Abstract
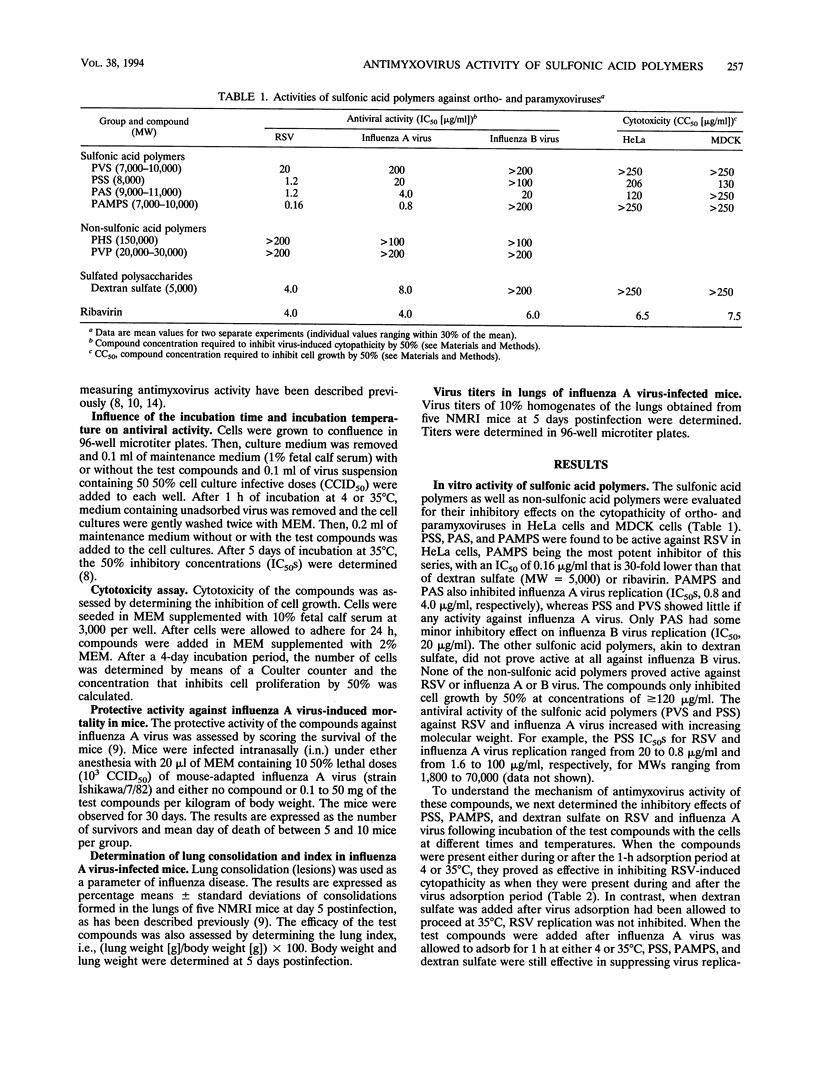
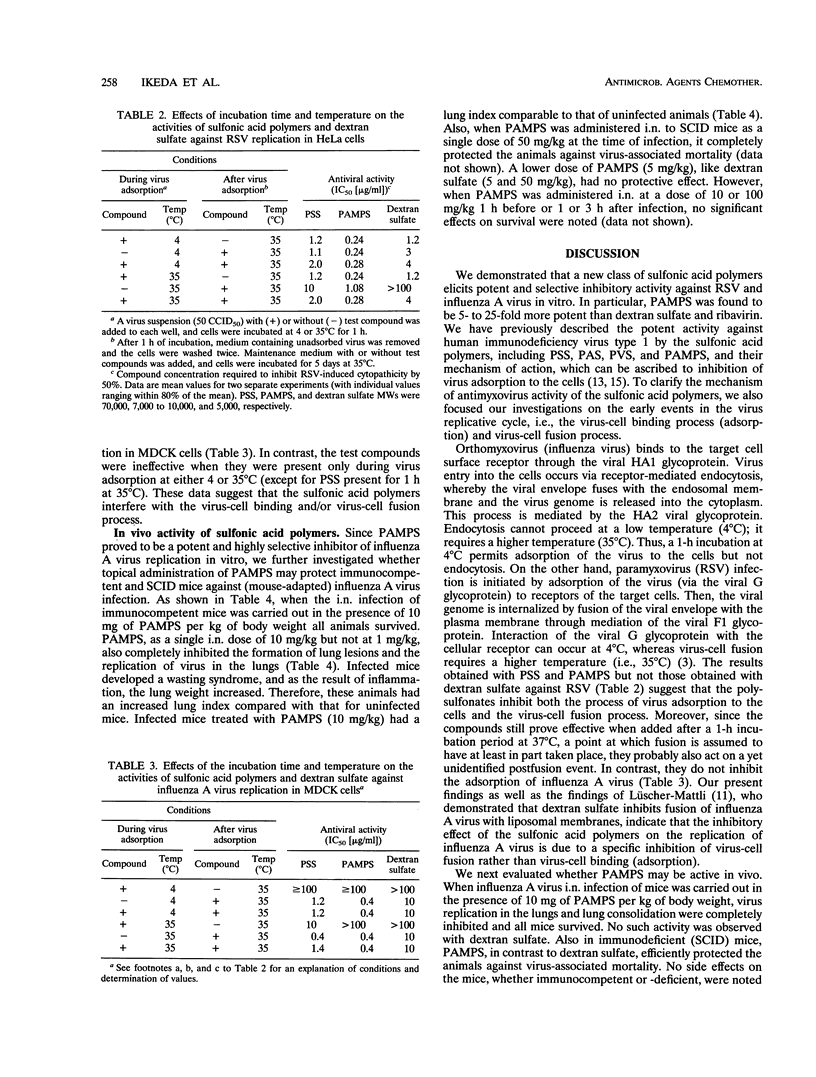
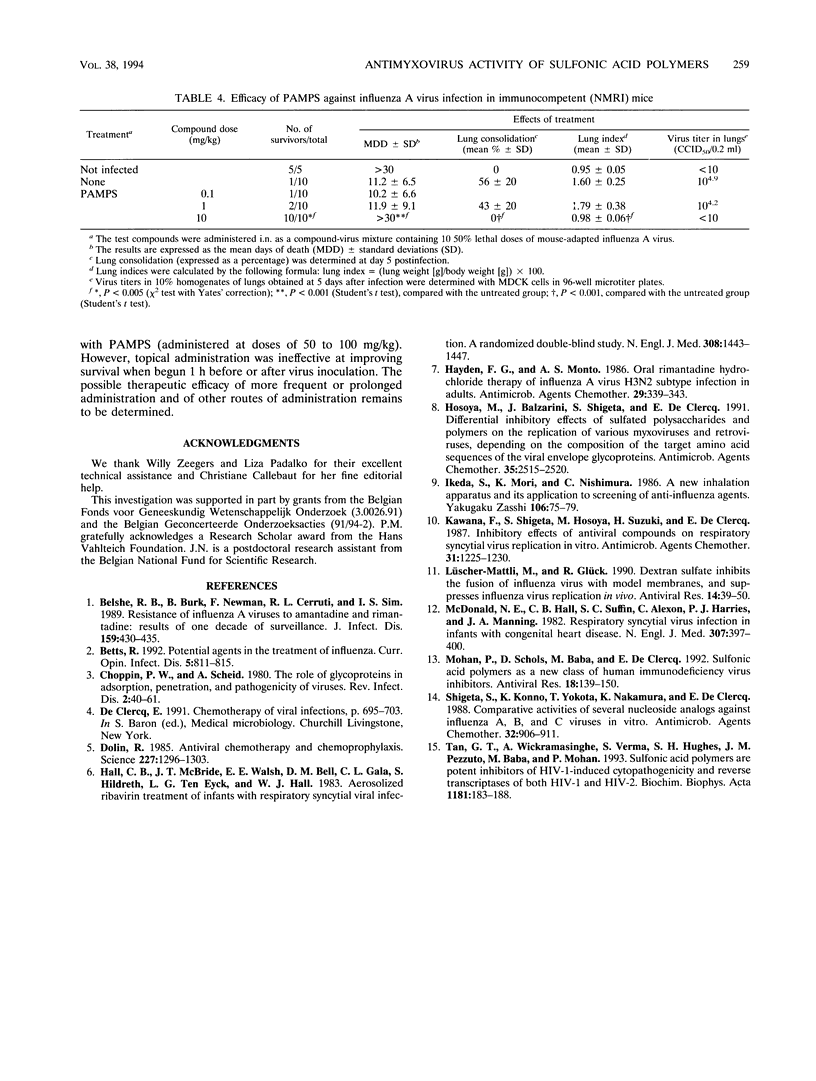
A series of sulfonic acid polymers were shown to be potent and selective inhibitors of respiratory syncytial virus (RSV) and influenza A virus. The compounds inhibit the replication of RSV and influenza A virus in HeLa and MDCK cells, at concentrations of 0.16 and 4.0 micrograms/ml, respectively, and are nontoxic to growing cells at concentrations of > 100 micrograms/ml. The mode of antiviral action of the sulfonic acid polymers can be ascribed to inhibition of virus-cell fusion (for influenza A virus) or inhibition of both virus-cell binding and fusion (for RSV). The sulfonic acid prototype PAMPS [poly(2-acrylamido-2-methyl-1-propanesulfonic acid)], when administered intranasally to mice as a single dose of 10 or 50 mg per kg of body weight at the time of infection, completely inhibited influenza A virus replication (in lungs) and virus-associated lung consolidation in immunocompetent mice and completely protected NMRI and SCID (severe combined immune deficiency) mice against influenza A virus-associated mortality. When administered 1 h before or after virus inoculation, no protective effect was observed at a dose of 10 or 100 mg/kg. Sulfonic acid polymers exert selective inhibitory effects on RSV and influenza A virus replication.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belshe R. B., Burk B., Newman F., Cerruti R. L., Sim I. S. Resistance of influenza A virus to amantadine and rimantadine: results of one decade of surveillance. J Infect Dis. 1989 Mar;159(3):430–435. doi: 10.1093/infdis/159.3.430. [DOI] [PubMed] [Google Scholar]
- Choppin P. W., Scheid A. The role of viral glycoproteins in adsorption, penetration, and pathogenicity of viruses. Rev Infect Dis. 1980 Jan-Feb;2(1):40–61. doi: 10.1093/clinids/2.1.40. [DOI] [PubMed] [Google Scholar]
- Dolin R. Antiviral chemotherapy and chemoprophylaxis. Science. 1985 Mar 15;227(4692):1296–1303. doi: 10.1126/science.2983421. [DOI] [PubMed] [Google Scholar]
- Hall C. B., McBride J. T., Walsh E. E., Bell D. M., Gala C. L., Hildreth S., Ten Eyck L. G., Hall W. J. Aerosolized ribavirin treatment of infants with respiratory syncytial viral infection. A randomized double-blind study. N Engl J Med. 1983 Jun 16;308(24):1443–1447. doi: 10.1056/NEJM198306163082403. [DOI] [PubMed] [Google Scholar]
- Hayden F. G., Monto A. S. Oral rimantadine hydrochloride therapy of influenza A virus H3N2 subtype infection in adults. Antimicrob Agents Chemother. 1986 Feb;29(2):339–341. doi: 10.1128/aac.29.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya M., Balzarini J., Shigeta S., De Clercq E. Differential inhibitory effects of sulfated polysaccharides and polymers on the replication of various myxoviruses and retroviruses, depending on the composition of the target amino acid sequences of the viral envelope glycoproteins. Antimicrob Agents Chemother. 1991 Dec;35(12):2515–2520. doi: 10.1128/aac.35.12.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S., Mori K., Nishimura C. [A new inhalation apparatus and its application to screening of anti-influenza agents]. Yakugaku Zasshi. 1986 Jan;106(1):75–79. doi: 10.1248/yakushi1947.106.1_75. [DOI] [PubMed] [Google Scholar]
- Kawana F., Shigeta S., Hosoya M., Suzuki H., De Clercq E. Inhibitory effects of antiviral compounds on respiratory syncytial virus replication in vitro. Antimicrob Agents Chemother. 1987 Aug;31(8):1225–1230. doi: 10.1128/aac.31.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher-Mattli M., Glück R. Dextran sulfate inhibits the fusion of influenza virus with model membranes, and suppresses influenza virus replication in vivo. Antiviral Res. 1990 Jul;14(1):39–50. doi: 10.1016/0166-3542(90)90064-e. [DOI] [PubMed] [Google Scholar]
- MacDonald N. E., Hall C. B., Suffin S. C., Alexson C., Harris P. J., Manning J. A. Respiratory syncytial viral infection in infants with congenital heart disease. N Engl J Med. 1982 Aug 12;307(7):397–400. doi: 10.1056/NEJM198208123070702. [DOI] [PubMed] [Google Scholar]
- Mohan P., Schols D., Baba M., De Clercq E. Sulfonic acid polymers as a new class of human immunodeficiency virus inhibitors. Antiviral Res. 1992 Jun;18(2):139–150. doi: 10.1016/0166-3542(92)90034-3. [DOI] [PubMed] [Google Scholar]
- Shigeta S., Konno K., Yokota T., Nakamura K., De Clercq E. Comparative activities of several nucleoside analogs against influenza A, B, and C viruses in vitro. Antimicrob Agents Chemother. 1988 Jun;32(6):906–911. doi: 10.1128/aac.32.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G. T., Wickramasinghe A., Verma S., Hughes S. H., Pezzuto J. M., Baba M., Mohan P. Sulfonic acid polymers are potent inhibitors of HIV-1 induced cytopathogenicity and the reverse transcriptases of both HIV-1 and HIV-2. Biochim Biophys Acta. 1993 Apr 30;1181(2):183–188. doi: 10.1016/0925-4439(93)90109-e. [DOI] [PubMed] [Google Scholar]



