the results could be interpreted with less statistical ambiguity. For instance, in the entire sample, percent sleep was significantly related to IL-6 and D-dimer in the bivariate correlation analysis, but not after taking covariates into account. To observe these relationships as significantly independent of covariates, 197 and 601 participants, respectively, would be required for IL-6 and D-dimer. The sample size also precluded controlling for a more extensive array of confounders. The study of such confounders is particularly important when studying the elderly, most of whom endorse medical problems and medication potentially affecting biomarkers. However, whether the relationship between poor sleep and biomarkers would be even stronger in institutionalized elderly and those with a higher prevalence of pre-existing CVD remains unclear.
Poor subjective sleep and insomnia might be viewed as surrogate markers of chronic stress, poor health and psychological distress [33]. Such arguing would, however, not necessarily discount the importance of insomnia as a valid marker for someone at risk for MI [33]. Moreover, controlling for medical comorbidity and role overload did not attenuate the significant predictive value of the PSQI for plasma VWF and D-dimer levels. The same was true when controlling for the Global Severity Index instead of role overload (not shown). We investigated 4 biomarkers of atherosclerosis covering important areas of pathophysiology in the process of atherosclerosis [9,12,15]. Nonetheless, biomarkers were not uniformly related to sleep and distinct relationships might exist, although such reasoning requires confirmation. Other biomarkers, particularly tumor necrosis factor-α and IL-1β, might have been revealed to be more accurate correlates of sleep. These proinflammatory cytokines are ultimately involved in eliciting sickness behavior that is, among other symptoms, characterized by perturbed sleep [44]. With regard to the role of inflammation in sickness behavior and cross-talking of inflammation and coagulation [45], also observed in our participants, the cross-sectional design precludes any inferences about the directionality of relationships between sleep and biomarkers.
To sum up, we found that subjective impairment in sleep was independently associated with markers of increased coagulation activation and endothelial dysfunction in elderly community-dwelling subjects. Impairment in objective sleep, particularly decreased percent sleep, was independently associated with increased inflammation activity in caregivers relative to controls. These associations may help explain the previously shown increased CVD risk in elderly poor sleepers in general and in Alzheimer caregivers in particular.
Table 4.
Crude and adjusted correlations between objective measures of sleep and biomarkers
| Percent sleep |
Number of awakenings |
Mean duration of awakenings |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| all | CGR | CTR | p | all | CGR | CTR | p | all | CGR | CTR | p | |
| IL-6 | −0.24∗∗ | −0.29∗∗ | −0.10 | 0.288 | 0.10 | 0.15 | 0.01 | 0.457 | 0.26∗∗ | 0.26∗ | 0.16 | 0.597 |
| −0.14 | −0.22∗ | 0.15 | 0.043 | 0.08 | 0.16 | −0.14 | 0.099 | 0.14 | 0.14 | 0.04 | 0.599 | |
| CRP | −0.12 | −0.18+ | 0.03 | 0.265 | 0.02 | 0.12 | −0.22 | 0.066 | 0.13 | 0.09 | 0.19 | 0.598 |
| −0.07 | −0.19+ | 0.22 | 0.028 | 0.03 | 0.15 | −0.19 | 0.066 | 0.08 | 0.10 | −0.08 | 0.345 | |
| D-dimer | −0.19∗ | −0.20+ | 0.02 | 0.221 | −0.01 | −0.03 | 0.02 | 0.786 | 0.24∗∗ | 0.28∗∗ | −0.14 | 0.022 |
| −0.08 | −0.11 | 0.09 | 0.281 | −0.01 | −0.03 | −0.07 | 0.841 | 0.05 | 0.10 | −0.16 | 0.159 | |
| VWF | −0.04 | −0.13 | 0.06 | 0.281 | 0.19∗ | 0.26∗ | 0.06 | 0.254 | −0.09 | −0.05 | −0.10 | 0.770 |
| <–0.01 | −0.11 | 0.04 | 0.410 | 0.17+ | 0.27∗ | 0.08 | 0.282 | −0.14+ | −0.11 | −0.08 | 0.842 | |
The columns show the bivariate (upper row) and adjusted (lower row) correlation coefficients with significance level for all participants, caregivers (CGR) and controls (CTR), and the p value for the comparison of the strength of the correlation coefficients between caregivers and controls. See table 3 footnotes for statistical adjustments of individual biomarkers.
p < 0.10,
p < 0.05,
p < 0.01.
Acknowledgements
The authors are grateful to Susan Calleran, M.A., Carolyn Swenerton, R.N., and Sharyn Wilenski, R.N., for data collection. The study was supported by grants NIA AG 15301, NIA AG 08415 and M01 RR00827 from the National Institutes of Health.
References
- 1.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease. Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 2.Wolk R, Gami AS, Garcia-Touchard A, Somers VK. Sleep and cardiovascular disease. Curr Probl Cardiol. 2005;30:625–662. doi: 10.1016/j.cpcardiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz S, McDowell Anderson W, Cole SR, Cornoni-Huntley J, Hays JC, Blazer D. Insomnia and heart disease: a review of epidemiologic studies. J Psychosom Res. 1999;47:313–333. doi: 10.1016/s0022-3999(99)00029-x. [DOI] [PubMed] [Google Scholar]
- 4.Mills PJ, Dimsdale JE. Sleep apnea: a model for studying cytokines, sleep, and sleep disruption. Brain Behav Immun. 2004;18:298–303. doi: 10.1016/j.bbi.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 5.von Känel R, Dimsdale JE. Hemostatic alterations in patients with obstructive sleep apnea and the implications for cardiovascular disease. Chest. 2003;124:1956–1967. doi: 10.1378/chest.124.5.1956. [DOI] [PubMed] [Google Scholar]
- 6.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med. 2008;177:369–375. doi: 10.1164/rccm.200608-1190PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 9.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 2002;252:283–294. doi: 10.1046/j.1365-2796.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 10.von Känel R, Loredo JS, Ancoli-Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest. 2007;131:733–739. doi: 10.1378/chest.06-2006. [DOI] [PubMed] [Google Scholar]
- 11.Lip GY, Blann A. von Willebrand factor: a marker of endothelial dysfunction in vascular disorders? Cardiovasc Res. 1997;34:255–265. doi: 10.1016/s0008-6363(97)00039-4. [DOI] [PubMed] [Google Scholar]
- 12.Whincup PH, Danesh J, Walker M, Lennon L, Thomson A, Appleby P, Rumley A, Lowe GD. von Willebrand factor and coronary heart disease: prospective study and meta-analysis. Eur Heart J. 2002;23:1764–1770. doi: 10.1053/euhj.2001.3237. [DOI] [PubMed] [Google Scholar]
- 13.von Känel R, Dimsdale JE, Ancoli-Israel S, Mills PJ, Patterson TL, McKibbin CL, Archuleta C, Grant I. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D-dimer in older caregivers of people with Alzheimer's disease. J Am Geriatr Soc. 2006;54:431–437. doi: 10.1111/j.1532-5415.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 14.Mausbach BT, Ancoli-Israel S, von Känel R, Patterson TL, Aschbacher K, Mills PJ, Ziegler MG, Dimsdale JE, Calleran S, Grant I. Sleep disturbance, norepinephrine, and D-dimer are all related in elderly caregivers of people with Alzheimer disease. Sleep. 2006;29:1347–1352. doi: 10.1093/sleep/29.10.1347. [DOI] [PubMed] [Google Scholar]
- 15.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, Rumley A, Lowe GD. Fibrin D-dimer and coronary heart disease: prospective study and meta-analysis. Circulation. 2001;103:2323–2327. doi: 10.1161/01.cir.103.19.2323. [DOI] [PubMed] [Google Scholar]
- 16.Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Hirsch IB, Siegler IC. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosom Med. 2002;64:418–435. doi: 10.1097/00006842-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 17.von Känel R, Mausbach BT, Patterson TL, Dimsdale JE, Aschbacher K, Mills PJ, Ziegler MG, Ancoli-Israel S, Grant I. Increased Framingham Coronary Heart Disease Risk Score in dementia caregivers relative to non-caregiving controls. Gerontology. 2008;54:131–137. doi: 10.1159/000113649. [DOI] [PubMed] [Google Scholar]
- 18.McKibbin CL, Ancoli-Israel S, Dimsdale J, Archuleta C, von Kanel R, Mills P, Patterson TL, Grant I. Sleep in spousal caregivers of people with Alzheimer's disease. Sleep. 2005;28:1245–1250. doi: 10.1093/sleep/28.10.1245. [DOI] [PubMed] [Google Scholar]
- 19.Creese J, Bédard M, Brazil K, Chambers L. Sleep disturbances in spousal caregivers of individuals with Alzheimer's disease. Int Psychogeriatr. 2008;20:149–161. doi: 10.1017/S1041610207005339. [DOI] [PubMed] [Google Scholar]
- 20.McCurry SM, Logsdon RG, Teri L, Vitiello MV. Sleep disturbances in caregivers of persons with dementia: contributing factors and treatment implications. Sleep Med Rev. 2007;11:143–153. doi: 10.1016/j.smrv.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist. 1990;30:583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- 22.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 23.Buysse DJ, Reynolds CF, 3rd, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–338. [PubMed] [Google Scholar]
- 24.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 25.Ancoli-Israel S: Actigraphy; in: Kryger MH, Roth T, Dement WC. (eds): Principles and Practice of Sleep Medicine. Philadelphia: Saunders; 2005. pp. 1459–1467. [Google Scholar]
- 26.Blackwell T, Redline S, Ancoli-Israel S, Schneider JL, Surovec S, Johnson NL, Cauley JA, Stone KL. Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep. 2008;31:283–291. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackwell T, Ancoli-Israel S, Gehrman PR, Schneider JL, Pedula KL, Stone KL. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28:1599–1605. doi: 10.1093/sleep/28.12.1599. [DOI] [PubMed] [Google Scholar]
- 28.Papoulis A. Probability and Statistics. Englewood Cliffs: Prentice-Hall International Editions; 1990. [Google Scholar]
- 29.Appels A, de Vos Y, van Diest R, Höppner P, Mulder P, de Groen J. Are sleep complaints predictive of future myocardial infarction? Act Nerv Super (Praha) 1987;29:147–151. [PubMed] [Google Scholar]
- 30.Eaker ED, Pinsky J, Castelli WP. Myocardial infarction and coronary death among women: psychosocial predictors from a 20-year follow-up of women in the Framingham Study. Am J Epidemiol. 1992;135:854–864. doi: 10.1093/oxfordjournals.aje.a116381. [DOI] [PubMed] [Google Scholar]
- 31.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207–216. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 32.Friedman GD, Ury HK, Klatsky AL, Siegelaub AB. A psychological questionnaire predictive of myocardial infarction: results from the Kaiser-Permanente epidemiologic study of myocardial infarction. Psychosom Med. 1974;36:327–343. doi: 10.1097/00006842-197407000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz SW, Cornoni-Huntley J, Cole SR, Hays JC, Blazer DG, Schocken DD. Are sleep complaints an independent risk factor for myocardial infarction? Ann Epidemiol. 1998;8:384–392. doi: 10.1016/s1047-2797(97)00238-x. [DOI] [PubMed] [Google Scholar]
- 34.von Känel R, Dimsdale JE. Effects of sympathetic activation by adrenergic infusions on hemostasis in vivo. Eur J Haematol. 2000;65:357–369. doi: 10.1034/j.1600-0609.2000.065006357.x. [DOI] [PubMed] [Google Scholar]
- 35.von Känel R, Kudielka BM, Abd-el-Razik A, Gander ML, Frey K, Fischer JE. Relationship between overnight neuroendocrine activity and morning haemostasis in working men. Clin Sci (Lond) 2004;107:89–95. doi: 10.1042/CS20030355. [DOI] [PubMed] [Google Scholar]
- 36.Briest W, Rassler B, Deten A, Leicht M, Morwinski R, Neichel D, Wallukat G, Ziegelhöffer T, Zimmer HG. Norepinephrine-induced interleukin-6 increase in rat hearts: differential signal transduction in myocytes and non-myocytes. Pflugers Arch. 2003;446:437–446. doi: 10.1007/s00424-003-1043-x. [DOI] [PubMed] [Google Scholar]
- 37.Keller P, Keller C, Robinson LE, Pedersen BK. Epinephrine infusion increases adipose interleukin-6 gene expression and systemic levels in humans. J Appl Physiol. 2004;97:1309–1312. doi: 10.1152/japplphysiol.00284.2004. [DOI] [PubMed] [Google Scholar]
- 38.DeRijk RH, Boelen A, Tilders FJ, Berkenbosch F. Induction of plasma interleukin-6 by circulating adrenaline in the rat. Psychoneuroendocrinology. 1994;19:155–163. doi: 10.1016/0306-4530(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 39.Schade R, Göhler K, Bürger W, Hirschelmann R. Modulation of rat C-reactive protein serum level by dexamethasone and adrenaline – comparison with the response of alpha 2-acute phase globulin. Agents Actions. 1987;22:280–287. doi: 10.1007/BF02009057. [DOI] [PubMed] [Google Scholar]
- 40.von Känel R. Psychological distress and cardiovascular risk: what are the links? J Am Coll Cardiol. 2008;52:2163–2165. doi: 10.1016/j.jacc.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Rudnicka AR, Rumley A, Lowe GD, Strachan DP. Diurnal, seasonal, and blood-processing patterns in levels of circulating fibrinogen, fibrin D-dimer, C-reactive protein, tissue plasminogen activator, and von Willebrand factor in a 45-year-old population. Circulation. 2007;115:996–1003. doi: 10.1161/CIRCULATIONAHA.106.635169. [DOI] [PubMed] [Google Scholar]
- 42.Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, Prolo P, Wong ML, Licinio J, Gold PW, Hermida RC, Mastorakos G, Chrousos GP. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- 43.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr Opin Hematol. 2007;14:55–61. doi: 10.1097/00062752-200701000-00011. [DOI] [PubMed] [Google Scholar]