Abstract
Pathogenesis of multiple myeloma is associated with an aberrant expression of pro-proliferative, pro-angiogenic and bone-metabolism modifying factors by malignant plasma cells. Given the frequently long time-span from diagnosis of early-stage plasma cell dyscrasias to overt myeloma and the mostly low proliferation rate of malignant plasma cells, we hypothesize these likewise to express a novel class of inhibitory factors of potential prognostic relevance. Bone morphogenic proteins (BMPs) represent possible candidates as they inhibit proliferation, stimulate bone formation, and have impact on the survival of cancer patients. We assessed expression of BMPs and their receptors by Affymetrix DNA-microarrays (n=779) including CD138-purified primary myeloma cell samples (n=635) of previously untreated patients. BMP6 is the only BMP expressed by malignant and normal plasma cells. Its expression is significantly lower in proliferating myeloma cells, myeloma cell lines, or plasmablasts. BMP6 significantly inhibits proliferation of myeloma cell lines, survival of primary myeloma cells, and in vitro angiogenesis. High BMP6-expression in primary myeloma cell samples delineates significantly superior overall survival for patients undergoing high-dose chemotherapy independent of conventional prognostic factors (ISS-stage, beta-2-microglobulin).
Keywords: Bone Morphogenetic Protein 6; biosynthesis; Cell Proliferation; Disease-Free Survival; Female; Gene Expression Profiling; Gene Expression Regulation, Neoplastic; Humans; Male; Multiple Myeloma; metabolism; mortality; pathology; Neoplasm Proteins; biosynthesis; Neovascularization, Pathologic; metabolism; mortality; pathology; Oligonucleotide Array Sequence Analysis; Plasma Cells; Survival Rate; Tumor Markers, Biological; biosynthesis
Keywords: Gene expression profiling, multiple myeloma, angiogenesis, proliferation, survival
Introduction
Multiple myeloma (MM) is an incurable malignant disease of clonal plasma cells which accumulate in the bone marrow (BM) causing clinical signs and symptoms related to the displacement of normal hematopoiesis, BM neovascularization, formation of osteolytic bone lesions, and production of monoclonal protein (Kyle and Rajkumar 2004; Vacca et al. 1994). Pathogenesis of MM is associated with an aberrant expression of pro-proliferative, pro-angiogenic and bone-metabolism modifying factors by malignant plasma cells (MM cells, MMCs) or their induced production in the BM microenvironment. Various of these factors have been identified by others and us (De Vos et al. 2006; Klein et al. 2003; Zhan et al. 2002; Hose et al. 2009b).
Given the frequently long time from first diagnosis of early-stage plasma cell dyscrasias to overt myeloma and the mostly low proliferation rate of MMCs (Witzig et al. 1999), we hypothesize these to express a novel class of inhibitory factors of potential prognostic relevance. Bone morphogenic proteins (BMPs) and especially BMP6 represent a possible candidate, as it is expressed in MMCs (Zhan et al. 2002) and inhibits proliferation of myeloma and memory B-cells (Ro et al. 2004; Kersten et al. 2005). It likewise stimulates osteoblast differentiation (Ebisawa et al. 1999), osteoclast development (Wutzl et al. 2006), and bone formation (Cheng et al. 2003). In different cancer entities, BMP6 shows contradictory impact on patients’ survival: whereas in renal cell carcinoma BMP6 acts as inhibitor of tumor growth (Kim et al. 2003), in prostate (Hamdy et al. 1997; Dai et al. 2005; Haudenschild et al. 2004) and breast cancer (Clement et al. 1999), BMP6-expression promotes tumor progression and metastasis. BMP6 has been described as pro-angiogenic factor in terms of endothelial cell migration and tube formation in vitro (Valdimarsdottir et al. 2002).
BMPs are members of the transforming growth factor-β superfamily, and act through binding to two different types of serine/threonine kinase receptors. Three type I receptors bind BMPs: activin-like kinase-2, (Alk-2, ACVR1), -3 (Alk-3, BMPR1A), and -6 (Alk-6, BMPR1B). Likewise, three type II receptors have been identified, i.e. BMP receptor II (BMPR2), activin type II receptor (ActRII, ACVR2) and activin type IIB receptor (ActRIIB, ACVR2B) (Ebisawa et al. 1999). Both, type I and type II receptors are required for signaling (Kawabata et al. 1998). All BMPs use BMPR2, but utilize different BMP type I receptors. BMP6 preferably binds to ACVR1 (Ro et al. 2004).
Intracellular BMP-signals are transduced mainly by small mothers against decapentaplegic proteins (SMADs). Eight different SMAD proteins have been identified in vertebrates, which can be subclassified into receptor-regulated SMADs, common-partner SMADs and inhibitory SMADs. In vertebrates, BMP signaling acts through SMAD1 and the close homologues SMAD5 and SMAD8. Phosphorylated SMAD1, -5 or -8 proteins form a complex with SMAD4, the only member of the common-partner SMADs, and translocate to the nucleus where they interact with other transcription factors (Massague 2000). Alternate BMP-signaling pathways include prostanoid-generation via COX-2 (Ren et al. 2007) and MAPK-dependant activation of p38 or the Ras- and Erk-pathway (Nohe et al. 2004; Du et al. 2007). Both pathways have been reported to be present in MMCs (Trojan et al. 2006; Hoang et al. 2006).
Assessing the expression of BMPs, its receptors and members of the signaling-transduction pathway, we found BMP6 to be the only BMP expressed in normal (BMPCs) and malignant plasma cells. In vitro, BMP6 binds to MMCs, blocks their proliferation, induces apoptosis and inhibits angiogenesis. BMP6-expression is a favorable prognostic factor in two independent cohorts of a total of 168 myeloma patients treated with high-dose chemotherapy (HDT) and autologous stem cell transplantation (ASCT). This is validated by an independent cohort of 345 patients treated within the total therapy 2 protocol of the Little Rock (LR)-group (Barlogie et al. 2006). The prognostic value of BMP6-expression is independent of classical prognostic parameters, i.e. serum-β2-microglobulin (B2M) and ISS (International Staging System). Thus, BMP6 exemplifies a novel class of prognostic pro-apoptotic and anti-angiogenic factors expressed by normal as well as malignant plasma cells.
Results
Expression of BMP6, BMP-receptors and downstream SMADs
Expression of BMPs, BMP-receptors and the members of the downstream signal-transduction chain were evaluated using U133 A+B (Heidelberg/Montpellier-group 1; HM1) and U133 2.0 plus (HM2) Affymetrix microarrays (see Table 1A–C, Table S1).
Table 1.
Presence of expression of BMP6, BMP receptors and downstream SMADs
| A | B | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene symbol | Probe set | BM present (%) (n=13) | PPC present (%) (n=12) | BMPC present (%) (n=14) | MGUS present (%) (n=12) | MM present (%) (n=233) | HMCL present (%) (n=40) | ND-WBM present (%) (n=7) | MM-WBM present (%) (n=57) |
| BMP6 | 206176_at | 0.0 | 16.7 | 92.9 | 100.0 | 97.9 | 85.0 | 0.0 | 70.2 |
| BMPR2a | 225144_at | 50.0 | 100.0 | 57.1 | 100.0 | 94.0 | 70.0 | 71.4 | 87.7 |
| ACVR1 | 203935_at | 0.0 | 66.7 | 57.1 | 58.3 | 84.5 | 72.5 | 0.0 | 57.9 |
| ACVR2A | 205327_s_at | 84.6 | 100.0 | 100.0 | 100.0 | 97.9 | 97.5 | 0.0 | 47.4 |
| ACVR2Ba | 236126_at | 0.0 | 0.0 | 0.0 | 20.0 | 12.5 | 50.0 | 14.3 | 26.3 |
| BMPR1A | 213578_at | 38.5 | 75.0 | 100.0 | 33.3 | 56.2 | 77.5 | 0.0 | 12.3 |
| SMAD1 | 210993_s_at | 0.0 | 0.0 | 0.0 | 33.3 | 42.9 | 82.5 | 71.4 | 75.4 |
| SMAD4 | 202527_s_at | 15.4 | 33.3 | 21.4 | 33.3 | 63.1 | 80.0 | 85.7 | 93.0 |
| SMAD5a | 225219_at | 71.4 | 100.0 | 100.0 | 100.0 | 98.8 | 100.0 | 28.6 | 59.6 |
| PTGS2 | 204748_at | 0.0 | 0.0 | 21.4 | 91.7 | 72.5 | 2.5 | 100.0 | 100.0 |
| C | |||||||||
| Gene symbol | Probe set | CD3 present (%) (n=5) | CD14 present (%) (n=5) | CD15 present (%) (n=5) | CD34 present (%) (n=5) | MSC-ND present (%) (n=7) | MSC-MGUS present (%) (n=5) | MSC-MM present (%) (n=7) | OC present (%) (n=7) |
| BMP6 | 202701_at | 0.0 | 0.0 | 0.0 | 0.0 | 28.6 | 60.0 | 28.6 | 0.0 |
| BMPR2a | 205290_s_at | 100.0 | 100.0 | 20.0 | 100.0 | 100.0 | 100.0 | 71.4 | 100.0 |
| ACVR1 | 211518_s_at | 100.0 | 100.0 | 0.0 | 100.0 | 100.0 | 100.0 | 71.4 | 100.0 |
| ACVR2A | 205431_s_at | 100.0 | 100.0 | 60.0 | 100.0 | 100.0 | 100.0 | 71.4 | 100.0 |
| ACVR2Ba | 206176_at | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 14.3 |
| BMPR1A | 209591_s_at | 100.0 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | 71.4 | 42.9 |
| SMAD1 | 220203_at | 0.0 | 40.0 | 0.0 | 100.0 | 100.0 | 100.0 | 85.7 | 85.7 |
| SMAD4 | 207865_s_at | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| SMAD5a | 221136_at | 100.0 | 100.0 | 20.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
Abbreviations: BM, bone marrow; BMP, bone morphogenic protein; BMPC, bone marrow plasma cell; HMCL, human myeloma cell line; MBC, memory B cell; MGUS, monoclonal gammopathy of unknown significance; MMC, multiple myeloma cell; MM-WBM, whole BM of multiple myeloma patients; ND-WBM, whole BM of normal donors; OC, osteoclast; PPC, polyclonal plasmablastic cell; SMAD, small mothers against decapentaplegic proteins.
Presence of expression of BMP6, BMP receptors and SMADs as judged by PANP in (A) BMPC, PPC, MBC, MMC and HMCL; (B) the WBM of normal donors (ND-WBM) as well as myeloma patients (MM-WBM) and (C) sub-fractions of the latter, respectively.
As absence and presence of gene expression by PANP can only be assessed for U133A and U133 2.0 plus arrays, for probe sets located on the U133B chip presented data are based on Heidelberg/Montpellier-group 2 only.
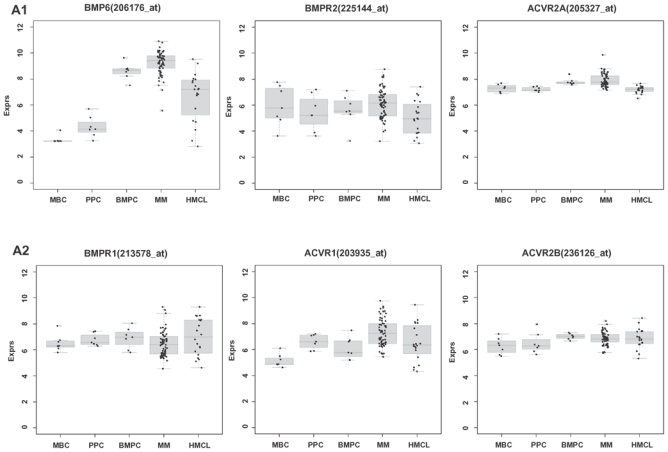
BMP6 is the only BMP expressed by normal and malignant plasma cells both in HM1 and HM2 (Table 1A,C, Supplementary Table S2). The mean BMP6-expression is significantly and by several orders of magnitude higher in BMPCs or MMCs compared to B-cell precursor cells (MBCs and polyclonal plasmablastic cells, PPCs; p<.001). Human myeloma cell lines (HMCLs) show a lower expression of BMP6 compared to BMPCs (p<.001 in HM1, Supplementary Table S1).
Of the BMP-receptors, four are expressed in BMPCs and MMCs. BMPR2 is present in most BMPCs and precursors without significant change throughout plasma cell differentiation, as well as in MMCs. ACVR1 is an early plasma cell marker, lacking expression in MBCs (Table 1B, Figure 1A), and ACVR2B is aberrantly expressed in MMCs of 12.5 % of patients (Table 1B). No significantly different gene expression could be found for BMP6 or BMP-receptors between MMCs from early-stage (monoclonal gammopathy of unknown significance (MGUS) and MMI) and advanced stage (MMII and MMIII) patients (Supplementary Table S1D).
Figure 1. Expression of BMP6, BMP-receptors and SMADs and validation of gene expression by flow cytometry and western blotting.
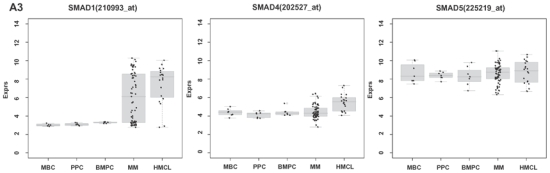
(A1–3) Expression of BMP6, ACVR1, BMPR2, BMPR1A, ACVR2A, ACVR2B, SMAD1, SMAD4 and SMAD5 in normal plasma cells (BMPCs), memory B-cells (MBCs), polyclonal plasmablastic cells (PPCs), myeloma cells (MMCs) and human myeloma cell lines (HMCLs) within the Heidelberg/Montpellier-group 1 (HM1). For HM2, see Supplementary Figure S1. SMAD8 is neither expressed in HM1 nor HM2. (B) To validate gene expression data, intracytoplasmatic expression of BMP6 was determined by flow cytometry. Shown are the cell lines (B1) XG-10, (B2) XG-11 and (B3) U266; the first shows a consistent absence of BMP6 by gene expression profiling, quantitative real-time PCR and flow cytometry, the other two a consistent expression. (B4) Expression of BMP6 by an exemplary myeloma patient (pMMC). Light gray line: control without primary antibody, black line: measurement with corresponding primary and secondary antibody. (C) Phosphorylation of downstream SMADs can be seen within 15 min. following incubation with BMP6. After pre-incubation with heparin, no SMAD-phosphorylation is detectable. Consistent with the literature, SMAD-2 is not phosphorylated. Actin was used as loading control.
Of the downstream signaling cascade, SMAD5 is expressed in all plasma cell precursor-, plasma cell- and 166/168 MMC samples (Table 1, Figure 1), whereas SMAD1 is aberrantly expressed in 100/233 MMC samples and 39/40 HMCLs. SMAD4 is expressed in 1/13 MBC, 4/12 PPC, 4/14 BMPC, and 147/233 MMC samples as well as 36/40 HMCLs. Thus, transducing SMAD1/SMAD4 or SMAD5/SMAD4 complexes are increasingly present from B-cell precursors over MMCs to cell lines. SMAD8 is not expressed.
Of the populations investigated within the whole bone marrow (WBM), only BMPCs, MMCs, and a sub-fraction of mesenchymal stromal cells (MSCs), express BMP6 (Table 1A,C). The low number of BMPCs and MSCs in the BM of normal donors could explain the lack of detectable BMP6-expression. Expression of BMP6 within the WBM correlates significantly (rs=.45, P=.001) with the percentage of plasma cell infiltration.
Validation of gene expression
By quantitative real-time PCR (qRT-PCR), BMP6 is expressed in 9/10 HMCLs (absent in XG-10) and 10/10 primary MMC samples, consistent with results by PANP/GEP (see methods). BMP6-receptors (BMPR2, ACVR1) are expressed in all HMCLs and primary MMC samples investigated. BMP6-expression measured by qRT-PCR and GEP correlates well for HMCLs (rs=0.78, P=.009) and MMCs (rs=0.56, P=.05).
By flow cytometry, intracellular BMP6-expression can be detected in 9/10 HMCLs and 3/3 primary MMC samples. In agreement with PANP/GEP and qRT-PCR, BMP6-expression is absent in XG-10. Exemplary data are shown in Figure 1B.
Performing enzyme-linked immunosorbent assays (ELISA), amounts of BMP6 around the level of the detection limit could be found in supernatants of HMCLs. For the highly BMP6-expressing HMCLs XG-11 and U266 (see Figure 1B) BMP6 levels of 47.59 and 57.51 pg/ml could be measured, respectively. In agreement with GEP, qRT-PCR and flow cytometry no BMP6 was detectable in supernatants of XG-10.
To determine whether BMP6 induces phosphorylation of downstream SMADs, phosphorylated SMAD1, -5, -8 were investigated by western blotting. Following incubation with BMP6, SMAD activation can be detected within 15 minutes. After pre-incubation with heparin, no SMAD-phosphorylation is detectable (Figure 1C, see below).
Biological and clinical correlations of BMP6-expression
BMP6-expression in MMCs inversely correlates (albeit weakly) with a gene expression based proliferation index (GPI; HM1 r=−0.45, P<.001, HM2 r=−0.35, P<.001). No correlation of the expression of BMP6 or BMP-receptors in MMCs with either the presence of the chromosomal aberration t(11;14), t(4;14), gain of 11q13, hyperdiploidy (as measured by our copy number score, see below), gain of 1q21, deletion of 17p, or deletion of 13q14.3 could be found. Likewise, no correlation could be found with expression of D-type cyclins (CCND1, CCND2, CCND3), or clinical parameters (including B2M, ISS, Durie-Salmon-stage, serum-albumin, data not shown). We likewise found no difference of the genetic or clinical markers in BMP6high and BMP6low patients.
MMCs bind BMP6 via membrane heparan-sulfate proteoglycans
For BMP-family members, a binding to heparin/heparan sulfates has been described (Irie et al. 2003). At the same time, we have shown heparan-sulfate binding members of the EGF-family to bind syndecan-1 (CD138), the only heparan-sulfate proteoglycan constantly present on the surface of BMPCs and MMCs (Mahtouk et al. 2006). Therefore, HMCLs were incubated with saturating concentrations of BMP6 and analyzed using flow cytometry. We could demonstrate BMP6 binding to 10/10 HMCLs. Binding in all cases is reduced by incubation of BMP6 with heparin, functioning as a competitor that likely captures away BMP6 (Figure 2, exemplary data). Together with the fact that BMP6-induced apoptosis is abrogated by heparin (see above), these data suggest BMP6 being a heparan-sulfate binding molecule.
Figure 2. Binding of BMP6 to human myeloma cell lines.
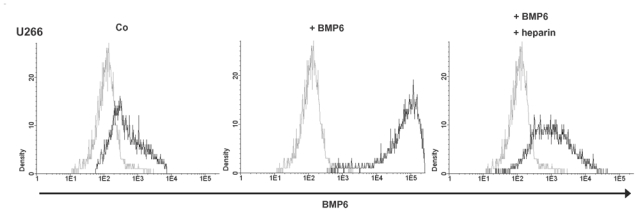
The myeloma cell line U266 was incubated with primary anti-BMP6 antibody and secondary PE-labeled antibody, pre-incubated with BMP6, or pre-incubated with BMP6 plus heparin, respectively. Light grey line: control, black line: BMP6, primary and secondary antibody ± heparin. A strong labeling of U266 was found, whereas binding is reduced by incubation with heparin.
BMP6 inhibits proliferation and induces apoptosis in HMCLs and primary MMCs
BMP6 significantly inhibits proliferation of all HMCLs investigated in a dose-dependent manner (Figure 3A). The maximum inhibition at 4 μg/ml ranged from 27.9 % (RPMI-8226) to 91.1 % (OPM-2). For 6/10 cell lines, a 50 % inhibition (IC50) could be obtained ranging from 0.08 (XG-11) - 2.15 (LP-1) μg/ml.
Figure 3. Inhibition of proliferation and induction of apoptosis of myeloma cell lines by BMP6 as well as survival of primary myeloma cells.
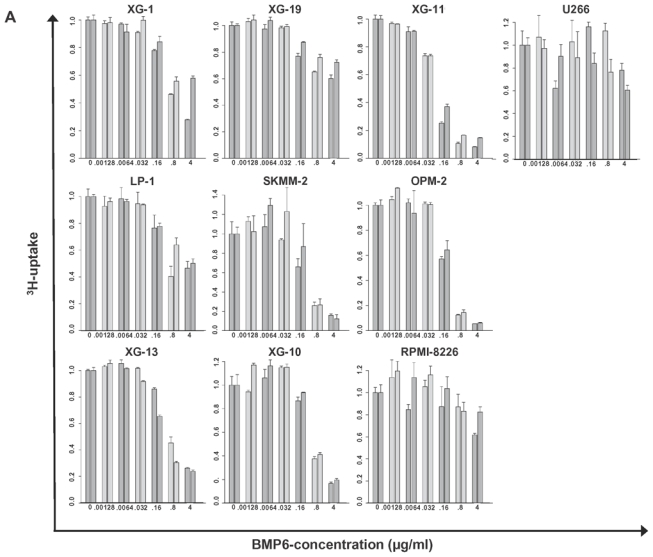
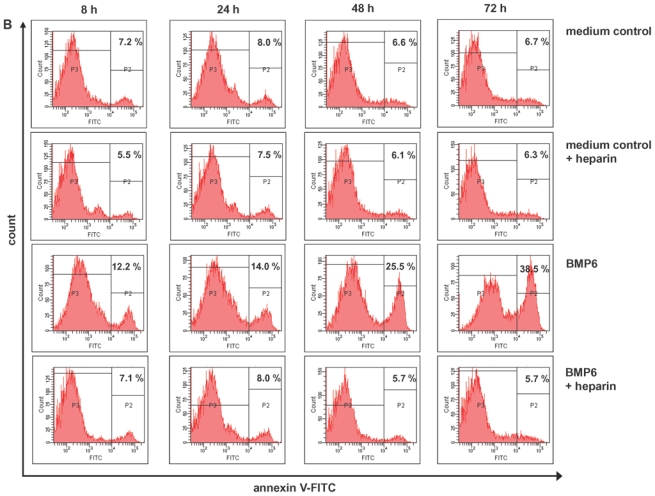
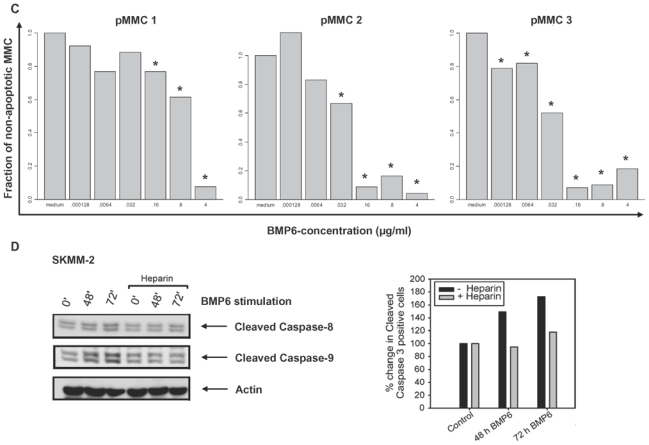
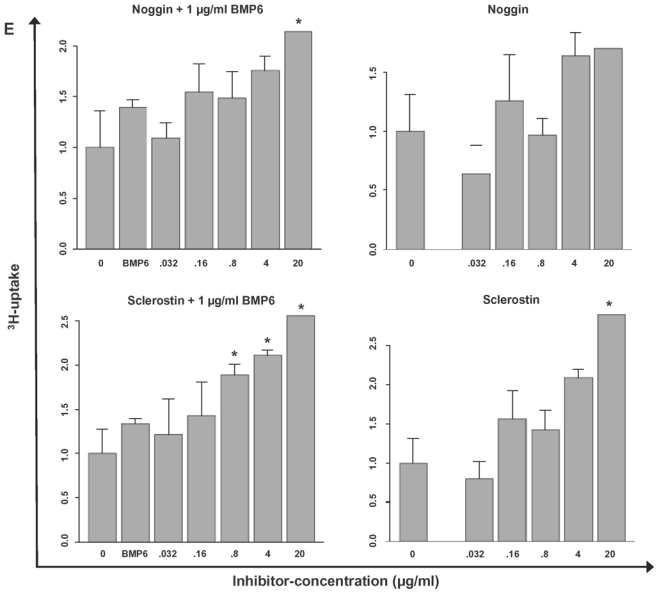
(A) Inhibition of proliferation of myeloma cell lines by BMP6 in graded concentrations vs. medium control measured by 3H-thymidine uptake. The IC50 (in μg/ml) and the maximal inhibition at 4 μg/ml (IMAX4 in %) are for XG-1 n.a./57.2 %, XG-10 0.6 μg/ml/81.9 %, XG-11 0.08 μg/ml/88.6 %, 23 XG-13 0.525 μg/ml/74.8 %, XG-19 n.a./33.7 %, OPM-2 0.175 μg/ml/94.3 %, RPMI-8226 n.a./27.9 %, SKMM-2 0.155 μg/ml/86 %, U266 n.a./30.7 %, LP-1 2.125 μg/ml/51.7 %. The two bars for each concentration correspond to two independent experiments. (B) Induction of apoptosis by BMP6 as determined by annexin V-staining after 8, 24, 48 and 72 h (3rd row). Apoptosis induction is abrogated by heparin-treatment (4th row), whereas heparin alone did not influence apoptosis rate (2nd row). (C) Survival of primary myeloma cells (pMMC) cultured within their bone marrow microenvironment is significantly inhibited by BMP6 as determined by staining with anti-CD138-FITC and propidium iodine. An asterisk (*) indicates a significant decrease between the medium control and the respective BMP6 concentration. (D) Increasing levels of cleaved caspase-3, -8 and -9 can be detected after BMP6 treatment for 48 and 72 h, respectively. This effect is abrogated by pre-treatment with heparin. Actin was used as loading control. (E) The proliferation rate of HMCLs can be increased using the BMP6-inhibitors noggin and sclerostin. As shown for U266, a highly BMP6-resistant and highly BMP6 expressing HMCL (see above), proliferation is concentration-dependently increased by both inhibitors, if cells are either co-exposed to exogenous BMP6, or endogenous BMP6-production is inhibited. Therefore, production of BMP6 by myeloma cells contributes to the inhibition of their growth in vitro. An asterisk (*) indicates a significant increase between the medium control and the respective inhibitor concentration (without exogenous BMP6) or between the BMP6-control and the respective inhibitor concentration (with 1 μg/ml BMP6).
Next, OPM-2 and XG-11 cells were cultured for 3 days with or without BMP6. Cell viability and apoptosis were determined by flow cytometric analysis of annexin V binding and propidium iodine (PI) uptake. BMP6 induced apoptosis after 8 h (12.1 % vs. 6.2 % control) to 72 h (38.5 % vs. 6.7 % control). Heparin-pretreatment inhibits BMP6-induced apoptosis (Figure 3B, exemplary data), likely explained by the heparin-induced competitive reduction of BMP6-binding to heparan-sulfate chains on plasma cells (see below). Heparin alone did not influence the apoptosis rate.
To test whether the survival of primary MMCs is inhibited as well, these were cultured within their BM microenvironment (negative fraction of plasma cell purification) and exposed to BMP6. After 6 days, cell viability was measured by CD138/PI flow cytometry. As shown in Figure 3C, BMP6 significantly inhibited the survival of 3/3 primary MMC samples. The maximal inhibition was 90 %, 93.6 %, and 94.6 %, respectively.
In terms of apoptosis-induction we could demonstrate increasing levels of cleaved caspase-3 (effector caspase), -8 and -9 (initiator caspases) after BMP6 treatment for 48 and 72 h, respectively. This effect is abrogated by pre-treatment with heparin (Figure 3D, exemplary data).
Next, we tested whether the production of BMP6 by MMCs themselves inhibits their proliferation. We therefore exposed HCMLs with the BMP6-inhibitors noggin (Kersten et al. 2006) and sclerostin (Kusu et al. 2003), respectively. Both, sclerostin and noggin exposure yielded a concentration dependant increase of proliferation. Exemplary data for U266 (high endogenous BMP6-production, very low sensitivity to exogenous BMP6; see Figure 2B, 3A) are shown in Figure 3E. As a control, we co-exposed the HMCLs with exogenous BMP6 (1 μg/ml) and graded concentrations of noggin or sclerostin, showing that indeed noggin and sclerostin significantly and concentration-dependently abrogated BMP6 mediated inhibition of myeloma cell proliferation (Figure 3E). Therefore, production of BMP6 by myeloma cells contributes to the inhibition of their growth in vitro.
Inhibition of in vitro tubule formation by BMP6
The angiogenic potential of BMP6 was investigated in the AngioKit assay with graded concentrations of BMP6. BMP6 significantly inhibited in vitro tubule formation with a strong inhibition already observed at 0.032 μg/ml (Figure 4, P=.04 and P=.001 in two independent experiments) compared to the medium control. The inhibition was as efficient as that provided by suramin, a usual tubule formation inhibitor.
Figure 4. Inhibition of in vitro induction of angiogenesis by BMP6.
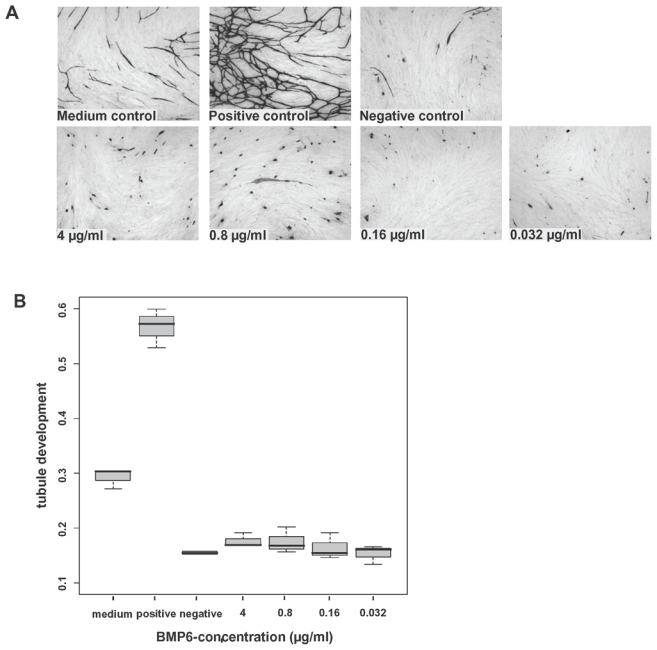
Inhibition of endothelial cell growth by BMP6 in the AngioKit model. Immunostaining with monoclonal anti-CD31 antibody. (A) Shown are medium control (RPMI-1640), positive control (vascular endothelial growth factor), negative control (suramin) and BMP6 in concentrations of 4, 0.8, 0.16 and 0.032 μg/ml, respectively. Original magnification ×40. (B) Boxplot summarizing the CD31 ELISA results. BMP6 significantly inhibits tubule formation down to the level of the negative control.
Prognostic value of BMP6, BMP-receptors and SMAD-expression
In a Cox-model as single continuous variable, BMP6-expression is significantly predictive for overall survival (OS) in the HM1 (P=.02), HM2 (P=.01) and the LR-data (P=.005). For event-free survival (EFS), it is only predictive in the LR-data (P=.004). In a Cox-model tested with B2M (as continuous variable), BMP6-expression appears as independent prognostic factor for OS in the HM1 (BMP6-expression (P=.02), B2M (P=.7)), HM2 (BMP6-expression (P=.03), B2M (P=.02)), and the LR-data (BMP6-expression (P=.01), B2M (P<.001)). The same holds true if BMP6-expression is tested with ISS in the HM1 (BMP6-expression (P=.04), ISS (P=.9)), HM2 (BMP6-expression (P=.03), ISS (P=.03)) and the LR-data (BMP6-expression (P=.02), ISS (P<.001)). In the LR-data, BMP6 remains an independent prognostic factor for EFS if tested with B2M (BMP6-expression (P=.02), B2M (P<.001)) or ISS (BMP6-expression (P=.01), ISS (P<.001)). Likewise, patients with BMP6high expressing MMCs show a significantly better OS compared to patients with BMP6low-expression (n=168, P=.02, HR 0.4, CI [0.18;0.87], Figure 5A). No prognostic value could be determined for EFS (P=.9, P=.9, and P=.9, respectively). Similar observations could be made with the patient cohort from the LR-group (n=345, OS, P=.03, HR 0.67, CI [0.46;0.97] and EFS, P=.15, Figure 5B). Genes coding for BMP-receptors or downstream SMADs had no prognostic value. In the WBM (n=57), BMP6-expression above the median (WBM-BMP6high) delineated a group with better EFS (n=49, P=.03, HR 0.45, CI [0.21;0.95]) and a tendency to better OS (P=.3, Figure 5C).
Figure 5. Effect of BMP6-expression on event-free and overall survival.
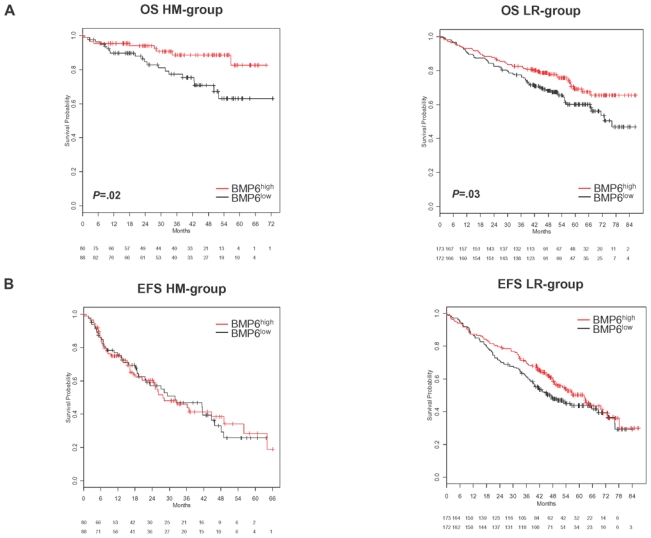
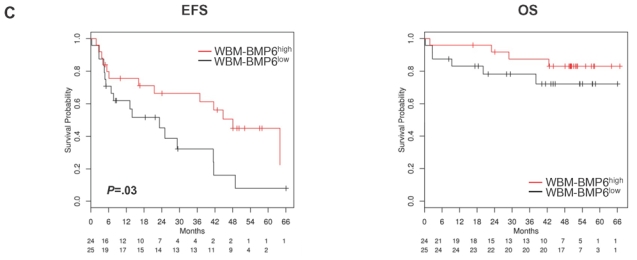
(A) Event-free (EFS) and (B) overall survival (OS) for our patients (HM-group; n=168) and the Little Rock-group (LR-group; n=345). All patients are treated with high-dose chemotherapy and autologous stem cell transplantation. Two groups of patients with high (BMP6high, greater or equal the median, red curve) and low (BMP6low, below the median, black curve) BMP6-expression. The OS is significantly superior for high BMP6-expression (P=.02 HM1 and HM2, P=.03 LR-group). (C) EFS and OS for BMP6-expression within the whole bone marrow (WBM; n=57). The EFS is significantly superior for the group of patients with BMP6-expression above the median (WBM-BMP6high, P=.03). This group shows also a tendency to better OS, which does not reach significance (P=.3), likely due to a low number of events.
Discussion
Expression of BMP6 and its receptors
BMP6-expression is a characteristic of normal and malignant plasma cells (Table 1A,C): from the populations present within the BM, only MMCs, BMPCs, and a sub-fraction of MSCs express BMP6 (Supplementary Table S2). The latter is in agreement with data from Kersten et al. who have shown stromal cells to express varying levels of BMP6 mRNA by conventional RT-PCR (Kersten et al. 2006). Indeed, BMP6-expression in MSCs in our data is by several orders of magnitude lower than in plasma cells as detected by gene expression profiling, qRT-PCR and flow cytometry (data not shown). BMP6-expression by malignant plasma cells is in agreement with data from Zhan et al. (Zhan et al. 2002). Unlike normal BM in a recent report (Kochanowska et al. 2007) and our data, BMP6-expression can be detected in 40/57 samples of myelomatous BM. The expression of BMP6 within the BM correlates significantly with the percentage of plasma cell infiltration; regarding the low frequency of MSCs in the BM which further deceases with age (Caplan 2007), thus, BMP6-expression in myelomatous WBM can be attributed to MMCs. However, in supernatants of HMCLs or BM sera of myeloma patients, only amounts of BMP6 at the level of the detection limit of the ELISA could be found. This could be explained by a significantly lower expression of BMP6 by proliferating cells (see below, Figure 1) and differences in the local concentrations of growth factors. A further possible explanation might be the high turnover of heparan-sulfate (syndecan-1) bound BMP6 (Figure 2).
BMP6 receptors (BMPR2, ACVR1), present in BMPCs and MMCs, have previously been detected by RT-PCR in 5/5 myeloma cell lines (Ro et al. 2004). Of the members of the SMAD signal-transduction cascade, SMAD5 is expressed in almost all samples during plasma cell development and within MMCs, whereas SMAD1, absent in BMPCs, is expressed at increasing rates from MMCs to HMCLs. SMAD4, the only common-partner SMAD, is expressed only in a minor fraction of BMPCs (21 %) but a high fraction of HMCLs (80 %; Table 1B). Thus, transducing SMAD1/SMAD4 or SMAD5/SMAD4 complexes are increasingly present from MBCc over MMCs to HMCLs (Table 1B). This is in agreement with the observation that BMP6-expression develops at the differentiation stage of plasma cells, whereas it is absent in MBCs and only expressed in a small minority of PPC samples. The picture is complicated by the fact that alternative BMP-signaling ways via prostanoid-generation (Ren et al. 2007) and MAPK (Nohe et al. 2004; Du et al. 2007) have been reported, both pathways being present in MMCs (Trojan et al. 2006; Hoang et al. 2006).
Biological implications
BMP6-expression is significantly lower in proliferating non-malignant (PPCs) or malignant (HMCLs) cells and correlates inversely with our GPI. In vitro, BMP6 significantly inhibits proliferation of HMCLs as well as primary MMCs and induces apoptosis in a time-dependant manner (Figure 3), in agreement with published data (Ro et al. 2004). Autocrine production of BMP6 reduces proliferation of HMCLs, as shown for U266. This effect can be abrogated using noggin or sclerostin as known BMP-inhibitors (Figure 3E). At the same time, BMP6 significantly inhibits in vitro tubule formation by human endothelial cells down to the level of the negative control (Figure 4), giving a further possible link with BM angiogenesis and thus prognosis. These were surprising results, as BMP6 has been described as agonist for endothelial cell migration and tube formation in vitro (Valdimarsdottir et al. 2002) using adenoviral transfected BMP-receptor expressing mouse embryonic endothelial- and bovine aortic endothelial cells. Differences might be explained by species dependent action of BMP6 and/or receptor expression or a difference in action of BMP6 on embryonic vs. adult endothelial cells.
With BMP6, BMPCs express a factor inhibiting proliferation and angiogenesis, as well as inducing apoptosis and bone formation. We hypothesize this i) to be a sign for an intense local interaction between BMPCs and the BM microenvironment, e.g. important during the settling of plasma cells within their BM niche. ii) As BMP6-expression is absent in MBCs, expressed in a small minority of PPC samples and appears at the differentiation stage of plasma cells, it might be part of a proliferative block appearing late during the development of terminal differentiated plasma cells. If this is the case, the maintenance of BMP6 expression in the vast majority of MMCs of newly-diagnosed patients could be interpreted as “anti-proliferative burden” in terms of reminiscence to the “plasma cell nature” of MMCs. MMCs in advanced stages would be expected to concomitantly loose BMP6-expression, as could be seen in HMCLs.
BMP6 and survival
For patients treated with HDT and ASCT, BMP6-expression as single continuous variable is significantly predictive for OS in the three independent data sets investigated. BMP6-expression remains significantly predictive for OS if tested with classical prognostic parameters, i.e. either B2M or ISS (in all three datasets). In the LR-data, BMP6-expression appears as independent prognostic factor for EFS if tested alone or with either B2M or ISS. Likewise, patients with BMP6-expression in MMCs above the median (BMP6high) show a superior OS vs. patients with expression below the median (BMP6low) in our data and the Arkansas-group (Figure 5). In the latter case, no differences in EFS could be observed. Thus, the height of BMP6-expression seemingly has a lower influence on the time to first relapse (disease progression). But, as prolonging OS, BMP6 seems to influence either the time to subsequent relapses, or the chemo-sensitivity in relapse. One might speculate that a slower increase in proliferation of BMP6high-MMCs during disease progression might reduce the probability of acquiring additional genetic lesions and changing to a more aggressive geno- and phenotype. A possible criticism is that different relapse treatments for BMP6high and BMP6low patient groups might have been used. Albeit it cannot be evaluated in a sufficiently high number of patients, this is, however, extremely unlikely, as i) the state of BMP6-expression has not been known during the treatment decisions, ii) no genetic or biochemical associations could be found for BMP6-expression (that might have driven implicitly different treatment strategies), and iii) two independent relapse strategies (in Germany/France and the USA, respectively) have been used. It is interesting to denote that a higher expression of BMP6 within the WBM (WBM-BMP6high) is associated with significantly superior EFS, despite the correlation of WBM-BMP6-expression and plasma cell infiltration. The OS is likewise superior, albeit, most likely due to a low number of events, not significantly (Figure 5C). In contrast to the BMP6-expression within MMCs (above), the BMP6-expression within the WBM assesses the integrated mean value of BMP6 expression of individual MMCs together with the percentage of MMC infiltration.
The inhibition of proliferation of all cell lines tested, induction of apoptosis in HMCLs and primary MMCs, as well as inhibition of in vitro angiogenesis give a possible biological explanation for an advantageous effect of BMP6high-expressing MMCs. It is interesting to denote that BMP6-expression seems to have either beneficial or detrimental effects on patients’ survival in different cancer types. Kim et al. have shown BMP6 to be present in the kidney and being a potent growth inhibitor in human renal carcinoma cell lines (Kim et al. 2003). In other cancers like prostate (Hamdy et al. 1997; Dai et al. 2005) or breast cancer (Clement et al. 1999), BMP6-expression promotes tumor progression and development of metastasis.
Conclusion
BMP6 exemplifies a novel class of factors independently prognostic for OS expressed by normal as well as malignant plasma cells that inhibits proliferation of MMCs and induction of angiogenesis.
Materials and Methods
Patients and healthy donors
Patients presenting with previously untreated MM (n=233) or MGUS (n=12) at the University Hospitals of Heidelberg and Montpellier as well as 14 healthy normal donors have been included in the study approved by the ethics committee after written informed consent. The first 65 patients comprise the HM1-, the 168 additional the independent HM2-group (see below). Patients were diagnosed, staged and response to treatment assessed according to standard criteria (Greipp et al. 2005; Durie BG 1986; Blade et al. 1998). For clinical parameters, see Supplementary Table S3. According or in analogy to the GMMG-HD3-trial (Goldschmidt et al. 2003), 168 patients underwent frontline HDT with 200 mg/m2 melphalan and ASCT. Survival data were validated by an independent cohort of 345 patients treated within the total therapy 2 protocol (Barlogie et al. 2006).
Samples
For an overview, see Supplementary Table S4. Bone marrow plasma cells were purified as previously published (Hose et al. 2009b). Aliquots of unpurified WBM of patients (n=57) and healthy donors (n=7) were obtained after NH4-lysis (Mahtouk et al. 2007). Alternate aliquots were subjected to FACS-sorting (FACSAria, Becton Dickinson) in CD3+-, CD14+-, CD15+-and CD34+-cells. Peripheral CD27+ MBCs were FACS-sorted as described (Moreaux et al. 2005). The HMCLs U266, RPMI-8226, LP-1, OPM-2 and SKMM-2 were purchased from DSMZ (Braunschweig, Germany), the XG-lines were generated at INSERM U847 (Montpellier, France) as published (Zhang et al. 1994). PPCs (Tarte et al. 2002), osteoclasts (OCs; (Moreaux et al. 2005)) and MSCs (Corre et al. 2007) were generated as published.
Interphase fluorescence in situ hybridization
Analyses were performed on CD138-purified plasma cells as described (Cremer et al. 2005) using probes for chromosomes 1q21, 9q34, 11q13, 13q14.3, 15q22, 17p13, 19q13, 22q11, and translocations t(4;14)(p16.3;q32.3), t(11;14)(q13;q32.3) (Kreatech Diagnostics, Berlin, Germany). Ploidy-status (excluding gains of 1q21), clonal/subclonal aberrations (i.e. present in ≥ 60 % vs. 20–59 % of assessed MMCs) were defined as published (Cremer et al. 2005).
Gene expression profiling
Gene expression profiling (GEP) was performed as previously published (Hose et al. 2009a). In brief, after RNA-extraction, labeled cRNA was generated using the small sample labeling protocol vII (Affymetrix, Santa Clara, CA, USA), and hybridized to U133 A+B GeneChip microarray (Affymetrix) for HM1, and U133 2.0 plus arrays for HM2, according to the manufacturer’s instructions. When different probe sets were available for the same gene, we chose the probe set yielding the maximal variance and the highest signal.
As validation, expression of BMP6 (Hs00233470_m1), BMPR2 (Hs00176148_m1) and Alk-2 (ACVR1, Hs00153836_m1, all Applied Biosystems, Darmstadt, Germany) was assessed by qRT-PCR using the ABI Prism 7700 Sequence Detection System (Mahtouk et al. 2005).
Preparation of cell lysates and western blotting
HMCLs were serum starved for 2 h in SynH medium (ABCell-Bio, Montpellier, France). With or without pre-treatment with 4 IU/ml heparin (Leo, Breda, The Netherlands) for 1 h, cells were stimulated with 0.5 μg/ml BMP6 (R&D Systems, Wiesbaden-Nordenstadt, Germany) and cell pellets re-suspended in Laemmli-buffer. Proteins were separated using a 10 % SDS-PAGE and transferred to nitrocellulose membranes (Schleicher&Schuell, Dassel, Germany). Membranes were probed with antibodies against phospho-SMAD2 (Ser465/467), and phospho-SMAD1(Ser463/465)/SMAD5(Ser463/465)/SMAD8(Ser426/428) from Cell Signaling Technology (Beverly, MA, USA). As loading-control, membranes were stripped and re-probed with an anti-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). For Caspase-detection, HMCLs were exposed to 0.75 μg/ml BMP6 for 48 and 72 h and treated as described above. Membranes were probed with antibodies against cleaved Caspase-8 and -9 (Cell Signaling Technology). Detection was performed using ECL (Amersham, Buckinghamshire, United Kingdom) according to the manufacturer’s instructions.
Intracellular staining for BMP6
Intracellular BMP6-expression of 10 HMCLs, 3 primary MMC samples was measured by flow cytometry. HMCLs were incubated with 3 μg/ml Brefeldin A (eBioscience, San Diego, CA, USA) overnight. Cells were stained according to the manufacturer’s instructions (eBioscience). For antibodies used, see below.
Measurement of proliferation by 3H-thymidine
Proliferation of HMCLs was investigated as previously published (De Vos et al. 2001). Cells were cultured in RPMI-1640 containing 10 % FCS and graded concentrations (0.00128, 0.0064, 0.032, 0.16, 0.8, 4 μg/ml) of BMP6. For the XG-lines, 2 ng/ml IL-6 (R&D Systems) was added. To test for the effect of an endogenous BMP6-production, graded concentrations (0.032, 0.16, 0.8, 4, 20 μg/ml) of the BMP6-inhibitors noggin or sclerostin (both R&D Systems) were added (with or without 1 μg/ml BMP6). Proliferation was evaluated after 54 h of culture: cells were pulsed with 37 kBq of 3H-thymidine for 18 h, harvested and 3H-thymidine uptake measured.
Apoptosis induction
OPM-2 and XG-11 were cultured in RPMI-1640 containing 10 % FCS and 2 ng/ml IL-6 (XG-11) with or without 1 μg/ml BMP6 and 4 IU/ml heparin (Braun, Melsungen, Germany), respectively. After 8, 24, 48 and 72 h, cells were stained for annexin V-FITC and PI according to the manufacturer’s instructions (Pharmingen, Heidelberg, Germany).
Flow cytometric analysis of Caspase-3 activation
Cells were treated as described above, and stained with the FITC-conjugated active Caspase-3 detection kit (Becton Dickinson) according to the manufacturer’s instructions. The intracellular fluorescence was determined by flow cytometry.
Survival of primary MMCs
Primary MMCs cultured together with their BM microenvironment of 3 newly-diagnosed patients were exposed to 0.00128, 0.0064, 0.032, 0.16, 0.8, and 4 μg/ml BMP6. After 6 days, cell viability was measured by CD138-FITC (IQ products, Groningen, Netherlands; clone B-A38)/PI (Pharmingen) staining and referred to the medium control (Jourdan et al. 1998). One μl of PI at 50 μg/ml was used.
In vitro assessment of angiogenesis
The angiogenic potential of BMP6 in concentrations of 0.0064, 0.032, 0.16, 0.8, and 4 μg/ml was investigated in the AngioKit assay (TCS Cellworks, Buckinghamshire, United Kingdom) as previously published (Hose et al. 2009b). RPMI-1640, vascular endothelial growth factor (2 ng/ml) and suramin (20 μM) served as medium, positive and negative controls, respectively.
Detection of BMP6 binding to myeloma cells by flow cytometry
Cells were incubated with 1 μg/ml BMP6 for 1 h at 4 °C and washed twice in PBS before incubation with the corresponding primary (R&D Systems; clone 74219) and secondary antibody (Dako, Hamburg, Germany). Subsequently, BMP6 was pre-incubated with 4 IU/ml heparin for 1 h at 4 °C. Staining without BMP6 was used as control. Analyses were performed by FACSAria and Infinicyt Software for obtaining overlays (Cytognos, Salamanca, Spain).
BMP6 ELISA
BMP6 levels were measured in culture supernatants of HMCLs (n=9) as well as BM sera of myeloma patients (n=10) using a human BMP6 ELISA kit (RayBiotech, Norcross, GA, USA).
Statistical analysis
Supplementary Material
Acknowledgments
We thank Katrin Heimlich, Maria Dörner and Gabriele Hoock for technical assistance. This work was supported in part by grants from the Hopp-Foundation, Germany, the University of Heidelberg, Germany, the National Centre for Tumor Diseases, Heidelberg, Germany, the Tumorzentrum Heidelberg/Mannheim, Germany, the European Myeloma Stem Cell Network (MSCNET) funded within the 6th framework program of the European Community, Novartis Pharma GmbH, Nuremberg, Germany, the Ligue Nationale Contre Le Cancer (équipe labellisée), Paris, France. It is also part of a national program called “Carte d’Identité des Tumeurs” (CIT) funded by the Ligue Nationale Contre le Cancer, France.
Footnotes
Conflict of interest
The authors declare no conflict of interests.
Reference List
- Barlogie B, Tricot G, Rasmussen E, Anaissie E, van RF, Zangari M, Fassas A, Hollmig K, Pineda-Roman M, Shaughnessy J, Epstein J, Crowley J. Blood. 2006;107:2633–2638. doi: 10.1182/blood-2005-10-4084. [DOI] [PubMed] [Google Scholar]
- Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, Gertz M, Giralt S, Jagannath S, Vesole D. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- Caplan AI. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC. J Bone Joint Surg Am. 2003;85-A:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- Clement JH, Sanger J, Hoffken K. Int J Cancer. 1999;80:250–256. doi: 10.1002/(sici)1097-0215(19990118)80:2<250::aid-ijc14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Corre J, Mahtouk K, Attal M, Gadelorge M, Huynh A, Fleury-Cappellesso S, Danho C, Laharrague P, Klein B, Reme T, Bourin P. Leukemia. 2007;21:1079–1088. doi: 10.1038/sj.leu.2404621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer FW, Bila J, Buck I, Kartal M, Hose D, Ittrich C, Benner A, Raab MS, Theil AC, Moos M, Goldschmidt H, Bartram CR, Jauch A. Genes Chromosomes Cancer. 2005;44:194–203. doi: 10.1002/gcc.20231. [DOI] [PubMed] [Google Scholar]
- Dai J, Keller J, Zhang J, Lu Y, Yao Z, Keller ET. Cancer Res. 2005;65:8274–8285. doi: 10.1158/0008-5472.CAN-05-1891. [DOI] [PubMed] [Google Scholar]
- De Vos J, Couderc G, Tarte K, Jourdan M, Requirand G, Delteil MC, Rossi JF, Mechti N, Klein B. Blood. 2001;98:771–780. doi: 10.1182/blood.v98.3.771. [DOI] [PubMed] [Google Scholar]
- De Vos J, Hose D, Reme T, Tarte K, Moreaux J, Mahtouk K, Jourdan M, Goldschmidt H, Rossi JF, Cremer FW, Klein B. Immunol Rev. 2006;210:86–104. doi: 10.1111/j.0105-2896.2006.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Yang S, Wang Z, Zhai C, Yuan W, Lei R, Zhang J, Zhu T. J Cell Biochem. 2007;19(103):1584–1597. doi: 10.1002/jcb.21547. [DOI] [PubMed] [Google Scholar]
- Durie BG. Semin Oncol. 1986;13:300–309. [PubMed] [Google Scholar]
- Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T. J Cell Sci. 1999;112:3519–3527. doi: 10.1242/jcs.112.20.3519. [DOI] [PubMed] [Google Scholar]
- Goldschmidt H, Sonneveld P, Cremer FW, van der HB, Westveer P, Breitkreutz I, Benner A, Glasmacher A, Schmidt-Wolf IG, Martin H, Hoelzer D, Ho AD, Lokhorst HM. Ann Hematol. 2003;82:654–659. doi: 10.1007/s00277-003-0685-2. [DOI] [PubMed] [Google Scholar]
- Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle RA, Lahuerta JJ, Ludwig H, Morgan G, Powles R, Shimizu K, Shustik C, Sonneveld P, Tosi P, Turesson I, Westin J. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- Hamdy FC, Autzen P, Robinson MC, Horne CH, Neal DE, Robson CN. Cancer Res. 1997;57:4427–4431. [PubMed] [Google Scholar]
- Haudenschild DR, Palmer SM, Moseley TA, You Z, Reddi AH. Cancer Res. 2004;64:8276–8284. doi: 10.1158/0008-5472.CAN-04-2251. [DOI] [PubMed] [Google Scholar]
- Hoang B, Zhu L, Shi Y, Frost P, Yan H, Sharma S, Sharma S, Goodglick L, Dubinett S, Lichtenstein A. Blood. 2006;107:4484–4490. doi: 10.1182/blood-2005-09-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hose D, Reme T, Meissner T, Moreaux J, Seckinger A, Lewis J, Benes V, Benner A, Hundemer M, Hielscher T, Shaughnessy JD, Jr, Barlogie B, Neben K, Kramer A, Hillengass J, Bertsch U, Jauch A, De VJ, Rossi JF, Mohler T, Blake J, Zimmermann J, Klein B, Goldschmidt H. Blood. 2009a;113:4331–4340. doi: 10.1182/blood-2008-09-178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hose D, Moreaux J, Meissner T, Seckinger A, Goldschmidt H, Benner A, Mahtouk K, Hillengass J, Reme T, de Vos J, Hundemer M, Condomines M, Bertsch U, Rossi JF, Jauch A, Klein B, Mohler T. Blood blood-2008. 2009b. [DOI] [PubMed] [Google Scholar]
- Irie A, Habuchi H, Kimata K, Sanai Y. Biochem Biophys Res Commun. 2003;308:858–865. doi: 10.1016/s0006-291x(03)01500-6. [DOI] [PubMed] [Google Scholar]
- Jourdan M, Ferlin M, Legouffe E, Horvathova M, Liautard J, Rossi JF, Wijdenes J, Brochier J, Klein B. Br J Haematol. 1998;100:637–646. doi: 10.1046/j.1365-2141.1998.00623.x. [DOI] [PubMed] [Google Scholar]
- Kawabata M, Imamura T, Miyazono K. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Kersten C, Dosen G, Myklebust JH, Sivertsen EA, Hystad ME, Smeland EB, Rian E. Exp Hematol. 2006;34:72–81. doi: 10.1016/j.exphem.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Kersten C, Sivertsen EA, Hystad ME, Forfang L, Smeland EB, Myklebust JH. BMC Immunol. 2005;6:9. doi: 10.1186/1471-2172-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IY, Lee DH, Lee DK, Kim BC, Kim HT, Leach FS, Linehan WM, Morton RA, Kim SJ. Clin Cancer Res. 2003;9:6046–6051. [PubMed] [Google Scholar]
- Klein B, Tarte K, Jourdan M, Mathouk K, Moreaux J, Jourdan E, Legouffe E, De Vos J, Rossi JF. Int J Hematol. 2003;78:106–113. doi: 10.1007/BF02983377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanowska I, Chaberek S, Wojtowicz A, Marczynski B, Wlodarski K, Dytko M, Ostrowski K. BMC Musculoskelet Disord. 2007;8:128. doi: 10.1186/1471-2474-8-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusu N, Laurikkala J, Imanishi M, Usui H, Konishi M, Miyake A, Thesleff I, Itoh N. J Biol Chem. 2003;278:24113–24117. doi: 10.1074/jbc.M301716200. [DOI] [PubMed] [Google Scholar]
- Kyle RA, Rajkumar SV. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- Mahtouk K, Cremer FW, Reme T, Jourdan M, Baudard M, Moreaux J, Requirand G, Fiol G, De VJ, Moos M, Quittet P, Goldschmidt H, Rossi JF, Hose D, Klein B. Oncogene. 2006;25:7180–7191. doi: 10.1038/sj.onc.1209699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahtouk K, Hose D, Raynaud P, Hundemer M, Jourdan M, Jourdan E, Pantesco V, Baudard M, De VJ, Larroque M, Moehler T, Rossi JF, Reme T, Goldschmidt H, Klein B. Blood. 2007;109:4914–4923. doi: 10.1182/blood-2006-08-043232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahtouk K, Hose D, Reme T, De Vos J, Jourdan M, Moreaux J, Fiol G, Raab M, Jourdan E, Grau V, Moos M, Goldschmidt H, Baudard M, Rossi JF, Cremer FW, Klein B. Oncogene. 2005;24:3512–3524. doi: 10.1038/sj.onc.1208536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- Moreaux J, Cremer FW, Reme T, Raab M, Mahtouk K, Kaukel P, Pantesco V, De Vos J, Jourdan E, Jauch A, Legouffe E, Moos M, Fiol G, Goldschmidt H, Rossi JF, Hose D, Klein B. Blood. 2005;106:1021–1030. doi: 10.1182/blood-2004-11-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohe A, Keating E, Knaus P, Petersen NO. Cell Signal. 2004;16:291–299. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Ren R, Charles PC, Zhang C, Wu Y, Wang H, Patterson C. Blood. 2007;109:2847–2853. doi: 10.1182/blood-2006-08-039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro TB, Holt RU, Brenne AT, Hjorth-Hansen H, Waage A, Hjertner O, Sundan A, Borset M. Oncogene. 2004;23:3024–3032. doi: 10.1038/sj.onc.1207386. [DOI] [PubMed] [Google Scholar]
- Tarte K, De Vos J, Thykjaer T, Zhan F, Fiol G, Costes V, Reme T, Legouffe E, Rossi JF, Shaughnessy J, Jr, Orntoft TF, Klein B. Blood. 2002;100:1113–1122. [PubMed] [Google Scholar]
- Trojan A, Tinguely M, Vallet S, Seifert B, Jenni B, Zippelius A, Witzens-Harig M, Mechtersheimer G, Ho A, Goldschmidt H, Jager D, Boccadoro M, Ladetto M. Swiss Med Wkly. 2006;136:400–403. doi: 10.4414/smw.2006.11467. [DOI] [PubMed] [Google Scholar]
- Vacca A, Ribatti D, Roncali L, Ranieri G, Serio G, Silvestris F, Dammacco F. Br J Haematol. 1994;87:503–508. doi: 10.1111/j.1365-2141.1994.tb08304.x. [DOI] [PubMed] [Google Scholar]
- Valdimarsdottir G, Goumans MJ, Rosendahl A, Brugman M, Itoh S, Lebrin F, Sideras P, ten DP. Circulation. 2002;106:2263–2270. doi: 10.1161/01.cir.0000033830.36431.46. [DOI] [PubMed] [Google Scholar]
- Witzig TE, Timm M, Larson D, Therneau T, Greipp PR. Br J Haematol. 1999;104:131–137. doi: 10.1046/j.1365-2141.1999.01136.x. [DOI] [PubMed] [Google Scholar]
- Wutzl A, Brozek W, Lernbass I, Rauner M, Hofbauer G, Schopper C, Watzinger F, Peterlik M, Pietschmann P. J Biomed Mater Res A. 2006;77:75–83. doi: 10.1002/jbm.a.30615. [DOI] [PubMed] [Google Scholar]
- Zhan F, Hardin J, Kordsmeier B, Bumm K, Zheng M, Tian E, Sanderson R, Yang Y, Wilson C, Zangari M, Anaissie E, Morris C, Muwalla F, van Rhee F, Fassas A, Crowley J, Tricot G, Barlogie B, Shaughnessy J., Jr Blood. 2002;99:1745–1757. doi: 10.1182/blood.v99.5.1745. [DOI] [PubMed] [Google Scholar]
- Zhang XG, Gaillard JP, Robillard N, Lu ZY, Gu ZJ, Jourdan M, Boiron JM, Bataille R, Klein B. Blood. 1994;83:3654–3663. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


