Abstract
In 1999, the World Health Organization switched from annual to semiannual recommendations for influenza vaccine composition. We compared the antigenic match between recommendations and circulating viruses before and after 1999. Vaccine match proportion for A/H3N2 viruses increased from 31% to 59% in the Southern hemisphere (P<0.05), and is now comparable to that in the Northern hemisphere. Vaccine match for influenza B decreased from ~100% to 33–54% in both hemispheres (P<0.05), following the unexpected resurgence of influenza B/Victoria in 1997. No estimate was available for influenza A/H1N1. We conclude that semi-annual vaccine recommendations are useful overall and discuss potential ways forward, including a recommendation for the improvement of vaccination policy and influenza surveillance in tropical areas.
Keywords: Influenza, vaccine match, WHO recommendations, antigenic characteristics, Southern Hemisphere, Northern Hemisphere, seasonality
1. Introduction
Large-scale influenza vaccination programs have been in place for many years in Europe, Japan, and the US. Annual immunization is the most widespread strategy to prevent influenza morbidity and mortality, especially in those at highest risk of complications, including seniors, children, and those with certain chronic conditions. [1] Three influenza subtypes circulate in the population (A/H3N2, A/H1N1 and B) and most commercial influenza vaccines include one representative strain for each of these subtypes. Gradual changes in the virus surface antigens generate new influenza strains that evade population immunity, and influenza vaccine composition must be updated periodically to reflect these changes. [2, 3] Occasionally, a novel influenza virus, such as the 2009 A/H1N1pandemic virus, emerges from the animal reservoir of influenza viruses and becomes transmissible among humans, requiring rapid development of a new pandemic vaccine and later incorporation of a new antigen into the seasonal influenza vaccine.
Since 1973, the World Health Organization (WHO) has issued annual recommendations for the influenza A/H3N2, A/H1N1 and B strains to be included in trivalent seasonal influenza vaccines. [4] The annual vaccine composition recommendations were based on surveillance of circulating strains in the previous twelve months, considerations regarding potential epidemic drift strains, vaccine strain production potential, and existing immunity within the population. [5] Until 1998, an annual recommendation was made every February, just in time for the vaccine to be produced and distributed for the following Northern Hemisphere influenza season (October-April). While the Northern Hemisphere population received a vaccine based on recommendations that were approximately eight months old, by the time the Southern Hemisphere received the same vaccine, around March or April of the following year, the vaccine recommendations were over 13 months old. Therefore, it was thought that the match between the strains included in the vaccine and the circulating strains was better in the Northern Hemisphere than in the Southern Hemisphere, although this was not formally evaluated. As a response to this perceived Northern Hemisphere bias, Southern Hemisphere-specific vaccine recommendations were implemented in October 1998, [6] and semi-annual influenza vaccine recommendations have been made since that time.
Despite these efforts, no published study has evaluated how well the WHO vaccine composition recommendations have matched the circulating strains worldwide, before or after the semiannual vaccine recommendations were implemented in 1999. In this study, we quantify the match between vaccine composition and circulating strains during seasonal epidemics in both Hemispheres, based on publicly available surveillance reports between 1991 and 2008.
2. Methods
We conducted a literature search to identify reports containing information on national or regional influenza virus surveillance (Pubmed search terms: national, regional, influenza, activity, surveillance, report), and perused publicly available data from national surveillance websites maintained by health authorities. Influenza virus surveillance data were collected for 1991–2008 for the United States (US) [7–16], Canada [17–31], and Europe [32], representing the Northern Hemisphere, and for Australia [33], New Zealand (1999, 2000 [34, 35]; 2001–2007 [36]), Latin America [37], and general global reports [38–48], representing the Southern Hemisphere. These data were collected separately for each location, year, and influenza subtype. This report presents primarily data for the A/H3N2 and B subtypes due to limited available surveillance data and less rapid antigenic change over the years for the A/H1N1 subtype.
To evaluate the match between influenza vaccine strains and circulating strains, we recorded the prevalence of each strain(s) within each of the 3 subtypes circulating in each season and location. Occasionally the reports did not specify the exact number or percent of the influenza isolates that matched a given strain, in which case the information was considered missing.
For influenza A/H3N2, we categorized strains (either naturally circulating or included in the vaccine) based on the antigenic clusters defined by Smith et al [2] and Russell et al. [49] For recent years when A/H3N2 antigenic clusters are less well defined, we considered that A/Fujian/411/2002, A/California/7/2004, and A/Wisconsin/67/2005 were distinct clusters, that strains A/Wellington/1/2004 and A/California/7/2004 belonged to the same cluster, as well as A/Brisbane/10/2007 and A/Wisconsin/67/2005. If a circulating A/H3N2 strain was in the same cluster as the vaccine strain, it was considered a match.
For influenza B, we considered two classically distinct virus lineages, Yamagata and Victoria, which are associated with different genetic and antigenic characteristics. [50, 51] If the circulating B strain was in the same lineage as the vaccine strain, it was considered a match. We note that our definitions of vaccine match for both influenza B and A/H3N2 are similar to those used in a recent Canadian study evaluating influenza vaccine effectiveness by subtype. [52]
To evaluate vaccine recommendations, we estimated the match proportion between circulating strains and vaccine strains by subtype, season and country. To compare vaccine match proportion between seasons and hemisphere, while taking into account potential geographical differences in sampling and vaccine match between countries, we used weighted t-tests for which weights were based on the number of influenza samples typed in each location and each season. We also conducted sensitivity analyses with a method that puts more emphasis on locations but disregards sampling issues (unweighted t-tests applied to average vaccine match proportion by hemisphere), and a method that disregards potential geographical heterogeneity but heavily relies on sampling (chi-square tests of aggregated virus surveillance data at the hemisphere and season level).
3. Results
Description of WHO influenza vaccine recommendations, 1990–2008
Table 1 lists the subtype-specific influenza vaccine composition recommendations issued by the WHO between 1990 and 2008. Nine recommendations were issued during the early part of our study period, 1990–1998, when influenza vaccine composition recommendations were made annually. Twenty recommendations (ten in the Northern hemisphere and ten in the Southern Hemisphere) were issued by the WHO during 1999–2008, when semi-annual composition recommendations were in place. Recommendations for vaccine composition in the Northern Hemisphere have been made between February 10th and March 1st since 1990, and between October 1st and 24th for the Southern Hemisphere since 1999. In three cases, incomplete recommendations were initially issued, twice without an H3N2 strain recommendation, and once without an H1N1 strain. In all three of these cases, an addendum was published two to four weeks later with an updated recommendation.
Table 1.
Influenza vaccine recommendations issued by WHO, 1990–2008. Changes in vaccine composition are highlighted when they correspond to a change in vaccine strain (A/H1N1), antigenic cluster (A/H3N2), or lineage (B; Yamagata (Yam) vs Victoria (Vic)). N=Northern Hemisphere, S=Southern Hemisphere
| Hemis phere | Year | A/H1N1 | A/H3N2 | B |
|---|---|---|---|---|
| N | 1990 | A/Singapore/6/86 | A/Guizhou/54/89 | B/Yamagata/16/88 (Yam) |
| N | 1991 | A/Singapore/6/86 | A/Beijing/353/89 | B/Yamagata/16/88 or B/Panama/45/90 (Yam) |
| N | 1992 | A/Singapore/6/86 | A/Beijing/353/89 | B/Yamagata/16/88 or B/Panama/45/90 |
| N | 1993 | A/Singapore/6/86 | A/Beijing/32/92 | B/Panama/45/90 (Yam) |
| N | 1994 | A/Singapore/6/86 | A/Shangdong/9/93 | B/Panama/45/90 |
| N | 1995 | A/Singapore/6/86 | A/Johannesburg/33/94 | B/Beijing/184/93 (Yam) |
| N | 1996 | A/Singapore/6/86 | A/Wuhan/359/95 | B/Beijing/184/93 |
| N | 1997 | A/Bayern/7/95 | A/Wuhan/359/95 | B/Beijing/184/93 |
| N | 1998 | A/Beijing/262/95 | A/Sydney/5/97 | B/Beijing/184/93 |
| S | 1999 | A/Beijing/262/95 | A/Sydney/5/97 | B/Beijing/184/93 |
| N | 1999 | A/Beijing/262/95 | A/Sydney/5/973 | B/Beijing/184/93 |
| S | 2000 | A/New Caledonia/20/99 | A/Moscow/10/99 | B/Beijing/184/93 |
| N | 2000 | A/New Caledonia/20/99 | A/Moscow/10/99 | B/Beijing/184/93 |
| S | 2001 | A/New Caledonia/20/99 | A/Moscow/10/99 | B/Sichuan/379/99 (Yam) |
| N | 2001 | A/New Caledonia/20/99 | A/Moscow/10/99 | B/Sichuan/379/99 |
| S | 2002 | A/New Caledonia/20/99 | A/Moscow/10/99 | B/Sichuan/379/99 |
| N | 2002 | A/New Caledonia/20/99 | A/Moscow/10/99 | B/Hong Kong/330/2001 (Vic) |
| S | 2003 | A/New Caledonia/20/99 | A/Moscow/10/99 | B/Hong Kong/330/2001 |
| N | 2003 | A/New Caledonia/20/99 | A/Moscow/10/99 | B/Hong Kong/330/2001 |
| S | 2004 | A/New Caledonia/20/99 | A/Fujian/411/2002 | B/Hong Kong/330/2001 |
| N | 2004 | A/New Caledonia/20/99 | A/Fujian/411/2002 | B/Shanghai/361/2002 (Yam) |
| S | 2005 | A/New Caledonia/20/99 | A/Wellington/1/2004 | B/Shanghai/361/2002 |
| N | 2005 | A/New Caledonia/20/99 | A/California/7/2004 | B/Shanghai/361/2002 |
| S | 2006 | A/New Caledonia/20/99 | A/California/7/2004 | B/Malaysia/2506/2004 (Vic) |
| N | 2006 | A/New Caledonia/20/99 | A/Wisconsin/67/2005 | B/Malaysia/2506/2004 |
| S | 2007 | A/New Caledonia/20/99 | A/Wisconsin/67/2005 | B/Malaysia/2506/2004 |
| N | 2007 | A/Solomon Islands/3/2006 | A/Wisconsin/67/2005 | B/Malaysia/2506/2004 |
| S | 2008 | A/Solomon Islands/3/2006 | A/Brisbane/10/2007 | B/Florida/4/2006 (Yam) |
| N | 2008 | A/Brisbane/59/2007 | A/Brisbane/10/2007 | B/Florida/4/2006 |
Since 1999, the A/H3N2 antigenic cluster represented in the vaccine has changed three times, the A/H1N1 component has changed three times, and the B component has changed five times (Table 1). No clear pattern has been seen in which hemisphere is driving the recommendation changes; 67% of A/H3N2 changes (2/3), 33% of A/H1N1 (1/3), and 60% of B (3 of 5), occurred first in the Southern Hemisphere recommendations.
Evaluation of match between WHO vaccine recommendations and circulating strains, 1990–2008
Based on a review of influenza surveillance reports between 1991 and 2008, we retrieved information on the antigenic characteristics of 33,565 A/H3N2 strains allowing categorization into 8 antigenic clusters. [2, 49] Of these A/H3N2 strains, 65% were isolated in the Northern Hemisphere and 35% in the Southern Hemisphere. A median of 760 A/H3N2 strains (range 25–4,334) were available for estimation of vaccine match each season in the Northern Hemisphere, while 658 isolates were available seasonally for the Southern Hemisphere (range 9–2,101). Table 2 provides information on the number of strains available for analysis by time period and Hemisphere.
Table 2.
Description of influenza viral surveillance data used in the evaluation of vaccine match, by influenza subtype, period (before and after the change in vaccine recommendations in 1999), and Hemisphere.
| Time period | Southern Hemisphere | Northern Hemisphere | ||
|---|---|---|---|---|
| Influenza A/H3N2 subtype | ||||
| Total number of isolates | Median number of isolates by season (min-max) | Total number of isolates | Median number of isolates by season (min-max) | |
| 1991–1998 (8 seasons) | 3,728 | 199 (9–1,636) | 2,877 | 252 (123–847) |
| 1999–2007 (9 seasons) | 7,947 | 842 (218–2,101) | 19,185 | 1,452 (25–4,334) |
| Influenza B subtype | ||||
| Total number of isolates | Median number of isolates by season (min-max) | Total number of isolates | Median number of isolates by season (min-max) | |
| 1996–1998 (3 seasons) | 669 | 43 (21–605) | 557 | 67 (37–288) |
|
| ||||
| 1999–2007 (9 seasons) | 3,530 | 355 (12–995) | 8,705 | 582 (64–2,324) |
We categorized influenza B viruses based on two broad antigenic families, Victoria and Yamagata. No B virus data from the Southern Hemisphere were available prior to 1996, therefore, we only considered the period 1996 to 2008 for the B viruses. Of the 13,461 influenza B viruses described between 1996 and 2008, 69% were isolated in the Northern Hemisphere, and 53% were categorized as Yamagata. A median of 488 influenza B isolates (range 37–2,324) were available for estimation of vaccine match each season in the Northern Hemisphere, while a median of 305 B isolates were available for the Southern Hemisphere (range 21–995). There was a trend towards increased sampling in recent years both for influenza A/H3N2 and B viruses: 68–94% of viruses included in the study were isolated after 1999, and the number of viruses available each season increased 4 to 8-fold after 1999, depending on the location and subtype (Table 2).
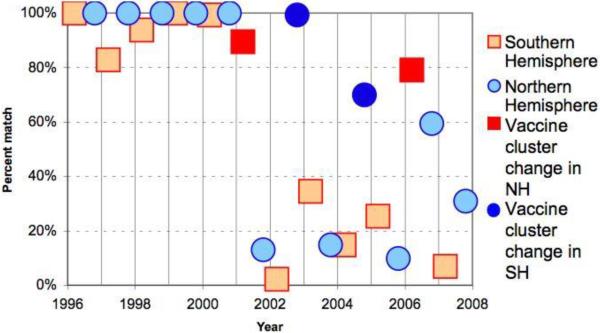
Prior to the semi-annual recommendations initiated in 1999, the annual match between the dominant circulating A/H3N2 strain and vaccine strain was either very high (>75%) or very low (<25%) in both hemispheres (Figure 1). A notable pattern prior to the implementation of the semi-annual vaccine recommendations is the substantial drop in vaccine match proportion for A/H3N2 in the Southern Hemisphere, immediately prior to a change in annual A/H3N2 vaccine strain recommendations for the Northern Hemisphere (1991, 1993, 1996, 1998 seasons). Prior to 1999, the average vaccine match proportion was 31% in the Southern Hemisphere, less than half that of the Northern Hemisphere over the same period (69%, P=0.02, Table 3). By contrast, after the semi-annual recommendations were implemented, the average match proportion increased in Southern Hemisphere to 59% (P<0.05 for difference in match proportion before and after 1999), and became similar to that in the Northern hemisphere (55%, P =0.77 for differences between hemispheres post-1999, Table 3).
Figure 1.
Percent of isolated and antigenically characterized A/H3N2 influenza viruses that matched the WHO recommended vaccine composition by hemisphere, 1990–2008, based on data described in Table 2. Percent match was calculated by dividing the number of A/H3N2 viruses that matched the vaccine strain (based on the definition of antigenic clusters as in [2, 49]) by the total number of antigenically characterized viruses.
Table 3.
Estimates of vaccine match proportion and 95% confidence intervals (CI) by influenza subtype, hemisphere, and period, 1991–2007 (H3N2) and 1996–2007 (B). Percent match was calculated by dividing the number of viruses that matched the vaccine strain (A/H3N2: based on the definition of antigenic clusters as in [2, 49]; B viruses matched the vaccine lineage (influenza B Yamagata or Victoria)) by the total number of antigenically characterized viruses.
| Time period | Southern Hemisphere Match proportion (95% CI) | Northern Hemisphere Match proportion (95% CI) |
|---|---|---|
| A/H3N2 subtype | ||
| 1991–1998 (8 seasons) Annual WHO recommendations | 31% (8%–53%) | 69% (47%–90%) |
| 1999–2007 (9 seasons) Semi-annual WHO recommendations | 59% (42%–75%) | 55% (37%–73%) |
| B subtype | ||
|---|---|---|
| 1996–1998 (3 seasons) Annual WHO recommendations | 93% (74%–100%) | 100% (100%–100%) |
| 1999–2007 (9 seasons) Semi-annual WHO recommendations | 54% (38%–71%) | 38% (21%–55%) |
There was little information identifying the circulating influenza B strains prior to the initiation of Southern Hemisphere vaccine recommendations. However, the available data from Canada, the US, and Australia suggest a degree of vaccine match close to 100% at the family level in both hemispheres prior to 1999 (Table 3, P=0.21 for differences between hemispheres). In recent years, however, vaccine match for influenza B significantly decreased in both hemispheres to 38–54% (P for change <0.05), and there was no measurable difference in matching proportion between hemispheres (P=0.21). The observed decline was mostly due to the global resurgence of the Victoria lineage in 1997 and frequent oscillations between Yamagata and Victoria since 1997 (every 1.5 to 2 years; Figure 2). We note that vaccine recommendations were nearly out of phase with the observed cycles of dominance of these B lineages. We conclude that more frequent semi-annual opportunities for updating the B strain included in the vaccine since 1999 have demonstrated little benefit for vaccine match.
Figure 2.
Percent of isolated and antigenically-characterized B influenza viruses that matched the WHO recommended vaccine viruses by hemisphere, 1996–2008, based on data described in Table 2. Percent match was calculated by dividing the number of B viruses that matched the vaccine lineage (influenza B Yamagata or Victoria) by the total number of antigenically characterized viruses.
Sensitivity analyses using different methods to compare vaccine match proportion over time and between hemispheres gave similar results. Vaccine match proportions and statistical significance remained unchanged when aggregating data at the hemisphere and season level, disregarding the effect of countries. When we gave the same weight to each country, irrespective of the number of specimens sampled, the vaccine match proportion estimates changed somewhat but the conclusions remained unchanged. In particular with this comparison method, A/H3N2 virus vaccine match was shown to improve in the Southern hemisphere after 1999, increasing from 50% to 75%, although the change was not statistically significant due to lack of power. For influenza B viruses, vaccine match was shown to decline from 100% to 50–52% in both hemispheres in recent years (P<0.05 for change), highly consistent with the results of our main analysis.
4. Discussion
Our study is the first to quantitatively evaluate influenza vaccine recommendations issued by the WHO since 1990. We demonstrate that the Southern Hemisphere recommendations put in place in 1999 substantially improved vaccine match in Southern Hemisphere countries, in particular for A/H3N2 viruses that are prone to more rapid antigenic changes than the other subtypes. [2, 3] The A/H3N2 vaccine match proportion in the Southern Hemisphere has significantly increased from 31% prior to implementation of the Southern Hemisphere recommendations to 69% in recent years, and is now equivalent to that in Northern Hemisphere countries based on publicly available data. Our data suggest that improvement in match between circulating and vaccine strains in the Southern Hemisphere is due to the use of more recent global influenza surveillance data to derive vaccine recommendations in that Hemisphere, rather than use of more locally-representative data. [5] These results are reassuring for Southern Hemisphere populations, who can now benefit from a better-matched and potentially more clinically effective vaccine, and for Northern Hemisphere populations who travel to the Southern Hemisphere during the influenza season and can now use an updated vaccine. [53, 54]
Vaccine match proportion decreased unexpectedly for influenza B viruses from 100% to 54%, concomitant with the implementation of Southern Hemisphere recommendations, a decrease which appears to be a coincidence related to changes in virus strain dynamics. While a single lineage of influenza B predominated globally between 1990 and 1997 (Yamagata lineage), there are now patterns of alternating dominance of two B lineages (Yamagata and Victoria lineages). These results highlight the difficulty in predicting the global dominance patterns of B viruses in recent years, rather than a failure of the Southern recommendations per se. In order to resolve this problem, it has been suggested that the annual vaccine be expanded to include representative antigens for both B lineages, or that the annual vaccine systematically alternate inclusion of the two lineages (Yamagata one year, Victoria the next) in an attempt to provide some residual protection against both lineages of B viruses. [55] Given the widespread circulation of A/H1N1 pandemic virus since April 2009, which is now incorporated into the seasonal vaccine recommendations for 2010, and the hurdles associated with influenza vaccine production, it seems unlikely that seasonal vaccines will be modified to contain two influenza B components in the near future. Although influenza B viruses are less likely to be responsible for widespread epidemics than A/H3N2 viruses (and perhaps A/H1N1 pandemic viruses in the coming years), B viruses occasionally predominate in a season and are particularly prevalent in children; therefore, protection from infection remains of public health importance.
One of the challenges in performing this evaluation was that the publicly available data were incomplete or lacking in some cases. In some reports, only the predominant strain type was mentioned, and we were unable to ascertain the identity of the less frequently isolated strains. In addition, we were unable to evaluate vaccine match for seasonal influenza A/H1N1 viruses, given that antigenic categories are not yet defined for this subtype, there are very few surveillance reports providing strain information, and vaccine recommendations are less frequently updated, resulting in very poor statistical power. Another challenge was that, although this paper focuses on a match between the WHO recommended vaccine strains and the circulating strains, some countries (e.g., New Zealand) choose to use strains that are different from the WHO recommendations during some years. As this is an evaluation of the WHO recommendations, alternate vaccine strain choices were not considered when calculating the percent match between vaccine strain recommendations and circulating strains within any one country or region. More importantly, we had very little data from tropical areas of the Southern and Northern Hemispheres, and our evaluation remains mostly limited to those temperate areas with publicly available data. Despite these caveats, we applied the same methodology to analysis of different years and different countries, so that our main results comparing vaccine match before and after 1999 are robust. In particular, the estimated lower vaccine match in Southern than Northern Hemisphere for influenza A/H3N2 before 1999 and improvement in recent years are robust to misspecifications of influenza strains or lack of data. These geographical and temporal differences in vaccine match are likely not overestimated, as small sample sizes tend to drive comparisons towards lack of statistical significance. Further, sensitivity analyses exploring various sampling and geographical issues suggest that our results are not sensitive to the specific methodology used. We note also that our observation of a poor match for influenza A/H3N2 in the Southern Hemisphere prior to 1999 is consistent with a descriptive analysis of influenza in Central and South America in the period when Southern Hemisphere recommendations did not exist. [37]
While our study suggests a reassuring improvement in vaccine match proportion for influenza A/H3N2 viruses, which are responsible for most influenza-related deaths, [56] further research is warranted to determine the clinical value of a good antigenic match between circulating strains and vaccine strains. Overall, vaccination is expected to prevent 80% of all laboratory-confirmed influenza infections in healthy people during years when the vaccine strains are antigenically-matched to the circulating strains, but vaccine efficacy decreases to 50% when there is a mismatch. [57] A detailed study investigated subtype-specific vaccine effectiveness in the 2006–07 season in Canada and reported high vaccine effectiveness against clinical A/H1N1 influenza infections (92%), when 98% of A/H1N1 isolates matched the vaccine component in that season. [52] Effectiveness was moderate (41%) against clinical A/H3N2 infections, while half of the A/H3N2 isolates matched the vaccine component at the antigenic cluster level. [52] Effectiveness was poor (19%) against B viruses, while none of the B isolates were matched to the vaccine component at the lineage level. [52] Similarly, results from a three-year US study demonstrate that statistically significant vaccine effectiveness was only observed during a season in which over 90% of the circulating isolates matched the vaccine strains. [58] Of note, both the Canadian and US studies used the same definition of vaccine antigenic match for influenza A/H3N2 and B components as ours. [52, 58] Additional research is needed to establish the degree of antigenic match between vaccine and circulating viruses required to elicit clinical protection. Of particular interest is whether previous seasonal vaccinations will provide cross-immunity to novel pandemic influenza viruses, like the recent 2009 A/H1N1 pandemic virus, which remains debated. [59]
Overall, our study highlights a relatively high and improved vaccine match proportion for A/H3N2 viruses in both hemispheres from 2000 onwards, averaging 55–60%. Can vaccine match be further improved? Possible reasons for vaccine strain mismatch include poor representation of epidemiologically important isolates due to limited geographic coverage of global influenza virus surveillance. Over the study period, 1990–2008, surveillance was heavily focused on temperate areas of the Northern Hemisphere, as illustrated by the fact that ~70% of changes in vaccine recommendations for influenza A/H3N2 and B were associated with viruses first isolated in this region. This may change in the coming years, given strengthening of surveillance efforts, especially in previously underrepresented tropical areas of Central and South America, Africa and Asia. Of particular note is the nomenclature used when describing “Northern” or “Southern” Hemispheric vaccine formulations. Only a tiny proportion of the global population actually resides in Southern temperate regions (estimated at 2% [60]); the vast majority residing in the tropics of both hemispheres (41%) and in Northern temperate regions (57%). Perhaps it would be more useful to think of recommendations specific to tropical countries, irrespective of whether these countries are located in Southern or Northern Hemisphere, but based on other variables predictive of their local influenza epidemiology and seasonality. These might include variables associated with land/sea/air connections to temperate areas or other factors more significant than their Southern or Northern latitude lines.
In addition to limited geographic coverage in surveillance, the time course of vaccine production may also prevent or delay changes in influenza vaccine recommendations and result in mismatch. On occasion, an antigenically-novel strain is recognized as epidemiologically important but gives a poor yield during preliminary vaccine production attempts, preventing revision of vaccine recommendations. This issue can only be resolved by development of new vaccine technologies that do not rely on the time-consuming process of virus growth on eggs. In addition, improving the timeliness of virus surveillance, together with real-time monitoring and interpretation of antigenic evolution globally, [2] may accelerate vaccine strain selection. In the long run, better predictive models of influenza antigenic changes and their epidemiological relevance will become available, although to date, the exact rules driving antigenic evolution and the interactions with other viral functions remain poorly understood. [61–63] Influenza genetic and antigenic data from the global influenza surveillance network should be widely and promptly shared with the scientific community to facilitate innovation in the vaccine strain selection process. [64–66]
Another area of improvement for vaccine match is the issue of the Tropics, where influenza seasonal vaccination can be underused or misused given lack of perceived disease burden. [67–69] It is thought that influenza may circulate year-round in tropical and subtropical areas, with periodic increases in influenza activity that may be linked to environmental factors like precipitation, humidity, or temperature. [70, 71] In interpandemic periods, new influenza antigenic variants may arise from year-round disease transmission in tropical areas, [61, 72] perhaps more specifically in East and Southeast Asia.[49] The biggest issue for influenza vaccination in the Tropics is the diversity of seasonal patterns and timing of influenza activity, where epidemics tend to be asynchronous with those in temperate areas of the Northern and Southern Hemisphere. [49, 71, 73, 74] For instance, Brazil is located in the Southern Hemisphere and uses Southern Hemisphere vaccine recommendations, despite experiencing very early epidemics in several of the Northern Tropical and Subtropical states. Consequently, vaccination is implemented too late in most years in those states, and modeling studies suggest that it would be more beneficial to use the Northern Hemisphere vaccine recommendations and vaccinate earlier in those states. Some locations with semi-annual influenza activity such as Hong-Kong, Taiwan or Singapore [69] may even benefit from semi-annual vaccination if vaccine composition was updated from one hemisphere recommendation to the next; however, local resources may limit this option. Unfortunately, there is no `one solution fits all' for the Tropics, and it is likely that each country or sub-region within a country will have to tailor its vaccination strategy to the specific local epidemiology, regardless of latitude. Rational vaccine policy decisions based on the best available local data are required to protect those living in the Tropics from influenza disease burden and optimize limited public health resources.
In conclusion, the semiannual influenza vaccine composition recommendations have resulted in a better match between circulating strains and vaccine strains for influenza A/H3N2, and have likely improved the effectiveness of the annual vaccination programs in the Southern Hemisphere. It is recommended that these semiannual recommendations continue, particularly in this era of global connectedness and emerging viral threats. Further work should focus on quantifying vaccine match for influenza A/H1N1 subtype, improving vaccine match for influenza B subtype, evaluating and optimizing vaccination strategies for Tropical regions, increasing real-time surveillance of influenza viruses globally and sharing of information, and developing a better understanding of influenza antigenic evolution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008 Aug 8;57(RR7):1–60. [PubMed] [Google Scholar]
- [2].Smith DJ, Lapedes AS, de Jong JC, Bestebroer TM, Rimmelzwaan GF, Osterhaus AD, et al. Mapping the antigenic and genetic evolution of influenza virus. Science. 2004 Jul 16;305(5682):371–6. doi: 10.1126/science.1097211. [DOI] [PubMed] [Google Scholar]
- [3].Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007 Sep 28;25(39–40):6852–62. doi: 10.1016/j.vaccine.2007.07.027. [DOI] [PubMed] [Google Scholar]
- [4].Kitler ME, Gavinio P, Lavanchy D. Influenza and the work of the World Health Organization. Vaccine. 2002 May 15;20(Suppl 2):S5–14. doi: 10.1016/s0264-410x(02)00121-4. [DOI] [PubMed] [Google Scholar]
- [5].Russell CA, Jones TC, Barr IG, Cox NJ, Garten RJ, Gregory V, et al. Influenza vaccine strain selection and recent studies on the global migration of seasonal influenza viruses. Vaccine. 2008 Sep 12;26(Suppl 4):D31–4. doi: 10.1016/j.vaccine.2008.07.078. [DOI] [PubMed] [Google Scholar]
- [6].Recommendation for the composition of influenza virus vaccines for use in 1999. Wkly Epidemiol Rec. 1998 Oct 2;73(40):305–8. [PubMed] [Google Scholar]
- [7].Molbak K, Jensen H, Ingholt L, Aaby P. Risk factors for diarrheal disease incidence in early childhood: a community cohort study from Guinea-Bissau. Am J Epidemiol. 1997 Aug 1;146(3):273–82. doi: 10.1093/oxfordjournals.aje.a009263. [DOI] [PubMed] [Google Scholar]
- [8].Molbak K, Hojlyng N, Gottschau A, Sa JC, Ingholt L, da Silva AP, et al. Cryptosporidiosis in infancy and childhood mortality in Guinea Bissau, west Africa. BMJ. 1993 Aug 14;307(6901):417–20. doi: 10.1136/bmj.307.6901.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Update: influenza activity--United States and worldwide, and the composition of the 1991–92 influenza vaccine. MMWR Morb Mortal Wkly Rep. 1991 Apr 12;40(14):231–4. 9–40. [PubMed] [Google Scholar]
- [10].Update: influenza activity--United States and worldwide, and composition of the 1992–93 influenza vaccine. MMWR Morb Mortal Wkly Rep. 1992 May 8;41(18):315–7. 23. [PubMed] [Google Scholar]
- [11].Update: influenza activity--United States, 1993–94 season. MMWR Morb Mortal Wkly Rep. 1994 Jan 14;43(1):1–3. [PubMed] [Google Scholar]
- [12].Update: influenza activity--United States and worldwide, 1994–95 season, and composition of the 1995–96 influenza vaccine. MMWR Morb Mortal Wkly Rep. 1995 Apr 21;44(15):292–5. [PubMed] [Google Scholar]
- [13].Update: influenza activity--United States and Worldwide, 1995–96 season, and composition of the 1996–97 influenza vaccine. MMWR Morb Mortal Wkly Rep. 1996 Apr 26;45(16):326–9. [PubMed] [Google Scholar]
- [14].Update: influenza activity--United States and worldwide, 1997–98 season, and composition of the 1998–99 influenza vaccine. MMWR Morb Mortal Wkly Rep. 1998 Apr 17;47(14):280–4. [PubMed] [Google Scholar]
- [15].Update: influenza activity--United States and worldwide, 1998–99 season, and composition of the 1999–2000 influenza vaccine. MMWR Morb Mortal Wkly Rep. 1999 May 14;48(18):374–8. [PubMed] [Google Scholar]
- [16].Gladstone BP, Iturriza-Gomara M, Ramani S, Monica B, Banerjee I, Brown DW, et al. Polymerase chain reaction in the detection of an 'outbreak' of asymptomatic viral infections in a community birth cohort in south India. Epidemiol Infect. 2008 Mar;136(3):399–405. doi: 10.1017/S0950268807008709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Influenza virus strain identification for the 1991–92 influenza season. Can Commun Dis Rep. 1992 Sep 25;18(18):141–4. [PubMed] [Google Scholar]
- [18].Influenza B virus strain identification and the rise of influenza A/Beijing/32/92-like strains during the 1992–1993 influenza season in Canada. Can Commun Dis Rep. 1993 Sep 30;19(18):148–52. [PubMed] [Google Scholar]
- [19].Influenza in Canada, 1993–1994 season. Can Commun Dis Rep. 1994 Nov 15;20(21):185–92. [PubMed] [Google Scholar]
- [20].Influenza in Canada. 1994–1995 season. Can Commun Dis Rep. 1995 Dec 15;21(23):205–12. [PubMed] [Google Scholar]
- [21].Influenza in Canada--1995–1996 season. Can Commun Dis Rep. 1996 Dec 1;22(23):193–9. [PubMed] [Google Scholar]
- [22].Influenza in Canada--1996–1997 season. Can Commun Dis Rep. 1997 Dec 15;23(24):185–92. [PubMed] [Google Scholar]
- [23].Buck P, Herman S, Scott C, Winchester B, Zabchuk P, Sockett P, et al. Influenza in Canada--1997–1998 season. Can Commun Dis Rep. 1998 Nov 1;24(21):169–76. [PubMed] [Google Scholar]
- [24].Pelletier L, Buck P, Zabchuk P, Winchester B, Tam T. Influenza in Canada--1998–1999 season. Can Commun Dis Rep. 1999 Nov 15;25(22):185–92. [PubMed] [Google Scholar]
- [25].Squires SG, Pelletier L, Zabchuk P, Winchester B, Tam T. Influenza in Canada--1999–2000 season. Can Commun Dis Rep. 2001 Jan 1;27(1):1–9. [PubMed] [Google Scholar]
- [26].Influenza in Canada: 2000–2001 season. Can Commun Dis Rep. 2002 Feb 1;28(3):17–28. [PubMed] [Google Scholar]
- [27].Macey JF, Tam TW, Li Y, Winchester B, Zabchuk P. Influenza in Canada: 2001–2002 season. Can Commun Dis Rep. 2003 Mar 15;29(6):45–59. [PubMed] [Google Scholar]
- [28].Influenza in Canada: 2003–2004 season. Can Commun Dis Rep. 2005 Jan 1;31(1):1–18. [PubMed] [Google Scholar]
- [29].Influenza in Canada--2004–2005 season. Can Commun Dis Rep. 2006 Mar 15;32(6):57–74. [PubMed] [Google Scholar]
- [30].Reyes F, Macey JF, Aziz S, Li Y, Watkins K, Winchester B, et al. Influenza in Canada: 2005–2006 season. Can Commun Dis Rep. 2007 Feb 1;33(3):21–41. [PubMed] [Google Scholar]
- [31].Reyes F, Aziz S, Li Y, Macey JF, Winchester B, Garner M, et al. Influenza in Canada: 2006–2007 season. Can Commun Dis Rep. 2008 Mar;34(3):1–25. [PubMed] [Google Scholar]
- [32].Influenza activity in Europe 2002–2007. European Influenza Surveillance Scheme Weekly Electronic Bulletin [Google Scholar]
- [33].National Influenza Surveillance - Annual Reports 1994–2005. Communicable Diseases Intelligence [Google Scholar]
- [34].Annual Summaries - 1999. Institute of Environmental Sciences and Research; Wellington: 2000. [Google Scholar]
- [35].Annual Summaries - 2000. Institute of Environmental Sciences and Research; Wellington: 2001. [Google Scholar]
- [36].Influenza Annual Reports 2000–2007. Public Health Surveillance - Information for New Zealand Public Health Action [Google Scholar]
- [37].Stamboulian D, Bonvehi PE, Nacinovich FM, Cox N. Influenza. Infect Dis Clin North Am. 2000 Mar;14(1):141–66. doi: 10.1016/s0891-5520(05)70222-1. [DOI] [PubMed] [Google Scholar]
- [38].Update: influenza activity--worldwide, March-August 1997. MMWR Morb Mortal Wkly Rep. 1997 Sep 5;46(35):815–8. [PubMed] [Google Scholar]
- [39].Update: influenza activity--worldwide, April-September 1998. MMWR Morb Mortal Wkly Rep. 1998 Oct 9;47(39):830–3. [PubMed] [Google Scholar]
- [40].Update: influenza activity--worldwide, May-September 1999. MMWR Morb Mortal Wkly Rep. 1999 Oct 8;48(39):883–6. [PubMed] [Google Scholar]
- [41].Influenza activity--United States and worldwide, April-October 2000. MMWR Morb Mortal Wkly Rep. 2000 Nov 10;49(44):1006–8. [PubMed] [Google Scholar]
- [42].Update: influenza activity--United States and worldwide, May-September 2001. MMWR Morb Mortal Wkly Rep. 2001 Sep 28;50(38):822–5. [PubMed] [Google Scholar]
- [43].Update: Influenza activity--United States and worldwide, June-September, 2002. MMWR Morb Mortal Wkly Rep. 2002 Oct 4;51(39):880–2. [PubMed] [Google Scholar]
- [44].Update: influenza activity--United States and worldwide, May-September 2003. MMWR Morb Mortal Wkly Rep. 2003 Sep 26;52(38):911–3. [PubMed] [Google Scholar]
- [45].Update: influenza activity--United States and worldwide, May-October 2004. MMWR Morb Mortal Wkly Rep. 2004 Oct 29;53(42):993–5. [PubMed] [Google Scholar]
- [46].Update: influenza activity--United States and worldwide, May 22-September 3, 2005, and 2005–06 season vaccination recommendations. MMWR Morb Mortal Wkly Rep. 2005 Sep 16;54(36):899–902. [PubMed] [Google Scholar]
- [47].Update: influenza activity--United States and worldwide, May 21-September 9, 2006. MMWR Morb Mortal Wkly Rep. 2006 Sep 22;55(37):1021–3. [PubMed] [Google Scholar]
- [48].Update: influenza activity--United States and worldwide, May 20-September 15, 2007. MMWR Morb Mortal Wkly Rep. 2007 Sep 28;56(38):1001–4. [PubMed] [Google Scholar]
- [49].Russell CA, Jones TC, Barr IG, Cox NJ, Garten RJ, Gregory V, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science. 2008 Apr 18;320(5874):340–6. doi: 10.1126/science.1154137. [DOI] [PubMed] [Google Scholar]
- [50].Kanegae Y, Sugita S, Endo A, Ishida M, Senya S, Osako K, et al. Evolutionary pattern of the hemagglutinin gene of influenza B viruses isolated in Japan: cocirculating lineages in the same epidemic season. J Virol. 1990 Jun;64(6):2860–5. doi: 10.1128/jvi.64.6.2860-2865.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology. 1990 Mar;175(1):59–68. doi: 10.1016/0042-6822(90)90186-u. [DOI] [PubMed] [Google Scholar]
- [52].Skowronski DM, De Serres G, Dickinson J, Petric M, Mak A, Fonseca K, et al. Component-specific effectiveness of trivalent influenza vaccine as monitored through a sentinel surveillance network in Canada, 2006–2007. J Infect Dis. 2009 Jan 15;199(2):168–79. doi: 10.1086/595862. [DOI] [PubMed] [Google Scholar]
- [53].Marti F, Steffen R, Mutsch M. Influenza vaccine: a travelers' vaccine? Expert Rev Vaccines. 2008 Jul;7(5):679–87. doi: 10.1586/14760584.7.5.679. [DOI] [PubMed] [Google Scholar]
- [54].Mutsch M, Tavernini M, Marx A, Gregory V, Lin YP, Hay AJ, et al. Influenza virus infection in travelers to tropical and subtropical countries. Clin Infect Dis. 2005 May 1;40(9):1282–7. doi: 10.1086/429243. [DOI] [PubMed] [Google Scholar]
- [55].Roos R. Experts consider 4-strain flu vaccine to fight B viruses. Center for Infectious Disease Research & Policy; Minneapolis: 2009. [Google Scholar]
- [56].Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med. 2005 Feb 14;165(3):265–72. doi: 10.1001/archinte.165.3.265. [DOI] [PubMed] [Google Scholar]
- [57].Jefferson TO, Rivetti D, Di Pietrantonj C, Rivetti A, Demicheli V. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2007;(2):CD001269. doi: 10.1002/14651858.CD001269.pub3. [DOI] [PubMed] [Google Scholar]
- [58].Belongia EA, Kieke BA, Donahue JG, Greenlee RT, Balish A, Foust A, et al. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J Infect Dis. 2009 Jan 15;199(2):159–67. doi: 10.1086/595861. [DOI] [PubMed] [Google Scholar]
- [59].Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-Reactive Antibody Responses to the 2009 Pandemic H1N1 Influenza Virus. N Engl J Med. 2009 Sep 10; doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- [60].The World Factbook. Central Intelligence Agency; Washington, DC: 2009. [Google Scholar]
- [61].Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC. The genomic and epidemiological dynamics of human influenza A virus. Nature. 2008 May 29;453(7195):615–9. doi: 10.1038/nature06945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hensley SE, Das SR, Bailey AL, Schmidt LM, Hickman HD, Jayaraman A, et al. Hemagglutinin receptor binding avidity drives influenza A virus antigenic drift. Science. 2009 Oct 30;326(5953):734–6. doi: 10.1126/science.1178258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Memoli MJ, Jagger BW, Dugan VG, Qi L, Jackson JP, Taubenberger JK. Recent human influenza A/H3N2 virus evolution driven by novel selection factors in addition to antigenic drift. J Infect Dis. 2009 Oct 15;200(8):1232–41. doi: 10.1086/605893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Plotkin JB, Dushoff J. Codon bias and frequency-dependent selection on the hemagglutinin epitopes of influenza A virus. Proc Natl Acad Sci U S A. 2003 Jun 10;100(12):7152–7. doi: 10.1073/pnas.1132114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bush RM, Bender CA, Subbarao K, Cox NJ, Fitch WM. Predicting the evolution of human influenza A. Science. 1999 Dec 3;286(5446):1921–5. doi: 10.1126/science.286.5446.1921. [DOI] [PubMed] [Google Scholar]
- [66].Salzberg S. The contents of the syringe. Nature. 2008 Jul 10;454(7201):160–1. doi: 10.1038/454160a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gordon A, Ortega O, Kuan G, Reingold A, Saborio S, Balmaseda A, et al. Prevalence and seasonality of influenza-like illness in children, Nicaragua, 2005–2007. Emerg Infect Dis. 2009 Mar;15(3):408–14. doi: 10.3201/eid1503.080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Chow A, Ma S, Ling AE, Chew SK. Influenza-associated deaths in tropical Singapore. Emerg Infect Dis. 2006 Jan;12(1):114–21. doi: 10.3201/eid1201.050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wong CM, Yang L, Chan KP, Leung GM, Chan KH, Guan Y, et al. Influenza-associated hospitalization in a subtropical city. PLoS Med. 2006 Apr;3(4):e121. doi: 10.1371/journal.pmed.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shek LP, Lee BW. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr Respir Rev. 2003 Jun;4(2):105–11. doi: 10.1016/s1526-0542(03)00024-1. [DOI] [PubMed] [Google Scholar]
- [71].Alonso WJ, Viboud C, Simonsen L, Hirano EW, Daufenbach LZ, Miller MA. Seasonality of influenza in Brazil: a traveling wave from the Amazon to the subtropics. Am J Epidemiol. 2007 Jun 15;165(12):1434–42. doi: 10.1093/aje/kwm012. [DOI] [PubMed] [Google Scholar]
- [72].Viboud C, Alonso WJ, Simonsen L. Influenza in tropical regions. PLoS Med. 2006 Apr;3(4):e89. doi: 10.1371/journal.pmed.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].de Mello WA, de Paiva TM, Ishida MA, Benega MA, Dos Santos MC, Viboud C, et al. The dilemma of influenza vaccine recommendations when applied to the tropics: the Brazilian case examined under alternative scenarios. PLoS One. 2009;4(4):e5095. doi: 10.1371/journal.pone.0005095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Laguna-Torres VA, Gomez J, Ocana V, Aguilar P, Saldarriaga T, Chavez E, et al. Influenza-like illness sentinel surveillance in Peru. PLoS One. 2009;4(7):e6118. doi: 10.1371/journal.pone.0006118. [DOI] [PMC free article] [PubMed] [Google Scholar]