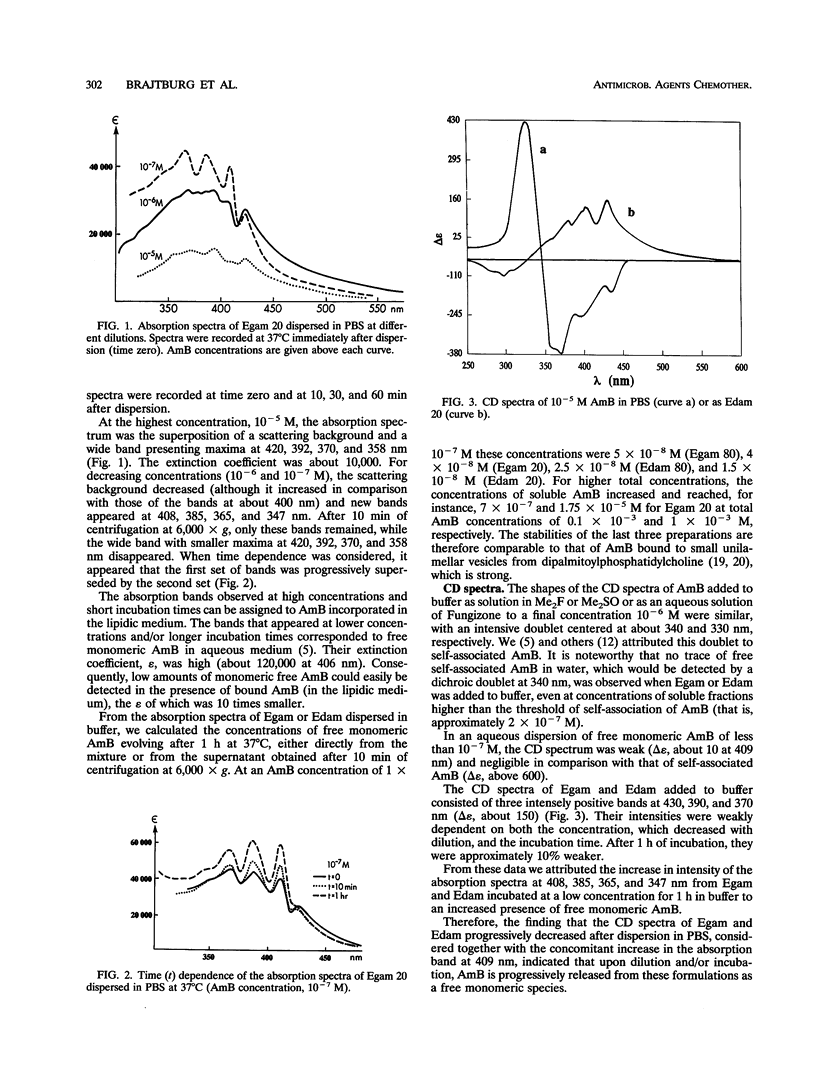
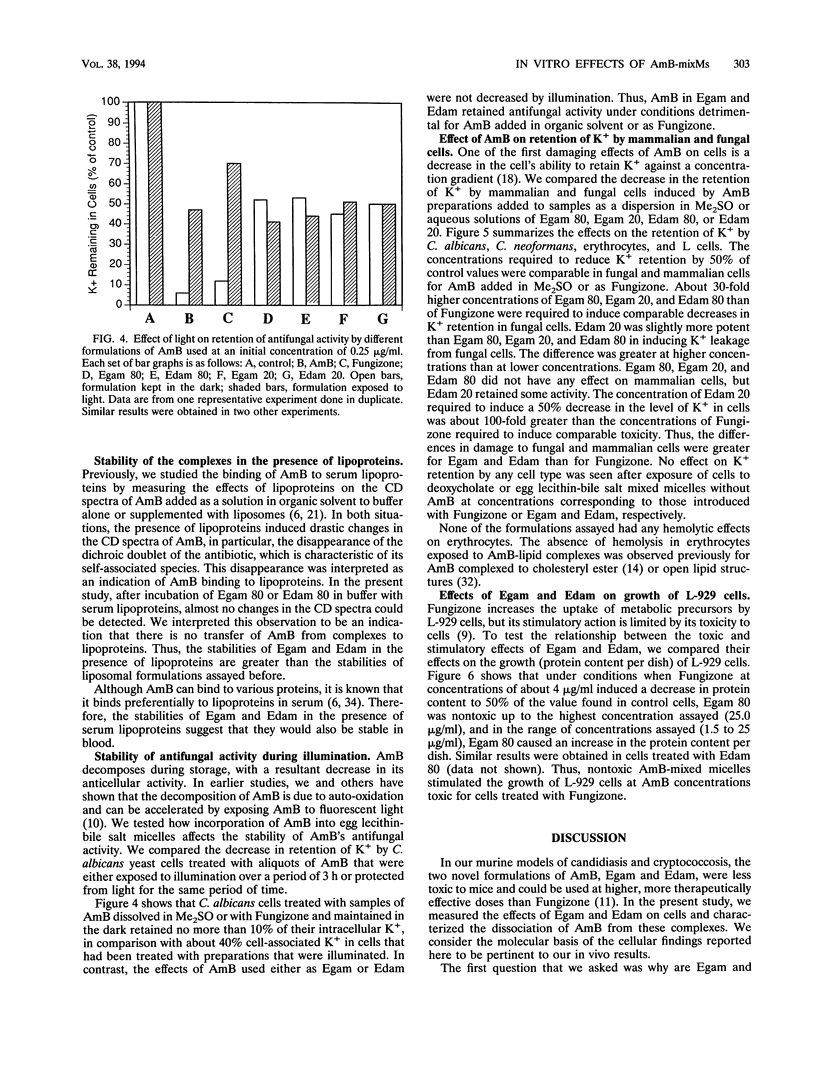
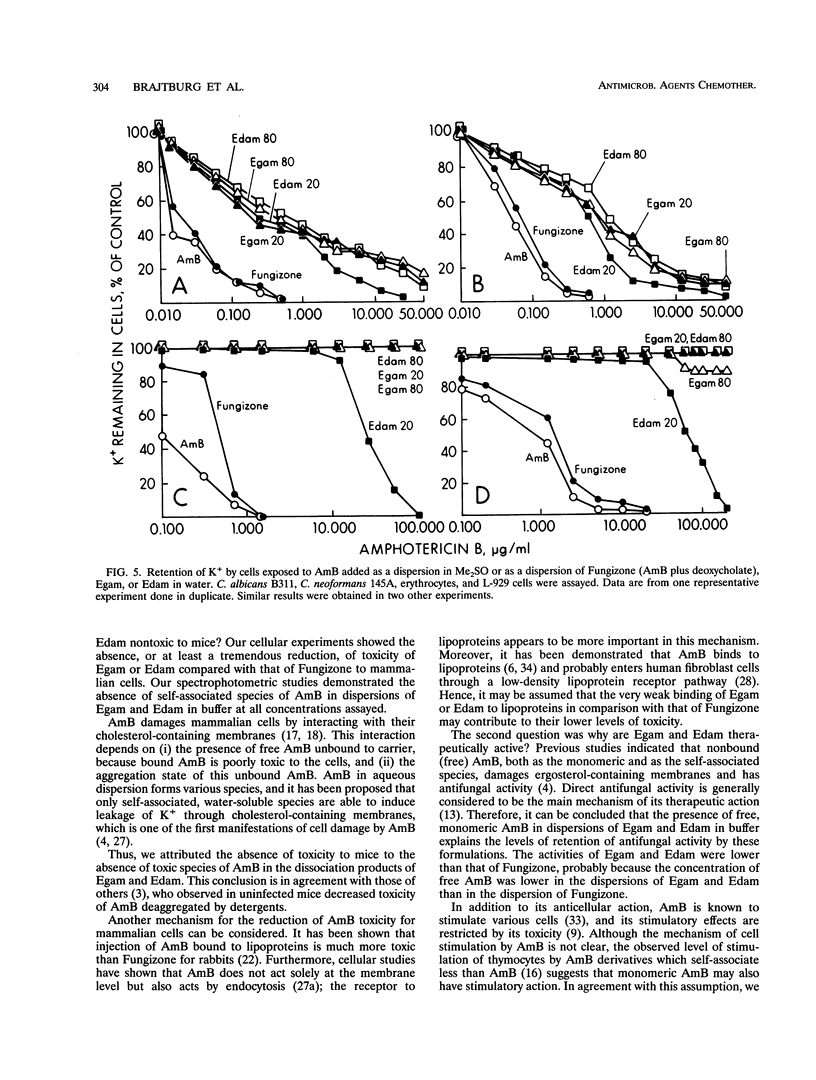
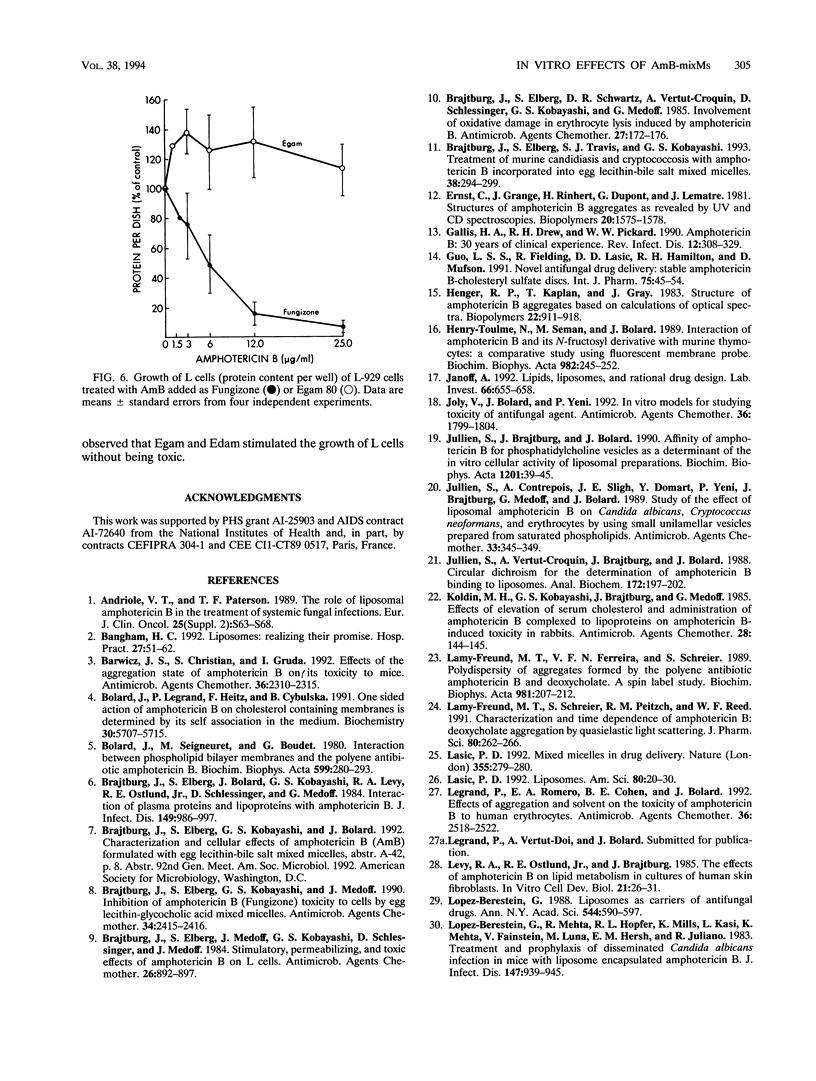
Abstract
The cellular activities of amphotericin B (AmB) used as Fungizone were compared with those of AmB complexed to either egg lecithin and glycocholate (Egam) or egg lecithin and deoxycholate (Edam). Under conditions in which leakage of K+ from erythrocytes and cultured L cells treated with Fungizone was almost complete, Egam and Edam containing concentrations of AmB severalfold greater than the concentration of AmB in Fungizone had no effect but retained the ability to decrease the level of retention of K+ in fungal cells. Analysis by absorption and circular dichroism spectroscopy demonstrated that when these formulations containing AmB at concentrations of less than 10(-5) M were added to buffer, the AmB dissociated slowly as monomers from Egam or Edam and dissociated rapidly as a mixture of monomers and self-associated species from Fungizone. We propose that in Egam and Edam, the absence of free AmB in the self-associated form reduces the toxicity of AmB to mammalian cells, whereas the presence of monomeric AmB results in the retention of the antifungal activities of these complexes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bangham A. D. Liposomes: realizing their promise. Hosp Pract (Off Ed) 1992 Dec 15;27(12):51-6, 61-2. doi: 10.1080/21548331.1992.11705537. [DOI] [PubMed] [Google Scholar]
- Barwicz J., Christian S., Gruda I. Effects of the aggregation state of amphotericin B on its toxicity to mice. Antimicrob Agents Chemother. 1992 Oct;36(10):2310–2315. doi: 10.1128/aac.36.10.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolard J., Legrand P., Heitz F., Cybulska B. One-sided action of amphotericin B on cholesterol-containing membranes is determined by its self-association in the medium. Biochemistry. 1991 Jun 11;30(23):5707–5715. doi: 10.1021/bi00237a011. [DOI] [PubMed] [Google Scholar]
- Bolard J., Seigneuret M., Boudet G. Interaction between phospholipid bilayer membranes and the polyene antibiotic amphotericin B: lipid state and cholesterol content dependence. Biochim Biophys Acta. 1980 Jun 20;599(1):280–293. doi: 10.1016/0005-2736(80)90074-7. [DOI] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Bolard J., Kobayashi G. S., Levy R. A., Ostlund R. E., Jr, Schlessinger D., Medoff G. Interaction of plasma proteins and lipoproteins with amphotericin B. J Infect Dis. 1984 Jun;149(6):986–997. doi: 10.1093/infdis/149.6.986. [DOI] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Kobayashi G. S., Medoff G. Inhibition of amphotericin B (Fungizone) toxicity to cells by egg lecithin-glycocholic acid mixed micelles. Antimicrob Agents Chemother. 1990 Dec;34(12):2415–2416. doi: 10.1128/aac.34.12.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Medoff J., Kobayashi G. S., Schlessinger D., Medoff G. Stimulatory, permeabilizing, and toxic effects of amphotericin B on L cells. Antimicrob Agents Chemother. 1984 Dec;26(6):892–897. doi: 10.1128/aac.26.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Schwartz D. R., Vertut-Croquin A., Schlessinger D., Kobayashi G. S., Medoff G. Involvement of oxidative damage in erythrocyte lysis induced by amphotericin B. Antimicrob Agents Chemother. 1985 Feb;27(2):172–176. doi: 10.1128/aac.27.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Travis S. J., Kobayashi G. S. Treatment of murine candidiasis and cryptococcosis with amphotericin B incorporated into egg lecithin-bile salt mixed micelles. Antimicrob Agents Chemother. 1994 Feb;38(2):294–299. doi: 10.1128/aac.38.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallis H. A., Drew R. H., Pickard W. W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990 Mar-Apr;12(2):308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- Henry-Toulmé N., Seman M., Bolard J. Interaction of amphotericin B and its N-fructosyl derivative with murine thymocytes: a comparative study using fluorescent membrane probes. Biochim Biophys Acta. 1989 Jul 10;982(2):245–252. doi: 10.1016/0005-2736(89)90061-8. [DOI] [PubMed] [Google Scholar]
- Janoff A. S. Lipids, liposomes, and rational drug design. Lab Invest. 1992 Jun;66(6):655–658. [PubMed] [Google Scholar]
- Joly V., Bolard J., Yeni P. In vitro models for studying toxicity of antifungal agents. Antimicrob Agents Chemother. 1992 Sep;36(9):1799–1804. doi: 10.1128/aac.36.9.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien S., Brajtburg J., Bolard J. Affinity of amphotericin B for phosphatidylcholine vesicles as a determinant of the in vitro cellular toxicity of liposomal preparations. Biochim Biophys Acta. 1990 Jan 15;1021(1):39–45. doi: 10.1016/0005-2736(90)90381-w. [DOI] [PubMed] [Google Scholar]
- Jullien S., Contrepois A., Sligh J. E., Domart Y., Yeni P., Brajtburg J., Medoff G., Bolard J. Study of the effects of liposomal amphotericin B on Candida albicans, Cryptococcus neoformans, and erythrocytes by using small unilamellar vesicles prepared from saturated phospholipids. Antimicrob Agents Chemother. 1989 Mar;33(3):345–349. doi: 10.1128/aac.33.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien S., Vertut-Croquin A., Brajtburg J., Bolard J. Circular dichroism for the determination of amphotericin B binding to liposomes. Anal Biochem. 1988 Jul;172(1):197–202. doi: 10.1016/0003-2697(88)90432-0. [DOI] [PubMed] [Google Scholar]
- Koldin M. H., Kobayashi G. S., Brajtburg J., Medoff G. Effects of elevation of serum cholesterol and administration of amphotericin B complexed to lipoproteins on amphotericin B-induced toxicity in rabbits. Antimicrob Agents Chemother. 1985 Jul;28(1):144–145. doi: 10.1128/aac.28.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy-Freund M. T., Ferreira V. F., Schreier S. Polydispersity of aggregates formed by the polyene antibiotic amphotericin B and deoxycholate. A spin label study. Biochim Biophys Acta. 1989 Jun 6;981(2):207–212. doi: 10.1016/0005-2736(89)90030-8. [DOI] [PubMed] [Google Scholar]
- Lamy-Freund M. T., Schreier S., Peitzsch R. M., Reed W. F. Characterization and time dependence of amphotericin B: deoxycholate aggregation by quasielastic light scattering. J Pharm Sci. 1991 Mar;80(3):262–266. doi: 10.1002/jps.2600800314. [DOI] [PubMed] [Google Scholar]
- Lasic D. D. Mixed micelles in drug delivery. Nature. 1992 Jan 16;355(6357):279–280. doi: 10.1038/355279a0. [DOI] [PubMed] [Google Scholar]
- Legrand P., Romero E. A., Cohen B. E., Bolard J. Effects of aggregation and solvent on the toxicity of amphotericin B to human erythrocytes. Antimicrob Agents Chemother. 1992 Nov;36(11):2518–2522. doi: 10.1128/aac.36.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. A., Ostlund R. E., Jr, Brajtburg J. The effects of amphotericin B on lipid metabolism in cultured human skin fibroblasts. In Vitro Cell Dev Biol. 1985 Jan;21(1):26–31. doi: 10.1007/BF02620910. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G. Liposomes as carriers of antifungal drugs. Ann N Y Acad Sci. 1988;544:590–597. doi: 10.1111/j.1749-6632.1988.tb40459.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G., Mehta R., Hopfer R. L., Mills K., Kasi L., Mehta K., Fainstein V., Luna M., Hersh E. M., Juliano R. Treatment and prophylaxis of disseminated infection due to Candida albicans in mice with liposome-encapsulated amphotericin B. J Infect Dis. 1983 May;147(5):939–945. doi: 10.1093/infdis/147.5.939. [DOI] [PubMed] [Google Scholar]
- Lyman C. A., Walsh T. J. Systemically administered antifungal agents. A review of their clinical pharmacology and therapeutic applications. Drugs. 1992 Jul;44(1):9–35. doi: 10.2165/00003495-199244010-00002. [DOI] [PubMed] [Google Scholar]
- Patterson T. F., Andriole V. T. The role of liposomal amphotericin B in the treatment of systemic fungal infections. Eur J Cancer Clin Oncol. 1989;25 (Suppl 2):S63–S68. [PubMed] [Google Scholar]
- Perkins W. R., Minchey S. R., Boni L. T., Swenson C. E., Popescu M. C., Pasternack R. F., Janoff A. S. Amphotericin B-phospholipid interactions responsible for reduced mammalian cell toxicity. Biochim Biophys Acta. 1992 Jun 30;1107(2):271–282. doi: 10.1016/0005-2736(92)90414-h. [DOI] [PubMed] [Google Scholar]
- Sullivan G. W., Carper H. T., Mandell G. L. Pentoxifylline modulates activation of human neutrophils by amphotericin B in vitro. Antimicrob Agents Chemother. 1992 Feb;36(2):408–416. doi: 10.1128/aac.36.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasan K. M., Brazeau G. A., Keyhani A., Hayman A. C., Lopez-Berestein G. Roles of liposome composition and temperature in distribution of amphotericin B in serum lipoproteins. Antimicrob Agents Chemother. 1993 Feb;37(2):246–250. doi: 10.1128/aac.37.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]



