Abstract
1,1-Bis(3′-indolyl)-1-(p-bromophenyl)methane (DIM-C-pPhBr) and the 2,2′-dimethyl analog (2,2′-diMeDIM-C-pPhBr) inhibit proliferation and induce apoptosis in SW480 colon and Panc28 pancreatic cancer cells. In this study, treatment with 10–20 μM concentrations of these compounds for 24 hr induced cleaved PARP and decreased survivin protein and mRNA expression in both cell lines. However, results of time course studies show that DIM-C-pPhBr and 2,2′-diMeDIM-C-pPhBr decrease survivin protein within 2 hr after treatment, whereas survivin mRNA levels were decreased only at later time points indicating activation of transcription-independent and -dependent pathways for downregulation of survivin. In addition, we also observed that γ-radiation inhibited pancreatic and colon cancer cell growth and this was associated with enhanced expression of survivin after 24 (SW480) or 24 and 48 (Panc28) hr and correlated with previous studies on the role of survivin in radiation-resistance. However, in cells cotreated with γ-radiation plus DIM-C-pPhBr or 2,2′-diMeDIM-C-pPhBr, induction of survivin by γ-radiation was inhibited after cotreatment with both compounds, suggesting applications for these drugs in combination cancer chemotherapy with γ-radiation.
Keywords: DIM analogs, survivin downregulation, gamma-radiation-induced survivin
INTRODUCTION
Survivin is a 16.5 kDa protein and a member of the inhibitor of apoptosis (IAP) family of proteins that suppress caspase-3, caspase-7 and caspase-9 and thereby inhibits both the extrinsic and intrinsic apoptotic pathways (1–3). In addition to the anti-apoptotic activity of survivin, this protein also acts as a subunit of the chromosomal passenger complex and plays a role in cell division and cell cycle control (4). Survivin expression in normal tissues is variable (5–9); however, several studies show that survivin is more highly expressed in precancerous and tumor tissue derived from most solid tumors and hematological malignancies (3, 5, 10, 11). Intuitively, overexpression of survivin and other IAPs is not unexpected since cancer cells and tumors typically exhibit deregulated proliferative and survival pathways. High levels of survivin expression in cancer cells are due, in part, to several factors which regulate survivin at the transcriptional and posttranscriptional level. For example, several cancer cell lines overexpress specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 which in turn increase survivin expression through interaction with GC-rich cis-elements in the survivin promoter (12–14). Nuclear factor kappa B (NFκB) is overexpressed in multiple tumor types and in some leukemia cell lines, expression of survivin is regulated by NFκB (15). In other leukemia cell lines, Krüppel-like factor 5 (KLF5) upregulates survivin expression and inhibits p53 which mediates suppression of survivin (16, 17).
Several studies report that survivin overexpression is a negative prognostic factor for cancer patient survival (3, 18–22). For example, increased nuclear (but not cytosolic) survivin expression was associated with a decreased overall survival for breast cancer patients (18). Survivin expression in tumors is not only a negative prognostic factor, but expression of this gene has also been linked to drug resistance associated with chemotherapy and radiotherapy (23–30). Resistance to antiandrogen and cis-platin therapy for treatment of prostate cancer is mediated by survivin (23, 24) and taxol resistance has also been linked to induction of survivin in cancer cell lines and tumors (25, 26). Radiotherapy is important for treating several types of tumors and radioresistance is related, in part, to induction of survivin in tumors undergoing radiotherapy (27–30). These observations suggest that survivin may be an important chemotherapeutic target for cancer chemotherapy and agents that decrease survivin expression could also serve to ameliorate drug- and radiotherapy-resistant tumors in which survivin expression is increased.
Research in this laboratory has identified a series of 1,1-bis(3′-indolyl)-1-(p-substituted phenyl) methanes (C-DIMs) that inhibit pancreatic, colon, prostate, bladder and breast cancer cell and tumor growth (31–36). C-DIMs containing p-phenyl, p-t-butyl and p-trifluoromethyl substituents activate peroxisome proliferator-activated receptor γ (PPARγ) (31–34), whereas p-methoxy and unsubstituted C-DIMs activate the orphan receptor Nur77 (35, 36). Other receptor-inactive C-DIMs including 1,1-bis(3′-indolyl)-1-(p-bromophenyl)methane (DIM-C-pPhBr) and the corresponding 2,2′-dimethyl derivative (2,2′-diMeDIM-C-pPhBr) also induce apoptosis in cancer cells through activation of ER stress (37, 38). In this study, we demonstrate that DIM-C-pPhBr and 2,2′-diMeDIM-C-pPhBr decrease survivin expression in pancreatic and colon cancer cells and in combination with radiotherapy, these compounds decrease radioresistance and inhibit radiation-induced survivin expression.
MATERIALS AND METHODS
Chemicals, antibodies, plasmids, and reagents
C-DIMs were synthesized in this laboratory from the condensation of indole or substituted indole plus a substituted benzaldehyde derivative and confirmed by gas chromatography-mass spectrometry as described previously (31, 37, 38). Cleaved poly (ADP-ribose) polymerase (PARP) antibody was obtained from Cell Signaling (Danvers, MA). Survivin antibody was purchased from R and D Systems (Minneapolis, MN); β-actin antibodies were purchased from Sigma-Aldrich (St. Louis, MO); and proteasome inhibitor MG132 was purchased from Calbiochem (San Diego, CA). The pSurvivin-269 constructs containing survivin promoter inserts (positions −269 to +49) linked to luciferase reporter gene was kindly provided by Dr. M. Zhou (Emory University, Atlanta, GA). Reporter lysis buffer and luciferase reagent for luciferase studies were purchased from Promega (Madison, WI).β-Galactosidase (β-gal) reagent was obtained from Tropix (Bedford, MA). Lipofectamine reagent was supplied by Invitrogen (Carlsbad, CA). Western lightning chemiluminescence reagent was from Perkin Elmer Life Sciences (Boston, MA).
Cell lines
SW480 human colon carcinoma cell lines were provided by Dr. Stanley Hamilton (M.D. Anderson Cancer Center, Houston, TX). Panc28 cell line was a generous gift from Dr. Paul Chiao, The University of Texas M.D. Anderson Cancer Center (Houston, TX). Human pancreatic Panc1 cancer cell line were obtained from the American Type Culture Collection (Manassas, VA). The L3.6pl cell line was developed at The University of Texas M.D. Anderson Cancer Center. Cell lines were maintained in DMEM/F-12 (Sigma, St. Louis, MO) supplemented with 2.2% sodium bicarbonate, 2.2% bovine serum albumin, 5% fetal bovine serum, and 10 ml/L of 100X antibiotic antimycotic solution (Sigma). Cells were maintained at 37°C in the presence of 5% CO2.
Cell proliferation assay
Pancreatic and colon cancer cells (3 × 104 per well) were plated in 12-well plates and allowed to attach for 24 hr. The medium was then changed to DMEM/Ham’s F-12 medium containing 2.5% charcoal-stripped FBS, and either vehicle (DMSO) or different concentrations of the compound were added. Cells were then trypsinized and counted after 24 hr using a Coulter Z1 cell counter. Each experiment was done in triplicate, and results were expressed as means ± SE for each set of experiments.
Transfection and luciferase assay
Colon cancer cells were plated in 12-well plates at 1×105 per well in DMEM/Ham’s F-12 media supplemented with 2.5% charcoal-stripped FBS. After 16 to 20 hr, reporter gene constructs [i.e. pSurvivin-269 (0.04 Ag) and β-gal (0.04 Ag)] were transfected by Lipofectamine (Invitrogen) according to the manufacturer’s protocol. Five hr after transfection, the transfection mix was replaced with complete media containing either vehicle (DMSO) or the indicated compound for 20 to 22 hr. Cells were then lysed with 100 μL of 1X reporter lysis buffer, and 30 μL of cell extract were used for luciferase and β-gal assays. Lumicount was used to quantitate luciferase and β-gal activities, and the luciferase activities were normalized to β-gal activity.
Western blots
Colon and pancreatic cancer cells were seeded in DMEM: Ham’s F-12 medium. Twenty-four hr later, cells were treated with either vehicle (DMSO) or the indicated compounds for 24 hr or pretreated with the proteasome inhibitor, MG132 (10 μM) for 1 hr and then treated with the compounds. Cells were lysed using high-salt buffer and Protease Inhibitor Cocktail. Protein lysates were separated on 12% SDS-PAGE 120 V for 4 hr. Proteins were transferred onto polyvinylidene difluoride (PVDF) membranes by wet electroblotting and the membranes were incubated with primary antibody. After washing with TBST, the PVDF membrane was incubated with secondary antibody in 5% TBST-Blotto and the membrane was washed with TBST for 10 min, incubated with chemiluminescence substrate for 1 min, and exposed to Kodak autoradiography film.
Irradiation
Colon and pancreatic cancer cells were plated and exposed to varying doses of γ-radiation generated from a Theratron 80 cobalt-60 teletherapy machine (Atomic Energy of Canada) with a dose rate of 80.166 cGy/minute (same as 0.8016 Gray per minute or 80.166 rads/minute), for a 30×30 cm field, at a source-surface distance of 80 cm. The irradiated cells were then treated with the indicated compounds after 8 hr, and cells were counted or lysates were obtained after the indicated treatment times.
Real Time PCR
Total RNA was isolated using the RNeasy Protect Mini kit (QIAGEN, Valencia, CA) according to the manufacturer’s protocol. RNA was eluted with 30 μL of RNase-free water and stored at −80°C. RNA was reverse-transcribed using Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s protocol. cDNA was prepared from the SW480 colon and Panc28 pancreatic cancer cell lines at different time intervals using a combination of oligodeoxythymidylic acid and dNTP mix (Applied Biosystems, Foster City, CA) and Superscript II (Invitrogen). Survivin primers (forward 5′-ATG GCC GAG GCT GGC TTC ATC-3′; reverse 5′-ACG GCG CAC TTT CTT CGC AGT T-3′) were acquired from IDT (Skokie, IL). Each PCR was carried out in triplicate in a 20-μL volume using SYBR Green Master mix (Applied Biosystems) for 15 min at 95°C for initial denaturing, followed by 40 cycles of 95°C for 30 sec and 60°C for 1 min in the ABI Prism 7500 sequence detection system (Applied Biosystems). Values for each gene were normalized to expression levels of TATA-binding protein (TBP).
RESULTS
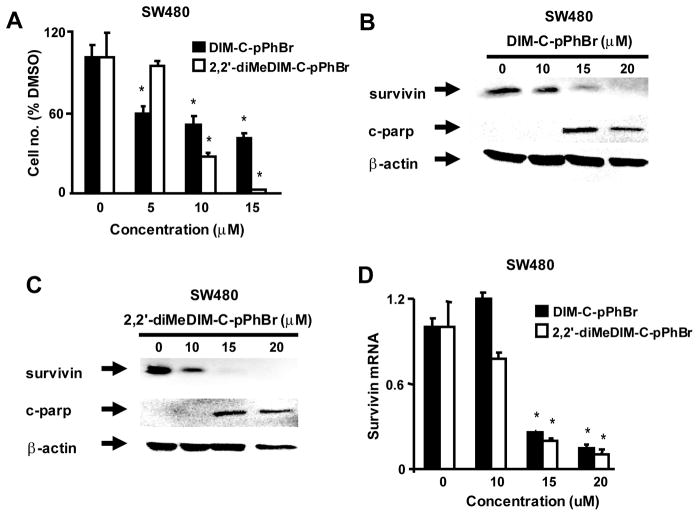
Treatment of colon and pancreatic cancer cells with DIM-C-pPhBr or 2,2′-diMeDIM-C-pPhBr induces apoptosis and ER stress (37, 38) and results in Figure 1A show that after treatment of SW480 cells with these compounds for 24 hr, there was significant concentration-dependent decrease in cell proliferation. Both C-DIMs decreased cell numbers at concentrations of 10 or 15μM and at a concentration of 5μM, DIM-C-pPhBr also inhibited SW480 cell growth, whereas 2,2′-diMeDIM-C-pPhBr was inactive. Figures 1B and 1C show that both compounds also induce caspase-dependent PARP cleavage (15 and 20 μM) and at these same concentrations there was a parallel decrease in survivin expression in SW480 cells. In addition, we also observed that both C-DIM compounds decreased survivin mRNA expression after treatment for 24 hr (Fig. 1D).
Figure 1.
Effects of C-DIMs on SW480 cell proliferation and expression of survivin and cleaved PARP (c-parp). Concentration-dependent effects on cell proliferation (A) and survivin and c-parp protein expression (B and C). Cells were treated with DMSO and different concentrations of DIM-C-pPhBr or 2,2′-diMeDIM-C-pPhBr for 24 hr. Cells were counted (A) or whole cell lysates were analyzed by western blots as described in the Materials and Methods. (D) Survivin mRNA levels. Cells were treated with DMSO or 10–20 μM of C-DIM compounds, and survivin mRNA levels were determined by real time PCR as described in the Materials and Methods. A significantly (p < 0.05) decreased response is indicated (*). β-Actin served as a loading control for all western blots in Figures 1–6.
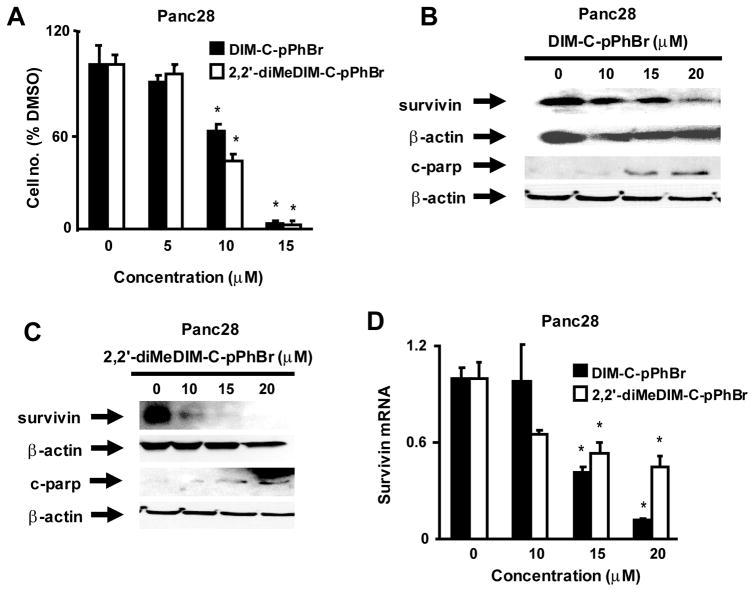
DIM-C-pPhBr and 2,2′-diMeDIM-C-pPhBr also inhibited Panc28 pancreatic cancer cell growth (Fig. 2A) and significant inhibition was observed at concentrations of 15–20 um. DIM-C-pPhBr and 2,2′-diMeDIM-C-pPhBr also induced PARP cleavage and decreased survivin protein levels in Panc28 cells (Figs. 2B and 2C) and this was also accompanied by decreased survivin mRNA levels. All of these responses were observed after treatment of SW480 or Panc28 cells for 24 hr. IC50 values for growth inhibition after treatment with DIM-C-pPhBr and 2,2′-diMeDIM-C-pPhBr were 10.8 and 8.3 μM (SW480) and 9.4 and 8.8 μM (Panc28), respectively.
Figure 2.
Effects of C-DIMs on Panc28 cell proliferation and expression of survivin and c-parp. Concentration-dependent effects on cell proliferation (A) and survivin and c-parp protein expression (B and C). Cells were treated with DMSO and different concentrations of DIM-C-pPhBr or 2,2′-diMeDIM-C-pPhBr for 24 hr. Cells were counted (A) or whole cell lysates were analyzed by western blots as described in the Materials and Methods. (D) Survivin mRNA levels. Cells were treated with DMSO or 10–20 μM of C-DIM compounds, and survivin mRNA levels were determined by real time PCR as described in the Materials and Methods. A significantly (p < 0.05) decreased response is indicated (*).
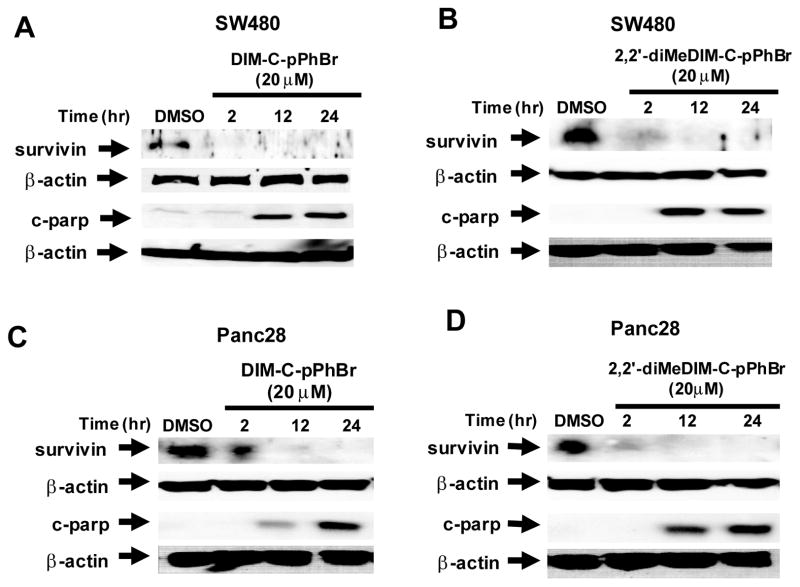
We also investigated the time dependent effects of DIM-C-pPhBr and 2,2′-diMeDIM-C-pPhBr on survivin protein and cleaved PARP expression using a relatively high concentration (20 μM) of both compounds in order to determine differences between the temporal expression of both proteins. In SW480 cells, 20 μM DIM-C-pPhBr and 2,2′-diMeDIM-C-pPhBr decreased levels of survivin protein within 2 hr after treatment, whereas cleaved PARP protein was observed only after treatment for 12 hr (Figs. 3A and 3B). Similar results were observed in Panc28 cells (Fig. 3C and 3D); however, the extent of survivin degradation after treatment for 2 hr was lower in Panc28 compared to SW480 cells. These results show that there was a lag between the loss of survivin and induction of cleaved PARP in both cell lines.
Figure 3.
Time course effects of DIM-C-pPhBr (20 μM) and 2,2′-diMeDIM-C-pPhBr (20 μM) on survivin and c-parp expression. SW480 cells were treated with DIM-C-pPhBr (A) or 2,2′-diMeDIM-C-pPhBr (B) and Panc28 cells were treated with DIM-C-pPhBr (C) or 2,2-diMeDIM-C-pPhBr (D) for 2, 12 or 24 hr. DMSO treatment served as a vehicle control. Whole cell lysates were analyzed by western blots as described in the Materials and Methods.
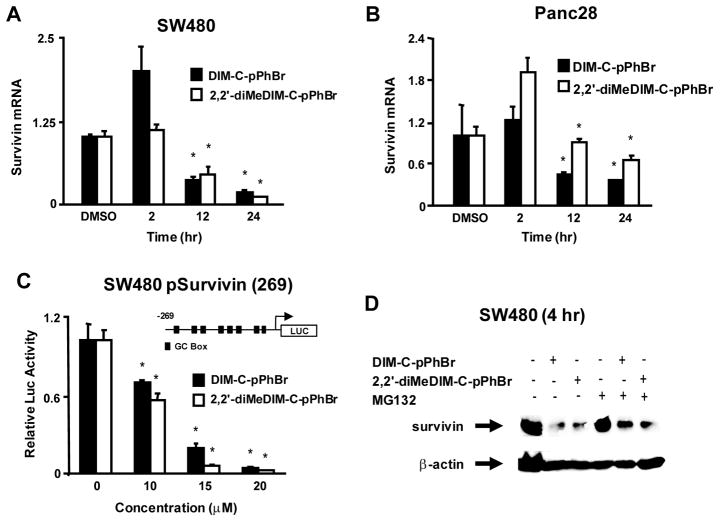
Figures 4A and 4B illustrate the effects of 20 μM DIM-C-pPhBr and 2,2′-diMeDIM-C-pPhBr on survivin mRNA levels in SW480 and Panc28 cells. Both compounds either had no effect or induced survivin mRNA levels after treatment of SW480 or Panc28 cells for 2 hr; significant inhibition of survivin mRNA levels was observed in SW480 and Panc28 cells after 12 hr; however, the magnitude of survivin mRNA repression was more pronounced in the colon cancer cells. Thus, the effects of DIM-C-pPhBr and 2,2′-diMeDIM-C-pPhBr on survivin mRNA levels were observed at later time points compared to the rapid downregulation (within 2 hr) of survivin protein (Fig. 3). We also confirmed that DIM-C-pPhBr and 2,2′-diMeDIM-C-pPhBr decreased luciferase activity in SW480 cells transfected with pSurvivin-269, a construct containing the −269 to +49 region of the survivin promoter (Fig. 4C). Results were not obtained in Panc28 cells due to low transfection efficiencies in this cell line. Since DIM-C-pPhBr and 2,2′-diMeDim-C-pPhBr decreased survivin protein but not mRNA levels within 2 hr after treatment, we investigated the effects of these compounds alone and in combination with the proteasome inhibitor MG132 after treatment of SW480 cells for 4 hr (Fig. 4D). The results show that C-DIM-dependent downregulation of survivin protein was partially reversed by the proteasome inhibitor, suggesting that activation of proteasomes contributed to the early decrease in survivin expression.
Figure 4.
C-DIM-dependent effects on survivin expression. Changes in survivin mRNA expression in SW480 (A) and Panc28 (B) cells. Cells were treated with 20 μM DIM-C-pPhBr or 2,2′-diMeDIM-C-pPhBr for 24 hr, and survivin mRNA levels were determined by real time PCR as described in the Materials and Methods. (C) Effects on the survivin promoter. SW480 cells were transfected with pSurvivin(269) and treated with DMSO or the C-DIM compounds. Luciferase activity was determined as described in the Materials and Methods. (D) Effects of MG132. SW480 cells were treated with DMSO, C-DIM compounds alone (20 μM), 10 μM MG132, or a combination of C-DIMs plus MG132 for 4 hr. Whole cell lysates were analyzed by western blots as described in the Materials and Methods. Significantly (p < 0.05) decrease mRNA levels or luciferase activity (compared to DMSO) is indicated (*).
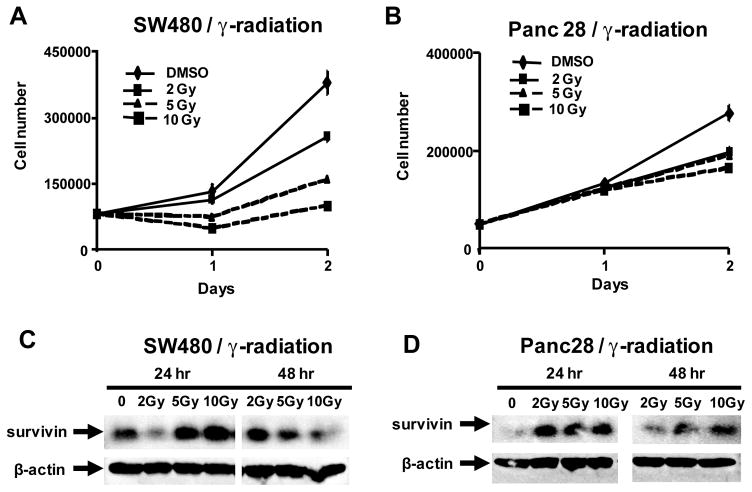
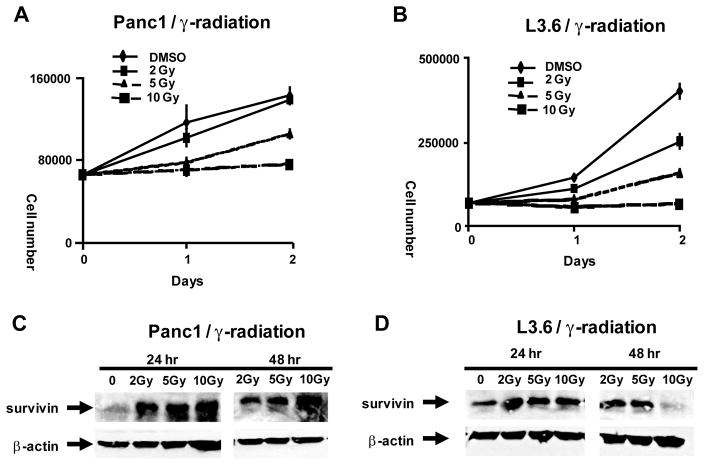
Since DIM-C-pPhBr and 2,2′-diMeDIM-C-pPhBr inhibit survivin protein expression, the interactions of these compounds with radiotherapy were investigated. Figure 5A illustrates the effects of γ-radiation on proliferation of SW480 cells. Cells were administered doses of 2, 5 and 10 Gy using a Theatron 80 cobalt-60 teletherapy instrument and the effects of radiation on cell growth were determined after 24 and 48 hr. This cell line was responsive to radiation-induced inhibition of cell growth and after 24 hr, significant inhibition was observed using 5 and 10 Gy (but not 2 Gy) and all three doses of radiation inhibited cell growth after 48 hr. Using a similar protocol, γ-radiation also inhibited Panc28 cell proliferation (Fig. 5B); however, this cell line was clearly more resistant to radiotherapy than SW480 cells over this time period and significant growth inhibition was observed only after 24 hr. The effects of γ-radiation on survivin expression were also investigated in SW480 (Fig. 5C) and Panc28 (Fig. 5D) cells and in both cell lines, 5 and 10 Gy induced survivin expression after radiation for 24 hr, whereas induction of survivin was either decreased or not observed after 48 hr. γ-Radiation also induced survivin and decreased proliferation of Panc1 and L3.6pl pancreatic cells and both cell lines were more responsive than Panc28 cells to the antiproliferative activity of γ-radiation (Fig. 7).
Figure 5.
Effects of γ-radiation of cell proliferation and survivin expression. SW480 (A) and Panc28 (B) cell growth and expression of survivin in SW480 (C) and Panc28 (D) cells. Cells were treated with DMSO (control) and irradiated with 2, 5 or 10 Gy for 24 or 48 hr, and cell number and survivin protein expression (from whole cell lysates) were determined as described in the Materials and Methods.
Figure 7.
Effects of γ-radiation on growth of Panc1 (A) and L3.6pl (B) pancreatic cancer cells and expression of survivin in Panc1 (C) and L3.6pl (D) cells. Experiments were performed as described in the Materials and Methods. β-Actin was used as a protein loading control.
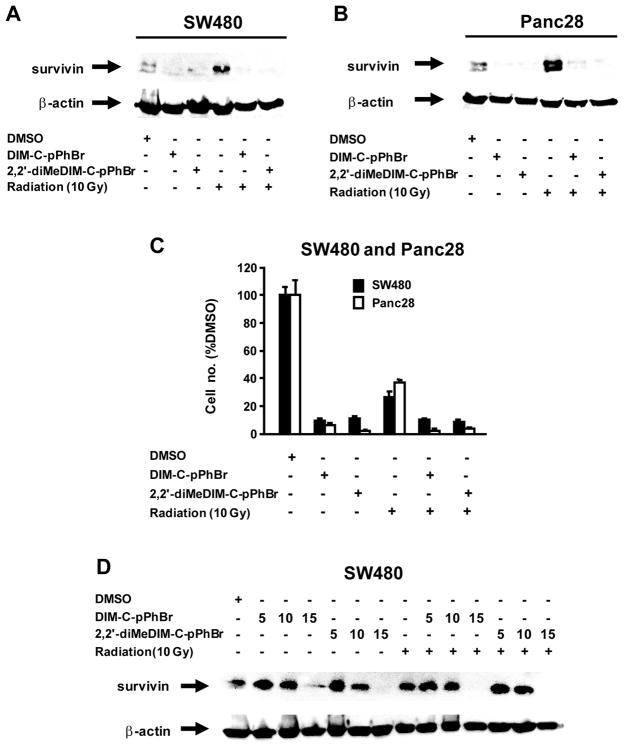
The combined effects of γ-radiation and C-DIM compounds on survivin expression are summarized in Figures 6A and 6B. Treatment of SW480 cells with 20μM DIM-C-pPhBr or 2,2′-diMeDIM-C-pPhBr for 24 hr showed that the C-DIMs alone decreased survivin, γ-radiation alone increased survivin and C-DIMs in combination with radiation decreased radiation-induced survivin expression. Similar effects were observed on cell numbers in SW480 or Panc28 cells (Fig. 6C); however, the high concentrations (20 μM) of the C-DIM alone significantly decreased cell proliferation so that the interactions with γ-radiation were not apparent. We also examined the effects of lower concentrations of DIM-C-pPhBr and 2,2′-diMeDIM-C-pPhBr (5, 10 and 15 μM) on inhibition of γ-radiation-induced induction of survivin in SW480 cells (Fig. 6D). Inhibition was only observed using 15 μM concentrations of C-DIMs and these concentrations alone also decreased levels of survivin protein. These results demonstrate that combinations of C-DIMs plus γ-radiation decrease radiation-induced survivin which plays a role in radioresistance.
Figure 6.
Interactions of γ-radiation and C-DIMs. (A) Effects of C-DIM on γ-radiation-induced survivin protein expression. Cells were treated with DMSO, C-DIMs (20 μM), radiation (10 Gy), or C-DIMs plus radiation for 24 hr. Whole cell lysates were analyzed by western blots as described in the Materials and Methods. Effects of C-DIMs and radiation on proliferation of SW480 (B) and Panc28 cells (C). Cells were treated with DMSO, C-DIMs and radiation as described in (A), and cell numbers were determined as described in the Materials and Methods. (D) Concentration-dependent effects of C-DIMs on γ-radiation-induced survivin protein expression. SW480 cells were treated with DMSO; 5, 10 or 15 μM DIM-C-pPhBr or 2,2′-diMeDIM-C-pPhBr alone; or in combination with γ-radiation for 24 hr. Whole cell lysates were analyzed by western blots as described in the Materials and Methods.
DISCUSSION
Overexpression of survivin in cancer cell lines and tumors coupled with the negative prognostic significance of this gene for the survival of patients with some tumors has heightened interest in survivin as a potential drug target (3, 5, 11). It is also important to evaluate the effects of both old and new drugs on survivin expression since it has been demonstrated that increased expression of survivin by taxol-like drugs and radiotherapy can lead to therapy resistance (25–30). Several reports show that diverse drugs can downregulate survivin expression and these include: vitamin D3 and related analogs in leukemia and breast cancer cells (39); PPARγ agonists in breast cancer cells (40); doxorubicin, histone deacetylase inhibitors and lovastatin (an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase) in colon cancer cells (41–43); cyclooxygenase-2 inhibitors in glioblastoma and pancreatic cancer cells (44) and γ-tocotrienol in human embryonic kidney A93 cells (45). The mechanisms of survivin downregulation by these drugs are dependent on cell context.
Previous studies in this laboratory showed that PPARγ-active C-DIMs downregulate survivin expression in MDA-MB-231 breast cancer cells after prolonged treatment; however, this was not accompanied by apoptosis (33). In contrast, DIM induced growth inhibition, apoptosis and downregulated survivin expression in MDA-MB-231 cells (46).
DIM-C-pPhBr and 2,2′-diMeDIM-C-pPhBr do not activate PPARγ or Nur77 but induced apoptosis in colon and pancreatic cancer cells, and activation of apoptosis by these compounds was due, in part, to ER stress-dependent upregulation of death receptor 5 (37, 38). In this study, we used these same compounds to investigate their effect on survivin expression in colon and pancreatic cancer cells and their potential interactions with γ-radiation. Like many other anticancer agents, C-DIMs potentially activate multiple pathways and we focused on some of the early responses induced by these compounds within 24 hr after treatment. Concentration-dependent studies indicate that 10–20 μM DIM-C-pPhBr and 2,2′-diMeDIM-C-pPhBr decrease survivin protein expression in SW480 and Panc28 cells (Figs. 1 and 2) and this is accompanied by caspase-dependent PARP cleavage. Using a relatively high concentration (20 μM) of the C-DIM compounds demonstrates that survivin downregulation occurs within 2 hr after treatment in both SW480 and Panc28 cells (Fig. 3), whereas in the same experiment PARP cleavage is not observed until a later time point. This does not necessarily completely uncouple loss of survivin with induction of caspase-dependent PARP cleavage but indicates at least that this rapid downregulation of survivin is not paralleled by PARP cleavage. Interestingly, we also observed that rapid downregulation of survivin protein in SW480 and Panc28 cells treated with DIM-C-pPhBr or 2,2′-diMeDIM-C-pPhBr was not accompanied by decreased survivin mRNA levels after 2 hr since decreased transcription was not observed until 12–24 hr after treatment in both cell lines (Fig. 4). Moreover, the proteasome inhibitor MG132 blocked C-DIM-induced downregulation of survivin protein after treatment of SW480 cells for 4 hr (Fig. 4D). These results suggest that the C-DIM compounds downregulate survivin expression by both transcription-independent and -dependent pathways, and current studies are focused on the mechanisms associated with activation of these pathways by C-DIMs.
Resistance to radiotherapy is a serious concern in cancer therapy and there is evidence that induction of survivin after radiation is a resistance factor (29, 30). In this study, we used a Theatron 80 cobalt-60 teletherapy instrument which emits γ-radiation to investigate whether this type of radiation does indeed increase survivin expression in SW480 and Panc28 cells. γ-Radiation with 2–10 Gy decreased proliferation of both Panc28 and SW480 cells; however, it was evident that the latter cells were more responsive to the growth inhibitory effects of γ-radiation (Figs. 5A and 5B). γ-Radiation decreased survivin protein expression in both cell lines after 24 hr and this was also observed in Panc28 cells after 48 hr (Figs. 5C and 5D). Similar but not identical responses were also observed in two additional pancreatic cancer cell lines (Panc1 and L3.6pl) and survivin protein was increased in these cells after 24 hr at doses of 2, 5 and 10 Gy (Fig. 7). These results clearly demonstrate that γ-radiation induces survivin expression in Panc28 pancreatic and SW480 colon cancer cells as previously reported in other cell lines (29, 30). Moreover, in irradiated SW480 and Panc28 cells, the increased expression of survivin was decreased in cells cotreated with 20 μM DIM-C-pPhBr and 2,2′-diMeDIM-C-pPhBr (Figs. 6A and 6B) and similar results were observed for 15 μM concentrations of these same compounds (Fig. 6D). Thus, like other drugs such as vitamin D3, PPARγ agonists, doxorubicin, lovastatin, histone deacetylase and cyclooxygenase inhibitors (39–45), the C-DIM compounds also downregulate survivin and block γ-radiation-induced expression of survivin. Previous studies with DIM-C-pPhBr and 2,2′-diMeDIM-C-pPhBr demonstrate their anticancer activity alone in both in vitro and in vivo (xenograft) pancreatic and colon cancer cells/tumors and this is due, in part, to their ER stress-dependent proapoptotic activity (38, 39). Results of this study demonstrate their potential clinical utility in combination with radiotherapy where the C-DIMs inhibit γ-radiation-induced survivin expression, a known marker of radiotherapy-resistance.
Acknowledgments
Funding: This research was supported by the National Institutes of Health (CA108718 and CA112337).
References
- 1.Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18:609–615. doi: 10.1016/j.ceb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Deveraux QL, Reed JC. IAP family proteins--suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 3.Stauber RH, Mann W, Knauer SK. Nuclear and cytoplasmic survivin: molecular mechanism, prognostic, and therapeutic potential. Cancer Res. 2007;67:5999–6002. doi: 10.1158/0008-5472.CAN-07-0494. [DOI] [PubMed] [Google Scholar]
- 4.Lens SM, Vader G, Medema RH. The case for Survivin as mitotic regulator. Curr Opin Cell Biol. 2006;18:616–622. doi: 10.1016/j.ceb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy: fulfilled promises and open questions. Carcinogenesis. 2007;28:1133–1139. doi: 10.1093/carcin/bgm047. [DOI] [PubMed] [Google Scholar]
- 6.Xing Z, Conway EM, Kang C, Winoto A. Essential role of survivin, an inhibitor of apoptosis protein, in T cell development, maturation, and homeostasis. J Exp Med. 2004;199:69–80. doi: 10.1084/jem.20031588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurbuxani S, Xu Y, Keerthivasan G, Wickrema A, Crispino JD. Differential requirements for survivin in hematopoietic cell development. Proc Natl Acad Sci U S A. 2005;102:11480–11485. doi: 10.1073/pnas.0500303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deguchi M, Shiraki K, Inoue H, et al. Expression of survivin during liver regeneration. Biochem Biophys Res Commun. 2002;297:59–64. doi: 10.1016/s0006-291x(02)02128-9. [DOI] [PubMed] [Google Scholar]
- 9.Chiou SK, Moon WS, Jones MK, Tarnawski AS. Survivin expression in the stomach: implications for mucosal integrity and protection. Biochem Biophys Res Commun. 2003;305:374–379. doi: 10.1016/s0006-291x(03)00724-1. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Cao Z, Yan H, Wood WC. Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer Res. 2003;63:6815–6824. [PubMed] [Google Scholar]
- 11.Zaffaroni N, Pennati M, Daidone MG. Survivin as a target for new anticancer interventions. J Cell Mol Med. 2005;9:360–372. doi: 10.1111/j.1582-4934.2005.tb00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Ling X, Pan D, et al. Molecular mechanism of inhibition of survivin transcription by the GC-rich sequence-selective DNA binding antitumor agent, hedamycin: evidence of survivin down-regulation associated with drug sensitivity. J Biol Chem. 2005;280:9745–9751. doi: 10.1074/jbc.M409350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F, Altieri DC. Transcriptional analysis of human survivin gene expression. Biochem J. 1999;344(Pt 2):305–311. doi: 10.1042/0264-6021:3440305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chadalapaka G, Jutooru I, Chintharlapalli S, et al. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008;68:5345–5354. doi: 10.1158/0008-5472.CAN-07-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawakami H, Tomita M, Matsuda T, et al. Transcriptional activation of survivin through the NF-κB pathway by human T-cell leukemia virus type I tax. Int J Cancer. 2005;115:967–974. doi: 10.1002/ijc.20954. [DOI] [PubMed] [Google Scholar]
- 16.Zhu N, Gu L, Findley HW, et al. KLF5 Interacts with p53 in regulating survivin expression in acute lymphoblastic leukemia. J Biol Chem. 2006;281:14711–14718. doi: 10.1074/jbc.M513810200. [DOI] [PubMed] [Google Scholar]
- 17.Raj D, Liu T, Samadashwily G, Li F, Grossman D. Survivin repression by p53, Rb and E2F2 in normal human melanocytes. Carcinogenesis. 2008;29:194–201. doi: 10.1093/carcin/bgm219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinnis AR, Luckett JC, Walker RA. Survivin is an independent predictor of short-term survival in poor prognostic breast cancer patients. Br J Cancer. 2007;96:639–645. doi: 10.1038/sj.bjc.6603616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karam JA, Lotan Y, Ashfaq R, Sagalowsky AI, Shariat SF. Survivin expression in patients with non-muscle-invasive urothelial cell carcinoma of the bladder. Urology. 2007;70:482–486. doi: 10.1016/j.urology.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Shariat SF, Ashfaq R, Karakiewicz PI, Saeedi O, Sagalowsky AI, Lotan Y. Survivin expression is associated with bladder cancer presence, stage, progression, and mortality. Cancer. 2007;109:1106–1113. doi: 10.1002/cncr.22521. [DOI] [PubMed] [Google Scholar]
- 21.Adida C, Recher C, Raffoux E, et al. Expression and prognostic significance of survivin in de novo acute myeloid leukaemia. Br J Haematol. 2000;111:196–203. doi: 10.1046/j.1365-2141.2000.02328.x. [DOI] [PubMed] [Google Scholar]
- 22.Adida C, Haioun C, Gaulard P, et al. Prognostic significance of survivin expression in diffuse large B-cell lymphomas. Blood. 2000;96:1921–1925. [PubMed] [Google Scholar]
- 23.Nomura T, Yamasaki M, Nomura Y, Mimata H. Expression of the inhibitors of apoptosis proteins in cisplatin-resistant prostate cancer cells. Oncol Rep. 2005;14:993–997. [PubMed] [Google Scholar]
- 24.Zhang M, Latham DE, Delaney MA, Chakravarti A. Survivin mediates resistance to antiandrogen therapy in prostate cancer. Oncogene. 2005;24:2474–2482. doi: 10.1038/sj.onc.1208490. [DOI] [PubMed] [Google Scholar]
- 25.Zaffaroni N, Pennati M, Colella G, et al. Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cell Mol Life Sci. 2002;59:1406–1412. doi: 10.1007/s00018-002-8518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling X, Bernacki RJ, Brattain MG, Li F. Induction of survivin expression by taxol (paclitaxel) is an early event, which is independent of taxol-mediated G2/M arrest. J Biol Chem. 2004;279:15196–15203. doi: 10.1074/jbc.M310947200. [DOI] [PubMed] [Google Scholar]
- 27.Rodel F, Hoffmann J, Distel L, et al. Survivin as a radioresistance factor, and prognostic and therapeutic target for radiotherapy in rectal cancer. Cancer Res. 2005;65:4881–4887. doi: 10.1158/0008-5472.CAN-04-3028. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 29.Asanuma K, Moriai R, Yajima T, et al. Survivin as a radioresistance factor in pancreatic cancer. Jpn J Cancer Res. 2000;91:1204–1209. doi: 10.1111/j.1349-7006.2000.tb00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodel C, Haas J, Groth A, Grabenbauer GG, Sauer R, Rodel F. Spontaneous and radiation-induced apoptosis in colorectal carcinoma cells with different intrinsic radiosensitivities: survivin as a radioresistance factor. Int J Radiat Oncol Biol Phys. 2003;55:1341–1347. doi: 10.1016/s0360-3016(02)04618-7. [DOI] [PubMed] [Google Scholar]
- 31.Chintharlapalli S, Papineni S, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through PPARγ-dependent and PPARγ-independent pathways. Mol Cancer Ther. 2006;5:1362–1370. doi: 10.1158/1535-7163.MCT-06-0002. [DOI] [PubMed] [Google Scholar]
- 32.Kassouf W, Chintharlapalli S, Abdelrahim M, Nelkin G, Safe S, Kamat AM. Inhibition of bladder tumor growth by 1,1-bis(3′-indolyl)-1-(p-substitutedphenyl)methanes: a new class of peroxisome proliferator-activated receptor γ agonists. Cancer Res. 2006;66:412–418. doi: 10.1158/0008-5472.CAN-05-2755. [DOI] [PubMed] [Google Scholar]
- 33.Vanderlaag K, Su Y, Frankel AE, et al. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit proliferation of estrogen receptor-negative breast cancer cells by activation of multiple pathways. Breast Cancer ResTreat. 2008;109:273–283. doi: 10.1007/s10549-007-9648-y. [DOI] [PubMed] [Google Scholar]
- 34.Ichite N, Chougule MB, Jackson T, Fulzele SV, Safe S, Singh M. Enhancement of docetaxel anticancer activity by a novel diindolylmethane compound in human non-small cell lung cancer. Clin Cancer Res. 2009;15:543–552. doi: 10.1158/1078-0432.CCR-08-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chintharlapalli S, Burghardt R, Papineni S, Ramaiah S, Yoon K, Safe S. Activation of Nur77 by selected 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes induces apoptosis through nuclear pathways. J Biol Chem. 2005;280:24903–24914. doi: 10.1074/jbc.M500107200. [DOI] [PubMed] [Google Scholar]
- 36.Cho SD, Yoon K, Chintharlapalli S, et al. Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and independent pathways. Cancer Res. 2007;67:674–683. doi: 10.1158/0008-5472.CAN-06-2907. [DOI] [PubMed] [Google Scholar]
- 37.Lei P, Abdelrahim M, Cho SD, Liu S, Chintharlapalli S, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through activation of c-Jun N-terminal kinase. Carcinogenesis. 2008;29:1139–1147. doi: 10.1093/carcin/bgn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lei P, Abdelrahim M, Cho SD, Liu X, Safe S. Structure-dependent activation of endoplasmic reticulum stress-mediated apoptosis in pancreatic cancer by 1,1-bis(3′-indoly)-1-(p-substituted phenyl)methanes. Mol Cancer Ther. 2008;7:3363–3372. doi: 10.1158/1535-7163.MCT-08-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li F, Ling X, Huang H, et al. Differential regulation of survivin expression and apoptosis by vitamin D3 compounds in two isogenic MCF-7 breast cancer cell sublines. Oncogene. 2005;24:1385–1395. doi: 10.1038/sj.onc.1208330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu M, Kwan T, Yu C, et al. Peroxisome proliferator-activated receptor γ agonists promote TRAIL-induced apoptosis by reducing survivin levels via cyclin D3 repression and cell cycle arrest. J Biol Chem. 2005;280:6742–6751. doi: 10.1074/jbc.M411519200. [DOI] [PubMed] [Google Scholar]
- 41.Esteve PO, Chin HG, Pradhan S. Molecular mechanisms of transactivation and doxorubicin-mediated repression of survivin gene in cancer cells. J Biol Chem. 2007;282:2615–2625. doi: 10.1074/jbc.M606203200. [DOI] [PubMed] [Google Scholar]
- 42.Nawrocki ST, Carew JS, Douglas L, Cleveland JL, Humphreys R, Houghton JA. Histone deacetylase inhibitors enhance lexatumumab-induced apoptosis via a p21Cip1-dependent decrease in survivin levels. Cancer Res. 2007;67:6987–6994. doi: 10.1158/0008-5472.CAN-07-0812. [DOI] [PubMed] [Google Scholar]
- 43.Kaneko R, Tsuji N, Asanuma K, Tanabe H, Kobayashi D, Watanabe N. Survivin down-regulation plays a crucial role in 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor-induced apoptosis in cancer. J Biol Chem. 2007;282:19273–19281. doi: 10.1074/jbc.M610350200. [DOI] [PubMed] [Google Scholar]
- 44.Pyrko P, Soriano N, Kardosh A, et al. Downregulation of survivin expression and concomitant induction of apoptosis by celecoxib and its non-cyclooxygenase-2-inhibitory analog, dimethyl-celecoxib (DMC), in tumor cells in vitro and in vivo. Mol Cancer. 2006;5:19. doi: 10.1186/1476-4598-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahn KS, Sethi G, Krishnan K, Aggarwal BB. γ-Tocotrienol inhibits nuclear factor-κB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J Biol Chem. 2007;282:809–820. doi: 10.1074/jbc.M610028200. [DOI] [PubMed] [Google Scholar]
- 46.Rahman KW, Li Y, Wang Z, Sarkar SH, Sarkar FH. Gene expression profiling revealed survivin as a target of 3,3′-diindolylmethane-induced cell growth inhibition and apoptosis in breast cancer cells. Cancer Res. 2006;66:4952–4960. doi: 10.1158/0008-5472.CAN-05-3918. [DOI] [PubMed] [Google Scholar]