Abstract
The goal of this work was to define cellular and molecular changes that occur during fracture healing as animals age. We compared the molecular, cellular, and histological progression of skeletal repair in juvenile (4 weeks old), middle-aged (6 months old), and elderly (18 months old) mice at 3, 5, 7, 10, 14, 21, 28, and 35 days post-fracture, using a non-stabilized tibia fracture model. Our histological and molecular analyses demonstrated that there was a sharp decline in fracture healing potential between juvenile and middle-aged animals, while a more subtle decrease in healing potential was apparent between middle-aged and elderly mice. By three days after fracture, chondrocytes expressing collagen type II, and osteoblasts expressing osteocalcin, were present in calluses of juvenile, but not middle-aged or elderly, mice. At day 5 immature chondrocytes and osteoblasts were observed in calluses of middle-aged and elderly mice. While at this time, chondrocytes in juvenile mice were expressing collagen type X (ColX) indicating that chondrocyte maturation was already underway. At day 7, chondrocytes expressing ColX were abundant in middle-aged mice while a small domain of ColX- positive chondrocytes were observed in elderly mice. Further, in juvenile and middle-aged mice, but not elderly mice, vascular invasion of the cartilage was underway by day 7. Juvenile mice had replaced nearly all of the cartilage by day 14, while cartilage was still present in the callus of middle-aged mice at day 21 and in elderly mice at day 28. In addition to these delays, histomorphometry revealed that elderly and middle-aged mice form less bone than juveniles (p<0.001), while cartilage production was unaffected (p>0.22). Collectively, these data suggest that enhancing cell differentiation, improving osteoblast function, and accelerating endochondral ossification may significantly benefit the elderly.
Keywords: fracture repair, aging, chondrocyte, osteoblasts, remodeling
Introduction
Skeletal injuries are common in the elderly, and fractures are associated with a high rate of morbidity and mortality (5, 24). In most healthy adults immobility associated with fracture healing does not significantly impact quality of life of the patient, but in elderly patients the period of convalescence required for fracture repair is a significant cause of post-trauma morbidity and mortality (12, 20). Although there is some disagreement (25), clinicians have suggested that advanced age might delay fracture healing (7, 13), which could further hinder recovery in the elderly. Understanding how the potential of fracture repair changes with age will identify novel therapeutic targets that can be exploited to improve fracture healing in the elderly. In turn, approaches designed to facilitate and accelerate healing in the elderly will lower the rate of morbidity and mortality.
Aging has a significant impact on skeletal repair. Previous studies of fracture healing in rats have shown that cartilage and bone formation, and cartilage resorption, are delayed in elderly animals (1), and there is evidence that accretion of mineral into the callus is reduced in older animals (15). Further, molecular analyses revealed that the onset of expression of skeletogenic factors, such as BMP-2 and Indian Hedgehog, are delayed during fracture repair in aged rats (16). These studies provide important insights into the age-dependent delay in fracture healing, yet the exact mechanism(s) underlying the delay is unknown. For example, altered skeletal repair resulting from delayed cell differentiation or decreased production of skeletal matrices are not resolved.
Our objective was to identify cellular mechanisms underlying changes in fracture repair that occur over the life span of an animal. The current work departs from previous studies in a variety of ways. We used molecular analyses to visualize gene expression patterns to allow assessment of stem cell differentiation prior to production of matrix components. We quantified cartilage and bone to determine the extent to which the potential to form skeletal tissues decreases with age. We examined juvenile, middle-aged, and elderly mice, to distinguish changes that occur as animals age into adulthood from those changes resulting from growing into old-age. Lastly, this work examines effects of age on fracture healing in a murine model that can be exploited in future studies to identify genomic loci underlying age-related defects in fracture repair (6). Our results suggest important avenues to address how aging affects skeletal regeneration.
Materials and Methods
Generation of Non-Stabilized Tibia Fractures
Animal procedures were approved by UCSF-IACUC. Juvenile, (4 weeks old, 20–23g), middle aged (6 months old, 25–30g), and elderly (18 months old, 28–34g) male 129J/B6 mice, derived from our colony and fed LabDiet 5001 (PMI Nutrition International Inc, St. Louis, MO), were used in this study. Ten animals of each age were assigned to the day 10 group, and 5 animals were assigned to the other time points. Due to post-surgical mortality the final number of animals analyzed is shown in Table 1. Mice were anesthetized with 2% avertin, and closed fractures of the mid-tibia were created by three-point bending (21). Fractures were not stabilized, and animals moved freely. Buprenex (0.1mg/kg) was given after fracture and when animals were in pain. Tibias were collected during the inflammatory (days 3 and 5), soft callus (days 7 and 10), hard callus (day 14), and remodeling phases (days 21 and 28 (and 35 in elderly mice)), fixed in 4% paraformaldehyde (4°C overnight), decalcified in 19% EDTA (10–14 days, confirmed by X-ray), and embedded in paraffin. Sagittal sections (10µm) through the entire callus were mounted on slides (3/slide). Depending on callus size 50-80 slides were collected.
Table 1.
Number of mice analyzed from each age group at each time point.
| Post-fracture | 3d | 5d | 7d | 10d | 14d | 21d | 28d | 35d |
|---|---|---|---|---|---|---|---|---|
| Age | ||||||||
| Juvenile | 3 | 3 | 5 | 5 | 4 | 5 | 3 | 0 |
| Middle-aged | 4 | 5 | 4 | 9 | 5 | 5 | 3 | 0 |
| Elderly | 3 | 4 | 4 | 7 | 6 | 5 | 4 | 5 |
Histological Analysis
Every tenth slide was stained with safranin-O/fast green (SO/FG) to visualize cartilage (red) (21). Adjacent slides were stained with modified Milligan’s trichrome (21), which stains bone blue and soft tissues red. In situ hybridization (21) to assess chondrocytes (Collagen type II (ColII)), hypertrophic chondrocytes (Collagen type X (ColX)), and osteoblasts (Osteocalcin (OC)) expression was performed on sections adjacent to histological analysis. Immunohistochemistry was performed to identify endothelial cells using an anti-Platelet-Endothelial Cell Adhesion Molecule antibody (PECAM) (8). Sections were incubated with anti-PECAM antibody (1:50; Pharmingen, San Diego, Ca) overnight at 4°C and standard horseradish peroxidase reaction (14).
Histomorphometry
Sections (every thirtieth) stained with SO/FG or trichrome were digitized using a Leica DMRB microscope, Optronics camera, and Adobe Photoshop. The number of pixels comprising each tissue component was used to estimate area. Callus size was determined by selecting the pixels in the callus using the lasso tool. Cartilage size was determined by selecting pixels stained by safranin-O. Size of newly-formed bone was determined by selecting pixels stained blue after trichrome staining and then deselecting those in cortical bone. Pixels/mm2 was determined using a 1mm scale bar. Total area of callus, and proportion of the callus comprised of cartilage and bone was determined by dividing the number of pixels by the number of pixels/mm2 (8). The volume of the callus, cartilage, and bone (TV, CV, or BV) was calculated using the equation for a conical frustum: TV, CV, or . Ai and Ai+1 are the area of callus, cartilage, or bone in the sequential sections; h is distance between sections (300µm), and n is total number of sections analyzed for each specimen. Proportion of newly formed bone (BV/TV) was calculated. We estimated star volume of the trabecular bone in callus (V*bone, (22)) at day 28 post-fracture on a section from each sample. Data were normalized to length of contralateral tibias to ameliorate differences due to size.
Statistics
Two-way ANOVA was performed to test the main effects of age and healing time and the interaction between age and healing time on TV, CV, BV, and BV/TV. One-way ANOVA was used to determine whether age affected TV, CV, BV, and BV/TV at each time point. Data are presented as the mean plus or minus one standard deviation.
Results
Effect of age on chondrocyte and osteoblast differentiation
Our results indicate that the timing of chondrocyte differentiation depends on age. At 3 days post-fracture, a periosteal reaction was evident in all mice to varying degrees. The periosteal reaction was robust in juvenile mice, but was slight in middle-aged and elderly mice (Fig. 1A, C, and E). In juvenile mice, some periosteal cells had become rounded (Fig. 1A) and differentiated into chondrocytes expressing ColII (n=3/3, Fig. 1B). In contrast, in the majority of middle-aged (n=3/4) and all of the elderly animals (n=3) there was no ColII expression (Fig. 1D, F), indicating that periosteal cells had not differentiated into chondrocytes. By day 5 a large amount of cartilage was present in calluses of juvenile mice (n=3/3, Fig. 3G, H). Chondrocytes expressing ColII and a small amount of cartilage matrix was observed in the all of the middle-aged mice (n=5/5, Fig. 1J, K) and in most elderly mice (n=2/3, Fig. 1M, N).
Fig. 1. Timing of chondrocyte differentiation and maturation is age-dependent.
(A) By day 3, the periosteum (bracket) adjacent to the fracture site had thickened in juvenile mice. A small locus of cells (dotted line) in the periosteum was stained by safranin-O, (B) and they were expressing ColII (red). In (C) middle-aged and (E) elderly mice, the periosteum (brackets) was slightly thickened, but (D, F) no ColII expression was evident. (G) By day 5, juvenile mice exhibited evidence of cartilage matrix production (light red) in the fracture callus, and (H) robust ColII expression was evident. (I) ColX expression (yellow) was detected in the ColII domain. In (J, K) middle-aged and (M, N) elderly mice chondrocytes stained by SO/FG and expressing ColII were evident at day 5, but (L, O) no ColX expression was observed. H, K, N (ColII) and I, L, O (ColX) are high magnifications of areas boxed in G, J, M. SO/FG=safranin-O/fast green staining. Scale bar A-F, H, I, K, L, N, O= 200 µm, G, J, M = 1mm.
Fig. 3. Timing of endochondral ossification is age dependent.
(A) Seven days after fracture a large amount of cartilage (red) was present in the fracture callus of juvenile mice, and (B) which coincided with a large area ColII expression. (C) Hypertrophic chondrocytes expressing ColX (yellow) were present throughout most of the cartilage at this time. (D) Foci of vascular invasion (arrows) were observed in the hypertrophic cartilage (dotted line). (E) Cartilage formed in the fracture callus of middle-aged mice, (F) exhibiting strong ColII expression. (G) At this time, ColX expression was detected in a portion of the ColII domain, and (H) vascular invasion (arrows) was seen in the hypertrophic cartilage (dotted line). (I) Cartilage formed in the fracture callus of elderly mice, and (J) ColII expression was robust throughout the callus. (K) ColX-expressing cells were observed in fracture calluses. (L) Blood vessels (arrowheads) were around the cartilage, but there was no evidence of vascular invasion of hypertrophic cartilage (dotted line). High magnification of ColII, ColX, and PECAM correspond to boxes in A, E, and I. Scale bars: A, E, I =1mm, B, C, D, F, G, H, J, K, L = 200 µm.
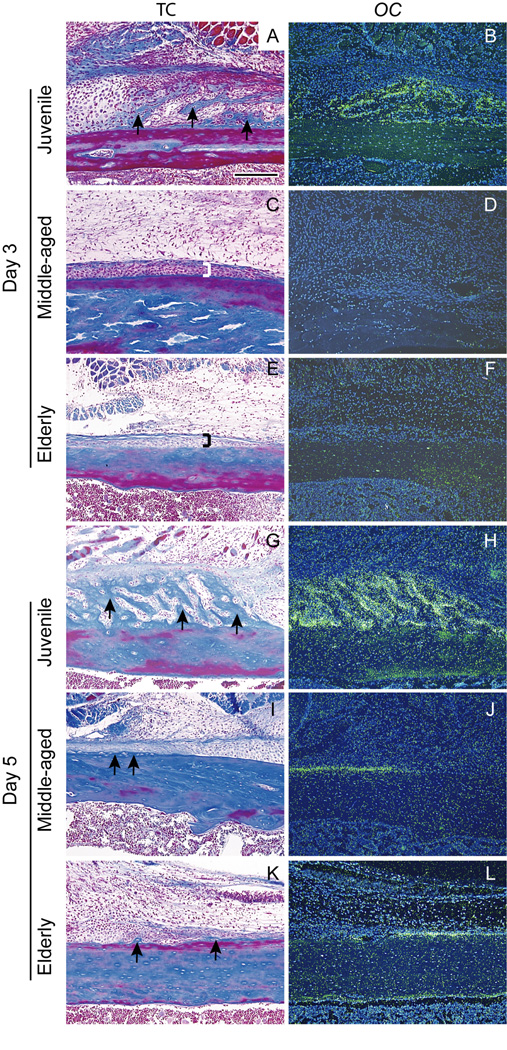
Differentiation of osteoblasts also exhibited an age-dependent delay. In juvenile mice, trabecular bone formation (Fig. 2A) and OC expression (Fig. 2B) were evident in the periosteum adjacent to the fracture site at 3 days post-fracture (n=3/3). In contrast, the periosteal reaction in middle-aged (Fig. 2C) and elderly (Fig. 2E) mice was minimal, and no differentiated osteoblasts (Fig. 2D, F) were observed at this time. At 5 days, new bone was observed in the periosteum of juvenile mice (Fig. 2G), which was accompanied by robust OC expression (Fig. 2H). In the majority of middle-aged (n=3/4) and elderly mice (n=2/3) a small amount of bone matrix was observed, and a few cells expressing OC were present in the periosteum at this time (Fig. 2I-L).
Fig. 2. Ontogeny of osteogenesis is age-dependent.
(A) In juvenile mice new bone (arrows) was apparent in the periosteum adjacent to the fracture site by day 3, and (B) osteocalcin (OC) transcripts (green) were detected by in situ hybridization. In (C) middle-aged and (E) elderly mice, the periosteum (brackets) adjacent to the fracture site had thickened slightly, but (D, F) no OC expression was observed in the periosteum at this time. (G) At day 5 after fracture new bone (arrows) was prominent in the periosteum of juvenile mice, accompanied by (H) robust OC expression (green). In contrast, (I, J) middle-aged and (K, L) elderly mice had only a small amount of new bone (arrows), and (J, L) slight OC expression (green) in the periosteum. TC = trichrome staining. Scale bar = 200µm.
Effects of age on chondrocyte maturation and endochondral ossification
Our data illustrate that maturation of chondrocytes in the cartilage and the onset of endochondral ossification were delayed as a result of aging. At 5 days post-fracture, hypertrophic chondrocytes expressing ColX were observed in the fracture callus of juvenile mice (n=2/2, Fig. 1I). However, no chondrocytes in calluses of middle-aged and elderly mice were expressing ColX, and they were not hypertrophic (Fig. 1L, O). At 7 days post-fracture, hypertrophic cartilage in juvenile mice was prominent (n=5/5, Fig. 3A, B, C). Many foci of vascular invasion and bone deposition were observed around cartilage islands indicating that endochondral ossification was underway at this time (Fig.3D, arrows). At this time, hypertrophic chondrocytes expressing ColX were present in cartilage islands of middle-aged mice (n=4/4, Fig. 3E, F, G), and vascular invasion was observed around some of these islands (Fig.3H, arrows). In elderly mice, only a small domain of hypertrophic chondrocytes was present in the cartilage (n=4/4, Fig.3I, J, K), and there was no evidence of vascular invasion (n=4/4, Fig. 3L). By day 10, endochondral ossification had begun in elderly mice (data not shown).
Age also affected the length of time required to complete endochondral ossification (Fig. 5B). By day 21, cartilage had been completely replaced with bone in calluses of juvenile mice. However, in the majority (n=4/5) of middle-aged mice a small amount of cartilage remained in the calluses, while in the elderly mice there was still a large amount of cartilage present in the calluses (n=5/5). By day 28, cartilage in the calluses of middle-aged mice had been completely replaced with bone (n=3/3), yet a small amount of cartilage was observed in the calluses of the elderly mice at this time (n=4/4); all of the cartilage was eventually replaced by bone in the elderly mice (day 35, n=5/5).
Fig. 5. Histomorphometric analyses.
(A) Volume of callus (TV). (B) Volume of cartilage (CV). (C) Volume of newly-formed bone (BV). (D) Proportion of newly-formed bone in the callus (BV/TV). Data shown as mean ±SD.  indicates significant differences, p<0.05.
indicates significant differences, p<0.05.
Effects of aging on fracture callus remodeling
By day 28, most of the fractured bone ends were absorbed and a thick shell of new bone ensheathing the callus was evident in juvenile mice (Fig. 4A, B). At this time, in middle-aged and elderly mice the callus was surrounded by a thin layer of bone (Fig. 4C-F), and remnants of cortical bone at the fracture ends were prominent (Fig. 4C, E). Juvenile mice had larger trabecular star volumes (0.270±0.255mm3) compared to middle-aged (0.041±0.017mm3) and elderly (0.007±0.010mm3) mice, which was not statistically significant (p<0.09) possibly due to the small number of animals analyzed, indicating that morphology of trabeculae in fracture calluses was affected by aging.
Fig. 4. Remodeling of fracture callus is age-dependent.
(A) At 28 days after fracture, a large amount of trabecular bone (blue) was present in the callus of juvenile mice, and fracture ends were largely absorbed by this time (arrowheads). (B) High magnification (corresponding to box in A) illustrates thick trabeculae (asterisks) inside the callus and a thick shell of new cortical bone (arrows). (C) Trabecular bone was present in the callus of middle-aged mice, and the ends of the fractured bone (arrowheads) were still evident at 28 days. (D) In the boxed region in C, the trabeculae (asterisks) inside the callus and the cortical shell (arrows) appear thinner than those in the juvenile mice. (E) At this time, elderly mice exhibited a small amount of bone in the callus, and the fractured bone ends were not resorbed (arrowheads). (F) High magnification of the box in E illustrates thin trabeculae (asterisks) throughout the callus, which was surrounded by a thin layer of cortical bone (arrows). TC = trichrome staining. Scale bars: A, C, E = 1mm, B, D, F = 200µm.
Histomorphometric analysis
In addition to the effects of aging on cell differentiation, endochondral ossification, and remodeling, we wanted to determine whether aging affected the ability of animals to form bone and cartilage during fracture repair. Two-way ANOVA demonstrated that age had significant effects on TV (Fig. 5A, p<0.001), BV (Fig. 5C, p<0.001), and BV/TV (Fig. 5D, p<0.001). The effect of age on both BV and BV/TV was significant between the juvenile and middle-aged mice (p<0.01), as well as between the juvenile and elderly mice (p<0.01). These findings suggested age affected the capability of animals to form the callus and bone within the callus. Juvenile mice had a higher potential for bone formation compared to middle-aged and elderly mice. In contrast, age did not appear to affect CV (Fig. 5B, p=0.228), indicating that the ability to form cartilage was not affected by aging. In addition, we also determined that the interaction between age and time had significant effects on TV (p<0.001), BV (p<0.001), and CV (p<0.001), which supports our conclusion that the time course of healing was delayed due to altered stem cell differentiation and cartilage maturation.
Discussion
The skeletal system manifests the effects of aging in many ways. In addition to changes in skeletal physiology, such as observed in osteoporosis and osteoarthritis, aging also affects the capacity for bone repair after injury. Numerous reports have indicated that fracture repair is delayed in aged animals (2, 16). As expected, our results demonstrate that fractures in juvenile mice heal more rapidly than the middle-aged and elderly. The decline in healing potential between juvenile and adult animals was dramatic and indicated a sharp decrease in regenerative ability occurs as animals age into adulthood. The age-related changes that we observed included a delay in the onset of the periosteal reaction, delays in cell differentiation, decreased bone formation, delayed angiogenic invasion of cartilage, a protracted period of endochondral ossification, and impaired bone remodeling. By using histomorphometry to quantify the extent of bone and cartilage formation we can conclude that aging significantly reduced the amount of bone but not cartilage that formed at the fracture site.
We observed a modest decrease in the healing capacity of elderly animals compared to middle-aged animals as well. Middle-aged mice had significantly larger calluses than elderly mice at days 5, 7, and 10 (Fig. 5A) indicating that callus formation and growth was delayed in the elderly animals. Further, maturation of chondrocytes in elderly mice lagged behind maturation in middle-aged animals. A hallmark of hypertrophic cartilage is the ability to induce vascular invasion thereby initiating endochondral ossification. In the middle-aged mice, blood vessels were observed in the cartilage by day 7 after fracture, but at this time no evidence of vascular invasion was seen in elderly mice. Cartilage resorption was also delayed in the elderly mice compared to middle-aged animals suggesting that the early delays in chondrocyte differentiation translate into a protracted process of endochondral ossification. Elderly mice were eventually able to heal their fractures, but our results illustrate that a decline in healing capacity continues throughout the life span of an animal. Whether this decline results from cell-autonomous changes in stem cells, or arises from other systemic changes as a result of age deserves investigation.
Candidate Targets for Improving Fracture Repair in the Elderly
The goal of our study was to compare the cellular events of the healing program of elderly animals with their younger counterparts in order to propose novel interventions to stimulate healing in the elderly. Stem cell differentiation is a crucial component of skeletal repair, and understanding the mechanism(s) leading to delayed cell differentiation will be important in determining why the regenerative potential of the skeleton is reduced as a consequence of age. Previous reports suggest that there are reductions in the number of stem cells capable of undergoing differentiation into osteoblasts (4, 10, 17, 19), which could result from decreased proliferate capacity of progenitor cells (3). Alternatively, mesenchymal stem cells in aged animals may preferentially differentiate into adipocytes rather than osteoblasts (11, 18). Thus, different scenarios need exploration to define how stem cell differentiation is altered.
Osteoblasts in aged animals also exhibit a decreased response to osteogenic stimuli as a consequence of aging (23). In our study, we observed significant differences in bone formation among juvenile, middle-aged and elderly mice at days 7 and 10 after fracture (Fig. 5C). These differences likely resulted from age-related impairments of the skeletogenic lineage. Such changes have been reported to be associated with decreases in bone mineral density (26), and identifying these changes will advance targeted therapies.
The coordination of vascular invasion of the cartilage callus and cartilage resorption is another area deserving focus. We observed delays in the timing of endochondral ossification in aged animals, which compliments previous work demonstrating that molecules involved in endochondral ossification remain elevated during fracture repair in adult rats (9, 16). Certainly, the delays in chondrocyte differentiation and maturation contribute to the delayed endochondral ossification, but determining if other steps that regulate this process are affected is essential for proposing methods to enhance the timing and rate of endochondral ossification. Accelerating stem cell differentiation, chondrocyte maturation, and endochondral ossification may have important consequences for elderly fracture patients by achieving mobility more rapidly and reducing musculoskeletal wasting.
Supplementary Material
(A) Normal tibial periosteum (bracket) of a 4-week-old juvenile mouse. (B) By day 3, the periosteum (bracket) adjacent to the fracture site was thickened in juvenile mice. A small locus of cells (dotted line) in the periosteum was stained by safranin-O (SO). (C) Normal tibial periosteum (bracket) of a middle-aged mouse, and (D) the extent to which the periosteum (bracket) had thickened near the fracture site. (E) Normal periosteum (bracket) of an elderly mouse and (F) thickened periosteum (bracket) near fracture site in an elderly mouse. Scale bar = 200 µm.
Acknowledgements
We would like to thank Dr. Ru-Fang Yeh for statistical analysis, and Dr. Richard Schneider for critical comments on the manuscript. This work was supported by a grant from the NIH-NIAMS (KO8-AR002164 to T.M.).
References
- 1.Aho AJ. Electron microscopic and histologic studies on fracture repair in old and young rats. Acta Chir Scand Suppl. 1966;357:162–165. [PubMed] [Google Scholar]
- 2.Bak B, Andreassen TT. The effect of aging on fracture healing in the rat. Calcif Tissue Int. 1989;45:292–297. doi: 10.1007/BF02556022. [DOI] [PubMed] [Google Scholar]
- 3.Bellows CG, Pei W, Jia Y, et al. Proliferation, differentiation and self-renewal of osteoprogenitors in vertebral cell populations from aged and young female rats. Mech Ageing Dev. 2003;124:747–757. doi: 10.1016/s0047-6374(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 4.Bergman RJ, Gazit D, Kahn AJ, et al. Age-related changes in osteogenic stem cells in mice. J Bone Miner Res. 1996;11:568–577. doi: 10.1002/jbmr.5650110504. [DOI] [PubMed] [Google Scholar]
- 5.Cauley JA, Thompson DE, Ensrud KC, et al. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11:556–561. doi: 10.1007/s001980070075. [DOI] [PubMed] [Google Scholar]
- 6.Churchill GA, et al. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat Genet. 2004;36:1133–1137. doi: 10.1038/ng1104-1133. [DOI] [PubMed] [Google Scholar]
- 7.Claes L, Grass R, Schmickal T, et al. Monitoring and healing analysis of 100 tibial shaft fractures. Langenbecks Arch Surg. 2002;387:146–152. doi: 10.1007/s00423-002-0306-x. [DOI] [PubMed] [Google Scholar]
- 8.Colnot C, Thompson Z, Miclau T, et al. Altered fracture repair in the absence of MMP9. Development. 2003;130:4123–4133. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai BJ, Meyer MH, Porter S, et al. The effect of age on gene expression in adult and juvenile rats following femoral fracture. J Orthop Trauma. 2003;17:689–698. doi: 10.1097/00005131-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 10.D'Ippolito G, Schiller PC, Ricordi C, et al. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 11.Duque G, Macoritto M. Kremer R: 1,25(OH)2D3 inhibits bone marrow adipogenesis in senescence accelerated mice (SAM-P/6) by decreasing the expression of peroxisome proliferator-activated receptor gamma 2 (PPAR[gamma]2) Experimental Gerontology. 2004;39:333–338. doi: 10.1016/j.exger.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Geerts WH, Heit JA, Clagett GP, et al. Prevention of venous thromboembolism. Chest. 2001;119:132S–175S. doi: 10.1378/chest.119.1_suppl.132s. [DOI] [PubMed] [Google Scholar]
- 13.Hee HT, Wong HP, Low YP, et al. Predictors of outcome of floating knee injuries in adults: 89 patients followed for 2–12 years. Acta Orthop Scand. 2001;72:385–394. doi: 10.1080/000164701753542050. [DOI] [PubMed] [Google Scholar]
- 14.Marcucio RS, Noden DM. Myotube heterogeneity in developing chick craniofacial skeletal muscles. Developmental Dynamics. 1999;214:178–194. doi: 10.1002/(SICI)1097-0177(199903)214:3<178::AID-AJA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Meyer J, Ralph A, Tsahakis PJ, Martin DF, et al. Age and ovariectomy impair both the normalization of mechanical properties and the accretion of mineral by the fracture callus in rats. Journal of Orthopaedic Research. 2001;19:428–435. doi: 10.1016/S0736-0266(00)90034-2. [DOI] [PubMed] [Google Scholar]
- 16.Meyer RA, Jr., Meyer MH, Tenholder M, et al. Gene expression in older rats with delayed union of femoral fractures. J Bone Joint Surg Am. 2003;85-A:1243–1254. doi: 10.2106/00004623-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Nishida S, Endo N, Yamagiwa H, et al. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab. 1999;17:171–177. doi: 10.1007/s007740050081. [DOI] [PubMed] [Google Scholar]
- 18.Nuttall ME, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Current Opinion in Pharmacology. 2004;4:290–294. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 19.O'Driscoll SW, Saris DB, Ito Y, et al. The chondrogenic potential of periosteum decreases with age. J Orthop Res. 2001;19:95–103. doi: 10.1016/S0736-0266(00)00014-0. [DOI] [PubMed] [Google Scholar]
- 20.Rose S, Maffulli N. Hip fractures. An epidemiological review. Bull Hosp Jt Dis. 1999;58:197–201. [PubMed] [Google Scholar]
- 21.Thompson Z, Miclau T, Hu D, et al. A model for intramembranous bone healing during fracture repair. Journal of Investigative Medicine. 2002;50:83A. [Google Scholar]
- 22.Vesterby A, Gundersen HJ, Melsen F. Star volume of marrow space and trabeculae of the first lumbar vertebra: sampling efficiency and biological variation. Bone. 1989;10:7–13. doi: 10.1016/8756-3282(89)90140-3. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Schwartz Z, Yaffe P, et al. The expression of transforming growth factorbeta and interleukin-1beta mRNA and the response to 1,25(OH)2D3' 17 beta-estradiol, and testosterone is age dependent in primary cultures of mouse-derived osteoblasts in vitro. Endocrine. 1999;11:13–22. doi: 10.1385/endo:11:1:13. [DOI] [PubMed] [Google Scholar]
- 24.White BL, Fisher WD, Laurin CA. Rate of mortality for elderly patients after fracture of the hip in the 1980's. J Bone Joint Surg Am. 1987;69:1335–1340. [PubMed] [Google Scholar]
- 25.Yokoyama K, Tsukamoto T, Aoki S, et al. Evaluation of functional outcome of the 2 floating knee injury using multivariate analysis. Arch Orthop Trauma Surg. 2002;122:43–45. doi: 10.1007/s00402-002-0406-7. [DOI] [PubMed] [Google Scholar]
- 26.Yudoh K, Nishioka K. Telomerized presenescent osteoblasts prevent bone mass loss in vivo. Gene Ther. 2004;11:909–915. doi: 10.1038/sj.gt.3302234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Normal tibial periosteum (bracket) of a 4-week-old juvenile mouse. (B) By day 3, the periosteum (bracket) adjacent to the fracture site was thickened in juvenile mice. A small locus of cells (dotted line) in the periosteum was stained by safranin-O (SO). (C) Normal tibial periosteum (bracket) of a middle-aged mouse, and (D) the extent to which the periosteum (bracket) had thickened near the fracture site. (E) Normal periosteum (bracket) of an elderly mouse and (F) thickened periosteum (bracket) near fracture site in an elderly mouse. Scale bar = 200 µm.