Abstract
Lack of chronic immune activation in the presence of persistent viremia is a key feature that distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection in natural hosts from pathogenic SIV and HIV infection. To elucidate novel mechanisms downmodulating immune activation in natural hosts of SIV infection, we investigated natural killer T (NKT) lymphocytes in sooty mangabeys. NKT lymphocytes are a potent immunoregulatory arm of the innate immune system that recognize glycolipid antigens presented on the nonpolymorphic MHC-class I-like CD1d molecules. In a cross-sectional analysis of 50 SIV-negative and 50 naturally SIV-infected sooty mangabeys, ligand α-galactosylceramide loaded CD1d tetramers co-staining with Vα24-positive invariant NKT lymphocytes were detected at frequencies ≥0.002% of circulating T lymphocytes in approximately half of the animals. In contrast to published reports in Asian macaques, sooty mangabey NKT lymphocytes consisted of CD8+ and CD4/CD8 double-negative T lymphocytes that were CXCR3-positive and CCR5-negative suggesting that they trafficked to sites of inflammation without being susceptible to SIV infection. Consistent with these findings, there was no difference in the frequency or phenotype of NKT lymphocytes between SIV-negative and SIV-infected sooty mangabeys. On stimulation with α-galactosylceramide loaded on human CD1d molecules, sooty mangabey NKT lymphocytes underwent degranulation and secreted IFN-γ, TNF-α, IL-2, IL-13, and IL-10, indicating the presence of both effector and immunoregulatory functional capabilities. The unique absence of CD4+ NKT lymphocytes in sooty mangabeys, combined with their IL-10 cytokine-secreting ability and preservation following SIV infection, raises the possibility that NKT lymphocytes might play a role in downmodulating immune activation in SIV-infected sooty mangabeys.
Introduction
While persistent immune activation is a strong prognosticator of disease progression in HIV-infected humans and SIV-infected Asian macaques, it is singularly lacking in non-progressive infection in natural hosts of SIV such as sooty mangabeys and African green monkeys [1], [2], [3], [4]. How natural hosts of SIV are able to contain chronic immune activation in the face of continuing viral replication and high viral loads remains a conundrum [5]. Immune activation is observed during acute SIV infection in sooty mangabeys and African green monkeys, but it is rapidly down-regulated to pre-SIV infection levels [6], [7], [8], [9], [10]. Mechanisms that have been implicated in down-modulation of immune activation in natural hosts include early induction of an anti-inflammatory response [11], absence of microbial translocation [12], paucity of CCR5+ CD4+ T lymphocytes [13], decreased responsiveness of plasmacytoid dendritic cells to SIV [14], and preservation of Th17 CD4+ T lymphocytes [15], [16]. The magnitude of SIV-specific T lymphocyte responses in sooty mangabeys during acute and chronic SIV infection is comparable to that in rhesus macaques [9], [17], [18] and hence, differences in the adaptive immune response to SIV are unlikely to be responsible for the differential immune activation of pathogenic and nonpathogenic SIV infection. The rapid activation of multiple pro-inflammatory cytokines and chemokines in acute HIV and SIV infection point to the early innate immune response as an important determinant of immune activation [9], [19].
Natural Killer T (NKT) lymphocytes are a small subset of T lymphocytes that are rapid responders of the innate immune system and mediate potent immunoregulatory and effector functions in a variety of disease settings [20], [21]. NKT lymphocytes recognize antigen presented by the non-polymorphic MHC Class-I-like CD1d molecules and are characterized by a restricted TCR repertoire due to the presence of an invariant TCR alpha chain paired with a limited number of TCR beta chains [20], [21]. In mice, the TCR of invariant NKT lymphocytes consists of a Vα14-Jα18 chain paired with Vβ8.2, Vβ7 or Vβ2, while human invariant NKT lymphocytes have a Vα24-Jα18 chain preferentially paired with Vβ11 [22], [23], [24]. Upon activation with glycolipids presented on CD1d molecules, NKT lymphocytes respond rapidly with production of a diverse array of cytokines including IL-10, TGF-β, and several Th1 and Th2 cytokines [25], [26]. Studies in mice suggest that the NKT lymphocytes transcribe cytokine genes even before activation and are therefore able to respond rapidly upon TCR stimulation [27]. Because NKT lymphocytes can produce a wide array of cytokines without the requirement of priming, they can modulate other arms of the innate and adaptive immune system and mediate diverse, often polar functions [28], [29], [30], [31]. Thus, while NKT lymphocytes can mediate anti-tumor [32] and anti-microbial effector activity against selected pathogens [33], [34], [35], [36], [37], [38], they also induce tolerance and play a predominantly immunoregulatory role in corneal graft tolerance, inhibition of autoimmune diabetes in NOD mice, and regulation of immunopathology in murine Mycobacterium bovis and lymphocytic choriomeningitis virus infections [37], [39], [40], [41], [42].
The precise role of NKT lymphocytes in HIV/SIV infection remains unresolved. CD4+ NKT lymphocytes in humans and macaques express CCR5 and are highly susceptible to HIV/SIV infection in vitro [43], [44], [45]. A selective and rapid loss of both CD4+ and CD4− NKT lymphocytes has been observed in the peripheral blood of HIV-infected humans [43], [46], [47], [48] with variable reconstitution after anti-retroviral therapy [49], [50], [51], [52], [53]. A recent study showed a similar decline of CD4+ NKT lymphocytes in SIV-infected pig-tailed macaques [54]. In addition to preferential infection of CD4+ NKT lymphocytes by R5-tropic HIV and SIV, Fas-mediated activation-induced apoptosis and tissue sequestration have been implicated as possible causal mechanisms of NKT lymphocyte depletion in pathogenic HIV/SIV infection [43], [45], [48], [49]. Although the consequences of NKT lymphocyte depletion in HIV infection are not known, it is speculated that loss of NKT lymphocytes might result in increased susceptibility to opportunistic infections as well as contribute to increased aberrant immune activation [48], [52].
In light of the immunoregulatory properties of NKT lymphocytes and their potential to down-regulate immune activation, we have investigated NKT lymphocytes in a natural host of SIV infection. In this study, we report on a detailed characterization of NKT lymphocytes in sooty mangabeys. We show that in contrast to published reports of NKT lymphocytes in Asian macaques, CD1d-restricted invariant NKT lymphocytes in sooty mangabeys do not express CD4 or CCR5. In a cross-sectional analysis, naturally SIV-infected sooty mangabeys had similar frequency and phenotype of circulating NKT lymphocytes compared to SIV-negative sooty mangabeys. Functionally, sooty mangabey NKT lymphocytes showed robust production of Th1 and Th2 cytokines, including IL-10, and underwent degranulation on NKT ligand-specific activation indicating that they had both effector and immunoregulatory functional capabilities. The IL-10 cytokine-secreting profile of sooty mangabey NKT lymphocytes along with their preservation in naturally SIV-infected mangabeys raises the possibility that NKT lymphocytes play an important role in down-regulating immune activation during natural SIV infection.
Results
Identification of NKT lymphocytes in sooty mangabeys
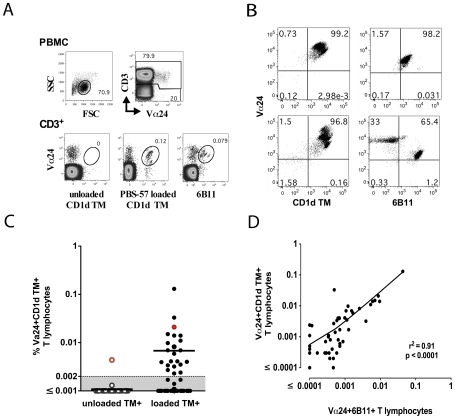
The majority of human CD1d-reactive NKT lymphocytes express the invariant Vα24-Jα18 TCR α-chain paired with the Vβ11 TCR β-chain and are identified by flow cytometric detection of Vα24 TCR-positive T lymphocytes that either bind to the 6B11 mAb directed against the invariant CDR3 region of the TCR α-chain [55], or that bind to human CD1d tetramers loaded with ligand α-galactosylceramide (α-GalCer) or its analog, PBS-57 [56], [57]. Using a similar approach in sooty mangabeys, staining of PBMC with anti-human Vα24 antibody and PBS-57 loaded CD1d tetramers (CD1d TM) or the 6B11 antibody, revealed a small but discrete population of Vα24+CD1d TM+ and Vα24+6B11+ NKT lymphocytes (Figure 1A). Limiting dilution cloning of sorted NKT lymphocytes in one SIV-negative sooty mangabey revealed that 97% of the NKT clones were concurrently Vα24+ and 6B11+ (Figure 1B top panel) confirming their invariant nature. A small subset of Vα24+CD1d TM+ cells were not 6B11+ (Figure 1B bottom panel), indicating the presence of a minor population of non-invariant NKT lymphocytes in sooty mangabeys, as have been reported in humans [58], [59], [60].
Figure 1. Identification of NKT lymphocytes in the peripheral blood of sooty mangabeys.
A) Representative dot plots showing gating strategy for identification of NKT lymphocytes. On the CD3+ T lymphocyte population, the co-staining for Vα24 and CD1d tetramers loaded with PBS-57 (CD1d TM) and 6B11 are shown in the bottom panel. Unloaded tetramers (shown in the left of bottom panel) served as a control for non-specific staining. B) Representative dot plots of sorted NKT clones in one SIV-negative sooty mangabey stained with anti-Vα24 and CD1d TM or 6B11 antibody. C) Frequency of peripheral blood NKT lymphocytes in 50 SIV-negative sooty mangabeys. Horizontal bar denotes mean. The background staining with unloaded CD1d TM for one animal (which was above 0.002%) and the corresponding Vα24+CD1d TM+ frequency are shown in red. D) Positive correlation between the frequency of Vα24+CD1d TM+ T lymphocytes and Vα24+6B11+ T lymphocytes in the peripheral blood of 50 SIV-negative sooty mangabeys.
In a cross-sectional analysis of fifty SIV-negative sooty mangabeys, Vα24+CD1d TM+ NKT lymphocytes ranged in frequencies from 0 to 0.13% of circulating T lymphocytes in the peripheral blood (Figure 1C). Owing to the rarity of circulating NKT lymphocytes, a minimum of 200,000 CD3+ T lymphocyte events were collected to ensure that detection of NKT lymphocytes at frequencies less than 0.01% reached a power of ≥80% at a P value <0.05. Additionally, the concurrent use of unloaded CD1d TM provided a valuable negative control for specificity of staining with PBS-57-loaded CD1d TM (Figure 1C). With the exception of one mangabey, the background staining with unloaded CD1d TM was well below 0.002% (Figure. 1C). Using an arbitrary positive cut-off value of ≥0.002%, ex vivo circulating NKT lymphocytes were detected at a mean frequency of 0.007% in 44% of the SIV-negative sooty mangabeys studied (Figure 1C). The frequency of NKT lymphocytes detected by the 6B11 antibody tended to be lower compared to detection with CD1d TM. However, there was a highly significant positive correlation between the frequency of peripheral blood Vα24+CD1d TM+ and Vα24+6B11+ NKT lymphocytes (Figure 1D), confirming a dominance of invariant NKT lymphocytes in the CD1d TM+ T lymphocyte population.
NKT lymphocytes in sooty mangabeys are comprised of CD8+ and CD/CD8 double negative (DN) T lymphocytes
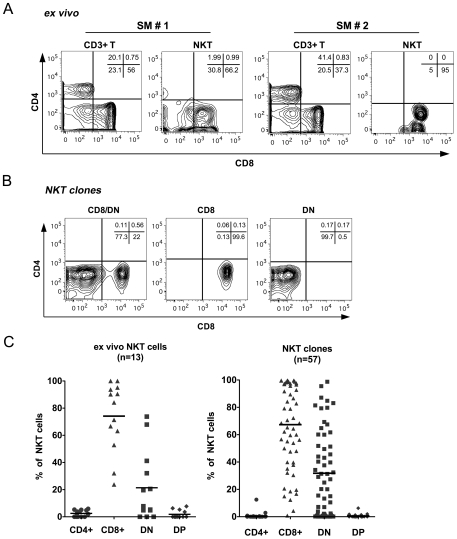
While most human and essentially all murine NKT lymphocytes consist of CD4+ and DN T lymphocyte subsets [24], [61], published reports in Asian macaques have shown the presence of predominantly CD8+ and CD4+ NKT lymphocyte subsets [45], [54], [62], [63]. Evaluation of CD4 and CD8 surface expression on ex vivo circulating NKT lymphocytes in 13 SIV-negative sooty mangabeys, revealed the presence of both CD8+ (mean ± SD; 79.3%±22.6) and DN (17.5%±21.6) subsets of NKT lymphocytes (Figure 2A & 2C left panel). In contrast to Asian macaques, CD4+ NKT lymphocytes were absent or detected at frequencies <5% of NKT cells in sooty mangabeys. Consistent with their phenotype, sooty mangabey NKT lymphocytes were significantly enriched for CD8+ T lymphocytes and had significantly lower number of CD4+ T lymphocytes compared to the circulating total T lymphocyte population (Table 1). The frequency of NKT lymphocytes within circulating CD8+ T lymphocytes ranged between 0% and 0.13% (mean 0.008%), while DN T lymphocytes contained 0% to 0.16% (mean 0.007%) of NKT lymphocytes.
Figure 2. T lymphocyte subset distribution of sooty mangabey NKT lymphocytes.
A) Representative contour plots showing surface expression of CD4 and CD8 molecules on ex vivo circulating T and Vα24+CD1d TM+ NKT lymphocytes in two sooty mangabeys (SM #1 and SM #2). B) Contour plots showing surface expression pattern of CD4 and CD8 molecules on representative NKT clones. C) Distribution of CD4+ (CD4+8−), CD8+ (CD8+4−), DN (CD4−8−) and DP (CD4+8+) T lymphocyte subsets in ex vivo NKT lymphocytes in 13 SIV-negative sooty mangabeys (left panel) and in 57 NKT clones derived from sorted 6B11+ T lymphocytes of one SIV-negative sooty mangabey (right panel).
Table 1. Distribution of T lymphocyte subsets in circulating NKT cells and total T lymphocytes in sooty mangabeys.
| Mean % ± SD@ | ||||
| CD4+ | CD8+ | CD4−CD8− | CD4+CD8+ | |
| CD3 + T cells | 35±7 | 45±7 | 19±4 | 0.5±0.4 |
| NKT cells | 2.5±2.3 | 74±25 | 21±25 | 1.8±2.8 |
| P-Value * | <0.0001 | <0.001 | 0.79 | 0.13 |
*Paired t-test.
@Mean % and Standard Deviation in 13 SIV-negative sooty mangabeys.
The CD4 and CD8 subset distribution of 57 NKT clones isolated from one SIV-negative sooty mangabey were similar to that of the ex vivo NKT lymphocytes (Figure 2B & 2C right panel). In addition to a mixed CD8+ and DN T lymphocyte phenotype, there were also a substantial number of clones that displayed a predominant or exclusive CD8+ or DN T lymphocyte phenotype (Figure 2C). Further analysis of CD8 expression using CD8α and CD8β antibodies revealed that NKT lymphocytes expressed CD8αα homodimers (data not shown). Consistent with findings on ex vivo PBMC, none of the NKT clones had a CD4+ T lymphocyte phenotype (Figure 2C).
Phenotypic characterization of sooty mangabey NKT lymphocytes
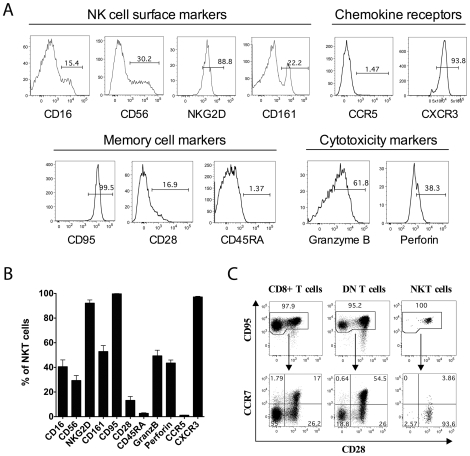
To investigate the properties of sooty mangabey NKT lymphocytes, we first examined the expression of NK cell markers, memory T lymphocyte markers and cytolytic molecules on sooty mangabey NKT clones (Figure 3A–B). Human and mouse NKT lymphocytes express the NK cell markers CD161A [64] and CD161C/NK1.1 respectively [65], while macaque NKT lymphocytes from the spleen were reported to express CD56 [63]. We examined the expression of the NK cell markers CD56, CD161, CD16, and NKG2D on sooty mangabey NKT clones. While the majority of mangabey NKT clones (92.1%±17.2) expressed NKG2D, there was considerable heterogeneity in the expression of CD161 (52.8%±32.6), CD16 (40.5%±36.6) and CD56 (29.3%±26.9%) within clones (Figure 3B). Analysis of the surface markers CD95, CD28, CD45RA, and CXCR3 showed that the majority of the NKT clones were CD95+CD28−CD45RA− and CXCR3+ (Figure 3A–B) indicating an effector memory phenotype consistent with findings in human NKT lymphocytes [43], [66]. In addition, the NKT clones contained intracellular granzyme B (49.3%±38.7) and perforin (43.5%±20) suggesting the potential for cytolytic activity (Figure 3A–B). This is in concordance with published reports of cytolytic activity in human NKT lymphocytes [61], [64], [67], [68].
Figure 3. Phenotypic characterization of sooty mangabey NKT clones.
A) Histogram plots gated on representative NKT clones showing surface expression of NK cell markers (CD16, CD56, NKG2D, and CD161), chemokine receptors (CCR5 and CXCR3), and memory markers (CD95, CD28, and CD45RA), and intracellular expression of cytolytic molecules (granzyme B and perforin). B) Mean % for each phenotypic marker on 5 to 71 NKT clones. Error bars denote standard error of mean (SEM). C) Memory phenotype of NKT lymphocytes in comparison to CD8+ and DN T lymphocytes in the peripheral blood of one SIV-negative sooty mangabey. Dot plots showing CD95 vs CD28 staining on each subset of T lymphocytes (top panel) and distribution of CCR7 and CD28 on the CD95+ memory lymphocytes (bottom panel).
Since the initial phenotypic characterization was performed on in vitro cultured NKT clones, we asked whether ex vivo circulating NKT lymphocytes displayed a similar phenotype. Peripheral blood NKT lymphocytes from one SIV-negative sooty mangabey from whom the NKT clones were derived showed a similar phenotype to clones with regards to a high frequency of CD95, NKG2D and CXCR3 surface expression (Table 2). However, in contrast to NKT clones, peripheral blood NKT lymphocytes were predominantly CD28+ (Table 2 and Figure 3C). This is consistent with loss of CD28 expression on tissue cultured human NKT lymphocytes [64], [67] as opposed to ex vivo human NKT lymphocytes [61], [68]. Ex vivo NKT lymphocytes also differed from NKT clones in having a paucity of cells expressing the NK markers CD56 and CD161, and the cytolytic molecules granzyme B and perforin (Table 2). In comparison to memory CD8+ and DN T lymphocytes, ex vivo NKT lymphocytes consisted of a homogenous CD95+CD28+CCR7− population, consistent with a transitional effector memory phenotype (Fig. 3C). The phenotypic differences between ex vivo NKT lymphocytes and NKT clones is likely a reflection of the activated status of NKT clones subsequent to in vitro stimulation with α-GalCer and IL-2.
Table 2. Phenotypic comparison of NKT clones and ex vivo circulating NKT lymphocytes from one SIV-negative sooty mangabey.
| % positive NKT lymphocytes | ||
| ex vivo | clones* | |
| CD95+CD28+ | 96.8 | 13.9 |
| CD95+CD28− | 3.2 | 85.7 |
| CXCR3+ | 95.4 | 97.1 |
| CCR5+ | ND | 1.2 |
| CD16+CD56+ | 0 | 10.4 |
| CD16+CD56− | 11.7 | 0.2 |
| CD16−CD56+ | 1.4 | 56 |
| CD161+ | 8.5 | 59.6 |
| NKG2D+ | 75 | 92 |
| NKG2A+ | 10.3 | ND |
| Granzyme B+ | 2.3 | 49.3 |
| Perforin+ | 1.7 | 43.4 |
*Mean % of 27 clones.
ND- not determined.
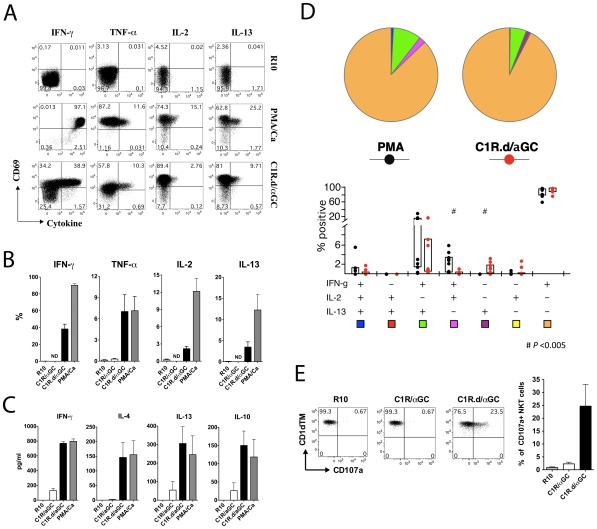
Sooty mangabey NKT clones secrete Th1 and Th2 cytokines, degranulate, and proliferate in a CD1d-restricted manner
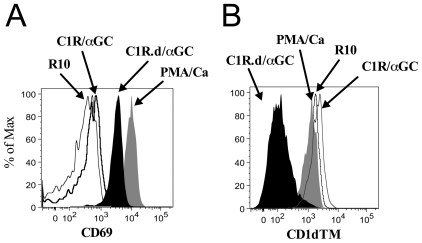
To investigate the functionality of sooty mangabey NKT lymphocytes, we first investigated the cytokine-secreting profile of NKT clones following stimulation with CD1d-transfected cell lines pulsed with α-GalCer (C1R.d/αGC). Stimulation with mock-transfected C1R cell lines pulsed with α-GalCer (C1R/αGC) served as a negative control. In vitro stimulation of NKT clones with either mitogen (PMA/Ca) or C1R.d/αGC resulted in up-regulation of CD69 indicating NKT cell activation (Figure 4A). There was a concomitant decrease in CD1d TM staining that was confined to the C1R.d/αGC stimulated lymphocytes (Figure 4B) indicating that the NKT lymphocyte TCR was down-regulated following ligand-specific stimulation.
Figure 4. Activation and TCR downregulation in sooty mangabey NKT clones.
Representative histogram plots on one NKT cell clone showing (A) surface expression of CD69 and (B) TCR detection by CD1d TM following 16 hours in vitro stimulation with the NKT-specific ligand α-GalCer presented on C1R cell lines expressing CD1d (C1R.d/α-GC), C1R cell lines pulsed with α-GalCer (C1R/α-GC), PMA/Ca or medium alone (R10).
At the end of a 16 to 24-hour stimulation period with C1R.d/αGC, production of the Th1 cytokines IFN-γ, TNF-α, and IL-2, and the Th2 cytokines IL-4, IL-13, and IL-10, was observed in NKT clones by intra-cellular cytokine flow cytometry (Figure 5A–B) and ELISA (Figure 5C). Interestingly, IL-17 production was not detected (data not shown). This contrasts with a recent report on emergence of IL-17 producing NKT lymphocytes in SIV-infected rhesus macaques [69]. Cytokine production following C1R.d/αGC stimulation was close to levels induced after PMA/Ca stimulation (Figure 5B–C) indicating the potency of NKT ligand-specific stimulation for activation of NKT clones. In contrast, stimulation with control C1R/αGC induced little or no cytokine production (Figure 5B–C). To determine whether both Th1 and Th2 cytokines were produced by the same NKT cell, we investigated concurrent production of IFN-γ, IL-2, and IL-13 by NKT clones stimulated with C1R.d/αGC and PMA/Ca (Figure 5D). More than 60% of cytokine-secreting NKT cells secreted only IFN-γ after C1R.d/αGC or PMA/Ca stimulation (Figure 5D). Concurrent production of IFN-γ and IL-13 was observed in <20% of cytokine-secreting NKT cells (Figure 5D). Comparison of mitogen stimulation with that of NKT ligand-specific stimulation showed significant differences with regards to cytokine production. Concurrent IFN-γ and IL-2 production in the absence of IL-13 was only seen after PMA/Ca but not C1R.d/αGC stimulation (Figure 5D). Furthermore, production of IL-13 alone in the absence of the Th1 cytokines IFN-γ and IL-2 was only seen after C1R.d/αGC stimulation (Figure 5D), suggesting the presence of discrete Th2 cytokine-secreting NKT lymphocyte subsets.
Figure 5. Th1 and Th2 cytokine production by sooty mangabey NKT clones.
A) Intracellular cytokine staining of one representative NKT clone with antibodies against IFN-γ, TNF-α, IL-2, and IL-13 following 16 hours in vitro stimulation with PMA/Ca or the specific ligand α-GalCer presented on C1R.d cells (C1R.d/α-GC). Medium alone (R10) served as a negative control. Dot plots gated on NKT lymphocytes show intracellular expression of recent activation marker CD69 versus each cytokine with stimulation conditions denoted on the right of each panel. Numbers denote the % of NKT cells in each quadrant. B) Mean frequency of cytokine-positive NKT cells. Data on 9 NKT clones shown. Error bars denote SEM. ND: Not determined. C) Cytokine ELISA for IFN-γ, IL-4, IL-13, and IL-10 on culture supernatants collected at 24 hours post-stimulation. Mean and SEM of four clones shown. D) Cytokine profile of the NKT clones with regards to the concurrent production of IFN-γ, IL-2, and IL-13. Data on 9 NKT clones after 16 hours of stimulation shown. Pie charts (top panel) showing the proportion of one-, two-, and three-functional responses in cytokine-secreting 6B11+ cells. Bar charts (bottom panel) showing the mean proportion of responding cells for each of seven functional combinations. Boxes represent interquartile ranges. E) Representative dot plots (on the left) gated on NKT clones showing CD107a surface expression in CD1d/α-GalCer stimulated NKT lymphocytes in comparison to cells stimulated with C1R/α-GalCer or medium alone for 4 hours. Mean frequency of NKT clones degranulating after 4-hour stimulation (on the right). Data on surface upregulation of CD107a on four NKT cell clones shown. Error bars denote SEM.
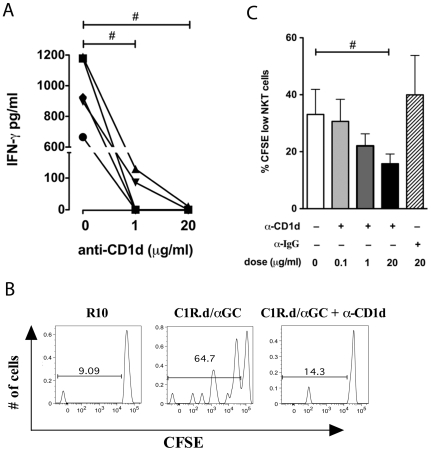
In addition to cytokine production, NKT clones also underwent degranulation upon stimulation with C1R.d/αGC, as evidenced by the surface expression of CD107a (Figure 5E). The degranulation was evident as early as four hours following stimulation, suggesting that sooty mangabey NKT clones were capable of rapid cytolytic activity. The CD1d-restricted nature of NKT lymphocyte activation was confirmed by partial to complete inhibition of cytokine secretion and proliferation in the presence of anti-CD1d blocking antibody (Figure 6). Addition of anti-human CD1d mAb (clone 42.1) to C1R.d cells prior to pulsing with α-GalCer, inhibited IFN-γ production by C1R.d/αGC-stimulated NKT clones in a dose-dependent manner (Figure 6A). Functional CD1d restriction of sooty mangabey NKT clones was further verified by examination of α-GalCer-induced proliferation. Proliferation of CFSE-labeled NKT clones was observed after a 5-day period of stimulation with C1R.d/αGC (Figure 6B). The proliferative response to C1R.d/αGC was significantly inhibited in the responding NKT clones treated with anti-CD1d in a dose-dependent manner (Figure 6B–C). No inhibition of proliferation was observed with control isotype Ab (Figure 6C).
Figure 6. Sooty mangabey NKT lymphocytes respond to NKT ligands in a CD1d-restricted manner.
A) IFN-γ ELISA on culture supernatants of NKT clones stimulated with C1R.d/α-GalCer (50 ng/ml) for 16 hours in the presence of anti-CD1d antibody (clone 42.1) at 0, 1, and 20 µg/ml. Data shows IFN- γ secretion from five clones. B) Representative histogram plots gated on NKT lymphocytes showing proliferation assessed by CFSE staining after 5 days of CD1d/α-GalCer stimulation in the presence or absence of anti-human CD1d mAb (clone 42.1). C) Dose-dependent reduction in proliferation of NKT cells with anti-CD1d. Mean and SEM of five NKT clones shown.
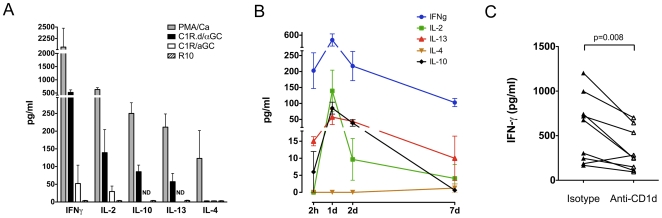
Cytokine responses of ex vivo peripheral blood NKT lymphocytes in sooty mangabeys mirror those of NKT clones
To determine whether the functional profile of in vitro expanded NKT clones reflected the properties of circulating NKT lymphocytes, we analyzed the cytokine secretion of ex vivo NKT lymphocytes in seven SIV-negative sooty mangabeys. The cytokine response of ex vivo peripheral blood NKT lymphocytes following NKT ligand-specific stimulation was assessed by ELISA. After a 24-hour stimulation period, supernatants of PBMC cultured with C1R.d/αGC showed production of IFN-γ, IL-2, IL-10, and IL-13 (Figure 7A). Stimulation with α-GalCer in the absence of CD1d (C1R/αGC) also resulted in low levels of IFN-γ and IL-2 production (Figure 7A). This may either reflect presentation of soluble α-GalCer by CD1d-positive APCs present in PBMC or a xenogenic response of mangabey PBMC to human-derived C1R cells. With the exception of IL-4, the cytokine secreting profile C1R.d/αGC-stimulated PBMC was similar to that of NKT clones. IL-4 was not detected in culture supernatants of PBMC stimulated with C1R.d/αGC, but was detected after PMA/Ca stimulation. A kinetic analysis of cytokine production over a 2 hour to 7-day period also failed to detect any IL-4 production after C1R.d/αGC stimulation (Figure 7B). IFN-γ was the dominant cytokine response, starting as early as 2 hours and peaking at one day (Figure 7B). In addition to IFN-γ, low levels of IL-13 and IL-10 were also detected at 2 hours. Similar to IFN-γ, production of IL-2, IL-13, and IL-10 peaked at 24 hours (Figure 7B).
Figure 7. Cytokine production by ex vivo peripheral blood NKT lymphocytes in sooty mangabeys.
A) Cytokine ELISA for IFN-γ, IL-2, IL-10, IL-13, and IL-4 with culture supernatants collected 24 hours post-stimulation of PBMC (mean and SEM of 4 to 7 animals). B) Kinetics of cytokine secretion in PMA/Ca and C1R.d/α-GC stimulated PBMCs at 2 hour, 1 day, 2 days, and 7 days post-stimulation. C) IFN-γ production in C1R.d/α-GC stimulated lymphocytes in the presence of anti-human CD1d (clone 42.1) blocking antibody or isotype control Ab.
In order to verify the specificity of the cytokine response on C1R.d/αGC stimulation, PBMC were stimulated in the presence of anti-CD1d blocking antibody. There was a significant 1.3-fold to 3-fold reduction in IFN-γ production in PBMC treated with anti-CD1d antibody in comparison to untreated PBMC (Figure 7C), indicating that the cytokine response on C1R.d/αGC stimulation PBMC was a result of NKT lymphocyte stimulation. There was no correlation between the ex vivo NKT lymphocyte frequencies and the amount of cytokine production (data not shown).
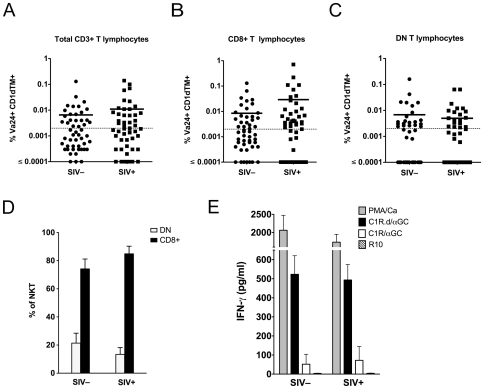
Peripheral blood NKT lymphocytes are not depleted in SIV-infected sooty mangabeys
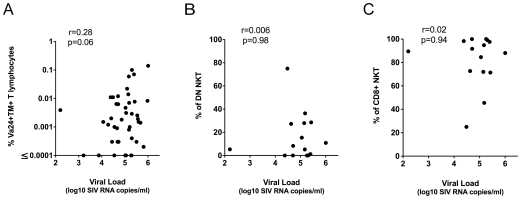
Previous studies have shown that CD4+ NKT lymphocytes are highly susceptible to in vitro HIV and SIV infection [43], [44], [45]. Selective depletion of peripheral blood NKT lymphocytes, not exclusively confined to CD4+ NKT cells, has been reported in HIV-infected humans [43], [46], [47] and SIV-infected pig-tailed macaques [54]. To assess if NKT lymphocytes are similarly depleted in sooty mangabeys with chronic SIV infection, we performed a cross-sectional analysis of the frequency of peripheral blood NKT lymphocytes in 50 SIV-negative and 50 naturally SIV-infected sooty mangabeys (Figure 8A–C). The median age of the SIV-negative sooty mangabeys was 11 years (range 4 to 20 years), while the median age of the SIV-infected mangabeys was 17 years (range 10 to 24 years). NKT lymphocyte frequencies >0.002% of circulating T lymphocytes were detected in 44% of SIV-negative sooty mangabeys (mean 0.007%) and in 52% of SIV-infected sooty mangabeys (mean 0.01%) and did not differ between the two groups (Figure 8A). The frequency of NKT lymphocytes within the CD8+ and DN T lymphocyte subsets were also similar between SIV-negative and naturally SIV-infected sooty mangabeys (Figure 8B–C), indicating that there was no depletion of CD4− NKT lymphocytes in SIV-infected sooty mangabeys. Consistent with these findings, we did not detect a difference in the proportion of CD8+ and DN NKT lymphocytes between SIV-negative and SIV-infected sooty mangabeys (Figure 8D). These data suggest that neither the frequency nor the phenotype of NKT lymphocytes are perturbed in sooty mangabeys with long-standing SIV infection. NKT lymphocytes in SIV-infected sooty mangabeys appear to be functionally intact as evidenced by comparable levels of IFN-γ production in SIV-infected and SIV-negative mangabeys following NKT ligand-specific stimulation (Figure 8E). Finally, we investigated the relationship between plasma SIV viremia and the frequency of NKT lymphocytes in SIV-infected sooty mangabeys (Figure 9A–C). Unlike pathogenic SIV infection where an inverse correlation between viral load and the frequency of total and CD4+ NKT lymphocytes was observed [54], we did not detect a similar correlation in sooty mangabeys (Figure 9A–C). A trend for a positive correlation between plasma SIV RNA and the total frequency of circulating invariant NKT lymphocytes did not reach statistical significance (Figure 9A). Moreover, the percentage of DN and CD8+ NKT lymphocytes showed no correlation with viral load (Figure 9B–C).
Figure 8. NKT lymphocytes are not depleted in SIV-infected sooty mangabeys.
Frequency of peripheral blood Vα24+CD1d TM+ NKT lymphocytes in 50 SIV-negative and 50 SIV-infected sooty mangabeys as a percentage of (A) total circulating T lymphocytes, (B) circulating CD8+ T lymphocytes, and (C) circulating DN T lymphocytes. Horizontal bar denotes mean. D) Percentage of CD8+ and DN T lymphocyte subsets in peripheral blood NKT lymphocytes in SIV− and SIV+ sooty mangabeys. E) IFN-γ ELISA on 24-hour culture supernatants of ex vivo PBMC stimulated with PMA/Ca, C1R.d/α-GC, C1R/α-GC, or medium alone (R10). Mean and SEM of data on six SIV-negative and six SIV-infected sooty mangabeys shown.
Figure 9. Relationship between plasma SIV RNA and NKT lymphocytes.
(A) Correlation between plasma SIV RNA and peripheral blood frequency of Vα24+CD1d TM+ NKT lymphocytes in 43 SIV-infected sooty mangabeys. (B) Percentage of DN NKT lymphocytes and (C) percentage of CD8+ NKT lymphocytes correlated with plasma SIV RNA in 15 SIV-infected sooty mangabeys. ‘r’ denotes correlation coefficient values determined by the Pearson correlation test.
Discussion
In this study we present results of a comprehensive analysis of the phenotype and function of ex vivo and clonal NKT lymphocytes in sooty mangabeys. To our knowledge this is the first report of NKT cells in a natural nonhuman primate host of the simian immunodeficiency virus. The mechanism of chronic immune activation in pathogenic lentiviral infection remains a central unresolved question of AIDS pathogenesis. We reasoned that, in light of their immunoregulatory function, NKT cells have the potential to play a role in modulating immune activation during HIV and SIV infection. Thus, a study of this unique T lymphocyte subset in natural hosts might provide useful insight into differences between pathogenic and nonpathogenic lentiviral infection. We show that sooty mangabey NKT lymphocytes are a functionally diverse population that rapidly produces a broad array of Th1 and Th2 cytokines, undergo degranulation, and proliferate when stimulated with α-GalCer presented on CD1d molecules indicating both effector and immunoregulatory functional capabilities. NKT lymphocytes were potent responders since these functional properties were apparent even on short-term stimulation of ex vivo PBMC, where NKT lymphocytes accounted for <1% of T lymphocytes. Circulating NKT lymphocytes in sooty mangabeys were characterized by a unique absence of CD4+ T lymphocytes, a feature that distinguished them from mice, humans, and Asian macaques. Consistent with this observation, NKT lymphocytes were neither depleted nor reduced in frequency in naturally SIV-infected sooty mangabeys and appeared to be functionally intact. Our data indicate that sooty mangabey NKT cells are resistant to SIV infection in vivo. Whether persistence of functionally intact systemic NKT cells helps to down-modulate and maintain low levels of immune activation in nonpathogenic natural SIV infection remains to be determined.
NKT lymphocytes comprise a small fraction of peripheral blood T lymphocytes in mice (mean 1.5%±0.8) and an even smaller fraction in humans (range 0.01 to 1%) [43], [70]. Published reports of NKT lymphocytes in nonhuman primates have been confined to three AIDS-susceptible Asian macaque species, namely, cynomolgus macaques, pig-tailed macaques, and rhesus macaques [45], [54], [62], [63], [69]. Ex vivo peripheral blood NKT lymphocyte frequencies averaging 0.4% in rhesus macaques and 0.19% in pig-tail macaques have been reported [54], [63]. While the frequencies of circulating NKT lymphocytes in sooty mangabeys were lower than those reported in SIV-negative Asian macaques, important phenotypic differences were noted. NKT cells in mice and humans include two major sub-populations: CD4+ and DN T lymphocytes [21], [70]. NKT cells in macaques include varying proportions of CD8+ and CD4+ T lymphocyte subsets but have a striking absence of DN T lymphocytes [45], [54], [62], [63]. Sooty mangabey NKT cells were phenotypically distinct from murine and human NKT cells, but similar to cynomolgus and rhesus macaques in having a dominance of CD8+ NKT lymphocytes. However, in contrast to macaques but similar to mice and humans, mangabey NKT cells contained DN T lymphocytes which accounted for as high as 74% of circulating NKT lymphocytes (mean 21%). The unique feature of circulating NKT lymphocytes in sooty mangabeys which distinguished them from mice, humans, and all three Asian macaque species, was the striking absence of CD4+ T lymphocytes. CD4+ single-positive or double-positive (CD4+CD8+) NKT lymphocytes account for approximately 50% of circulating NKT lymphocytes in humans [61], [68] and 20–80% of NKT lymphocytes in macaques [45], [54]. Similar to murine and human NKT lymphocytes, sooty mangabey NKT lymphocytes expressed high levels of NKG2D and moderate to high levels of CD161 [71], [72], [73], [74]. The effector memory phenotype and dominant expression of CXCR3 on ex vivo NKT cells in sooty mangabeys suggested that similar to humans, they had the ability to traffic to extra-lymphoid sites of inflammation [66], [75]. Unlike humans and macaques, the majority of NKT cells in sooty mangabeys did not express surface CCR5. In vitro experiments have shown that human and macaque CD4+ NKT lymphocytes are preferred targets of HIV and SIV infection in which the viruses replicate more rapidly in comparison to conventional CD4+ T lymphocytes [43], [45]. The lack of CD4 and CCR5 on sooty mangabey NKT cells suggests that they are not susceptible to SIV infection, despite recruitment to inflammatory sites.
Is there a functional consequence to the lack of CD4+ NKT cells in sooty mangabeys? Studies in humans have shown that DN NKT cells mainly produce Th1 cytokines while the CD4+ NKT cells produce both Th1 and Th2 cytokines [61], [68]. However, despite these differences, a recent study reported that human CD4+ NKT lymphocyte subsets preferentially induced Th1 responses, whereas the DN subset induced a shift toward Th2 responses through NKT-DC cross-talk along with elimination of IL-12-producing DC by direct NKT cytolysis [76]. Thus the association between phenotype and cytokine-secreting profile of NKT lymphocytes is not necessarily a simple one. Despite the absence of CD4+ NKT cells, ex vivo NKT lymphocytes and NKT cell clones in sooty mangabeys produced a wide array of Th1 and Th2 cytokines, including IL-10. At the single-cell level, Th2 cytokine secretion was mediated by a heterogeneous population of NKT cells (Figure 5D). Thus, IL-13 was produced by both IFN-γ-secreting and non-IFN-γ-secreting NKT cells. Moreover, sooty mangabey DN NKT lymphocytes produced both Th1 and Th2 cytokines. These observations reinforce the concept that functional differences in NKT lymphocytes are not solely associated with their CD4/CD8 phenotype. Other factors, such as the cytokine microenvironment and NKT-APC interaction are likely to determine the Th1/Th2 bias of a responding NKT lymphocyte.
The limited published data on functionality of NKT lymphocytes in AIDS-susceptible macaque species does not allow for a complete comparative analysis of the function of sooty mangabey and macaque NKT lymphocytes. In one study on long-term cultured splenic NKT lymphocytes in rhesus macaques, cytokine secretion in response to α-GalCer stimulation was dominated by TGF-β and IL-13 production, with low levels of IFN-γ and little or no IL-2, IL-4 or IL-10 secretion, although IL-10 was produced intracellularly [77]. In contrast, we observed that sooty mangabey NKT clones readily produced high levels of IFN-γ along with IL-2, IL-4, IL-10, and IL-13 on α-GalCer stimulation. Moreover, with the exception of IL-4, the same pattern of cytokine secretion was also observed on α-GalCer stimulation of ex vivo PBMC that contained low frequencies of NKT lymphocytes. IFN-γ and IL-2 secretion was apparent as early as two hours following α-GalCer stimulation of ex vivo PBMC. Early IFN-γ secretion by NKT lymphocytes might help in recruitment and activation of downstream effectors such as NK cells and CD8+ T lymphocytes, as well as enhance DC maturation, thereby promoting immune responses to pathogens. Studies in mice have demonstrated efficient induction of CD8+ T cell responses by NKT lymphocytes through early IFN-γ production during acute virus infection [33], [38], [78]. IL-2, on the other hand, is a primary growth factor for antigen-activated T lymphocytes and also acts as the major inducer for the development of suppressive Treg cells. Indeed, activation of NKT lymphocytes with α-GalCer has been demonstrated to enhance IL-2 production by NKT lymphocytes and subsequent expansion of Tregs [79], [80]. In this regard it is interesting that a recent comparative study of acute SIV infection in African green monkeys (AGM) and pig-tailed macaques showed an earlier increase of Tregs in AGM that correlated with resolution of immune activation [15]. Likewise, IL-4 and IL-10 produced by NKT lymphocytes can induce Treg cells [81], and IL-13 can act on myeloid cells to induce TGF-β [82]. Thus, the broad array of cytokines produced by sooty mangabey NKT lymphocytes indicate that they have the capability to mediate both effector functions and suppress immunopathology. The potent production of IL-10 by sooty mangabey NKT lymphocytes, a feature notably absent in rhesus macaques [77], suggests that there may be species-specific functional differences in NKT lymphocytes. In light of these findings, it is tempting to speculate that the presence of functionally intact NKT cells in SIV-infected sooty mangabeys could help to blunt non-specific immune activation in sooty mangabeys as opposed to rhesus macaques. While the low levels of circulating invariant NKT lymphocytes in sooty mangabeys raises questions about their overall impact on systemic immune activation, it is possible that the presence of higher frequencies of tissue-resident NKT cells at mucosal effector sites could help blunt hyper-immune activation.
In summary, we have characterized the phenotype and function of sooty mangabey NKT cells and demonstrated that they are a heterogeneous population comprised chiefly of CD8+ and DN T lymphocyte subsets with a CXCR3-positive memory phenotype, and expression of NKG2D and CD161 as the prominent NK cell markers. We show that CD1d-restricted sooty mangabey NKT cells are multifunctional and resistant to SIV infection. By virtue of their cytolytic and Th1 cytokine-secreting ability, combined with IL-10 production, it is possible that sooty mangabey NKT cells facilitate early induction of an appropriate antiviral immune response while concurrently dampening non-specific chronic immune activation. Further studies are required to investigate the role of these T cell subsets in modulation of immune responses in pathogenic and nonpathogenic SIV infection.
Materials and Methods
Ethics Statement
All animals were maintained in accordance with Emory University's Institutional Animal Care and Use Committee and federal guidelines for animal care.
Animals
Blood samples used in this study were obtained from fifty SIV-uninfected and fifty naturally SIV-infected sooty mangabeys. All animals were housed at the Yerkes National Primate Research Center (YNPRC), Atlanta.
Sample collection
Sooty mangabey blood was collected at YNPRC in heparin vacutainer tubes (Becton Dickinson Vacutainer systems, Franklin Lakes, NJ), and shipped overnight on ice to the New England Primate Research Center (NEPRC) where it was processed the following day. Peripheral blood mononuclear cells (PBMC) were separated by density gradient centrifugation (Lymphocyte Separation Medium; MP Biomedicals Inc., Solon, OH) at 1500 rpm for 45 minutes and used for phenotyping and in vitro assays.
Immunophenotyping and flow cytometry of NKT lymphocytes
Multicolor flow cytometric analysis was performed on ex vivo and in vitro expanded cells according to standard procedures using anti-human mAbs that cross-react with sooty mangabeys. NKT lymphocyte ligand PBS-57-loaded and unloaded human CD1d Tetramers (CD1d TM) conjugated with APC were obtained from the NIH Tetramer core facility. The following antibodies were obtained from BD Biosciences unless stated otherwise: anti-Vα24–PE (clone C15; Immunotech), 6B11–FITC (6B11), anti-CD3–APC-Cy7 (SP34-2), anti-CD4–Qdot605 (T4/19Thy5D7; custom/NHP Resource), anti-CD8–Alexa Fluor 700 (RPA-T8), anti-CD56–PE-Cy7 (NCAM16.2), anti-CD16–Alexa Fluor 700 (3G8; Invitrogen), anti-CD161–APC (DX12), anti-NKG2D–PE (ON72; Beckman Coulter), anti-CD95–PE-Cy5 (DX2), anti-CD28–PE TexasRed (CD28.2; Immunotech), anti-CD45RA–PE-Cy7 (L48), anti-CCR7–biotin (150503; custom), anti-CCR5–APC (3A9), anti-CXCR3–PE (1C6), anti-CD69–PE TexasRed (TP1.55.3; Beckman Coulter), anti-CD107a–PE (H4A3), anti-Perforin–FITC (B56), anti-GranzymeB–APC (GB12; Caltag), anti-IFN-γ–PE-Cy7 (B27), anti-IL-2–APC (MQ1-17H12), anti-IL-13–FITC (PVM13-1; eBioscience), anti-TNF-α–Alexa Fluor 700 (MAb11).
For identification of NKT cells, PBMCs were surface stained for CD3 and anti-Vα24 combined with PBS-57 loaded CD1d TM or 6B11 antibody. APC-labeled unloaded CD1d TM controls were used in all experiments. Surface staining was carried out by standard procedures. Briefly, 2 to 4 million PBMC re-suspended in 100 µl wash buffer (PBS with 2% FBS) were initially incubated with tetramers for 20 min at 4°C followed by addition of surface antibodies and further incubation for 30 min at 4°C. After washing, the cells were fixed in 2% paraformaldehyde. All intracellular cytokine staining (ICS) assays were carried out on cells that were stimulated overnight. Following 16 h incubation, cells were washed in PBS containing 2% FCS and 0.5 mM EDTA, stained for surface markers in wash buffer for 30 min at 4°C, washed and then fixed and permeabilized using the Invitrogen Fix/Perm reagents (CALTAG™). Permeabilized cells were stained intracellularly with the requisite antibodies. Cells were then washed in wash buffer and fixed in 2% paraformaldehyde. Flow cytometric acquisition was performed on an LSR-II cytometer driven by the FACS DiVa software (version 5.2; BD). At least 200,000 T lymphocyte events were collected. Analysis of the acquired data was performed using FlowJo software (version 8.8.3; TreeStar, Ashland, OR). For experiments measuring three-functional responses, Boolean gating was used to partition cells into specific response categories and PESTLE v1.6.2 (provided kindly by Dr Mario Roederer) and SPICE 5.05 software (provided free of charge by NIAID/NIH in collaboration with Dr. Mario Roederer) was used to analyze data.
Medium and Reagents
The complete medium (R10 medium) used throughout was RPMI medium 1640 (Cellgro, Herndon, VA) supplemented with 10% FCS (Sigma-Aldrich, St. Louis, MO), 1% 1 M HEPES, 2 mM L-glutamine (Cellgro), 50 IU/ml penicillin (Cellgro), 50 µg/ml streptomycin (Cellgro). The NKT-ligand α-galactosylceramide (α-GalCer, Diagnocine LLC, Hackensack, NJ) was used at 100 ng/ml. The anti-human CD1d antibody (Clone 42.1) described earlier [83] was used at 0.1–20 µg/ml. Recombinant human IL-2 (Roche) was used at 10–50 IU/ml of medium for the expansion and maintenance of NKT cell clones.
In vitro expansion of NKT lymphocytes
6B11-positive lymphocytes were sorted on a FACSAria cell sorter (BD Biosciences, San Jose, CA, USA) and cloned by limiting dilution at 3 and 10 cells per well in 96-well, round-bottom, polystyrene plates (Corning, NY, USA). Cells were incubated in R10 medium containing 5 µg/ml ConA and 100 ng/ml of α-GalCer along with 100,000 cells/well of human feeder PBMC irradiated at 3000 rads. After two days, ConA was removed and 50 IU/ml recombinant human IL-2 was added. Wells with cell outgrowth were re-stimulated and expanded over a 2–4 week period. The presence of NKT clones was confirmed by staining with anti-Vα24, PBS-57 loaded CD1dTM or 6B11 mAb. Positive NKT clones were maintained in complete media supplemented with 50 IU/ml IL-2. For functional analyses, the clones were gradually switched to resting stage (10 IU/ml IL-2) 48 h prior to the assay as previously described [84].
Functional Analysis of NKT lymphocytes
For NKT cell activation assays, 2×104 NKT cells or 105 PBMCs were plated in a 96-well flat-bottom plate with either medium alone or with an equal number of stimulator cells that had been treated with α-GalCer at a final concentration of 100 ng/ml. 25 ng/ml PMA (Sigma-Aldrich, St. Louis, MO) with 1 µg/ml calcium ionophore (PMA/Ca) was used as positive control stimulus. 50,000 CD1d-transfected C1R B cell line (C1R.d) were γ-irradiated at 10,000 rads and used as APCs for the presentation of α-GalCer as previously described [67]. Irradiated mock-transfected C1R cells served as a negative control stimulus for NKT cells. For stimulation of clones with CD1d transfectants, IL-2 was included in the culture medium at a final concentration of 10 IU/ml. Typically, proliferation and cytokine release assays on NKT cell clones were performed 3–4 wk after the last re-stimulation. All measurements were performed in triplicate.
CFSE proliferation assay
NKT cells were re-suspended at a concentration of ≤5×106/ml in RPMI 1640 (Cellgro, Herndon, VA) and labeled with 2 µM CFSE (Molecular Probes, Eugene, OR) by incubation for 10 min at 37°C in a 5% CO2 incubator. The CFSE label was quenched with addition of 1x volume of 100% FBS. After incubation for 1 min at RT, the dye was diluted further with RPMI- 1640 and the cells were washed twice before culturing in flat-bottom 96-well plates. FACS analysis was performed after 5 days of incubation.
Statistical Analysis
Paired and unpaired t-tests were used for comparisons of grouped and ungrouped samples respectively. The Pearson test was performed for correlation analysis. P<0.05 was considered statistically significant. All statistical analyses were performed using the GraphPad Prism software version 5.0b (GraphPad Software, Inc., La Jolla, CA).
Acknowledgments
We thank Tracy Meeker of the Yerkes National Primate Research Center for coordinating the sooty mangabey blood sample collections, the Emory University CFAR virology core for plasma SIV RNA measurements, and the flow cytometry core facility staff at the New England Primate Research Center for cell sorting and flow cytometric analysis. We thank the NIH Tetramer Core Facility for providing PBS-57-loaded and empty human CD1d Tetramers. We acknowledge Dr. Mario Roederer and NIAID/NIH for providing the SPICE software program.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Public Health Service grants RR00168, RR00165, DK066917, AI049809 and AI084810. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chakrabarti LA, Lewin SR, Zhang L, Gettie A, Luckay A, et al. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J Virol. 2000;74:1209–1223. doi: 10.1128/jvi.74.3.1209-1223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silvestri G, Sodora DL, Koup RA, Paiardini M, O'Neil SP, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 3.Kaur A, Di Mascio M, Barabasz A, Rosenzweig M, McClure HM, et al. Dynamics of T- and B-lymphocyte turnover in a natural host of simian immunodeficiency virus. J Virol. 2008;82:1084–1093. doi: 10.1128/JVI.02197-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandrea I, Sodora DL, Silvestri G, Apetrei C. Into the wild: simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol. 2008;29:419–428. doi: 10.1016/j.it.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sodora DL, Allan JS, Apetrei C, Brenchley JM, Douek DC, et al. Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nat Med. 2009;15:861–865. doi: 10.1038/nm.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandrea IV, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol. 2007;179:3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon SN, Klatt NR, Bosinger SE, Brenchley JM, Milush JM, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol. 2007;179:3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estes JD, Gordon SN, Zeng M, Chahroudi AM, Dunham RM, et al. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J Immunol. 2008;180:6798–6807. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meythaler M, Martinot A, Wang Z, Pryputniewicz S, Kasheta M, et al. Differential CD4+ T-lymphocyte apoptosis and bystander T-cell activation in rhesus macaques and sooty mangabeys during acute simian immunodeficiency virus infection. J Virol. 2009;83:572–583. doi: 10.1128/JVI.01715-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lederer S, Favre D, Walters KA, Proll S, Kanwar B, et al. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 2009;5:e1000296. doi: 10.1371/journal.ppat.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest. 2005;115:1082–1091. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 13.Pandrea I, Apetrei C, Gordon S, Barbercheck J, Dufour J, et al. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007;109:1069–1076. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 15.Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaur A, Grant RM, Means RE, McClure H, Feinberg M, et al. Diverse host responses and outcomes following simian immunodeficiency virus SIVmac239 infection in sooty mangabeys and rhesus macaques. J Virol. 1998;72:9597–9611. doi: 10.1128/jvi.72.12.9597-9611.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Metcalf B, Ribeiro RM, McClure H, Kaur A. Th-1-type cytotoxic CD8+ T-lymphocyte responses to simian immunodeficiency virus (SIV) are a consistent feature of natural SIV infection in sooty mangabeys. J Virol. 2006;80:2771–2783. doi: 10.1128/JVI.80.6.2771-2783.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 21.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koseki H, Asano H, Inaba T, Miyashita N, Moriwaki K, et al. Dominant expression of a distinctive V14+ T-cell antigen receptor alpha chain in mice. Proc Natl Acad Sci U S A. 1991;88:7518–7522. doi: 10.1073/pnas.88.17.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178:1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci U S A. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the 'Swiss-Army knife' of the immune system. Curr Opin Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda JL, Gapin L, Baron JL, Sidobre S, Stetson DB, et al. Mouse V alpha 14i natural killer T cells are resistant to cytokine polarization in vivo. Proc Natl Acad Sci U S A. 2003;100:8395–8400. doi: 10.1073/pnas.1332805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 29.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, et al. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 30.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 31.Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci U S A. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–127. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ilyinskii PO, Wang R, Balk SP, Exley MA. CD1d mediates T-cell-dependent resistance to secondary infection with encephalomyocarditis virus (EMCV) in vitro and immune response to EMCV infection in vivo. J Virol. 2006;80:7146–7158. doi: 10.1128/JVI.02745-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grubor-Bauk B, Simmons A, Mayrhofer G, Speck PG. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant V alpha 14-J alpha 281 TCR. J Immunol. 2003;170:1430–1434. doi: 10.4049/jimmunol.170.3.1430. [DOI] [PubMed] [Google Scholar]
- 35.Ashkar AA, Rosenthal KL. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J Virol. 2003;77:10168–10171. doi: 10.1128/JVI.77.18.10168-10171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson TR, Hong S, Van Kaer L, Koezuka Y, Graham BS. NK T cells contribute to expansion of CD8(+) T cells and amplification of antiviral immune responses to respiratory syncytial virus. J Virol. 2002;76:4294–4303. doi: 10.1128/JVI.76.9.4294-4303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diana J, Griseri T, Lagaye S, Beaudoin L, Autrusseau E, et al. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity. 2009;30:289–299. doi: 10.1016/j.immuni.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–930. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonoda KH, Faunce DE, Taniguchi M, Exley M, Balk S, et al. NK T cell-derived IL-10 is essential for the differentiation of antigen-specific T regulatory cells in systemic tolerance. J Immunol. 2001;166:42–50. doi: 10.4049/jimmunol.166.1.42. [DOI] [PubMed] [Google Scholar]
- 40.Watte CM, Nakamura T, Lau CH, Ortaldo JR, Stein-Streilein J. Ly49 C/I-dependent NKT cell-derived IL-10 is required for corneal graft survival and peripheral tolerance. J Leukoc Biol. 2008;83:928–935. doi: 10.1189/jlb.0807579. [DOI] [PubMed] [Google Scholar]
- 41.Chen YG, Choisy-Rossi CM, Holl TM, Chapman HD, Besra GS, et al. Activated NKT cells inhibit autoimmune diabetes through tolerogenic recruitment of dendritic cells to pancreatic lymph nodes. J Immunol. 2005;174:1196–1204. doi: 10.4049/jimmunol.174.3.1196. [DOI] [PubMed] [Google Scholar]
- 42.Dieli F, Taniguchi M, Kronenberg M, Sidobre S, Ivanyi J, et al. An anti-inflammatory role for V alpha 14 NK T cells in Mycobacterium bovis bacillus Calmette-Guerin-infected mice. J Immunol. 2003;171:1961–1968. doi: 10.4049/jimmunol.171.4.1961. [DOI] [PubMed] [Google Scholar]
- 43.Motsinger A, Haas DW, Stanic AK, Van Kaer L, Joyce S, et al. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J Exp Med. 2002;195:869–879. doi: 10.1084/jem.20011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleuridor R, Wilson B, Hou R, Landay A, Kessler H, et al. CD1d-restricted natural killer T cells are potent targets for human immunodeficiency virus infection. Immunology. 2003;108:3–9. doi: 10.1046/j.1365-2567.2003.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Motsinger A, Azimzadeh A, Stanic AK, Johnson RP, Van Kaer L, et al. Identification and simian immunodeficiency virus infection of CD1d-restricted macaque natural killer T cells. J Virol. 2003;77:8153–8158. doi: 10.1128/JVI.77.14.8153-8158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandberg JK, Fast NM, Palacios EH, Fennelly G, Dobroszycki J, et al. Selective loss of innate CD4(+) V alpha 24 natural killer T cells in human immunodeficiency virus infection. J Virol. 2002;76:7528–7534. doi: 10.1128/JVI.76.15.7528-7534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Vliet HJ, von Blomberg BM, Hazenberg MD, Nishi N, Otto SA, et al. Selective decrease in circulating V alpha 24+V beta 11+ NKT cells during HIV type 1 infection. J Immunol. 2002;168:1490–1495. doi: 10.4049/jimmunol.168.3.1490. [DOI] [PubMed] [Google Scholar]
- 48.Unutmaz D. NKT cells and HIV infection. Microbes Infect. 2003;5:1041–1047. doi: 10.1016/s1286-4579(03)00185-0. [DOI] [PubMed] [Google Scholar]
- 49.van der Vliet HJ, van Vonderen MG, Molling JW, Bontkes HJ, Reijm M, et al. Cutting edge: Rapid recovery of NKT cells upon institution of highly active antiretroviral therapy for HIV-1 infection. J Immunol. 2006;177:5775–5778. doi: 10.4049/jimmunol.177.9.5775. [DOI] [PubMed] [Google Scholar]
- 50.Moll M, Snyder-Cappione J, Spotts G, Hecht FM, Sandberg JK, et al. Expansion of CD1d-restricted NKT cells in patients with primary HIV-1 infection treated with interleukin-2. Blood. 2006;107:3081–3083. doi: 10.1182/blood-2005-09-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasan S, Poles MA, Horowitz A, Siladji EE, Markowitz M, et al. Function of NKT cells, potential anti-HIV effector cells, are improved by beginning HAART during acute HIV-1 infection. Int Immunol. 2007;19:943–951. doi: 10.1093/intimm/dxm055. [DOI] [PubMed] [Google Scholar]
- 52.Yang OO, Wilson SB, Hultin LE, Detels R, Hultin PM, et al. Delayed reconstitution of CD4+ iNKT cells after effective HIV type 1 therapy. AIDS Res Hum Retroviruses. 2007;23:913–922. doi: 10.1089/aid.2006.0253. [DOI] [PubMed] [Google Scholar]
- 53.Moll M, Kuylenstierna C, Gonzalez VD, Andersson SK, Bosnjak L, et al. Severe functional impairment and elevated PD-1 expression in CD1d-restricted NKT cells retained during chronic HIV-1 infection. Eur J Immunol. 2009;39:902–911. doi: 10.1002/eji.200838780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernandez CS, Chan AC, Kyparissoudis K, De Rose R, Godfrey DI, et al. Peripheral NKT cells in simian immunodeficiency virus-infected macaques. J Virol. 2009;83:1617–1624. doi: 10.1128/JVI.02138-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Exley MA, Hou R, Shaulov A, Tonti E, Dellabona P, et al. Selective activation, expansion, and monitoring of human iNKT cells with a monoclonal antibody specific for the TCR alpha-chain CDR3 loop. Eur J Immunol. 2008;38:1756–1766. doi: 10.1002/eji.200737389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med. 2000;191:1895–1903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Goff RD, Zhou D, Mattner J, Sullivan BA, et al. A modified alpha-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods. 2006;312:34–39. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 58.Gadola SD, Dulphy N, Salio M, Cerundolo V. Valpha24-JalphaQ-independent, CD1d-restricted recognition of alpha-galactosylceramide by human CD4(+) and CD8alphabeta(+) T lymphocytes. J Immunol. 2002;168:5514–5520. doi: 10.4049/jimmunol.168.11.5514. [DOI] [PubMed] [Google Scholar]
- 59.Van Rhijn I, Young DC, Im JS, Levery SB, Illarionov PA, et al. CD1d-restricted T cell activation by nonlipidic small molecules. Proc Natl Acad Sci U S A. 2004;101:13578–13583. doi: 10.1073/pnas.0402838101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaulov A, Yue S, Wang R, Joyce RM, Balk SP, et al. Peripheral blood progenitor cell product contains Th1-biased noninvariant CD1d-reactive natural killer T cells: implications for posttransplant survival. Exp Hematol. 2008;36:464–472. doi: 10.1016/j.exphem.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kashiwase K, Kikuchi A, Ando Y, Nicol A, Porcelli SA, et al. The CD1d natural killer T-cell antigen presentation pathway is highly conserved between humans and rhesus macaques. Immunogenetics. 2003;54:776–781. doi: 10.1007/s00251-002-0527-8. [DOI] [PubMed] [Google Scholar]
- 63.Gansuvd B, Goodwin J, Asiedu CK, Jiang XL, Jargal U, et al. Invariant natural killer T cells from rhesus macaque spleen and peripheral blood are phenotypically and functionally distinct populations. J Med Primatol. 2008;37:1–11. doi: 10.1111/j.1600-0684.2007.00222.x. [DOI] [PubMed] [Google Scholar]
- 64.Exley M, Porcelli S, Furman M, Garcia J, Balk S. CD161 (NKR-P1A) costimulation of CD1d-dependent activation of human T cells expressing invariant V alpha 24 J alpha Q T cell receptor alpha chains. J Exp Med. 1998;188:867–876. doi: 10.1084/jem.188.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Makino Y, Kanno R, Ito T, Higashino K, Taniguchi M. Predominant expression of invariant V alpha 14+ TCR alpha chain in NK1.1+ T cell populations. Int Immunol. 1995;7:1157–1161. doi: 10.1093/intimm/7.7.1157. [DOI] [PubMed] [Google Scholar]
- 66.Kim CH, Johnston B, Butcher EC. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among V alpha 24(+)V beta 11(+) NKT cell subsets with distinct cytokine-producing capacity. Blood. 2002;100:11–16. doi: 10.1182/blood-2001-12-0196. [DOI] [PubMed] [Google Scholar]
- 67.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells. J Exp Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campillo-Gimenez L, Cumont MC, Fay M, Kared H, Monceaux V, et al. AIDS Progression Is Associated with the Emergence of IL-17-Producing Cells Early After Simian Immunodeficiency Virus Infection. J Immunol. 2010;184:984–992. doi: 10.4049/jimmunol.0902316. [DOI] [PubMed] [Google Scholar]
- 70.Hammond KJ, Pelikan SB, Crowe NY, Randle-Barrett E, Nakayama T, et al. NKT cells are phenotypically and functionally diverse. Eur J Immunol. 1999;29:3768–3781. doi: 10.1002/(SICI)1521-4141(199911)29:11<3768::AID-IMMU3768>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 71.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, et al. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 72.Loza MJ, Metelitsa LS, Perussia B. NKT and T cells: coordinate regulation of NK-like phenotype and cytokine production. Eur J Immunol. 2002;32:3453–3462. doi: 10.1002/1521-4141(200212)32:12<3453::AID-IMMU3453>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 73.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, et al. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 74.Vilarinho S, Ogasawara K, Nishimura S, Lanier LL, Baron JL. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc Natl Acad Sci U S A. 2007;104:18187–18192. doi: 10.1073/pnas.0708968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas SY, Hou R, Boyson JE, Means TK, Hess C, et al. CD1d-restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. J Immunol. 2003;171:2571–2580. doi: 10.4049/jimmunol.171.5.2571. [DOI] [PubMed] [Google Scholar]
- 76.Liu TY, Uemura Y, Suzuki M, Narita Y, Hirata S, et al. Distinct subsets of human invariant NKT cells differentially regulate T helper responses via dendritic cells. Eur J Immunol. 2008;38:1012–1023. doi: 10.1002/eji.200737838. [DOI] [PubMed] [Google Scholar]
- 77.Gansuvd B, Hubbard WJ, Hutchings A, Thomas FT, Goodwin J, et al. Phenotypic and functional characterization of long-term cultured rhesus macaque spleen-derived NKT cells. J Immunol. 2003;171:2904–2911. doi: 10.4049/jimmunol.171.6.2904. [DOI] [PubMed] [Google Scholar]
- 78.Grubor-Bauk B, Arthur JL, Mayrhofer G. Importance of NKT cells in resistance to herpes simplex virus, fate of virus-infected neurons, and level of latency in mice. J Virol. 2008;82:11073–11083. doi: 10.1128/JVI.00205-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu R, La Cava A, Bai XF, Jee Y, Price M, et al. Cooperation of invariant NKT cells and CD4+CD25+ T regulatory cells in the prevention of autoimmune myasthenia. J Immunol. 2005;175:7898–7904. doi: 10.4049/jimmunol.175.12.7898. [DOI] [PubMed] [Google Scholar]
- 80.Jiang S, Game DS, Davies D, Lombardi G, Lechler RI. Activated CD1d-restricted natural killer T cells secrete IL-2: innate help for CD4+CD25+ regulatory T cells? Eur J Immunol. 2005;35:1193–1200. doi: 10.1002/eji.200425899. [DOI] [PubMed] [Google Scholar]
- 81.Roelofs-Haarhuis K, Wu X, Gleichmann E. Oral tolerance to nickel requires CD4+ invariant NKT cells for the infectious spread of tolerance and the induction of specific regulatory T cells. J Immunol. 2004;173:1043–1050. doi: 10.4049/jimmunol.173.2.1043. [DOI] [PubMed] [Google Scholar]
- 82.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Exley M, Garcia J, Wilson SB, Spada F, Gerdes D, et al. CD1d structure and regulation on human thymocytes, peripheral blood T cells, B cells and monocytes. Immunology. 2000;100:37–47. doi: 10.1046/j.1365-2567.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Exley MA, Balk SP, Wilson SB. Isolation and functional use of human NK T cells. Curr Protoc Immunol. 2003 doi: 10.1002/0471142735.im1411s52. Chapter 14: Unit 14 11. [DOI] [PubMed] [Google Scholar]