Abstract
Accumulating evidence suggests that metabotropic glutamate receptors (mGluRs) are involved in both cocaine reinforcement and the reinstatement of cocaine-seeking behavior. In the present experiments, rats were trained to self-administer cocaine under fixed ratio (for cocaine priming-induced reinstatement) or second order (for cocaine cue-induced reinstatement) schedules of reinforcement. Lever pressing was then extinguished followed by a reinstatement phase where operant responding was promoted by either cocaine itself or cocaine-associated light cues. Results indicated that systemic administration of the mGluR5 antagonists 2-Methyl-6-(phenylethynyl)pyridine (MPEP: 1 and 3 mg/kg i.p.) or 3-((2-Methyl-1,3-thiazol-4-yl)ethynyl)pyridine (MTEP: 0.1 and 1 mg/kg i.p.) dose-dependently attenuated reinstatement of drug seeking induced by a systemic priming injection of 10 mg/kg cocaine. Systemic administration of MTEP (0.1 and 1 mg/kg i.p) also dose-dependently attenuated cocaine cue-induced reinstatement of drug seeking. Systemic administration of neither MPEP nor MTEP influenced the reinstatement of sucrose seeking, which indicates that the effects of these compounds on cocaine seeking were reinforcer specific. Additionally, administration of MPEP (1 μg/0.5μl) into the nucleus accumbens shell, a brain region that plays a critical role in cocaine seeking, attenuated cocaine priming-induced reinstatement of drug seeking. These results add to a growing literature indicating that mGluR antagonists attenuate the reinstatement of cocaine seeking. Importantly, the current findings also suggest that activation of mGluR5s specifically in the nucleus accumbens shell promotes the reinstatement of cocaine seeking.
Keywords: relapse, addiction, psychostimulant, glutamate, metabotropic, mGluR5, MPEP, MTEP
INTRODUCTION
An extensive pre-clinical literature indicates that cocaine-induced alterations in limbic glutamate transmission play a critical role in the reinstatement of cocaine seeking, an animal model of relapse. However, these studies have focused primarily on the role of ionotropic glutamate receptors in the reinstatement of cocaine seeking [1–4]. Examination of the role of metabotropic glutamate receptors (mGluRs) in cocaine reinstatement is of interest since these receptors also regulate neuronal excitability and plasticity [5,6].
Eight different mGluR subunits have been identified to date and classified into three main groups based on sequence homology, pharmacology and coupling to intracellular effectors. For example, activation of group I mGluRs, which includes mGluR5 receptors, stimulates Gq/phospholipase C (PLC), resulting in the generation of diacylglycerol (DAG) and inositol triphosphate (IP3) [7,8]. A growing body of evidence indicates that mGluR5 receptors, which are highly expressed in cortical and limbic nuclei including the nucleus accumbens [9], modulate cocaine-mediated behaviors. Initial evidence indicated that genetic deletion of mGluR5 in mice blocked the acquisition of cocaine self-administration without adversely affecting operant responding for food [10]. Subsequent pharmacological studies demonstrated that the systemic administration of the group I mGluR antagonist, MPEP, attenuated the expression of behavioral sensitization to cocaine [11], cocaine-induced conditioned place preference [12,13] and reinforcing effects of cocaine [14–19].
Relapse (or reinstatement) of cocaine-seeking can be modeled in monkeys and rodents by re-exposure to cocaine-associated cues or the administration of a priming injection of cocaine among animals in which cocaine self-administration behavior has been extinguished [20]. Systemic injection of the mGluR5 antagonist, MPEP, attenuated both cue- [21] and priming- [17] induced reinstatement of cocaine seeking. The goal of the present experiments was to replicate these cocaine reinstatement studies and extend them by using MTEP, a non-competitive mGluR5 antagonist that is more potent and selective than MPEP [22–24]. Moreover, we also assessed the effect of administration of MPEP into the nucleus accumbens shell, a brain region known to play an important role in the reinstatement of cocaine seeking [25–28].
MATERIALS AND METHODS
Animals and housing
Male Sprague Dawley rats (Rattus norvegicus), weighing 250–300 g, were obtained from Taconic Laboratories (Germantown, N.Y., USA) and housed individually with food and water available ad libitum. The colony was maintained on a 12h light/dark cycle with lights on at 7:00 am. All behavioral training and testing was done during the light cycle. All experimental protocols were conducted in accordance with guidelines from the National Institutes of Health (NIH) and approved by the Boston University School of Medicine Institutional Animal Care and Use Committee.
Materials
All experiments used Med-Associates (Georgia, VT), modular testing instrumentation enclosed within ventilated, sound attenuating chambers. The testing apparatus was equipped with response levers, stimulus lights, sucrose pellet dispensers and injection pumps for the delivery of intravenous drug infusions.
Surgery
Prior to surgery, rats were anesthetized with an i.p. injection of 80 mg/kg ketamine and 12 mg/kg xylazine (Sigma, St. Louis, MO). An indwelling silastic catheter (inner diameter 0.33 mm, outer diameter 0.64 mm) was inserted in to the right jugular vein and sutured in place. The catheter was threaded subcutaneously to a mesh backmount platform (CamCaths, Cambridge UK). Catheters were flushed daily with 0.2 ml of the antibiotic solution Timentin (ticarcillin disodium/potassium clavulanate, 0.93 mg/ml) dissolved in heparinized saline. Catheters were sealed with plastic obturators when not in use.
Following catheter insertion some rats were placed in a stereotaxic apparatus and guide cannulae (14 mm, 24 gauge, Plastics One) for microinjections were implanted bilaterally, dorsal to the shell subregion of the nucleus accumbens. Coordinates for the ventral ends of the guide cannulae relative to bregma according to the Paxinos and Watson atlas [29] were as follows: +1.0 mm A/P; +/−1.0 mm M/L; −5.0 mm D/V. Cannulae were cemented in place by affixing dental acrylic cement to stainless steel screws secured in the skull. Stainless steel obturators (14 mm, 33 gauge) were inserted into the guide cannulae after surgery. Cannulae were implanted 2 mm above the nucleus accumbens shell. MPEP microinjections were delivered using stainless steel 33 gauge microinjectors. The tips of the microinjectors extended 2 mm below the guide cannulae in order to administer MPEP or vehicle into the nucleus accumbens shell.
Cocaine self-administration and extinction
Following a 7-day recovery period from surgery, rats were trained either for cocaine-priming induced reinstatement or for cue-induced reinstatement. Rats did not undergo any prior food restriction or training of any sort. For testing mGluR5 involvement in cocaine-priming induced reinstatement, rats were trained initially using a fixed ratio (FR) 1 schedule of reinforcement. Each session began with the i.v. administration of 59 μl cocaine (0.25 mg) to fill the catheter (little or none of this non-contingent injection reached the systemic circulation). Following one week of training under the FR1 schedule, rats were switched to an FR5 schedule. All rats received one week of training under an FR1 schedule of cocaine self-administration, at which point all subjects received a minimum of 20 cocaine infusions per session. Throughout the self-administration phase, rats were restricted to a maximum of 30 infusions per session with a 60 second timeout following each infusion. Each daily training session (FR1 and FR5) lasted for two hours. During this timeout active lever responses had no scheduled consequences. Responses made on the inactive lever, which had no scheduled consequences, were also recorded during all self-administration sessions. Rats were allowed to self-administer cocaine for a total of 21 days. This was sufficient for rats to attain stable levels of self-administration, defined as at least 20 cocaine infusions per session (corresponding to 20 active lever pressed under FR1 and 100 active lever presses under FR5).
After a total of 21 days of cocaine self-administration, rats whose responses fulfilled the criteria stated above were switched to an extinction phase during which cocaine was replaced with saline. In this study, all rats fulfilled these criteria for stable self-administration and were switched to the extinction phase followed by reinstatement testing phase. Daily 2-hour extinction sessions were conducted until responding was less than 10% of the responses maintained by cocaine self administration. This initial extinction criterion was typically met in 4–5 days.
To determine the effect of mGluR5 antagonists on cue-induced reinstatement, rats were initially allowed to self-administer cocaine under a fixed ratio schedule (FR1 for 1 week, FR5 for 3 weeks) followed by a second-order schedule of training (10 days) for a total of 38 days. For this experiment, cocaine infusions were always paired with a red cue light over the active lever was illuminated for a total duration of 10 seconds. At the completion of training using a fixed ratio schedule, rats were transferred to a second-order schedule of training [FI5(FR5)] for a period of 10 days. During this phase, they were required to respond on the active lever for a fixed interval of 5 minutes. Each five responses executed on the active lever resulted in illumination of the red cue light over the active lever for 10 sec. When the fixed interval of 5 minutes was completed, rats were required to execute 5 additional responses in order to obtain a cocaine infusion, which was paired with the light cue. Self-administration sessions were 2 hours in duration. In this experiment, seven rats were removed from the analysis because they either failed to achieve 20 cocaine infusions per session or perform 400 active lever responses under the second-order schedule of cocaine self-administration.
After a total of 38 days of cocaine self-administration the rats belonging to the cue-induced reinstatement experimental group were switched to the extinction phase. Response extinction was carried out in the absence of the cocaine-paired cue. Responding on either lever had no programmed consequences. Daily 2-hour extinction sessions were conducted until responding was less than 10% of the responses maintained by cocaine self administration. This initial extinction criterion was typically met in 4–5 days.
Reinstatement
Following extinction, animals entered the reinstatement phase of the experiment. For all reinstatement testing, satisfaction of the response requirements for each component resulted in saline rather than cocaine infusions. Each reinstatement test session for both cocaine-priming induced and cue-induced reinstatement was followed by additional extinction sessions until the extinction criterion was met (a minimum of two sessions following systemic administration of MPEP, MTEP or vehicle). During testing of cocaine priming-induced reinstatement (10 mg/kg cocaine, i.p.), the mGluR5 selective allosteric modulators MPEP (0.3, 1.0 or 3 mg/kg, i.p.), MTEP (0.1 or 1 mg/kg) or their vehicle (1% DMSO, 1 ml/kg, i.p.) was administered 30 minutes prior to a systemic priming injection of cocaine. The doses of MPEP and MTEP were derived from previous studies [11,12,15,16] as was the delay period between MPEP or MPEP administration and the onset of the test session [15]. Animals were placed in the operant chambers immediately following the cocaine injection. Microinjections of MPEP (1 μg/0.5 μl or 0.1 μg/0.5 μl) or its vehicle (0.5 μl of 1% DMSO) were administered directly into the shell of the nucleus accumbens in a separate group of rats. These rats were given a systemic cocaine injection 20 minutes following the intracranial microinjection and immediately placed in the operant chambers for behavioral testing. All drug and vehicle injections were counterbalanced across reinstatement sessions. The dose of MPEP for microinjection as well as the interval between intra-cranial MPEP administration and the onset of the test session were derived from previous studies (Backstrom and Hyytia, 2007; Lu et al. 2005).
Using this experimental design, the rats underwent a series of extinction and reinstatement sessions. Using this paradigm, extinction of the ability to induce reinstatement is a concern. However, we have previously shown that reinstatement of cocaine seeking persists for at least 20 days after the initial extinction of cocaine self-administration [30]. In all cases, the drug and vehicle treatments were counterbalanced across reinstatement days. All subjects demonstrated stable drug seeking throughout the reinstatement phase of these experiments.
Cue induced reinstatement was triggered by presentation of cue alone. During reinstatement sessions, MTEP (0.1 or 1 mg/kg) or its vehicle (1% DMSO) was administered systemically, 30 minutes prior to reinstatement. Reinstatement was triggered by a non-contingent presentation of the red cue light over the active lever for 40 seconds. Following presentation of the cue light, levers were extended and reinstatement was assessed using the same second-order schedule as during the self-administration phase except satisfaction of the response requirements resulted in saline rather than cocaine infusions (the cue light was illuminated after every 5 lever press). Reinstatement sessions lasted for 2 hours. Each reinstatement test session was followed by extinction sessions until the extinction criterion was satisfied, with a minimum of two extinction sessions between each reinstatement session. All drug and vehicle injections were counterbalanced across reinstatement sessions.
Microinjection Procedures
The obturators were removed from the microinjection guide cannulae and replaced by 33 gauge stainless steel injectors, which extended 2 mm below the ends of the guide cannulae into the structure of interest. Bilateral microinjections were made over a period of 120 seconds in a volume of 0.5 μl/side. The microinjectors were left in place for 2 minutes (to allow for diffusion of reagent away from injector tip) and then removed.
Reinstatement of sucrose seeking
Potential nonspecific rate-suppressing effects of injections of the tested compounds were evaluated by assessing the influence of MPEP and MTEP on reinstatement of sucrose pellet-reinforced responding. Rats were maintained on 3 pellets of rat chow (18 gm) (Harlan Teklad, Wilmington, DE) per day for the duration of all phases of the sucrose reinstatement experiments. Rats were trained initially to press a lever under an FR1 schedule for sucrose pellets (Research Diets Inc., New Brunswick, NJ) delivery in daily 1-hour sessions. Following a week of FR1 training, rats were switched to an FR5 schedule of reinforcement. During the self-administration phase, animals were restricted to a maximum of 30 sucrose pellets during each hour-long session.
After 2 weeks of sucrose-maintained responding on the FR5 schedule, rats underwent an extinction phase during which sucrose pellets were removed from the dispenser and responding on the active lever no longer resulted in sucrose delivery. Once lever pressing decreased to 10% or less of the responding maintained by contingent sucrose reinforcement, animals began reinstatement testing. Rats were tested with the lowest dose of the mGluR5 antagonists that were effective in attenuating cocaine-priming induced reinstatement. A group of rats were administered either MPEP (1 mg/kg, i.p.) or vehicle (1%DMSO,1ml/kg, i.p.). A separate group of rats were administered MTEP (0.1 mg/kg, i.p.) or vehicle (1 ml/kg of 1% DMSO, i.p.). Drug and vehicle administrations were made 30 minutes prior to the reinstatement of sucrose-pellet seeking session. For reinstatement testing, rats were placed in the operant chambers and the session began with the delivery of a non-contingent delivery of a sucrose pellet prime. The experimenter remotely administered one sucrose pellet every 2 minutes thereafter for the first 10 minutes of the reinstatement session. Each 1-hour reinstatement session was followed by extinction sessions until active lever responding again decreased to 10% or less of the response rate maintained by sucrose. Similar to the cocaine seeking experiments, at least two test reagent free extinction days elapsed prior to the next reinstatement test session.
Verification of Cannula Placements
Following the completion of all microinjection experiments, animals were given an overdose of pentobarbital (100 mg/kg, i.p.) and perfused intracardially with 0.9% saline followed by 10% formalin. The brain was then removed and 100 μm coronal sections were taken at the level of the nucleus accumbens using a Vibratome (Technical Products International, St. Louis, MO). The sections were mounted on gelatin-coated slides and stained with Cresyl violet. An individual unaware of the animal’s behavioral response determined cannula placements as well as potential mechanical damage. The microinjection area also was examined for potential signs of drug-induced toxicity, including overt neuronal loss and associated gliosis. Animals with cannula placements outside of the areas of interest, or with excessive mechanical damage, were excluded from subsequent data analysis.
Drugs
Cocaine hydrochloride was obtained from the National Institute on Drug Abuse drug supply program (Research Triangle Park, NC). Ketamine, xylazine, sodium pentobarbital and DMSO were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO). 250 mg/ml stock solutions of MPEP hydrochloride (Tocris; Ellisville, MO) or MTEP (Calbiochem; San Diego, CA) were dissolved in 100% DMSO and stored at −20° C and later diluted in sterile saline (0.9% w/v) to the required final concentrations, resulting in vehicle concentration of 1% DMSO.
RESULTS
Cocaine priming- and cue-induced reinstatement of drug seeking
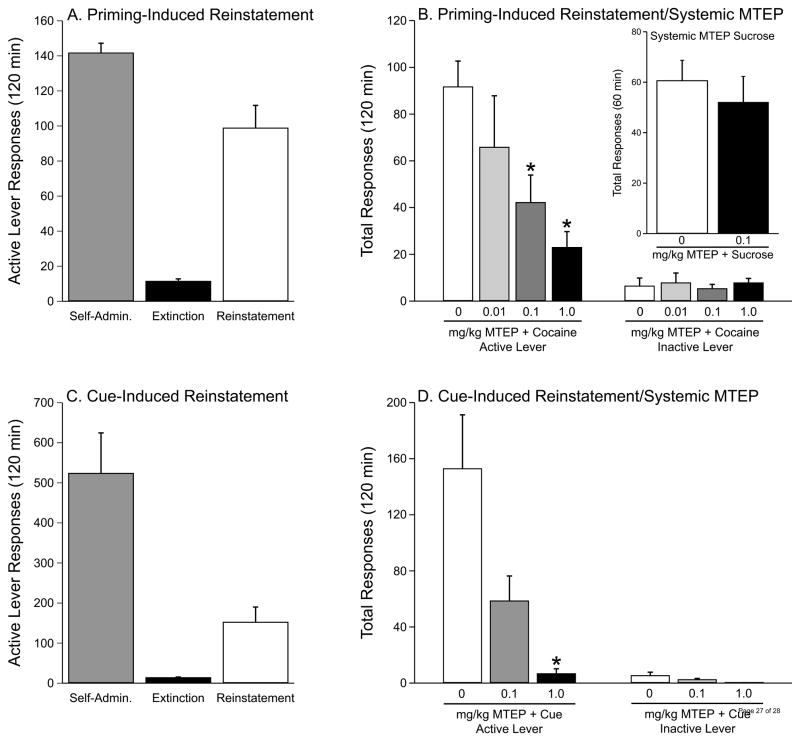
The data depicted in Figures 1A and 1C show the last day of self-administration, extinction and reinstatement of drug seeking induced by either cocaine itself (panel A) or cocaine-associated cues (panel C). Following a total of 21 days of cocaine self-administration under an FR (cocaine-priming induced reinstatement group) (panel A) or 28 FR + 10 days under a second order (cue-induced reinstatement group) (panel C) schedule of reinforcement, all subjects entered the extinction phase during which self-administration behavior was extinguished by replacing the cocaine with 0.9% saline. For rats self-administering under a second order schedule, response extinction was carried out in the absence of light cues and responses on either lever had no programmed consequences. The extinction phase continued until responding on the active lever was <15% of the response rate maintained by cocaine self-administration. The extinction criterion was reached in (mean±SEM) 5.46±0.0.456 days (panel A) or 4.27±0.48 days (panel C). Following extinction, reinstatement of cocaine seeking was precipitated by the administration of 10 mg/kg cocaine, i.p. (panel A) or cocaine-associated cue light (panel C).
Figure 1.
The mGluR5 selective antagonist MTEP attenuates reinstatement of cocaine seeking. A) Mean number of active lever responses on last day of cocaine self-administration, extinction and during the reinstatement of drug seeking by a cocaine priming injection (10 mg/kg, i.p.). B) Responses on active and inactive levers following the administration of vehicle (1% DMSO) or varying concentrations of MTEP. MTEP dose-dependently attenuated cocaine priming-induced reinstatement of cocaine seeking. The asterisks represent significant differences from vehicle (p< 0.05, Tukey’s HSD). Inset: Systemic MTEP had no influence on the reinstatement of sucrose seeking. C) Average of active lever responses on last day of cocaine self-administration using a second-order schedule of reinforcement [FI5(FR5)]. Mean of active lever responses on the last day of extinction and during cue-induced reinstatement of cocaine seeking also are depicted. D) MTEP dose-dependently attenuated cue-induced reinstatement of cocaine seeking. The asterisk represents a significant difference from vehicle pretreatment (p<0.05, Tukey’s HSD). All data are expressed as mean (± SEM) active or inactive lever pressing per session.
Systemic administration of the mGluR5 antagonist, MTEP, dose-dependently attenuates cocaine priming-induced and cocaine cue-induced reinstatement of drug seeking
The data depicted in Figure 1B show the effect of MTEP (0, 0.01, 0.1 or 1.0 mg/kg, i.p.) on the reinstatement of cocaine seeking induced by a systemic priming injection of 10 mg/kg cocaine (i.p.). The total active lever presses were analyzed with a one-way analysis of variance (ANOVA), which revealed a significant main effect of treatment [F(3,33)=6.753, p<0.0013]. Subsequent pairwise analyses (Tukey’s HSD, p<0.05) showed that the total active responses were significantly different between the MTEP (0.1 and 1.0 mg/kg) and the vehicle (1% DMSO) treatments. In contrast, a one-way ANOVA indicated no significant treatment effect in terms of inactive lever responses. There were 5–13 animals per treatment (1% DMSO vehicle n=13, MTEP 1mg/kg n=8; MTEP 0.1mg/kg n=8 and MTEP 0.01mg/kg n=5). In order to assess the reinforcer specificity of this MTEP effect, we also assessed the effect of systemic MTEP (0.1 mg/kg) on the reinstatement of sucrose seeking (see Figure 1B inset). These data were analyzed with a t-test, which indicated a lack of a significant treatment effect. There were 5–6 animals per treatment.
Figure 1D shows the effect of MTEP (0, 0.1 or 1.0 mg/kg, i.p.) on cocaine cue-induced reinstatement of drug seeking. A one-way ANOVA showed a significant main effect of treatment [F(2,22)=4.331, p<0.0274]. Subsequent pairwise analyses (Tukey’s HSD, p<0.05) showed that the total active responses were significantly different between the 1.0 mg/kg MTEP and the vehicle (1% DMSO) treatments. Analysis of the inactive lever responses indicated no significant treatment effect. There were 4–11 animals per treatment (0.1 mg/kg MTEP n=7, 1.0 mg/kg MTEP n=4, 1% DMSO vehicle n=11).
Systemic or intra-accumbal shell administration of MPEP dose-dependently attenuates cocaine priming-induced reinstatement of drug seeking
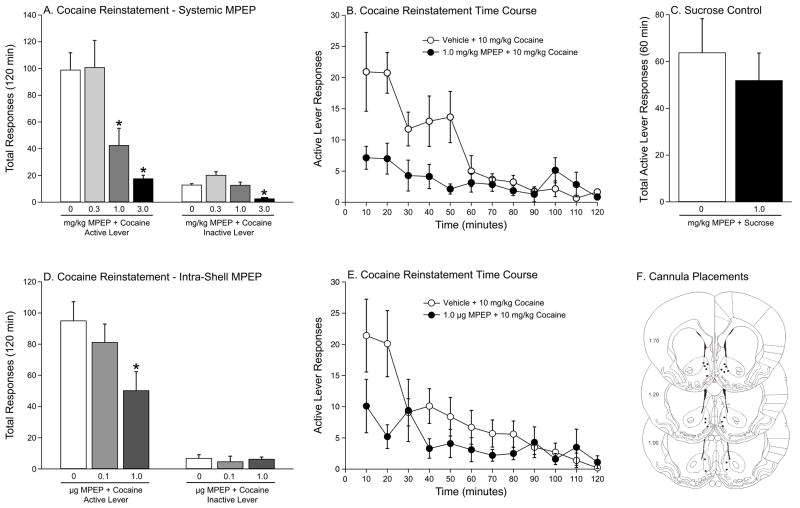
We next wanted to assess the effect of administration of an mGluR5 antagonist into the nucleus accumbens shell on the reinstatement of cocaine seeking. Previous work identified the effective dose range for intra-accumbal administration of the mGluR5 antagonist, MPEP [31]. To our knowledge, MTEP has not been administered intra-cranially previously. Before administering MPEP into the nucleus accumbens shell, we wanted to confirm that it produced similar effects as MTEP when administered systemically. Therefore, animals were administered vehicle (n=12), 0.3 mg (n=6), 1.0 mg (n=7) or 3.0 mg/kg (n=8) MPEP (i.p.) prior to a priming injection of cocaine (10 mg/kg, i.p.) during the reinstatement phase. The total active and inactive lever presses during reinstatement following the systemic administration of vehicle or MPEP prior to a systemic priming injection of cocaine are shown in Figure 2A. The total active lever presses were analyzed with a one-way ANOVA, which revealed a significant main effect of treatment [F(3,32)=9.783, p<0.0001]. Subsequent pairwise analyses (Tukey’s HSD, p<0.05) showed that the total active responses were significantly different between the MPEP (1.0 and 3.0 mg/kg) and vehicle treatments. The time courses of the active lever responses in the vehicle plus cocaine and 1.0 mg/kg MPEP plus cocaine treatments are summarized in Figure 2B.
Figure 2.
Systemic and intra-accumbens shell administration of MPEP, a non-competitive negative modulator of mGluR5 receptors, attenuates cocaine priming-induced reinstatement of cocaine seeking. A) Systemic MPEP dose-dependently blocks priming-induced reinstatement of cocaine seeking. MPEP also attenuated inactive lever responses at the highest dose tested (3 mg/kg). However, the lowest effective dose of MPEP (1.0 mg/kg) had no effect on inactive lever responses. B) Time course of active lever responses in the vehicle (1% DMSO) + cocaine and 1.0 mg/kg MPEP + cocaine treatments. C) The lowest dose of MPEP (1.0 mg/kg) effective in blocking reinstatement of cocaine seeking did not affect reinstatement of sucrose seeking. D) Intra-accumbens shell microinjection of MPEP dose-dependently attenuated cocaine priming-induced reinstatement of drug seeking. E) Time course of active lever responses in the vehicle (1% DMSO) + cocaine and 1.0 μg MPEP + cocaine treatments. F) Coronal sections of the brain at the level of the nucleus accumbens depicting locations cannulae within the shell of the accumbens. All data are expressed as mean (± SEM). Asterisks represent significant differences from vehicle pretreatment (p<0.05, Tukey’s HSD).
Several measures were used to evaluate potential nonspecific rate suppressing effects of MPEP. The modular testing chambers were equipped with an inactive lever, responses on which often are used as a measure of nonspecific alterations in lever pressing during the reinstatement phase. These data were analyzed with a one-way ANOVA, which showed a significant treatment effect in terms of inactive lever presses [F(3,32)=5.289, p<0.0049]; a Tukey’s test (p<0.05) indicated a significant difference between the vehicle and 3.0 mg/kg MPEP treatments (see Figure 2A). These results suggest that the highest dose of MPEP (3.0 mg/kg) may produce non-specific behavioral disruptions. In contrast, the lower dose of MPEP (1.0 mg/kg) significantly reduced active, but not inactive, lever responding (see Figure 2A). Even though 1.0 mg/kg MPEP did not have a significant influence on responding on the inactive lever, the low number of inactive lever presses limits the utility of this measure to meaningfully assess potential rate suppressant drug effects. Therefore, we also assessed the effect of 1.0 mg/kg MPEP on the reinstatement of sucrose seeking, where non-contingent administration of a sucrose pellet prime reinstates responding previously maintained by sucrose reinforcement. A separate cohort of rats was administered i.p. injections of saline and 1.0 mg/kg (n=8) MPEP prior to a one hour test session where reinstatement of sucrose seeking was initiated by non-contingent administration of sucrose pellets. The active lever responses obtained during the reinstatement phase are depicted in Figure 2C. These data were analyzed with a paired t-test, which revealed no significant treatment effect. These sucrose control results indicate that systemic MPEP, which impairs the reinstatement of cocaine seeking but not sucrose seeking at a dose of 1.0 mg/kg, does not produce nonspecific rate-suppressing effects.
Next, we assessed the effect of administration of MPEP directly into the nucleus accumbens shell on cocaine priming-induced reinstatement of drug seeking. Rats were administered vehicle (n=12), 0.1 μg (n=6) or 1.0 μg (n=10) into the accumbens shell prior to a priming injection of cocaine (10 mg/kg, i.p.) during the reinstatement phase. The total active and inactive lever presses during reinstatement following the administration of vehicle or MPEP into the shell prior to a systemic priming injection of cocaine are shown in Figure 2D. The total active lever presses were analyzed with a one-way ANOVA, which revealed a significant main effect of treatment [F(2,27)=3.671, p<0.04]. Subsequent pairwise analyses (Tukey’s HSD, p<0.05) showed that the total active responses were significantly different between the 1.0 μg MPEP and vehicle treatments. Analysis of inactive lever pressing indicated no significant treatment effect. The time courses of the active lever responses in the vehicle plus cocaine and 1.0 μg MPEP plus cocaine treatments are summarized in Figure 2E. Cannula placements in the nucleus accumbens shell are shown in Figure 2F.
DISCUSSION
The present results indicate that systemic administration of mGluR5 antagonists attenuated cocaine priming-induced and cocaine cue-induced reinstatement of drug seeking. In addition, microinjection of MPEP directly into the nucleus accumbens shell significantly decreased drug seeking induced by a cocaine priming injection. These effects were reinforcer specific since neither of the mGluR5 antagonists (MPEP or MTEP) influenced the reinstatement of sucrose seeking. These data suggest that, at certain doses for mGluR5 receptors, MPEP and MTEP specifically disrupt cocaine seeking in the absence of non-specific motor disruption.
Consistent with the current findings, previous work showed that systemic MPEP attenuated cocaine priming-induced reinstatement in squirrel monkeys [17] as well as cocaine cue-induced reinstatement of drug seeking in rats [21]. However, these effects cannot be definitively linked to mGluR5 antagonism since MPEP also is a positive allosteric modulator of mGluR4s, although the doses of MPEP used in the current study have been shown to be selective for mGluR5 relative to mGluR4 [32]. MPEP also is a weak NMDA receptor antagonist [33,34]. It is unlikely that the effects of MPEP in the current and previous studies were due to effects at NMDA receptors since the NMDA receptor open channel blocker, dizocilpine (MK801), had no effect on cocaine reinstatement in squirrel monkeys [17] and actually enhanced the reinstatement of cocaine seeking when administered into the rat nucleus accumbens [35,36]. Moreover, the present results showed that the highly selective mGluR5 antagonist MTEP [22,34] attenuated the reinstatement of drug seeking induced by either cocaine cues or a cocaine priming injection. Together, these findings suggest that MPEP and MTEP influence the reinstatement of cocaine seeking via selective antagonism of mGluR5 receptors.
As noted above, the effective doses of MPEP and MTEP used in the present study attenuated the reinstatement of cocaine seeking without producing general rate suppressing effects. Thus, 1.0 mg/kg MPEP and MTEP decreased active lever responding during the reinstatement phase without influencing inactive lever pressing. In contrast, 3.0 mg/kg MPEP reduced both active and inactive lever responding during reinstatement, indicating a generalized disruption of behavior. Moreover, the effects of MPEP and MTEP also are reinforcer specific, in that 1.0 mg/kg MPEP or MTEP had no influence on the reinstatement of sucrose seeking (present results) and 1 μg MPEP administered directly into the nucleus accumbens had no effect on cue-induced reinstatement of cocaine seeking [31].
There are several mechanisms whereby mGluR5 receptors, which are expressed predominantly postsynaptically in the striatal complex [37], may modulate cocaine-mediated behaviors. mGluR5s augment NMDA receptor function and are in turn positively modulated by NMDA receptors [6,38]. However, as noted above, the role of NMDA receptors in cocaine seeking remains unclear [17,35,39].
Accumulating evidence suggests that the Homer family of postsynaptic scaffolding proteins, which influence mGluR trafficking and signal transduction, play a critical role in cocaine-induced neuronal and behavioral plasticity [40]. For example, mice with genetic deletions of Homer1 or Homer2 expressed a neurochemical and behavioral phenotype similar to control mice that received a sensitizing regimen of repeated cocaine injections [41]. Similarly, antisense oligonucleotide-induced reduction of Homer1 expression in the nucleus accumbens resulted in a sensitization-like increase in the behavioral activating effects of cocaine [42]. Interestingly, acute administration of cocaine transiently increased Homer1a immediate early gene transcription in the nucleus accumbens, an effect that was not observed after repeated cocaine injections [43]. In contrast to the immediate early gene Homer isoform, withdrawal from a sensitizing regimen of cocaine injections decreased Homer1b/c and Homer2a/b protein expression in the nucleus accumbens [44–46], which is in agreement with behavioral and neurochemical data indicating that persistent decreases in constitutive Homer isoforms are necessary for the expression of neuroplasticity induced by repeated cocaine injections [47]. Extinction of cocaine self-administration also resulted in decreased synaptosomal Homer1b/c and mGluR5 in the nucleus accumbens shell [48]. Taken together, these results suggest that Homer-mediated changes in mGluR5 trafficking and function may underlie cocaine-mediated behaviors including the extinction and reinstatement of cocaine seeking.
Activation of group I mGluRs also increase the endocytosis of AMPA glutamate receptors [49,50] via interactions with Homer proteins [51]. Recent evidence indicates that a cocaine challenge injection following an extended period of forced abstinence after repeated cocaine injections prompted the internalization of AMPA receptor subunits [52] and depressed AMPA-mediated synaptic transmission [53]. Consonant with these results, impairing the endocytosis of GluR2-containing AMPA receptors in the nucleus accumbens promoted the reinstatement of cocaine seeking [54]. Moreover, impairment of accumbal synaptic depression induced by cocaine self-administration was reversed by activation of mGluR5 [55]. Collectively, these data suggest that interactions between mGluR5 and Homer in the nucleus accumbens may contribute to changes in AMPA receptor trafficking associated with cocaine-induced neuronal and behavioral plasticity.
Homer proteins enable endocannabinoid synthesis and release following activation of group I mGluRs [56,57]. Stimulation of postsynaptic mGluR5s in the nucleus accumbens also results in endocannabinoid-mediated inhibition of glutamate release [58]. Given evidence that a cannabinoid receptor (CB1) antagonist attenuated both priming- and cue-induced reinstatement of cocaine seeking [59], it is possible that mGluR5 receptors mediate cocaine-mediated behaviors by influencing endocannabinoid and glutamate transmission in the nucleus accumbens.
In conclusion, the present results indicate that blocking mGluR5 receptor transmission in the nucleus accumbens shell attenuates the reinstatement of cocaine seeking. Although the exact mechanism(s) whereby mGluR5 antagonists modulate cocaine seeking requires further investigation, the present results underscore the potential use of mGluR5 antagonists as therapeutic targets in the treatment of cue- or cocaine-induced relapse of cocaine seeking.
Acknowledgments
This research was supported by grants from the National Institutes of Health (NIH) to RCP (R01 DA15214 and K02 DA18678). KRF was partially supported by a National Research Service Award (NRSA) from the NIH (F30 DA19304), as well as an NIH training grant (T32 GM008541-7). HDS was also partially supported by an NRSA from the NIH (DA16824). The authors thank Natasha D’Agostini for assistance with some of the sucrose reinstatement experiments and Audrey Pierce for administrative assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–9. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 3.Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47 Suppl 1:61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–42. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellone C, Luscher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell Mol Life Sci. 2008;65:2913–23. doi: 10.1007/s00018-008-8263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grueter BA, McElligott ZA, Winder DG. Group I mGluRs and long-term depression: potential roles in addiction? Mol Neurobiol. 2007;36:232–44. doi: 10.1007/s12035-007-0037-7. [DOI] [PubMed] [Google Scholar]
- 7.Kim CH, Lee J, Lee JY, Roche KW. Metabotropic glutamate receptors: phosphorylation and receptor signaling. J Neurosci Res. 2008;86:1–10. doi: 10.1002/jnr.21437. [DOI] [PubMed] [Google Scholar]
- 8.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–37. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 9.Testa CM, Standaert DG, Landwehrmeyer GB, Penney JB, Jr, Young AB. Differential expression of mGluR5 metabotropic glutamate receptor mRNA by rat striatal neurons. J Comp Neurol. 1995;354:241–52. doi: 10.1002/cne.903540207. [DOI] [PubMed] [Google Scholar]
- 10.Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–4. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 11.Dravolina OA, Danysz W, Bespalov AY. Effects of group I metabotropic glutamate receptor antagonists on the behavioral sensitization to motor effects of cocaine in rats. Psychopharmacology (Berl) 2006;187:397–404. doi: 10.1007/s00213-006-0440-1. [DOI] [PubMed] [Google Scholar]
- 12.McGeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47:240–2. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- 13.Herzig V, Capuani EM, Kovar KA, Schmidt WJ. Effects of MPEP on expression of food-, MDMA- or amphetamine-conditioned place preference in rats. Addict Biol. 2005;10:243–9. doi: 10.1080/13556210500223272. [DOI] [PubMed] [Google Scholar]
- 14.Platt DM, Rowlett JK, Spealman RD. Attenuation of cocaine self-administration in squirrel monkeys following repeated administration of the mGluR5 antagonist MPEP: comparison with dizocilpine. Psychopharmacology (Berl) 2008;200:167–76. doi: 10.1007/s00213-008-1191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology (Berl) 2005;179:255–61. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- 16.Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology (Berl) 2005;179:247–54. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- 17.Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. Attenuation of Behavioral Effects of Cocaine by the Metabotropic Glutamate Receptor 5 Antagonist 2-Methyl-6-(phenylethynyl)-pyridine in Squirrel Monkeys: Comparison with Dizocilpine. J Pharmacol Exp Ther. 2005;312:1232–40. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- 18.Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–33. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 19.Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, Koob GF, Skoubis PD, Markou A. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann N Y Acad Sci. 2003;1003:415–8. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- 20.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 21.Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–86. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- 22.Cosford ND, Roppe J, Tehrani L, Schweiger EJ, Seiders TJ, Chaudary A, Rao S, Varney MA. [3H]-methoxymethyl-MTEP and [3H]-methoxy-PEPy: potent and selective radioligands for the metabotropic glutamate subtype 5 (mGlu5) receptor. Bioorg Med Chem Lett. 2003;13:351–4. doi: 10.1016/s0960-894x(02)00997-6. [DOI] [PubMed] [Google Scholar]
- 23.O’Leary DM, Movsesyan V, Vicini S, Faden AI. Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism. Br J Pharmacol. 2000;131:1429–37. doi: 10.1038/sj.bjp.0703715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidbreder CA, Bianchi M, Lacroix LP, Faedo S, Perdona E, Remelli R, Cavanni P, Crespi F. Evidence that the metabotropic glutamate receptor 5 antagonist MPEP may act as an inhibitor of the norepinephrine transporter in vitro and in vivo. Synapse. 2003;50:269–76. doi: 10.1002/syn.10261. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 Dopamine Receptors in the Shell, but Not the Core, of the Nucleus Accumbens Reinstates Cocaine-Seeking Behavior in the Rat. European Journal of Neuroscience. 2006;23:219–28. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- 26.Anderson SM, Schmidt HD, Pierce RC. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology. 2006:1452–61. doi: 10.1038/sj.npp.1300922. [DOI] [PubMed] [Google Scholar]
- 27.Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intranucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1997. [Google Scholar]
- 30.Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:132–8. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- 31.Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–80. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- 32.Mathiesen JM, Svendsen N, Brauner-Osborne H, Thomsen C, Ramirez MT. Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and MPEP. Br J Pharmacol. 2003;138:1026–30. doi: 10.1038/sj.bjp.0705159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasparini F, Bilbe G, Gomez-Mancilla B, Spooren W. mGluR5 antagonists: discovery, characterization and drug development. Curr Opin Drug Discov Devel. 2008;11:655–65. [PubMed] [Google Scholar]
- 34.Lea PMt, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 2006;12:149–66. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Famous KR, Schmidt HD, Pierce RC. When administered into the nucleus accumbens core or shell, the NMDA receptor antagonist AP-5 reinstates cocaine-seeking behavior in the rat. Neurosci Lett. 2007;420:169–73. doi: 10.1016/j.neulet.2007.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–25. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paquet M, Smith Y. Group I metabotropic glutamate receptors in the monkey striatum: subsynaptic association with glutamatergic and dopaminergic afferents. J Neurosci. 2003;23:7659–69. doi: 10.1523/JNEUROSCI.23-20-07659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106:579–87. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- 39.Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–67. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- 40.Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol. 2008;75:112–33. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, Rohrer J, Griffin W, 3rd, Toda S, Champtiaux NP, Berry T, Tu JC, Shealy SE, During MJ, Middaugh LD, Worley PF, Kalivas PW. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–13. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 42.Ghasemzadeh MB, Permenter LK, Lake R, Worley PF, Kalivas PW. Homer1 proteins and AMPA receptors modulate cocaine-induced behavioural plasticity. Eur J Neurosci. 2003;18:1645–51. doi: 10.1046/j.1460-9568.2003.02880.x. [DOI] [PubMed] [Google Scholar]
- 43.Ghasemzadeh MB, Windham LK, Lake RW, Acker CJ, Kalivas PW. Cocaine activates Homer1 immediate early gene transcription in the mesocorticolimbic circuit: differential regulation by dopamine and glutamate signaling. Synapse. 2009;63:42–53. doi: 10.1002/syn.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–52. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ary AW, Szumlinski KK. Regional differences in the effects of withdrawal from repeated cocaine upon Homer and glutamate receptor expression: a two-species comparison. Brain Res. 2007;1184:295–305. doi: 10.1016/j.brainres.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 46.Ghasemzadeh MB, Mueller C, Vasudevan P. Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 47.Szumlinski KK, Abernathy KE, Oleson EB, Klugmann M, Lominac KD, He DY, Ron D, During M, Kalivas PW. Homer isoforms differentially regulate cocaine-induced neuroplasticity. Neuropsychopharmacology. 2006;31:768–77. doi: 10.1038/sj.npp.1300890. [DOI] [PubMed] [Google Scholar]
- 48.Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Neuroadaptations in the cellular and postsynaptic group 1 metabotropic glutamate receptor mGluR5 and Homer proteins following extinction of cocaine self-administration. Neurosci Lett. 2009;452:167–71. doi: 10.1016/j.neulet.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–85. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 50.Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Keuren-Jensen K, Cline HT. Visual experience regulates metabotropic glutamate receptor-mediated plasticity of AMPA receptor synaptic transmission by homer1a induction. J Neurosci. 2006;26:7575–80. doi: 10.1523/JNEUROSCI.5083-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–35. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–8. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha JH, Pierce RC. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28:11061–70. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–9. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, Mackie K, Piomelli D. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- 57.Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. A key role for diacylglycerol lipase-alpha in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol Pharmacol. 2007;72:612–21. doi: 10.1124/mol.107.037796. [DOI] [PubMed] [Google Scholar]
- 58.Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–4. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]