Abstract
Objective
The matrix metalloproteinases (MMPs) have been implicated in the aggressive course of non-small cell lung cancer (NSCLC). However, there are a large number of MMP subtypes with diverse proteolytic substrates and different induction pathways. This study tested the hypothesis that a differential MMP profile would exist between NSCLC and normal lung and that MMP patterns would differ between NSCLC histologic type.
Methods
NSCLC samples and remote normal samples were obtained from patients with stage I or II NSCLC with either squamous cell (n=22) or adenocarcinoma (n=19) histology. Absolute concentrations for each of the MMP subclasses; collagenases (MMP-1, 8, -13), gelatinases (MMP-2,-9), lysins (MMP-2, -7) and elastase (MMP-12) were determined by a calibrated and validated multiplex suspension array.
Results
Overall, MMP levels were significantly increased in NSCLC compared to normal. For example, MMP-1 and MMP-7 increased by approximately 10 fold in NSCLC (p<0.05). Moreover, a different MMP portfolio was observed between NSCLC histologic types. For example MMP-1,-8,-9 and -12 increased by over 4-fold in squamous cell versus adenocarcinoma (p<0.05). In those patients who recurred within 3 years of resection, 3-fold higher levels of MMP-8 and -9 were observed (p<0.05).
Conclusion
Increased levels of a number of MMP types occur with NSCLC, but the MMP profile was distinctly different between histologic types and in those patients with recurrence. These different MMP profiles may be important in the mechanistic basis for the natural history of different NSCLC types, as well as identifying potential prognostic and therapeutic targets.
Keywords: matrix metalloproteinases, lung cancer, multiplex, recurrence
Introduction
Matrix metalloproteinases (MMPs) are a large family of structurally and functionally related zinc endopeptidases which collectively can degrade virtually all extracellular matrix (ECM) components and can regulate the tumor microenvironment through processing substrates including growth factors and their receptors, cell adhesion molecules, cytokines, chemokines, apoptotic ligands and angiogenic factors.1-2 The potential role and regulation of MMPs have been the subject of a number of past studies in lung cancer.1-12 However, these past studies usually focused upon a single MMP type or class, and therefore the pattern of MMP expression in lung cancer remained poorly understood. Moreover, while the diversity and complexity of the MMP family are becoming well recognized, the initial strategy for MMPs in the context of cancer therapeutics was that of broad-spectrum, global MMP inhibitors.13-16 These initial broad-spectrum MMP inhibitors were not successful, and the underlying reasons for this were multi-factorial and likely included poor specificity and adverse side effects. One outcome from these past MMP pharmacological studies was the recognition that more targeted therapeutic approaches would be required. Thus, studies which more carefully quantify a more diverse number of MMPs in the context of cancer, such as non-small cell lung cancer (NSCLC) are warranted. Accordingly, the overall goal of the present study was to quantify a large number of MMP types, from different MMP classes, in samples taken from nonsmall cell lung cancer (NSCLC) and compare these to normal regions from the same patient. Moreover, this study examined whether and to what degree different MMP portfolios would emerge in NSCLC of different histological types and in patients with early recurrence.
Materials and Methods
Patients
Snap frozen lung specimens were taken from the targeted resection region from patients with either stage I or II NSCLC (22 squamous cell; 19 adenocarcinoma) or from the remote, normal region. These samples were retained under cryogenic conditions in the Hollings Cancer Center, MUSC tissue repository. The adenocarcinoma cohort consisted of 16 patients with stage I and 3 patients with stage II (4 males; 15 females). The squamous cell cohort included 16 patients with stage I and 6 patients with stage II (18 males; 4 females). Prior to tissue sampling at the time of tumor resection, all patients had signed an informed consent for tissue banking purposes, and this specific study was approved by the Institutional Review Board of the Medical University of South Carolina, Charleston, South Carolina (HR# 18229). The data obtained from these banked samples included the histology type (squamous cell carcinoma, adenocarcinoma) as well as whether recurrence had occurred within 3 years of the initial resection. Recurrence was defined as loco-regional (recurrence of tumor within the lung or mediastinal lymph nodes ipsilateral to the side of surgery) or distant.
Sample Preparation
The lung samples were processed in a simultaneous fashion using a homogenization and extraction protocol which has been demonstrated previously to successfully remove both high and low molecular weight proteins, such as MMPs, from solid tissue samples.17-19 This method utilizes a homogenization process, an extraction buffer and a centrifugation method by which a complete uniform yield of intracellular and extracellular protein is obtained. Briefly, a uniform weight (50 mg) of the frozen samples were placed in ice-cold extraction/homogenization buffer (buffer volume used is 1:6 w/v; containing 10mM cacodylic acid pH 5.0, 0.15 M NaCl, 20 mM ZnCl, 1.5 mM NaN3, and 0.01% Triton X-100 (v/v)) and allowed to thaw on ice. We have demonstrated previously that this approach stabilizes proteins and proteases during the extraction process.18,20 Stainless steel beads (5 mm) were placed in the samples, and the beads were oscillated at a high frequency (30 Hz; 2 cycles, 5 min each) resulting in full tissue disruption and uniform homogneization (TissueLyserII, Cat#85300, Qaigen). This homogenization approach utilizes a full cell disruption technique in order to fully extract intracellular and extracellular proteins from a solid tissue sample. The homogenate was then centrifuged (800 × g, 10min, 4°C) (model 5810 or 5417c, Eppendorf, Westbury, NY) and the supernatant removed to a fresh tube and stored on ice. Protein concentrations were determined in duplicate from the final extracts by the Bradford method (Bio-Rad Protein Assay, Hercules, CA) and then titrated to a uniform protein concentration (100 ug), aliquoted and stored at −80°C until subjected to multiplex suspension array analysis.
Analytical Measurements
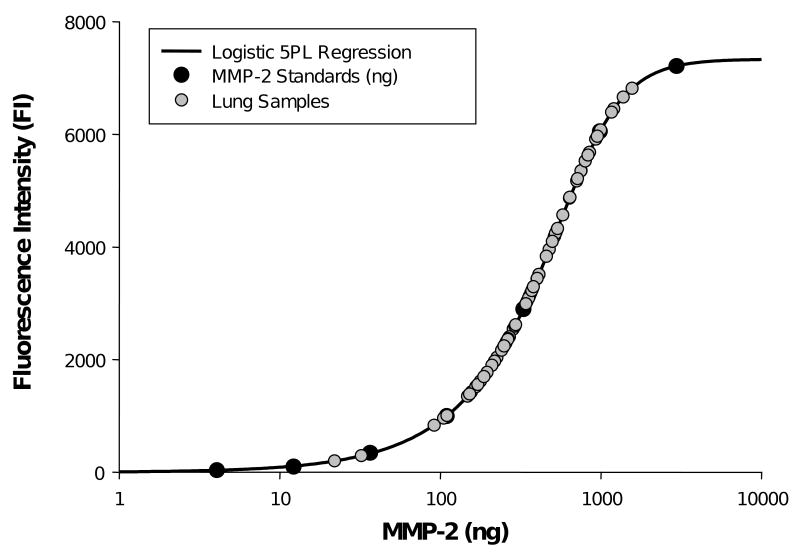
The protein titrated samples were thawed on ice and subjected to multiplex suspension array (Human Fluorokine MAP MMP Kit, R&D Systems, LMP000) in which all samples could be measured in simultaneous fashion, thereby minimizing inter-assay coefficient of variation. The titrated samples were subjected to this assay in triplicate and these triplicate values were then examined with respect to intra-assay coefficient of variation. For repeated samples that were outside of a 15% confidence interval (3 occurrences in the present study), the entire homogenization and assay was repeated. The multiplex array was previously validated and calibrated using internal controls for each MMP type.21 The matrix was assembled in order to measure representative subtypes from each class of MMPs which included the collagenases (MMP-1, MMP-8, MMP-13), the gelatinases (MMP-2 and MMP-9), the stromelysins/matrilysins (MMP-3/MMP-7) and the elastases (MMP-12). The relative fluorescence obtained for each distinct MMP (Bio-Plex 200, BioRad Laboratories) was converted to an absolute concentration using standards that were included in each assay and the specifications for each reagent and sensitivity are shown in Table 1. The coefficient of variation for these assays was 15% or less. For all of the MMP multiplex assays, the sample readings fell within the targeted dynamic range for the analyte of interest. A representative standard curve, and the sample results for MMP-2 is shown in Figure 1. All MMP assays were blinded to region, histology, and status of recurrence.
Table 1. Multiplex matrix metalloproteinase (MMP) antisera and sensitivity.
| Analyte | Manufacturer | Catalog Number | Sensitivity |
|---|---|---|---|
| MMP-1 | R&D Systems | LMP901 | 4.44 pg/mL |
| MMP-2 | R&D Systems | LMP902 | 25.4 pg/mL |
| MMP-3 | R&D Systems | LMP513 | 1.3 pg/mL |
| MMP-7 | R&D Systems | LMP907 | 16.9 pg/mL |
| MMP-8 | R&D Systems | LMP908 | 8.9 pg/mL |
| MMP-9 | R&D Systems | LMP911 | 7.4 pg/mL |
| MMP-12 | R&D Systems | LMP919 | 1.2 pg/mL |
| MMP-13 | R&D Systems | LMP511 | 15.9 pg/mL |
Figure 1.
A representative 5-parameter (5-PL) logistic curve developed for MMP-2 using a wide range of MMP-2 recombinant control standards (black circles). The 5-PL uses the equation where y=d+(a-d)/(1+(x/c) ˆb) ˆg) in which x= concentration of standard, y si the response variable, a is the estimated response at 0 concentration, b is the slope of the tangent midpoint, c is the midrange of concentration or midpoint, d is the estimated response at infinite concentration, and g is an asymmetry factor. This non-linear regression fitting is optimal for response variables such as fluorescence emission.22 The lung samples which were loaded using identical protein concentrations (gray circles) all fell within the dynamic range of the standards and the 5-PL equation.
Data Analysis
The fluorescence emissions for each set of MMP standards were first fit to a 5-parameter logistic (5-PL) equation (Figure 1), following a conventional format for nonlinear fluorescence profiles.22 Using this 5-PL curve fit, the fluorescence emissions for the samples were converted to actual MMP values, and then finally to MMP content/mg of wet sample weight. The comparisons of MMP profiles by region and by histologic type, pair-wise comparisons were performed using a t-statistic. For the purposes of comparisons of MMP profiles by both region and histologic type, analysis of variance (ANOVA) was performed in which region and histology were considered main treatment effects. For the purposes of examining the relative MMP profiles to recurrence rates, a categorical analysis was performed. First, the samples were stratified to recurrence or no recurrence and the relative MMP values compared between these stratifications. Since these data were not normally distributed, a Wilcoxon test was performed. All statistical procedures were performed using STATA statistical software (STATA Intercooled V 8.0. College Station, TX). Results are presented as mean ± standard error of the mean (SEM). Values of p<0.05 were considered to be statistically significant.
Results
The summary of MMP levels, by region (normal, NSCLC) and by histologic type are presented in Table 2. The MMP types examined were categorized by sub-groups as shown. For the MMP collagenase subgroup, MMP-1 and MMP-8 increased by over 3-fold. Relative levels for MMP-13 appeared increased in the NSCLC region, but due to a high degree of variation between samples, this did not reach statistical significance. For the MMP gelatinase subgroup, MMP-2 levels were increased by approximately 3-fold in the NSCLC region. In the lysin and the elastase subgroups, MMP-7 and MMP-12 respectively, were increased by over 10-fold in the NSCLC region. Moreover, a different MMP portfolio was observed between NSCLC histologic types. For example MMP-1,-8,-9 and -12 increased by over 4-fold in squamous cell versus adenocarcinoma. In light of the fact that MMP levels changed as a function of region (normal, NSCLC) and histologic type, ANOVA was performed in order to identify main effects and potential interactions (Table 3). From this analysis, it was demonstrated that a significant region and histologic type interaction occurred for MMP-1, -8, and MMP-12, with MMP-9 approaching statistical significance. This analysis further confirmed the univariate analysis in that changes in the levels of specific MMP types were affected by NSCLC as well as by histology. Finally, MMP levels in patients whose tumor did not recur were compared to MMP concentrations in patients whose tumor did recur within 3 years (Table 4). Recurrence was evenly divided between distant and loco-regional. From this approach, increased levels for MMP-8 and MMP-9 were observed within the NSCLC region in those patients with recurrence.
Table 2. Matrix metalloproteinase (MMP) content in normal and non-small cell lung cancer specimens. MMP types are presented by MMP class.
| Region | Histology Type | |||||
|---|---|---|---|---|---|---|
| MMP Type | Normal | NSCLC | p- value | AdenoCa | Sq Cell Ca | p-value |
| Collagenases | ||||||
| MMP-1 | 1.3±0.9 | 33.7±10.9 | 0.0025 | 8.3±2.4 | 55.7±19.2 | 0.0115 |
| MMP-13 | 0.1 ±0.1 | 7.9±5.3 | 0.0731 | 1.8±0.6 | 13.1±9.8 | 0.1347 |
| MMP-8 | 85.2±10.8 | 296.4±84.0 | 0.0084 | 53.3±22.6 | 506.4±141.9 | 0.0023 |
| Gelatinases | ||||||
| MMP-2 | 57.3±7.5 | 161.0±58.8 | 0.0437 | 79.7±20.3 | 231.3±107.0 | 0.0888 |
| MMP-9 | 216.3±24.3 | 185.5±37.8 | 0.2479 | 89.5±28.6 | 268.4±61.4 | 0.0065 |
| Lysins | ||||||
| MMP-3 | 0.7±0.1 | 38.6±30.8 | 0.1130 | 1.3±0.4 | 70.8±57.1 | 0.1183 |
| MMP-7 | 7.9±1.5 | 75.1±15.8 | 0.0001 | 87.4±23.0 | 63.5±21.9 | 0.4559 |
| Elastases | ||||||
| MMP-12 | 1.4±1.2 | 24.4±6.5 | 0.0006 | 7.8±2.9 | 38.7±11.0 | 0.0062 |
| Sample Size (n) | 41 | 41 | 19 | 22 | ||
Values reported in pg/mg wet sample weight.
AdenoCa = adenocarcinoma
Sq Cell Ca = squamous cell carcinoma
Table 3. Analysis of variance results for main effects (region and histology) and interactions.
| MMP Type | Region | Histology | Interaction | |||
|---|---|---|---|---|---|---|
| Collagenases | F-statistic | p-value | F-statistic | p-value | F-statistic | p-value |
| MMP-1 | 8.67 | 0.0043 | 5.58 | 0.0206 | 4.71 | 0.0330 |
| MMP-13 | 1.97 | 0.1644 | 1.11 | 0.2955 | 1.11 | 0.2961 |
| MMP-8 | 6.34 | 0.0138 | 10.16 | 0.0021 | 6.91 | 0.0103 |
| Gelatinases | ||||||
| MMP-2 | 2.83 | 0.0966 | 2.21 | 0.1413 | 1.18 | 0.2801 |
| MMP-9 | 0.71 | 0.4037 | 5.67 | 0.0197 | 3.07 | 0.0837 |
| Lysins | ||||||
| MMP-3 | 1.32 | 0.2541 | 1.30 | 0.2582 | 1.26 | 0.2653 |
| MMP-7 | 19.04 | 0.0000 | 0.34 | 0.5593 | 0.91 | 0.3444 |
| Elastases | ||||||
| MMP-12 | 12.45 | 0.0007 | 7.07 | 0.0095 | 5.34 | 0.0235 |
Table 4. Recurrence and tumor MMP Content*.
| MMP Type | No Recurrence | Recurrence | p-value |
|---|---|---|---|
| Collagenases | |||
| MMP-1 | 30.3±12.2 | 43.2±24.4 | 0.5963 |
| MMP-13 | 1.4±0.4 | 25.5±19.5 | 0.3977 |
| MMP-8 | 148.2±37.4 | 700.4±268.0 | 0.0057 |
| Gelatinases | |||
| MMP-2 | 97.6±18.5 | 334.1±211.3 | 0.3315 |
| MMP-9 | 132.0±22.9 | 331.4±119.4 | 0.0158 |
| Lysins | |||
| MMP-3 | 6.2±2.3 | 126.7±114.1 | 0.2392 |
| MMP-7 | 83.7±20.2 | 50.3±18.0 | 0.6995 |
| Elastases | |||
| MMP-12 | 25.6±8.5 | 21.0±7.4 | 0.5562 |
| Sample Size (n) | 30 | 11 |
Values reported in pg/mg wet sample weight.
Discussion
The matrix metalloproteinases (MMPs) constitute a large family of proteolytic enzymes, which were historically considered to have similar proteolytic portfolios and induction pathways. However, it is now recognized that specific MMP types can be induced by an array of biological signaling pathways and biophysical events and that this induction is not a uniform process. Moreover, the profile for MMPs, in general, is highly diverse, and specific MMPs can exhibit unique biological effects on extracellular matrix proteins, cell adhesion, cytokine processing and cell growth characteristics. Much of this new insight into MMP biology has been brought about by past studies which have examined MMP regulation and expression patterns in cancer, and more specifically lung carcinoma.1,2,13 However, past clinical studies which have examined relative MMP expression and/or levels in lung cancer specimens have focused upon one MMP type or a single class of MMPs.3-12 In addition, past studies have utilized semi-quantitative approaches or examined MMP levels in relative terms, rather than in absolute levels. As a consequence, the absolute changes in MMP levels across a wide spectrum of MMP types and classes in a focused set of lung cancer specimens, such as non-small cell lung cancer (NSCLC), remained unknown. Accordingly, the present study used a quantitative approach in order to measure absolute MMP levels from all of the classes of soluble MMP types in NSCLC and normal lung samples. The unique findings from this study were two-fold. First, a diverse number of MMP types are increased in absolute amounts in NSCLC, and these levels change as a function of histologic type. Second, the absolute levels for certain MMP types were increased in samples taken from patients with tumor recurrence. Taken together, these findings demonstrate that a full portfolio of MMP types can be consistently measured in NSCLC samples, which may hold both prognostic and therapeutic relevance.
A number of past studies have successfully isolated RNA and protein from tumor samples, and profiled a limited number of MMPs.23-26 For example, protein was successfully extracted from thyroid tumors and relative MMP-1 and MMP-9 measurements were performed using a targeted enzyme linked immunoassay (ELISA).35 This past study demonstrated an association between higher levels of MMP-1 and MMP-9 to thyroid histology type. The present study utilized a reproducible protein extraction method and a unique MMP multiplex approach that allowed for quantification of 8 individual MMP protein levels simultaneously. Through this quantitative approach, the results demonstrated important differences in absolute MMP abundance profiles between NSCLC and normal lung samples, as well as between distinct NSCLC histological profiles. For example, absolute levels of MMPs within the collagenase class (MMP-1, -8,-13) increased significantly in NSCLC, and increased levels of MMP-1 and MMP-8 were particularly pronounced in squamous cell carcinoma samples. Past studies have identified that this class of MMPs can play a role in a number of biological processes relevant to cancer progression which includes growth, migration, invasion, apoptosis, and angiogenesis.1-5,25,26 However, there are a number of considerations which must be taken into account when evaluating absolute MMP levels and to what extent these levels may be reflective of changes in overall proteolytic potential and thereby influencing relevant biological events. In general terms, net MMP proteolytic activity is dependent upon transcriptional and post-translational events, which include MMP synthesis, secretion and activation.1,2 The present study demonstrated absolute levels of specific MMP types changed in NSCLC, but could not differentiate between the proform and active form of these MMPs. In past immunohistochemical studies, a similar limitation exists whereby changes in the relative abundance of certain MMP types could be identified in a tumor specimen, but the activational state of the MMP could not be discerned.4-7,10,11 While the net changes in proteolytic activity could not be determined in the present study, the absolute changes in MMP levels which were observed in the NSCLC samples are likely reflective of changes in upstream induction pathways. Specifically, MMPs are tightly regulated at the transcriptional level,27,28 in which both ligand and adhesion mediated receptor interactions alter intracellular formation of transcription factors which specifically bind to regulatory elements within the MMP promoter region. Thus, the absolute changes in MMP profiles with NSCLC which were observed in the present study, are likely the result of altered transcription pathways that are reflective of the underlying cancer process. Indeed, MMP expression profiles have been demonstrated to be directly associated with relevant process of tumor biology which include local growth and prognosis.1,5,7,11,23,25,26 The present study provides for a proof of concept that profiling of a large array of MMPs in NSCLC is possible, and could be expanded to reveal specific subsets of MMPs which hold both diagnostic and therapeutic relevance.
While past studies have examined one or several MMP types in NSCLC specimens,3-12 these past investigations have utilized semi-quantitative approaches and therefore direct comparisons to the present study are difficult, where absolute protein levels of a full spectrum of MMP types were quantified in normal and NSCLC regions. However, in general, past studies have identified that a relative increase in MMP-1, -2, -7 and -12 have occurred in NSCLC, consistent with the current study.3-12 Moreover, in the present study, a differential MMP profile could be demonstrated between histological types of NSCLC, which can be difficult when utilizing immunohistological and morphometric approaches. In the present study, absolute levels of MMP-9 were increased in squamous cell carcinoma but not in adenocarcinoma. A past study by Pinto suggests that the increased levels of MMP-9 in this histological type of NSCLC can be associated with poor prognosis.10 Taken together, these results suggest that simultaneous quantitative measurements of a full portfolio of MMPs in NSCLC specimens may provide for distinct tumor profiling, and thereby develop specific thresholds for MMP protein levels for prognostic use. However, further examination of how MMP profiles differ between histological types of NSCLC and are related to prognosis will be required. Furthermore, the results from the present study using MMP protein measurements using a high sensitivity multiplex array will need to be performed in parallel with histological studies in order to provide cell and spatial information. For example, Thomas, et al.11 demonstrated a higher expression of certain MMP types in adenocarcinoma using morphometric scoring of immunohistochemical stained specimens. Furthermore, since the multiplex array provides for a high sensitivity approach for measuring MMPs in small sample volumes, then future studies which examine the relationship between MMP levels in NSCLC samples to that of a simultaneously obtained plasma sample would be of prognostic relevance. Indeed, initial studies of profiling of MMPs in a collected blood sample have shown prognostic potential in several different cancers.29-34 For example, plasma MMP levels (particularly MMP -2, -7 and -9) have been studied in a variety of cancers, including colon cancer,29,30 breast cancer,31 gastric cancer,32 and renal cell carcinoma.33 Moreover, increased circulating MMP-9 levels have been identified in patients with NSCLC.34 Indeed, the relative increase in plasma MMP-9 levels reported in this past study parallel the magnitude of absolute changes in MMP-9 levels which were observed in the present study with respect to the squamous cell NSCLC samples. However, these past studies and the present study only provide associative results, and a prospective study which simultaneously measures the relationship between circulating and tumor levels of MMPs in patients with NSCLC to that of clinical outcomes would be warranted.
The present study measured absolute levels of MMPs in NSCLC samples and identified that certain MMPs changed dramatically whereas other MMP types remained unaltered when compared to normal samples. In addition, the levels of certain MMP types appeared to be selectively increased in certain histological types. Through this type of broad MMP profiling, it may be possible to identify which MMPs may be specifically expressed in NSCLC as well as in a specific histological type, and thereby allow for the formation of hypothesis based, directed pharmacological studies. While MMPs in general have been identified as potential targets for anticancer pharmacology, the use of broad spectrum MMP inhibitors have not met with success.13-16 Postulated reasons for the failure of broad spectrum MMP inhibition in the context of NSCLC, is that inhibition of certain MMP types which are not contributory to cancer progression will result in toxic side effects, initiation or progression of disease, and poor patient outcome. In the present study, absolute levels of MMP-7 were increased by 10-fold in NSCLC, and this MMP type has been identified for more specific pharmacological inhibition in the context of cancer progression.13,35,36 MMP-7 is one of only a few MMPs actually secreted by tumor cells. It regulates cell proliferation and apoptosis by cleaving the ectodomain of heparin binding-epidermal growth factor precursor.1 MMP-7 cleaves E-cadherin, a cell-adhesion molecule, and deregulation of E-cadherin is associated with cancer progression.2 Cleavage also triggers the epithelial-to-mesenchymal transition (EMT). However, the relationship between the biological function of certain MMP types and the quantitative measurements performed in the present study remain associative at best. Nevertheless, due to the unique functionality of certain MMP types and the marked overexpression pattern observed in the present set of NSCLC specimens, a postulate for further study is that pharmacological regulation of certain MMP types is required in certain forms of NSCLC, and that MMP profiling would provide a means to tailor pharmacotherapy. The differential profile observed in the present study and the diversity of biological functions of different MMP types, underscores a likely reason for disappointing results when broad-spectrum MMP inhibitors were evaluated.14-16,35,36
MMP-2,4,9 MMP-7,5,12 MMP-9,4,6,10 and MMP-127,8 overexpression have been related to poor outcome in patients with NSCLC. However, different analysis techniques, semi-quantitative analyses, differing stages and histologies make comparisons difficult. We found increased levels of MMP-8 and MMP-9 in patients who recurred within 3 years of surgery compared to those who did not (Table 4). Overexpression of several MMPs tested (MMP-2, MMP-3, MMP-9, MMP-13) have been associated with EMT, a fundamental biological process where epithelial cells lose their polarity, cell-cell adhesion, and adopt a mesenchymal morphology appropriate for migration.1 Tumor staining for MMP-9 in resected adenocarcinoma of the lung has been shown to be strongly related to survival,10 and in another study detection of homogeneous MMP-9 in non-small cell lung cancer cells was an independent prognostic factor for shortened cancer-related survival.6 More patients with recurrence must be studied to ascertain whether a MMP expression profile can be constructed that will predict early recurrence of resected adenocarcinoma and squamous cell carcinoma of the lung.
There are several limitations of the present study which must be recognized. First, this study quantified MMP types in a small number of samples from squamous and adenocarcinoma NSCLC. Thus, the study was underpowered to perform a number of relationships between tumor biology and outcomes. Nevertheless, our preliminary results suggest that a relationship between recurrence and certain MMP levels are likely to exist in the context of NSCLC. Second, there are over 26 MMP types which have been described, and the present study therefore only examined a limited number. However, this study constructed the multiplex array to encompass representative MMPs from the major classes of MMP types, and those that have been reported in past NSCLC histological studies. Future studies which expand the sample size and MMP portfolio would be appropriate. Third, a critical control point of MMP activity is the tissue inhibitors of the MMPs (TIMPs), and can regulate MMP activity by binding MMPs in a 1:1 stoichiometry. The biological functions of the TIMPs are also complex.37 For example, elevated levels of TIMP-1 have been associated with a poor prognosis in non-small cell lung cancer.38,39 Thus, a future study which builds upon the MMP multiplex assay through quantification of the four known TIMPs would be an important future direction. Fourth, this study measured MMP profiles from whole lung samples, and therefore the specific cell types and relative MMP distribution throughout the tumor could not be determined. Nevertheless, the present study demonstrated quantitative differences in MMP levels of early stage adenocarcinoma and squamous cell carcinoma, as well as tumors that have recurred within 3 years of resection. These new results underscore the diversity of MMPs in NSCLC and suggest that profiling this proteolytic system would hold clinical significance.
Acknowledgments
This study was supported by NIH grants HL59165, HL81691, and a Merit Award from the Veterans' Affairs Health Administration. SAS participated in this project as a recipient of a medical student fellowship award from the American Association of Thoracic Surgery.
Footnotes
Presented at American Association for Thoracic Surgery Boston, MA, May 12, 2009
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Noël A, Jost M, Maquoi E. Matrix metalloproteinases at cancer tumor-host interface. Sem Cell Dev Biol. 2008:52–60. doi: 10.1016/j.semcdb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Egebad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 3.Pritchard SC, Nicolson MC, Lloret C, McKay JA, Ross VG, Kerr KM, et al. Expression of matrix metalloproteinases 1, 2, 9 and their tissue inhibitors in stage II non-small cell lung cancer: implications for MMP inhibition therapy. Oncol Rep. 2001;8:421–24. [PubMed] [Google Scholar]
- 4.Kodate M, Kasai T, Hashimoto H, Yasumoto K, Iwata Y, Manabe H. Expression of matrix metalloproteinase (gelatinase) in T1 adenocarcinoma of the lung. Pathol Int. 1997;47:461–69. doi: 10.1111/j.1440-1827.1997.tb04525.x. [DOI] [PubMed] [Google Scholar]
- 5.Leinonen T, Pirinen R, Böhm J, Johansson R, Ropponen K, Kosma VM. Expression of matrix metalloproteinases 7 and 9 in non-small cell lung cancer. Relation to clinicopathological factors, β-catenin and prognosis. Lung Cancer. 2006;51:313–21. doi: 10.1016/j.lungcan.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Sienel W, Hellers J, Morresi-Hauf A, Lichtinghagen R, Mutschler W, Jochum M, et al. Prognostic impact of matrix metalloproteinase-9 in operable non-small cell lung cancer. Int J Cancer. 2003;103:647–51. doi: 10.1002/ijc.10841. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann HS, Hansen G, Riehter G, Taege C, Simm A, Silber RE, et al. Matrix metalloproteinase-12 expression correlates with local recurrence and metastatic disease in non-small cell lung cancer patients. Clin Cancer Res. 2005;11:1086–92. [PubMed] [Google Scholar]
- 8.Heist RC, Marshall AL, Liu G, Zhou W, Su L, Neuberg D, et al. Matrix metalloproteinase polymorphisms and survival in stage I non-small cell lung cancer. Clin Cancer Res. 2006;12:5448–53. doi: 10.1158/1078-0432.CCR-06-0262. [DOI] [PubMed] [Google Scholar]
- 9.Passlick B, Sienel W, Seen-Hibler R, Wöckel W, Thetter O, Mutschler W, et al. Overexpression of matrix metalloproteinase 2 predicts unfavorable outcome in early-stage non-small cell lung cancer. Clin Cancer Res. 2000;6:3944–48. [PubMed] [Google Scholar]
- 10.Pinto CA, de Oliveira Carvalho PE, Antonângelo L, Garippo A, da Silva AG, Soares F, et al. Morphometric evaluation of tumor matrix metalloproteinase 9 predicts survival after surgical resection of adenocarcinoma of the lung. Clin Cancer Res. 2003;9:3098–3104. [PubMed] [Google Scholar]
- 11.Thomas P, Khokha R, Shepherd FA, Feld R, Tsao MS. Differential expression of matrix metalloproteinases and their inhibitors in non-small cell lung cancer. J Pathol. 2000;190:150–56. doi: 10.1002/(SICI)1096-9896(200002)190:2<150::AID-PATH510>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Liu HL, Zhang T, Li X, Huang J, Wu B, Huang X, et al. Predictive value of MMP-7 expression for response to chemotherapy and survival in patients with non-small cell lung cancer. Cancer Sci. 2008;99:2185–92. doi: 10.1111/j.1349-7006.2008.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overall CM, Kleifeld O. Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–39. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 14.Leighl NB, Paz-Ares L, Douillard JY, Peschel C, Arnold A, Depierre A, et al. Randomized phase III study of matrix metalloproteinase inhibitor BMS-275291 in combination with paclitaxel and carboplatin in advanced non-small lung cancer: National Cancer Institute of Canada – Clinical Trials Group Study BR.18. J Clin Oncol. 2005;23:2831–39. doi: 10.1200/JCO.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 15.Bissett D, O'Byrne KJ, von Pawel J, Gatzemeier U, Price A, Nicolson M, et al. Phase III study of matrix metalloproteinase inhibitor prinomastat in non-small cell lung cancer. J Clin Oncol. 2005;23:842–49. doi: 10.1200/JCO.2005.03.170. [DOI] [PubMed] [Google Scholar]
- 16.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–92. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 17.Ikonomidis JS, Jones JA, Barbour JR, Stroud RE, Clark LL, Kaplan BS, Zeeshan A, Bavaria JE, Gorman JH, Spinale FG. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with Marfan Syndrome. Circulation. 2006 Jul 4;114:I365–70. doi: 10.1161/CIRCULATIONAHA.105.000810. [DOI] [PubMed] [Google Scholar]
- 18.Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation. 2000;102(16):1944–1949. doi: 10.1161/01.cir.102.16.1944. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee R, Lowry AS, Allen RA, Stroud MR, Wharton JM, Ikonomidis JS, Crumbley AJ, III, Spinale FG, Gold MR. Selective induction of matrix metalloproteinases and tissue inhibitor of metalloproteinases in atrial and ventricular myocardium in patients with atrial fibrillation. Am J Cardiol. 2006 Feb 15;97(4):532–7. doi: 10.1016/j.amjcard.2005.08.073. [DOI] [PubMed] [Google Scholar]
- 20.Deschamps AM, Yarbrough WM, Squires CE, Allen RA, Dowdy KB, McLean JE, Mingoia JT, Sample JA, Mukherjee R, Spinale FG. Trafficking of the membrane type-1 matrix metalloproteinase (MT1-MMP) in ischemia and reperfusion: relation to interstitial MT1-MMP activity. Circulation. 2005 Mar 8;111(9):1166–74. doi: 10.1161/01.CIR.0000157149.71297.3A. [DOI] [PubMed] [Google Scholar]
- 21.Ford RL, Mains IM, Hilton EJ, Reeves ST, Stroud RE, Crawford FA, Ikonomidis JS, Spinale FG. Endothelin-A receptor inhibition after cardiopulmonary bypass: cytokines and receptor activation. Ann Thorac Surg. 2008 Nov;86(5):1576–83. doi: 10.1016/j.athoracsur.2008.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottschalk PG, Dunn JR. The five-parameter logistic: a characterization and comparison with the four-parameter logistic. Anal Biochem. 2005 Aug 1;343(1):54–65. doi: 10.1016/j.ab.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 23.Simi L, Andreani M, Davini F, Janni A, Pazzagli M, Serio M, Orlando C. Simultaneous measurement of MMP9 and TIMP1 mRNA in human non small cell lung cancers by multiplex real time RT-PCR. Lung Cancer. 2004 Aug;45(2):171–9. doi: 10.1016/j.lungcan.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Safranek J, Pesta M, Holubec L, Kulda V, Dreslerova J, Vrzalova J, Topolcan O, Pesek M, Finek J, Treska V. Expression of MMP-7, MMP-9, TIMP-1 and TIMP-2 mRNA in lung tissue of patients with non-small cell lung cancer (NSCLC) and benign pulmonary disease. Anticancer Res. 2009 Jul;29(7):2513–7. [PubMed] [Google Scholar]
- 25.Buergy D, Weber T, Maurer GD, Mudduluru G, Medved F, Leupold JH, Brauckhoff M, Post S, Dralle H, Allgayer H. Urokinase receptor, MMP-1 and MMP-9 are markers to differentiate prognosis, adenoma and carcinoma in thyroid malignancies. Int J Cancer. 2009 Aug 15;125(4):894–901. doi: 10.1002/ijc.24462. [DOI] [PubMed] [Google Scholar]
- 26.Gentner B, Wein A, Croner RS, Zeittraeger I, Wirtz RM, Roedel F, Dimmler A, Dorlaque L, Hohenberger W, Hahn EG, Brueckl WM. Differences in the gene expression profile of matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) in primary colorectal tumors and their synchronous liver metastases. Anticancer Res. 2009 Jan;29(1):67–74. [PubMed] [Google Scholar]
- 27.Munshi HG, Stack MS. Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev. 2006 Mar;25(1):45–56. doi: 10.1007/s10555-006-7888-7. [DOI] [PubMed] [Google Scholar]
- 28.Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40(67):1362–78. doi: 10.1016/j.biocel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Tutton MG, George ML, Eccles SA, Burton S, Swift RI, Abulafi AM. Use of plasma MMP-2 and MMP-9 levels as a surrogate for tumour expression in colorectal cancer patients. Int J Cancer. 2003;107:541–50. doi: 10.1002/ijc.11436. [DOI] [PubMed] [Google Scholar]
- 30.Langenkiöld M, Holmdahl L, Falk P, Ivarsson ML. Increased plasma MMP-2 protein expression in lymph node-positive patients with colorectal cancer. Colorectal Dis. 2005;20:245–52. doi: 10.1007/s00384-004-0667-4. [DOI] [PubMed] [Google Scholar]
- 31.Ranuncolo SM, Armanasco E, Cresta C, De Kier Bal, Joffe E, Puricelli L. Plasma MMP-9 (92 kDa – MMP) activity is useful in the followup and in the assessment of prognosis in breast cancer patients. Int J Cancer. 2003;106:745–51. doi: 10.1002/ijc.11288. [DOI] [PubMed] [Google Scholar]
- 32.Wu CY, Wu MS, Chiang EP, Chen YJ, Chen CJ, Chi NH, et al. Plasma matrix metalloproteinase-9 level is better than serum matrix metalloproteinase-9 level to predict gastric cancer evolution. Clin Cancer Res. 2007;13:2054–60. doi: 10.1158/1078-0432.CCR-06-2299. [DOI] [PubMed] [Google Scholar]
- 33.Ramankulov A, Lein M, Johannsen M, Schrader M, Miller K, Jung K. Plasma matrix metalloproteinase-7 as a metastatic marker and survival predictor in patients with renal cell carcinoma. Cancer Science. 2008;99:1188–94. doi: 10.1111/j.1349-7006.2008.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iizasa T, Fujisawa T, Suzuki M, Motohashi SI, Yasufuku K, Yasakawa T, et al. Elevated levels of circulating plasma matrix metalloproteinase-9 in non-small cell lung cancer patients. Clin Cancer Res. 1999;5:149–53. [PubMed] [Google Scholar]
- 35.Overall CM, Kleifeld O. Towards third generation matrix metalloproteinase inhibitors for cancer therapy. Br J Cancer. 2006;94:941–46. doi: 10.1038/sj.bjc.6603043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–27. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 37.Visse R, Nagase H. Matrix metalloproteinase and tissue inhibitors of metalloproteinases. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 38.Aljada IS, Ramnath N, Donohue K, Harvey S, Brooks JJ, Wiseman SM, et al. Upregulation of the tissue inhibitor of metalloproteinase-1 protein is associated with progression of human non-small cell lung cancer. J Clin Oncol. 2004;22:3218–29. doi: 10.1200/JCO.2004.02.110. [DOI] [PubMed] [Google Scholar]
- 39.Gouyer V, Conti M, Devos P, Zerimech F, Copin MC, Créme E, et al. Tissue inhibitor of metalloproteinase 1 is an independent predictor of prognosis in patients with nonsmall cell lung carcinoma who undergo resection with curative intent. Cancer. 2005;103:1676–84. doi: 10.1002/cncr.20965. [DOI] [PubMed] [Google Scholar]