Abstract
Pain and fatigue are recognized as critical symptoms that impact quality of life (QOL) for cancer patients. The barriers to pain and fatigue relief have been classified into three categories: patient, professional and system barriers. The overall objective of this trial is to test the effects of the “Passport to Comfort” intervention on reducing barriers to pain and fatigue management for ambulatory care cancer patients. This intervention demonstrates innovation by translating the evidence-based guidelines for pain and fatigue as developed by the National Comprehensive Cancer Network (NCCN) into practice. This quasi-experimental, comparative study utilizes a Phase 1 control group of usual care followed sequentially by a Phase 2 intervention group in which educational and systems change efforts were directed toward improved pain and fatigue management. This paper reports on the patient barriers and the patient education intervention to overcome these barriers. A sample of 187 cancer patients with breast, lung, colon, or prostate cancers, and pain and/or fatigue of ≥ 4 (moderate to severe), were recruited. Patients in the intervention group received four educational sessions on pain/fatigue assessment and management, whereas patients in the control group received usual care. Pain and fatigue barriers and patient knowledge were measured at baseline, one month, and three months post-accrual. Patients in the intervention group experienced significant improvements in pain and fatigue measures immediately post-intervention, and these improvements were sustained over time. The “Passport to Comfort” intervention was effective in reducing patient barriers to pain and fatigue management, as well as increasing patient knowledge regarding pain and fatigue.
Keywords: Barriers, pain, fatigue
Introduction
Barriers to Symptom Management
Major deficiencies in the treatment of cancer symptoms such as pain and fatigue have been documented extensively over the past two decades. Pain and fatigue are recognized as impacting all dimensions of patient’s quality of life.1 One framework often applied to understanding the reasons for unrelieved symptoms is to examine the barriers to their relief. After two decades of effort to advance symptom management, sources have documented that reducing these barriers requires more than publication of guidelines or staff education.2,3 Allard and colleagues analyzed 33 studies of educational interventions for cancer pain and found that attitudes and knowledge about cancer pain were essential in improving pain management.4
The “Passport to Comfort” intervention model aimed to reduce barriers to optimal symptom relief. Patient outcomes were selected, as the investigators believe that patient, professional and systems barriers ultimately impact in the patient’s outcomes and their experience of symptoms. The final analysis at the conclusion of the five-year project will further analyze the dissemination of the interventions into ongoing usual care. The intervention was designed and implemented in response to the overwhelming evidence of the impact of these barriers on symptom management, and the intent to move beyond descriptive studies to test innovative models that would eliminate these barriers. The primary hypothesis was that the “Passport” intervention would promote significantly greater reductions of patient-related barriers to pain and fatigue management, and greater increase in knowledge of pain and/or fatigue assessment and management over time.
The literature has consistently documented that patients’ beliefs and actions play a key role in the undertreatment of pain.5,6 Patients are reluctant to report their pain for reasons including fear of side effects, fatalism about the possibility of achieving pain control, fear of distracting physicians from treating cancer, and belief that pain is indicative of progressive disease.6-11 There are also significant healthcare professional barriers to adequate pain relief. Physicians, nurses, and other members of the interdisciplinary team often fail to adequately assess the patient’s pain or to recognize patient barriers.12-14 Professionals lack knowledge of the principles of pain relief, side effect management, or understanding of key concepts like addiction, tolerance, dosing, and communication.15-18 System barriers include legal and regulatory structures that interfere with the provision of optimal care as well as inadequate reimbursement for pain services. System barriers can be internal, such as low referrals to supportive care services, as well as external system barriers such as reimbursement and regulatory constraints.19 Synthesis of pain literature by the Institute of Medicine and the National Cancer Policy Board have continued to document the importance of these system barriers on the ultimate outcomes of patient care.1,20
The symptom of fatigue has emerged as a high priority concern in cancer.21,22 While fatigue is the most common symptom of cancer, it is also the least understood. Cancer-related fatigue is reported by 60% to 99% of cancer patients and has been described as one of the most significant quality-of-life (QOL) issues in cancer care.23-25 Patients with fatigue have significantly lower QOL, cognitive function and physical performance. Similar to pain, numerous barriers to effective fatigue management have been documented.
Despite its prevalence and intensity, patients are reluctant to report fatigue and have little expectation that it can be relieved.1,25,26 A study conducted with 576 outpatients revealed that patients who experience fatigue do not report it to their doctors because they feel it is inevitable (43%), unimportant (34%) or untreatable (27%).25 Patients do not regard fatigue as a valid problem about which to complain unless they are asked about it by a healthcare provider.23,24 Fatigue often prompts patients to interrupt treatment schedules or stop treatment altogether, compromising the effectiveness of therapy, thereby potentially hindering the opportunity to treat the cancer.27-29 Fatigue is a symptom of cancer that is also poorly understood by professionals.22,30 Fatigue is also a symptom that is not routinely assessed in the clinical setting by healthcare providers.26 Consequently, cancer-related fatigue has been under-reported, under-diagnosed and under-treated. Even when patients report their fatigue, it may not be taken seriously by providers.31 Studies have reported that few patients ever receive treatment or advice from providers about how to manage their fatigue.21,25,30 Providers may erroneously assume that cancer-related fatigue is the same as the fatigue that healthy persons experience in everyday living.21,32 Thus, providers may not appreciate the significant negative effects of cancer-related fatigue.33,34 Providers may be unwilling to initiate discussion about fatigue with the patient, particularly if they are unaware of available treatments or believe that there is little they can do to manage fatigue.1 A major barrier to fatigue management is the lack of knowledge about the underlying causes of fatigue.1
Finally, there are institutional and system barriers related to fatigue assessment and management. Documentation of fatigue assessment and management in the medical record is not a common requirement in most healthcare institutions and is not required by organizations that accredit hospitals and nursing homes, such as the Joint Commission. As a consequence, assessment and management of fatigue is often not a priority and the health care provider is not reminded that fatigue should be assessed and documented routinely.28-30 The use of physical therapy to combat deconditioning is a good example of a potentially useful intervention affected by institutional/system barriers. Unfortunately, because it is burdensome to get a physician’s order, many patients are not referred. Healthcare reimbursement may also be a barrier, affecting the availability of medications, prescription practices, or referral patterns such as for psychiatric or relational support, physical therapy, nutritional support, or erythropoietin therapy.34
Educational Interventions
The effectiveness of education programs in addressing the barriers to symptom management has been evaluated in numerous studies and a systematic review with meta-analyses.35 Wells et al. 36 provided a brief pain education program to 64 patients with cancer-related pain and their caregivers at a comprehensive cancer center and a VA cancer clinic. They were then all randomized to one of three groups: 1) usual care, 2) pain hot line, and 3) weekly provider-initiated follow-up calls for one month following education. Long-term outcomes were evaluated monthly for six months. While continued access to the pain hot line or the weekly follow-up calls did not affect long-term outcomes, the brief pain education program did improve patient and caregiver knowledge and beliefs.
Another study conducted by Miaskowski and colleagues37 randomized cancer patients to a PRO-SELF intervention (n=93) or to standard care (n=81). Patients receiving the psychoeducational intervention by trained nurses were taught how to use a pillbox and given written instructions on how to communicate unrelieved pain to their physician as well as needed analgesic prescription changes. Patients also received two follow-up home visits and three phone calls by the nurses who coached them on how to improve their pain management. The standard care group received three home visits by the nurses, as well as three phone calls between visits. Results demonstrated that using a psychoeducation intervention involving nurse coaching within the context of self-care can improve the patient’s management of their pain.
Bennett and colleagues35 recently conducted a systematic review and meta-analysis of experimentally randomized and non-randomized controlled clinical trials identified from six databases from inception to November 2007. Twenty one trials, of which 19 were randomized, met inclusion criteria and, fifteen were used in the meta-analysis for a total of 3501 patients. In comparison to usual care/control, educational interventions improved knowledge and attitudes by one half point on a 0-5 scale (weighted mean difference 0.52, 95% CI 0.04-1.0), reduced average pain intensity by over one point on a 0-10 scale (WMD -1.1, -1.8 to -0.41), and reduced worst pain intensity by just under one point (WMD -0.78, -1.21 to -0.35). The authors concluded that patient-based educational interventions can result in modest but significant benefits in the management of cancer pain, and are probably underused alongside more traditional analgesic approaches.
In studies of education by Given et al.,38 Allison et al.,39 Ream et al.,40 and Godino et al.,41 a decrease in fatigue was found in the experimental groups. Given and colleagues38 conducted a randomized controlled trial with 113 cancer patients with mixed diagnoses providing an educational intervention that included teaching, counseling and support, coordination, and communication. All education was provided by nurses to patients receiving chemotherapy, and having pain and fatigue. Patients in the intervention group reported improved physical and social functioning as well as a significant reduction in number of symptoms. In a psychoeducational study conducted by Allison et al.,39 head and neck cancer patients (n=50) reported improvement in their fatigue as a result of the education. The intervention consisted of teaching patients how to cope with their cancer by focusing on two areas, the enhancement of a sense of personal control and, learning emotional and instrumental coping responses. The didactic component consisted of an eight-chapter workbook along with an audio cassette or CD containing verbal directions. Results showed significant improvements in physical, social, functioning and global quality of life, reduced fatigue (P=0.01), and sleep disturbance. Ream et al.40 conducted an intervention pilot study (Beating Fatigue) (n=8) comprised of four parts, 1) assessment/monitoring, 2) education, 3) coaching in the management of fatigue, and 4) provision of emotional support. Patients reported less fatigue and improved emotional well-being. Godino and colleagues41 studied whether or not nursing education decreased the perception of fatigue in patients with gastric or colon cancer. Patients were randomized into either standard care (n=17) or the experimental group (n=23) that received individualized intervention over three sessions. The sessions covered nutrition, stress management, rest and sleep, activity to maintain energy, lifestyle changes and adjustment. These sessions were conducted by a trained nurse using one-on-one education, training and counseling, as well as audio-visual and computerized education materials. Results showed that the intervention group experienced a decrease in fatigue levels while the control group experienced and increase in fatigue.
According to these studies and recent literature reviews,42,43 cancer patients benefit from psychoeducational interventions for their fatigue and the information learned is applied in their daily life.
The literature has documented that there are significant patient, professional and system barriers to the management of pain and fatigue based on clinical practice guidelines. Interventions are needed to improve symptom management for cancer patients. In response to overwhelming evidence of the impact of these barriers on symptom management, the National Cancer Institute (NCI) issued a call for proposals with the intent of moving beyond descriptive studies to test innovative models that would eliminate these barriers. This paper reports on comparative results from one of the projects supported by this NCI initiative and conducted at a NCI-designated comprehensive cancer center to test the effects of the “Passport to Comfort” intervention on pain and fatigue management. The primary hypothesis was that the “Passport” intervention would promote significantly greater reductions of patient-related barriers to pain and fatigue management and greater increase in knowledge of pain and/or fatigue assessment and management over time.
Methods
Design
This quasi-experimental, comparative study was designed in three phases (Table 1). Phase 1 assessed patient pain and fatigue management in usual care to describe the current status of pain and fatigue management in the sample population and setting. In Phase 2, all patients were offered the intervention. During the final phase now in progress (Phase 3), the intervention is being translated to a realistic model of care for use into the clinic setting so that it can be maintained after the project concludes. This design was selected instead of a randomized controlled trial (RCT) as the investigators believe it would be difficult to randomize and maintain a pure control/non-intervention group at the same time patient, professional and system changes were being made; thus contamination of the control group would be likely. The project demonstrates innovation by translating the evidence-based guidelines for pain and fatigue as developed by the National Comprehensive Cancer Network (NCCN) into clinical practice.10,11 This paper presents comparative analyses between the Phase 1 sample (control group) and Phase 2 sample (experimental group), and findings focused on the impact of intervention on patient-related barriers to pain and fatigue management.
Table 1.
Research Phases
| Phase 1 | Phase 2 | Phase 3 |
|---|---|---|
| Usual Care | Education Intervention | Support Intervention |
| Patient Barriers | Patient Barriers | |
| No intervention. Data collected to obtain baseline information. |
|
|
|
||
| Professional Barriers | Professional Barriers | |
|
|
|
| System Barriers | System Barriers | |
|
|
|
Sample
Study participants were recruited from the Ambulatory Care Clinic at an NCI-designated comprehensive cancer center. Eligibility criteria included a diagnosis of breast, colon, lung, or prostate cancer, time since diagnosis of at least one month, an expected prognosis of six months or greater, and subjective pain and/or fatigue rating of ≥ 4 (moderate to severe pain) on a numeric scale of 0-10 (0=none; 10=worst imaginable). Stratification was conducted to reflect a sample of the four diagnoses that were proportional to the cancer center population.
Procedure
The study design was reviewed and approved by the Institutional Review Board. Written informed consent was obtained from all participants prior to participation in this study. Each patient received four educational sessions. At each session, information pertaining to pain assessment, pain management, fatigue assessment, and fatigue management was provided. A teaching material packet was also provided to the patient, and the packet included the NCCN patient version guidelines for pain and fatigue and “tip sheets” which were one page supplemental education sheets on topics of nutrition, sleep disturbance, emotional issues, and exercise. Following the education and beginning at one month post-accrual, all patients were retained and supported through bi-weekly phone contacts. For both the usual care and intervention groups, all outcome measures were collected at baseline and repeated later at one and three months post-accrual. Figure 1 presents the attrition across both phases and all three time points.
Figure 1.
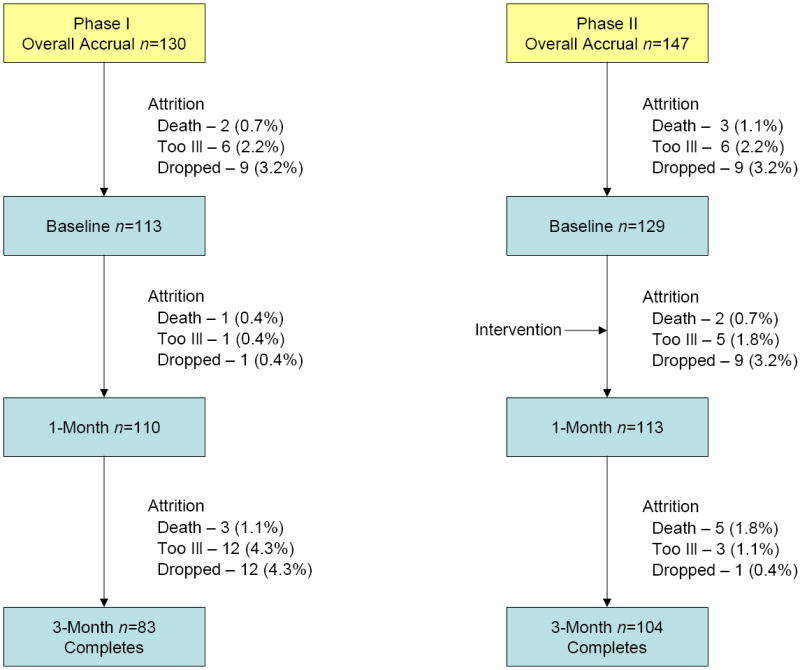
Total attrition for both phases, n=90 (32.5%)
Outcome Measures
The Demographic and Treatment Data Tool was designed to capture key disease and treatment variables of importance in describing the population and for analysis of influencing variables. Demographic and treatment data such as age, gender, disease type, stage of disease, treatments, and performance status (KPS) were collected at baseline.
The Barriers Questionnaire (BQ II) was developed by Ward and colleagues44 to measure patient-reported barriers to pain management. Significant (t=-2.16, P<0.05) construct validity and a factor analysis revealed four factors: (1) physiologic effects, (2) fatalism, (3) communication and (4) harmful effects. The BQ-II total has an internal consistency reliability coefficient alpha of 0.89 (n=134), and a range of 0.75-0.85 for the subscales.44
The Patient Pain Knowledge Tool was drafted by the investigators based on the NCCN Pain Guidelines. This 15-item questionnaire was used as an assessment of patient’s knowledge and beliefs. The questions were based on contents from the NCCN pain guidelines and asked subjects to identify whether each statement regarding pain is true or false. This tool was written at a low literacy level and to be less burdensome for subjects to complete. Internal consistency reliability analysis was performed, and Cronbach’s alpha was 0.67.
The Piper Fatigue Scale (PFS) is a 22-item, self-report scale that measures four dimensions of subjective fatigue (behavioral/severity [6-items], sensory [5-items], cognitive/mood [6-items], and affective meaning [5-items]) confirmed by principal axes factor analysis with oblique rotation (2). Internal consistency (Cronbach’s alpha) reliabilities were strong (0.83-0.97) for the PFS and its subscales across various cultural, languages, and diagnostic groups and were 0.89-0.97 in this sample. Each item is measured on a 0-10 numeric rating scale, and higher scores indicate more fatigue. Mild (1-3), moderate (4-6) and severe (7-10) levels have been validated with declines in physical functioning (MOS-SF-36 Physical Functioning subscale and total scores).45 Five additional items, not included in the scale’s scoring, assess perceived causes, relief measures, additional fatigue descriptors, presence of other symptoms, and duration of fatigue.45
The Fatigue Barriers Scale (FBS) is an investigator-developed 13-item scale designed to elicit patient beliefs and attitudes that might serve as barriers to effective fatigue assessment and management. It was developed based on an extensive literature review and clinical experience.1,46 Patients completed this scale at baseline. In this study, the reliability estimates for the subscales were (Cronbach’s alpha): Beliefs/Attitudes (0.30), Good Patient (0.65), and Fatalism (0.54). For the total scale (0.73), the reliability coefficient indicated good reliability for a new scale.
The Patient Fatigue Knowledge Tool is an investigator-developed scale that contains 15 true and false statements about fatigue designed to assess a patient’s knowledge about what fatigue is, and how it can be assessed, measured and treated. It was developed based on the NCCN fatigue guidelines, and was designed to capture key patient-related knowledge barriers.
Statistical Methods
The study was powered to detect a moderate effect size of .3 for the outcomes of pain and fatigue intensity, barriers to pain management (BQII), the Piper Fatigue Scale, Quality of Life, patient knowledge of pain and fatigue, and adherence to pain and fatigue management. Sample sizes were determined for specific study instruments to detect significance in the interaction effect of the repeated measures ANOVA statistical design at a power of approximately 0.80, with a two-tailed alpha of 0.05 at various effect sizes, assuming a correlation of 0.7 between repeated measures. Sample size calculations were based upon means and standard deviations available in Drs. Ferrell and Piper’s studies, as well as upon discriminating group difference cores for the BQ-II. A total of 130 patients were accrued to the Phase 1 (usual care) group and 20 dropped out because they were too ill (n=7), deceased (n=3), did not wish to continue (n=10). Of the 83 patients retained for analysis in Phase 1, 27 did not provide three-month data (due to illness, death, or personal reasons), and their missing data were imputed. Of the 147 Phase 2 (intervention) patients accrued, 43 dropped out because they were too ill (n=14), died (n=10), or did not want to continue (n=19). All of the 104 patients retained for analysis provided complete data for all measurement periods. The association between percent of missing data and study group was not significant. Likewise, there was not a significant association between reason for dropout and study group. The data were analyzed using SPSS (v. 15.0). A missing values analysis was conducted using SPSS MVA, and missing values were imputed based on the analysis. Descriptive statistics were computed for all variables in the study. Contingency table analysis with the chi-square statistic or t-test was conducted on selected demographic and treatment variables to examine the equivalence of study groups.
Prior to data analysis, instruments were scored according to standardized scoring instructions and reliability was tested for this sample of patients. The primary hypothesis indicates that the intervention group will report significantly greater reductions of patient-related barriers to pain and fatigue as well as greater increase in knowledge of pain and fatigue. Measures related to pain were tested only on patients who had pain of intensity at least equal to 4 out of 10, or patients who had both pain and fatigue at that level of intensity (n=83). Measures related to fatigue were tested only on patients who had fatigue at least equal to 4 out of 10, or patients who had both fatigue and pain at that level of intensity (n=167). The statistical tests utilized were two by three repeated measures ANOVAs in which the between group variable was study group and the within group variable was the baseline, 1, and 3 month measure of barriers subscales and overall scores (BQII, Fatigue Barriers Scale), the knowledge of pain and fatigue test scores, and the Piper Fatigue Scale. Significance tests were not conducted at the item level of the instruments used, but descriptive statistics are included for selected items to provide clinical context. The effect of the intervention on pain and fatigue was examined using hierarchical regression analysis, in which either pain or fatigue at one month was regressed first on age, gender, number of years since diagnosis, cancer stage, type of cancer (dummy coded); and then on study group and pain (or fatigue) at baseline.
Results
Demographics
A total of 83 patients were included in the usual care (Phase 1) group and 104 patients in the intervention (Phase 2) group (Table 2). Twenty patients had pain only, 105 patients had fatigue only, and 62 patients had both pain and fatigue at the levels required to meet the inclusion criterion. The majority of patients was female, Caucasian (non-Hispanic), married, and living with someone; the sample included 34% ethnic minorities. Nearly half of the patients had some college education or were college-graduates. In terms of diagnoses, 73 patients had breast cancer, 39 had colon cancer, 46 had lung cancer, and 29 had prostate cancer. Seventy-seven percent of patients were undergoing chemotherapy at study accrual. In terms of symptom intensity, the intervention group was more likely to have both pain and fatigue. Intensity for both symptoms dropped at one and three months for both groups, but there were no significant differences over time by group.
Table 2.
Baseline Demographic & Clinical Characteristics
| Variable | Usual Care (n=83) n (%) | Intervention (n=104) n (%) | Total (n=187) n (%) |
|---|---|---|---|
| Age | |||
| Mean (SD) | 60 (12) | 59 (12) | 59.7 (11.9) |
| Types of Cancer | |||
| • Breast | 33 (40) | 40 (39) | 73 (39) |
| • Colon | 19 (23) | 20 (19) | 39 (21) |
| • Lung | 18 (22) | 28 (27) | 46 (25) |
| • Prostate | 13 (16) | 16 (15) | 29 (16) |
| Nature of Symptoms | |||
| • Pain | 14 (17) | 6 (6) | 20 (11) |
| • Fatigue | 45 (54) | 60 (58) | 105 (56) |
| • Both | 24 (29) | 38 (37) | 62 (33) |
| Gender | |||
| • Male | 31 (37) | 44 (42) | 75 (40) |
| • Female | 52 (63) | 60 (58) | 112 (60) |
| Race/Ethnicity | |||
| • Caucasian | 54 (65) | 69 (67) | 123 (66) |
| • African American | 9 (11) | 12 (12) | 21 (11) |
| • Hispanic/Latino | 14 (17) | 12 (12) | 26 (14) |
| • Other | 6 (7) | 10 (10) | 16 (9) |
| Education | |||
| • HS or less | 35 (43) | 32 (31) | 67 (36) |
| • College | 32 (39) | 51 (49) | 83 (45) |
| • Post Graduate | 15 (18) | 21 (20) | 36 (19) |
| Marital Status | |||
| • Married | 51 (61) | 69 (66) | 120 (64) |
| • Other | 32 (39) | 35 (34) | 67 (36) |
| Lives Alone | 75 (90) | 89 (86) | 164 (88) |
| No | 8 (10) | 15 (14) | 23 (12) |
| • Yes | |||
| Cancer Stage | |||
| • I-II | 15 (19) | 27 (26) | 42 (23) |
| • III-IV | 66 (82) | 76 (74) | 142 (77) |
| Disease Status | |||
| • Recently diagnosed | 33 (40) | 63 (61) | 96 (52) |
| • Completed treatment | 2 (2) | 11 (11) | 13 (7) |
| • Recurrence | 34 (42) | 23 (22) | 57 (31) |
| • Other | 13 (16) | 6 (6) | 19 (10) |
| Treatments | |||
| Chemotherapy | |||
| • Yes | 62 (77) | 82 (79) | 144 (78) |
| Radiation | |||
| • Yes | 6 (7) | 8 (8) | 14 (8) |
| Supportive Services | |||
| • Yes | 21 (25) | 14 (14) | 35 (19) |
| Pain intensity (0-10) a | n =38 | n =44 | n =82 |
| Mean (SD) | |||
| • Baseline | 5.5 (1.4) | 5.3 (1.3) | 5.6 (1.5) |
| • One month | 4.0 (2.3) | 3.8 (2.4) | 4.1 (2.5) |
| • Three months | 4.6 (3.0) | 3.5 (3.2) | 4.2 (3.1) |
| Fatigue intensity (0-10) b | n=69 | n=98 | n =167 |
| Mean (SD) | |||
| • Baseline | 6.2 (1.5) | 6.0 (1.3) | 6.0 (1.4) |
| • One month | 3.5 (2.2) | 5.6 (1.8) | 4.8 (2.3) |
| • Three months | 3.9 (2.1) | 4.8 (2.1) | 4.5 (2.2) |
Patients with pain only (≥ 4.0) or pain and fatigue (≥ 4.0): Interaction effect: F(2,160)=0.076, P=0.328.
Patients with fatigue only or with pain and fatigue (≥ 4.0): Interaction effect: F(2,330)=1.90, P=0.155.
Impact of Intervention on Patient Barriers to Pain Management
A total of 83 patients had pain or both pain and fatigue that was of moderate to severe intensity (≥4). Considering both groups, specific items that are barriers to pain management at baseline (scores > 2.0 on the 0-5 scale) include: fear of addiction, belief that drowsiness or confusion caused by pain medications are difficult to control, that the chronic use of pain medications renders it ineffective later, and that the chronic use of pain medications masks new pain or body changes.
The intervention group demonstrated significantly more improvements than the usual care group on three of the four subscales (physiological concerns, fatalism, and belief in harmful effects) and the total BQ-II score over time, and these decreases were immediate. At both one and three months, barriers were significantly higher in the usual care group than in the intervention group (Table 3).
Table 3.
Select item and subscale scores from the BQ IIa
| Usual Care n=38 Mean (SD) | Intervention n=44 Mean (SD) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 1 month | 3 months | Baseline | 1 month | 3 months | ||
| BQ-II Items (0-5) | |||||||
| Danger in becoming addicted to pain medications | 3.8 (1.3) | 3.2 (1.7) | 3.3 (1.3) | 3.1 (1.7) | 1.5 (1.8) | 1.3 (1.8) | |
| Drowsiness from pain medications is uncontrollable | 3.1 (1.3) | 2.4 (1.6) | 2.7 (1.3) | 2.7 (1.8) | 1.7 (1.8) | 1.6 (1.8) | |
| Pain medications cause confusion | 2.5 (1.6) | 2.2 (1.6) | 1.9 (1.7) | 1.9 (1.7) | 1.3 (1.6) | 1.2 (1.7) | |
| Body gets used to pain medications so it won’t work anymore | 3.3 (1.5) | 3.4 (1.6) | 3.0 (1.4) | 2.5 (1.7) | 1.5 (1.7) | 1.6 (1.7) | |
| Pain medication blocks awareness of new pain | 2.6 (1.5) | 2.9 (1.7) | 2.3 (1.6) | 2.5 (1.7) | 1.4 (1.8) | 1.2 (1.6) | |
| People get addicted to pain medications | 2.8 (1.6) | 2.8 (1.7) | 2.3 (1.0) | 2.6 (1.6) | 1.1 (1.6) | 1.1 (1.5) | |
| Taking pain medications now means that it won’t work when pain becomes worse | 2.0 (1.6) | 2.5 (1.9) | 2.2 (1.1) | 1.8 (1.7) | .75 (1.4) | .77 (1.4) | |
| Pain medications prevents knowledge of what’s going on with body | 2.4 (1.8) | 2.8 (1.6) | 3.3 (1.1) | 2.6 (1.7) | 1.2 (1.7) | 1.2 (1.6) | |
| Using pain medications now means it won’t work when it is needed later | 2.2 (1.7) | 2.3 (1.7) | 2.1 (1.1) | 1.7 (1.7) | .66 (1.1) | .61 (1.2) | |
| Pain medications can mask changes in health | 2.5 (1.6) | 2.9 (1.8) | 2.8 (1.1) | 2.3 (1.7) | 1.1 (1.4) | 1.1 (1.5) | |
| Pain medication is very addictive | 3.0 (1.6) | 3.0 (1.7) | 2.4 (1.0) | 2.8 (1.5) | 1.2 (1.6) | 1.1 (1.7) | |
| Usual Care n=38 Mean (SD) | Intervention n=44 Mean (SD) | Group by Time Interaction P-value | |||||
| Baseline | 1 month | 3 month | Baseline | 1 month | 3 months | ||
| BQ-II Scale Scores | |||||||
| Physical Effects | 2.3 (.94) | 2.5 (.97) | 2.2 (.59) | 1.9 (.92) | 1.1 (1.1) | 1.1 (1.2) | <.001 |
| Fatalism | 1.4 (.73) | 1.8 (1.2) | 1.1 (.92) | 1.4 (1.2) | .69 (.85) | .62 (.88) | .001 |
| Communication | 1.2 (1.2) | 1.6 (1.1) | .92 (.87) | 1.2 (1.0) | .84 (.91) | .83 (.84) | .017 |
| Harmful Effects | 2.5 (1.1) | 2.5 (1.2) | 2.4 (.90) | 2.1 (1.1) | 1.0 (1.3) | .95 (1.3) | <.001 |
| Total BQ-II Score | 2.0 (.83) | 2.2 (.92) | 1.9 (.56) | 1.7 (.69) | .97 (.84) | .93 (.88) | <.001 |
Patients with pain only (intensity ≥ 4.0) or pain and fatigue (intensity ≥ 4.0), n=82, were included in this analysis. Item means were provided for detailed context but did not undergo statistical testing for group differences over time.
Overall knowledge score for the usual care group at baseline was 73%, versus 78% for the intervention group. In the intervention group, knowledge about pain increased significantly to 87% at one month and 88% at three months, and this increase was statistically significant when compared to the usual care group. Despite this improvement, two areas of lack of knowledge persisted for the intervention group. These include knowledge that cancer pain can only be treated with medication, and that pain medications can be stopped suddenly if not needed anymore. Table 4 provides select items (<70% correct) and total scores on patient pain knowledge.
Table 4.
Patient Pain Knowledge, Percent Correct on Selected Items and Overall Score
| Knowledge of Pain Items | Usual Care n=38 Mean (SD) | Intervention n=44 Mean (SD) | Group by Time Interaction P-value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 1 month | 3 months | Baseline | 1 month | 3 months | ||
| 1. Cancer is the most common cause of pain | 45 (.50) | 51 (.51) | 44 (.53) | 43 (.50) | 66 (.48) | 80 (.41) | |
| 2. Taking opioids leads to addiction | 53 (.51) | 46 (.51) | 67 (.50) | 73 (.45) | 89 (.32) | 89 (.32) | |
| 3. Side effects of pain meds can be treated and prevented | 66 (.48) | 84 (.37) | 89 (.33) | 84 (.37) | 93 (.25) | 84 (.37) | |
| 4. Cancer pain can only be tx w/meds | 68 (.47) | 65 (.48) | 89 (.33) | 70 (.46) | 68 (.47) | 75 (.44) | |
| 5. Most common side effect of morphine is constipation | 68 (.47) | 59 (50) | 89 (.33) | 80 (.41) | 91 (.29) | 95 (.21) | |
| 6. Need to increase dose of pain med sign of addiction | 61 (.50) | 54 (.51) | 78 (.44) | 66 (.48) | 93 (.25) | 86 (.35) | |
| 7. Stop pain med suddenly w/o worry about side effects | 63 (.49) | 54 (.51) | 44 (.53) | 59 (.50) | 73 (.45) | 68 (.47) | |
| Total Knowledge of Pain Score (% correct) | .73 (.13) | .69 (.15) | .78 (.16) | .78 (.12) | .87 (.09) | .88 (.10) | 0.001 |
Patients with pain only (intensity ≥4.0) or pain and fatigue (intensity ≥4.0) were included in this analysis.
Impact of the Intervention on Patient Barriers to Fatigue Management
A total of 167 patients had fatigue or both fatigue and pain of moderate to severe intensity (≥4). Considering both groups, aspects of fatigue demonstrating a score of ≥ 6 (0-10 scale) included interference with work and enjoyable activities, symptom intensity, a sensation of unpleasantness, disagreeability, negativity, a feeling of weakness, listlessness, tiredness, lack of energy, and overall emotional distress. While sensory fatigue dropped significantly at one and three months for the intervention group, it did not change over time for the usual care group, and this difference was statistically significant (Table 5).
Table 5.
Select Item and Subscale Scores from the Piper Fatigue Scale a
| Scale | Usual Care n=69 Mean (SD) | Intervention n=98 Mean (SD) | Group by Time Interaction P-value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 1 month | 3 months | Baseline | 1 month | 3 months | ||
| Piper Fatigue Scale (PFS) Items (0-10) | |||||||
| Fatigue interfered with work | 6.2 (3.2) | 5.9 (3.4) | 5.0 (3.1) | 5.8 (3.2) | 5.0 (2.8) | 4.0 (3.0) | |
| Fatigue interfered with enjoyable activities | 7.0 (2.8) | 6.3 (3.2) | 6.0 (3.2) | 6.7 (2.8) | 5.8 (2.9) | 5.0 (3.0) | |
| Intensity of fatigue | 6.3 (2.6) | 5.8 (3.1) | 5.1 (2.6) | 5.4 (2.3) | 4.9 (2.2) | 4.2 (2.3) | |
| Fatigue was unpleasant | 6.4 (2.5) | 6.2 (2.8) | 5.5 (2.7) | 6.2 (2.7) | 5.9 (2.5) | 5.1 (2.4) | |
| Fatigue was disagreeable | 6.4 (2.6) | 6.2 (2.8) | 5.6 (2.7) | 6.0 (2.9) | 5.6 (2.6) | 5.1 (2.4) | |
| Fatigue had negative effect | 6.1 (2.6) | 6.0 (2.7) | 5.2 (2.6) | 6.4 (2.8) | 5.9 (2.5) | 5.2 (2.5) | |
| Fatigue made me feel weak | 6.3 (2.6) | 6.2 (2.6) | 5.5 (2.8) | 6.8 (2.5) | 5.7 (2.8) | 4.2 (3.0) | |
| Fatigue made me feel listless | 6.1 (2.6) | 6.2 (2.4) | 5.7 (2.7) | 5.9 (2.3) | 5.1 (2.3) | 4.3 (2.5) | |
| Fatigue made me feel tired | 6.9 (2.4) | 6.9 (2.5) | 5.8 (2.9) | 7.0 (2.5) | 6.3 (2.7) | 5.1 (2.8) | |
| Fatigue made me feel unenergetic | 7.2 (2.3) | 7.0 (2.5) | 5.8 (2.8) | 7.0 (2.3) | 6.0 (2.4) | 5.1 (2.6) | |
| Fatigue was emotionally distressing | 6.6 (2.6) | 6.5 (2.9) | 5.3 (2.7) | 6.2 (2.5) | 5.6 (2.3) | 4.8 (2.7) | |
| PFS Subscale Scores | |||||||
| Sensory | 6.4 (2.2) | 6.2 (2.3) | 5.5 (2.8) | 6.4 (2.1) | 5.4 (2.3) | 4.4 (2.5) | .025 |
| Affective Meaning | 6.0 (2.4) | 5.7 (2.5) | 5.4 (2.6) | 5.8 (2.4) | 5.2 (2.2) | 4.7 (2.2) | .696 |
| Cognitive/Mood | 4.9 (2.0) | 4.9 (2.2) | 4.6 (2.1) | 5.1 (2.2) | 4.5 (2.0) | 3.9 (2.1) | .070 |
| Behavioral Severity | 6.0 (2.6) | 5.7 (3.0) | 5.5 (3.1) | 5.8 (2.5) | 5.1 (2.5) | 4.2 (2.5) | .080 |
| Total Score | 5.8 (2.0) | 5.6 (2.3) | 5.3 (2.4) | 5.7 (2.0) | 5.0 (2.0) | 4.3 (2.1) | .053 |
Patients with fatigue only (intensity ≥4.0) or fatigue and pain (intensity ≥4.0) were included in this analysis.
Considering both groups, seven items stand out as particular barriers to fatigue management at baseline (scores > 2.0 on the 0-5 scale). These items include: inevitability of fatigue, no effective treatments for it, meaning of worsening underlying disease, less important compared to cancer itself, and concerns about being perceived as a complainer. The usual care group was significantly different than the intervention group on total scores on fatigue barriers over time. At both one and three months, fatigue management barriers were significantly higher in the usual care group than in the intervention group. Overall, patients demonstrated a high level of knowledge (85% to 91%) about fatigue across time. The only lack of knowledge is found regarding the relationship between exercise and energy use as well as staying in bed when tired. Both groups demonstrated lack of knowledge regarding the need for exercise and activity to reduce fatigue. Patients strongly felt that activity should be limited and exercise avoided which is in sharp contrast to the strong evidence supporting exercise as an important aspect of fatigue management (Table 6).
Table 6.
Key Barriers to Fatigue: FBS and Selected Knowledge Items
| Usual Care n=69 Mean (SD) | Intervention n=98 Mean (SD) | Group by Time Interaction P-value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 1 month | 3 months | Baseline | 1 month | 3 months | ||
| Fatigue Barriers Scale (FBS) Items (0-5) | |||||||
| Fatigue is inevitable result of treatment | 4.3 (1.0) | 4.5 (.87) | 4.3 (1.0) | 3.9 (1.6) | 4.2 (1.3) | 4.3 (1.1) | |
| There are many effective treatments for fatigue | 2.5 (1.6) | 2.6 (1.7) | 2.6 (1.6) | 2.5 (1.5) | 1.3 (1.4) | 1.4 (1.4) | |
| Fatigue means my disease is getting worse | 1.5 (1.7) | 3.1 (1.9) | 1.8 (1.7) | 2.0 (1.8) | 1.4 (1.5) | 1.5 (1.4) | |
| Treating fatigue is not as important as treating illness | 3.3 (1.7) | 1.8 (1.4) | 3.2 (1.6) | 2.3 (1.8) | 1.6 (1.8) | 1.4 (1.8) | |
| Fatigue means my treatment is not working | 1.1 (1.6) | 3.4 (1.8) | 1.3 (1.5) | 1.6 (1.9) | .89 (1.3) | .94 (1.3) | |
| Talking about fatigue means I am a complainer | 1.6 (1.7) | 3.3 (1.7) | 1.9 (1.8) | 2.3 (2.0) | 1.8 (1.8) | 1.9 (1.8) | |
| My doctor will ask me about fatigue | 2.6 (1.8) | 2.6 (1.8) | 2.2 (1.8) | 3.1 (1.8) | 3.5 (1.8) | 3.5 (1.8) | |
| Total FBS Score | 28.1 (10.6) | 28.5 (11.9) | 27.3 (8.3) | 29.9 (16.1) | 23.0 (11.9) | 23.5 (12.0) | 0.002 |
| Knowledge of Fatigue Items (% Correct) | |||||||
| Exercising takes more energy | 71% (.46) | 69% (.47) | 79% (.41) | 35% (.48) | 26% (.44) | 23% (.43) | |
| If tired, should stay in bed all day | 80% (.41) | 72% (.45) | 79% (.41) | 70% (.46) | 92% (.28) | 85% (.36) | |
| Total Knowledge of Fatigue Score | .91 (.10) | .90 (.11) | .89 (.17) | .85 (.13) | .92 (.06) | .91 (.07) | <0.001 |
Impact of the Intervention on Pain and Fatigue Intensity
Demographic and clinical variables accounted for 25% of the variance in one-month pain intensity (P=0.003), while study group and baseline pain intensity accounted for another 7% of the variance (P=0.025), and the full model (R2=32%) was significant (F(9,71)=3.78, P=0.001). Those with breast cancer had significantly less pain at three months than those with prostate cancer (beta = -0.495), and the higher the baseline pain intensity, the higher it was at three months (beta = 0.268, P=0.012). The same model did not explain fatigue score at one month nearly as well. Demographic and clinical variables accounted for only 7% of the variance in one-month fatigue intensity (P=0.118), while study group and baseline fatigue accounted for 6% of the variance (P=0.006). The entire model accounted for 13% of the variance (P=0.010). Younger patients expressed significantly more fatigue (beta = -0.209), baseline fatigue was significantly positively associated with one-month fatigue (beta = 0.181), and the usual care group had significantly more fatigue (beta = -0.155).
Discussion
One limitation of this study was that the analysis for pain was underpowered due to the fewer than expected number of patients who met the criterion for pain intensity. A significant effect on pain over time may have demonstrated if the sample size of 55 per group had been achieved.
A second limitation is that this study was implemented at one national comprehensive cancer center outpatient setting located in Southern California where patients commonly are referred for treatment and second opinions, usually late in the course of their treatment and disease process. As a consequence, the findings may not be generalizable to other geographic areas or inpatient settings, or to other populations, such as newly diagnosed patients with early stage disease, survivors without evidence of disease, or patients with other types of malignancies. Although ethnic minorities were represented in this study, due to the small sample size we were unable to determine statistical differences by ethnicity.
Another limitation is the heterogeneity of sample population with four different cancer diagnoses. This strategy limited the number of participants accrued into each diagnosis group, which in turn prohibited generalizations of study results due to the smaller sample size in each diagnosis group.
The “Passport to Comfort” intervention is one of the first reported trials to utilize the NCCN supportive care guidelines to reduce barriers to pain and fatigue management in ambulatory care cancer patients. Study findings show that the model was effective in reducing patient barriers as well as increasing knowledge about pain and fatigue management. The literature has consistently documented that patients play a key role in the undertreatment of pain.9,10 Patients are reluctant to report their pain for reasons including fear of side effects, fatalism about the possibility of achieving pain control, fear of distracting physicians from treating cancer, and belief that pain is indicative of progressive disease.7 In this study, the “Passport” intervention was effective in improving patient’s beliefs on common and pervasive pain barriers, such as fear of addiction, tolerance, and beliefs that side effects of pain medications are difficult to control. Furthermore, these statistically significant improvements were observed immediately post-intervention as well as sustained over time.
Findings in the pain literature also suggest that strategies in reducing barriers to pain management require attention to knowledge as well as attitudes about pain.47-49 In this study, the intervention was effective in improving overall patient knowledge about pain assessment and management. Overall knowledge scores were increased for items such as opioids leading to addiction, beliefs that side effects from pain medications cannot be controlled, and that the need to increase medication dose is a sign of addiction. However, study findings suggest that some lack of knowledge persisted for patients in the intervention group, and these included the belief that pain can only be managed with pharmacologic agents. A potential explanation for this finding may be related to healthcare professionals’ lack of knowledge in non-pharmacologic management of pain, which have been shown to be effective in the pain literature.16,20
Findings from this study show that the “Passport” intervention was effective in decreasing sensory fatigue ratings, and this positive effect was immediate and sustained over time. In this study, the intervention was successful in improving perceptions of fatigue barriers. Common beliefs such as inevitability of fatigue, lack of effective treatment for fatigue, the lower priority given to managing fatigue, and concerns of being a complainer when reporting fatigue were all improved for the intervention group. In terms of patients’ fatigue knowledge, both the intervention and usual care group demonstrated high levels of knowledge. However, misconceptions persisted on the relationship between exercise and energy use and staying in bed when tired. This finding suggests that patients are more likely to utilize inactivity as a strategy to manage fatigue. This perception underscores the lack of translation of the strong empirical evidence of the benefits of physical activity on fatigue into usual practice and patient education.50-53
Over the past decade, several studies have demonstrated that it is possible to overcome patient barriers to pain and fatigue management.49,54 These model programs have emphasized patient teaching interventions including the use of pain and fatigue assessment tools, strategies to dispel misconceptions, and patient coaching regarding the reporting and documenting of their symptoms. Most of these interventions were successful in reducing barriers to symptom management that are persists in clinical practice settings. The “Passport” intervention is unique in aspects including the translation of the NCCN pain and fatigue guidelines into an educational intervention for reducing misconceptions and increasing patient knowledge and the incorporation of a design to integrate the intervention into usual care. These unique characteristics of the intervention will aid in the translation of the model into actual clinical settings so that it can be maintained after project conclusion.
Acknowledgments
This work was supported by grant R-01 CA115323 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Institutes of Health. Symptom management in cancer: pain, depression, and fatigue. 2002 Available from: http://consensus.nih.gov/ta/022/022_statement.htm.
- 2.Davis D, O’Brien MA, Freemantle N, et al. Impact of formal continuing medical education: do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes? JAMA. 1999;282(9):867–874. doi: 10.1001/jama.282.9.867. [DOI] [PubMed] [Google Scholar]
- 3.Slotnick HB. How doctors learn: physicians’ self-directed learning episodes. Acad Med. 1999;74(10):1106–1117. doi: 10.1097/00001888-199910000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Allard P, Maunsell E, Labbe J, Dorval M. Educational interventions to improve cancer pain control: a systematic review. J Palliat Med. 2001;4(2):191–203. doi: 10.1089/109662101750290227. [DOI] [PubMed] [Google Scholar]
- 5.Cleeland CS, Janjan NA, Scott CB, Seiferheld WF, Curran WJ. Cancer pain management by radiotherapists: a survey of radiation therapy oncology group physicians. Int J Radiat Oncol Biol Phys. 2000;47(1):203–208. doi: 10.1016/s0360-3016(99)00276-x. [DOI] [PubMed] [Google Scholar]
- 6.Miaskowski C, Dodd MJ, West C, et al. Lack of adherence with the analgesic regimen: a significant barrier to effective cancer pain management. J Clin Oncol. 2001;19(23):4275–4279. doi: 10.1200/JCO.2001.19.23.4275. [DOI] [PubMed] [Google Scholar]
- 7.Pargeon KL, Hailey BJ. Barriers to effective cancer pain management: a review of the literature. J Pain Symptom Manage. 1999;18(5):358–368. doi: 10.1016/s0885-3924(99)00097-4. [DOI] [PubMed] [Google Scholar]
- 8.Potter VT, Wiseman CE, Dunn SM, Boyle FM. Patient barriers to optimal cancer pain control. Psychooncology. 2003;12(2):153–160. doi: 10.1002/pon.627. [DOI] [PubMed] [Google Scholar]
- 9.Ward S, Donovan HS, Owen B, Grosen E, Serlin R. An individualized intervention to overcome patient-related barriers to pain management in women with gynecologic cancers. Res Nurs Health. 2000;23(5):393–405. doi: 10.1002/1098-240x(200010)23:5<393::aid-nur6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Ward S, Hughes S, Donovan H, Serlin RC. Patient education in pain control. Support Care Cancer. 2001;9(3):148–155. doi: 10.1007/s005200000176. [DOI] [PubMed] [Google Scholar]
- 11.Ward SE, Goldberg N, Miller-McCauley V, et al. Patient-related barriers to management of cancer pain. Pain. 1993;52(3):319–324. doi: 10.1016/0304-3959(93)90165-L. [DOI] [PubMed] [Google Scholar]
- 12.Ferrell BR, Grant M, Ritchey KJ, Ropchan R, Rivera LM. The pain resource nurse training program: a unique approach to pain management. J Pain Symptom Manage. 1993;8(8):549–556. doi: 10.1016/0885-3924(93)90084-9. [DOI] [PubMed] [Google Scholar]
- 13.McCaffery M, Ferrell B, O’Neil-Page E, Lester M, Ferrell B. Nurses’ knowledge of opioid analgesic drugs and psychological dependence. Cancer Nurs. 1990;13(1):21–27. [PubMed] [Google Scholar]
- 14.McCaffery M, Ferrell BR, Pasero C. Nurses’ personal opinions about patients’ pain and their effect on recorded assessments and titration of opioid doses. Pain Manag Nurs. 2000;1(3):79–87. doi: 10.1053/jpmn.2000.9295. [DOI] [PubMed] [Google Scholar]
- 15.Elliott TE, Elliott BA. Physician attitudes and beliefs about use of morphine for cancer pain. J Pain Symptom Manage. 1992;7(3):141–148. doi: 10.1016/s0885-3924(06)80005-9. [DOI] [PubMed] [Google Scholar]
- 16.Elliott TE, Murray DM, Elliott BA, et al. Physician knowledge and attitudes about cancer pain management: a survey from the Minnesota cancer pain project. J Pain Symptom Manage. 1995;10(7):494–504. doi: 10.1016/0885-3924(95)00100-d. [DOI] [PubMed] [Google Scholar]
- 17.Ferrell B, Virani R, Grant M, Vallerand A, McCaffery M. Analysis of pain content in nursing textbooks. J Pain Symptom Manage. 2000;19(3):216–228. doi: 10.1016/s0885-3924(00)00107-x. [DOI] [PubMed] [Google Scholar]
- 18.Rabow MW, Hardie GE, Fair JM, McPhee SJ. End-of-life care content in 50 textbooks from multiple specialties. JAMA. 2000;283(6):771–778. doi: 10.1001/jama.283.6.771. [DOI] [PubMed] [Google Scholar]
- 19.Agency for Health Care Policy and Research. Clinical practice guideline for cancer pain management, No. 9. Rockville, MD: US Department of Health and Human Services; 1994. [Google Scholar]
- 20.Gee RE, Fins JJ. Barriers to pain and symptom management, opioids, health policy, and drug benefits. J Pain Symptom Manage. 2003;25(2):101–103. doi: 10.1016/s0885-3924(02)00693-0. [DOI] [PubMed] [Google Scholar]
- 21.Vogelzang NJ, Breitbart W, Cella D, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue Coalition. Semin Hematol. 1997;34(3 Suppl 2):4–12. [PubMed] [Google Scholar]
- 22.Winningham ML, Nail LM, Burke MB, et al. Fatigue and the cancer experience: the state of the knowledge. Oncol Nurs Forum. 1994;21(1):23–36. [PubMed] [Google Scholar]
- 23.Cella D, Peterman A, Passik S, Jacobsen P, Breitbart W. Progress toward guidelines for the management of fatigue. Oncology (Williston Park) 1998;12(11A):369–377. [PubMed] [Google Scholar]
- 24.Schwartz AL, Nail LM, Chen S, et al. Fatigue patterns observed in patients receiving chemotherapy and radiotherapy. Cancer Invest. 2000;18(1):11–19. doi: 10.3109/07357900009023057. [DOI] [PubMed] [Google Scholar]
- 25.Stone P, Richardson A, Ream E, et al. Cancer-related fatigue: inevitable, unimportant and untreatable? Results of a multi-centre patient survey. Cancer Fatigue Forum. Ann Oncol. 2000;11(8):971–975. doi: 10.1023/a:1008318932641. [DOI] [PubMed] [Google Scholar]
- 26.Nail LM. Fatigue in patients with cancer. Oncol Nurs Forum. 2002;29(3):537. doi: 10.1188/onf.537-546. [DOI] [PubMed] [Google Scholar]
- 27.Ancoli-Israel S, Moore PJ, Jones V. The relationship between fatigue and sleep in cancer patients: a review. Eur J Cancer Care (Engl) 2001;10(4):245–255. doi: 10.1046/j.1365-2354.2001.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Jong N, Courtens AM, Abu-Saad HH, Schouten HC. Fatigue in patients with breast cancer receiving adjuvant chemotherapy: a review of the literature. Cancer Nurs. 2002;25(4):283–297. doi: 10.1097/00002820-200208000-00004. quiz 298-299. [DOI] [PubMed] [Google Scholar]
- 29.Payne JK. The trajectory of fatigue in adult patients with breast and ovarian cancer receiving chemotherapy. Oncol Nurs Forum. 2002;29(9):1334–1340. doi: 10.1188/02.ONF.1334-1340. [DOI] [PubMed] [Google Scholar]
- 30.Visser MR, Smets EM. Fatigue, depression and quality of life in cancer patients: how are they related? Support Care Cancer. 1998;6(2):101–108. doi: 10.1007/s005200050142. [DOI] [PubMed] [Google Scholar]
- 31.Grant M, Golant M, Rivera L, Dean G, Benjamin H. Developing a community program on cancer pain and fatigue. Cancer Pract. 2000;8(4):187–194. doi: 10.1046/j.1523-5394.2000.84012.x. [DOI] [PubMed] [Google Scholar]
- 32.Glaus A, Crow R, Hammond S. A qualitative study to explore the concept of fatigue/tiredness in cancer patients and in healthy individuals. Support Care Cancer. 1996;4(2):82–96. doi: 10.1007/BF01845757. [DOI] [PubMed] [Google Scholar]
- 33.Curt GA. Impact of fatigue on quality of life in oncology patients. Semin Hematol. 2000;37(4 Suppl 6):14–17. doi: 10.1016/s0037-1963(00)90063-5. [DOI] [PubMed] [Google Scholar]
- 34.Nail LM. Long-term persistence of symptoms. Semin Oncol Nurs. 2001;17(4):249–254. doi: 10.1053/sonu.2001.27916. [DOI] [PubMed] [Google Scholar]
- 35.Bennett M, Bagnall A, Closs S. How effective are patient-based educational interventions in the management of cancer pain? Systematic review and meta-analysis. Pain. 2009;143(3):192–199. doi: 10.1016/j.pain.2009.01.016. Epub 2009 Mar 12. [DOI] [PubMed] [Google Scholar]
- 36.Wells N, Hepworth J, Murphy B, Wujcik D, Johnson R. Improving cancer pain management through patient and family education. J Pain Symptom Manage. 2003;25(4):344–356. doi: 10.1016/s0885-3924(02)00685-1. [DOI] [PubMed] [Google Scholar]
- 37.Miaskowski C, Dodd M, West C, et al. Randomized clinical trial of the effectiveness of a self-care intervention to improve cancer pain management. J Clin Oncol. 2004;22(9):1713–1720. doi: 10.1200/JCO.2004.06.140. [DOI] [PubMed] [Google Scholar]
- 38.Given B, Given CW, McCorkle R, et al. Pain and fatigue management: results of a nursing randomized clinical trial. Oncol Nurs Forum. 2002;29(6):949–956. doi: 10.1188/02.ONF.949-956. [DOI] [PubMed] [Google Scholar]
- 39.Allison PJ, Edgar L, Nicolau B, et al. Results of a feasibility study for a psychoeducational intervention in head and neck cancer. Psychooncology. 2004;13(7):482–485. doi: 10.1002/pon.816. [DOI] [PubMed] [Google Scholar]
- 40.Ream E, Richardson A, Alexander-Dann C. Facilitating patients’ coping with fatigue during chemotherapy – pilot outcomes. Cancer Nurs. 2002;25(4):300–308. doi: 10.1097/00002820-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Godino C, Jodar L, Duran A, Martinez I, Schiaffino A. Nursing education as an intervention to decrease fatigue perception in oncology patients. Eur J Oncol Nurs. 2006;10(2):150–155. doi: 10.1016/j.ejon.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Nijs E, Ros W, Grijpdonck M. Nursing intervention for fatigue during the treatment of cancer. Cancer Nurs. 2008;31(3):191–206. doi: 10.1097/01.NCC.0000305721.98518.7c. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell S, Beck S, Hood L, Moore K, Tanner E. Putting evidence into practice: evidence-based interventions for fatigue during and following cancer and its treatment. Clin J Oncol Nurs. 2008;11(1):99–113. doi: 10.1188/07.CJON.99-113. [DOI] [PubMed] [Google Scholar]
- 44.Gunnarsdottir S, Donovan HS, Serlin RC, Voge C, Ward S. Patient-related barriers to pain management: the Barriers Questionnaire II (BQ-II) Pain. 2002;99(3):385–396. doi: 10.1016/S0304-3959(02)00243-9. [DOI] [PubMed] [Google Scholar]
- 45.Piper BF, Dibble SL, Dodd MJ, et al. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25(4):677–684. [PubMed] [Google Scholar]
- 46.Passik SD. Impediments and solutions to improving the management of cancer-related fatigue. J Natl Cancer Inst Monogr. 2004;32:136. doi: 10.1093/jncimonographs/lgh030. [DOI] [PubMed] [Google Scholar]
- 47.Ferrell BR, Grant M, Chan J, Ahn C, Ferrell BA. The impact of cancer pain education on family caregivers of elderly patients. Oncol Nurs Forum. 1995;22(8):1211–1218. [PubMed] [Google Scholar]
- 48.Ferrell BR, Rhiner M, Ferrell BA. Development and implementation of a pain education program. Cancer. 1993;72(11 Suppl):3426–3432. doi: 10.1002/1097-0142(19931201)72:11+<3426::aid-cncr2820721608>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 49.Miaskowski C, Dodd M, West C, et al. Randomized clinical trial of the effectiveness of a self-care intervention to improve cancer pain management. J Clin Oncol. 2004;22(9):1713–1720. doi: 10.1200/JCO.2004.06.140. [DOI] [PubMed] [Google Scholar]
- 50.Conn VS, Hafdahl AR, Porock DC, McDaniel R, Nielsen PJ. A meta-analysis of exercise interventions among people treated for cancer. Support Care Cancer. 2006;14(7):699–712. doi: 10.1007/s00520-005-0905-5. [DOI] [PubMed] [Google Scholar]
- 51.Courneya KS, Mackey JR, Bell GJ, et al. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 52.Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005;23(16):3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- 53.Schmitz KH, Holtzman J, Courneya KS, et al. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 54.West CM, Dodd MJ, Paul SM, et al. The PRO-SELF(c): Pain Control Program--an effective approach for cancer pain management. Oncol Nurs Forum. 2003;30(1):65–73. doi: 10.1188/03.ONF.65-73. [DOI] [PubMed] [Google Scholar]


