Abstract
Understanding the properties of viruses capable of establishing infection during perinatal transmission of HIV-1 is critical for designing effective means of limiting transmission. We previously demonstrated that the newly transmitted viruses (in infant) were more fit in growth, as imparted by their envelope glycoproteins, than those in their corresponding mothers. Here, we further characterized the viral envelope glycoproteins from six mother-infant transmission pairs and determined whether any specific envelope functions correlate with HIV-1 subtype C perinatal transmission. We found that most newly transmitted viruses were less susceptible to neutralization by their maternal plasma compared to contemporaneous maternal viruses. However, the newly transmitted variants were sensitive to neutralization by pooled heterologous plasma but in general were resistant to IgG1 b12. Neither Env processing nor incorporation efficiency was predictive of viral transmissibility. These findings provide further insight into the characteristics of perinatally transmissible HIV-1 and may have implications for intervention approaches.
Keywords: HIV-1 subtype C, perinatal transmission, envelope glycoproteins, neutralization, autologous or heterologous antibodies, envelope processing and incorporation
Introduction
Mother-to-child transmission (MTCT) of HIV-1 is the primary mode of pediatric HIV-1 infection and remains a significant problem in developing countries where anti-retroviral therapy (ART) is still not widely available. HIV-1 infected children account for 20% of all HIV-1 related deaths (Luzuriaga and Sullivan, 2002). More than two thirds of new HIV-1 infections occur in sub-Saharan Africa and 60% of these infections are with HIV-1 subtype C with a significant number of infections in infants and children. HIV-1 subtype C is currently responsible for the vast majority of new HIV-1 infections worldwide (http://www.unaids.org). However, factors that contribute to the continuing expansion of subtype C viruses have yet to be clearly elucidated.
It has been shown that subtype C viruses display biological properties, such as restricted CCR5 usage and reduced replicative capacity that distinguish it from other subtypes (Abebe et al., 1999; Abraha et al., 2009; Ball et al., 2003; Bjorndal et al., 1999; Neilson et al., 1999; Zhang et al., 2006). Such differences may contribute to the rapid spread and predominance of subtype C HIV-1 in sub-Saharan Africa (Abraha et al., 2009; Ndung’u, Renjifo, and Essex, 2001). Since many of these parameters are influenced by the HIV-1 envelope (Env) glycoprotein, we and others have suggested that subtype C viruses might have Env glycoproteins that are atypical in structure or function compared to viruses from other HIV-1 subtypes (Cilliers et al., 2003; Zhang et al., 2006). Whether the observed differences in cellular tropism, transmission and pathogenetic outcome between subtype C and other subtypes correlate with biological or genetic properties of the Env glycoprotein has not been resolved. Previous studies of HIV-1 transmission, including sexual transmission and MTCT, have mainly focused on evaluating the genotypic and antigenic properties of transmitted variants but have provided little information on potential correlations between Env function and MTCT (Derdeyn et al., 2004; Dickover et al., 2006; Haaland et al., 2009; Kliks et al., 1994; Rainwater et al., 2007; Scarlatti et al., 1993a; Wolinsky et al., 1992; Wu et al., 2006). In addition, our understanding of perinatal transmission in infants is mainly derived from studies of subtype B or other non-subtype C HIV-1. The applicability of such findings to subtype C remains to be substantiated. Given the high prevalence of subtype C infections, a complete understanding of virus transmission and identification of factors that may affect the transmission of subtype C HIV-1 are of importance.
Selective transmission of a few maternal variants has been the dominant feature during MTCT (Ahmad et al., 1995; Dickover et al., 2001; Scarlatti et al., 1993b; Wolinsky et al., 1992; Zhang et al., 2002). However, the basis of the observed genetic bottleneck remains poorly defined and the factors governing variant selection are largely unknown. The bottleneck transmission has been attributed to various factors including specific viral selection (Wolinsky et al., 1992) and neutralization resistance of the transmitted viruses (Dickover et al., 2006; Kliks et al., 1994; Scarlatti et al., 1993a; Wu et al., 2006). Recently, we demonstrated that in infected mother-infant pairs (MIPs), the newly transmitted viruses, those in infants, were selected to have higher ex vivo fitness, as imparted by their envelope glycoproteins, than viruses from their corresponding chronically infected maternal donors. Our study showed that the Env V1-V5 region was sufficient to confer the higher replicative fitness phenotype (Kong et al., 2008). Genetic analyses indicated a significant viral bottleneck during perinatal transmission among the MIPs analyzed (Zhang et al., submitted for publication). Since all the mother and infants are anti-retroviral naïve, these MIPs provide a setting in which to examine the biological properties of transmitted viruses and investigate the correlates of subtype C HIV-1 perinatal transmission without additional selective pressure. Here, we further explored the relationships between Env biological functions such as synthesis and processing, incorporation, or susceptibility to antibody neutralization and the likelihood to achieve transmission through the maternal-infant bottleneck. We found that in the majority of cases, the newly transmitted viruses from the infant were less susceptible to neutralization by their maternal plasma compared with contemporaneous maternal viruses, but no correlation between Env processing or incorporation efficiency and transmissibility was detected. These results, in conjunction with our previous studies, suggest that viruses with the higher replication fitness, and lower susceptibility to neutralization by the maternal plasma were selectively transmitted during subtype C HIV-1 perinatal transmission. These findings provide further insight into the characteristics of perinatally transmissible HIV-1 and also provide important information relevant to the development of effective vaccine intervention strategies.
Materials and Methods
Patient information and sample collection
Six MIPs labeled 2617, 1449, 1084, 2669, 2873, 1984 from our previous study (Zhang et al., 2006) were characterized in the study. The mothers were known to be HIV-1 positive at the time of delivery and all subjects were asymptomatic and were not yet eligible for ART at the time. The babies were all breast-fed and drug naïve. Maternal samples at delivery were defined as baseline and infant baseline samples were referred to the first postpartum HIV-1 PCR positive time point. The baseline HIV-1 serological status of the mother and HIV-1 infection in infants was determined as previously described (Zhang et al., 2006).
Cell cultures
293T, COS-1 and TZM-bl ( NIH AIDS Research and Reference Reagent Program catalog no. 8129) cells were maintained in Dulbecco’s modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS, Hyclone) and 100 μg/ml penicillin-streptomycin. PBMC were purified using Lymphoprep (Life Technology) from a HIV-seronegative donor and then propagated in RPMI 1640 containing 10% FBS, 100 μg/ml penicillin-streptomycin and 5 μg/ml of phytohemagglutinin (Sigma, St. Louis, MO) for 40 hrs before being infected with viruses.
Env glycoprotein expression constructs and proviral expression constructs
The V1-V5 region of env gene was amplified by nested PCR from uncultured patient PBMC, cloned into the pGEM-T Easy vector and sequenced as described previously (Zhang et al., 2006). The preexisting, genetically characterized Env V1-V5 clones were used as the source of genetic materials for generation of the Env chimeras. Approximately 20 representative Env clones, based on Env V1-V5 length and putative N-glycan number, as well as sequence diversity, were selected from each MIP baseline viral population (Kong et al., 2008). The Env V1-V5 region from selected clones was amplified from the pGEM-T Easy vector by using primers containing restriction enzyme sites Dra III and AvrII: sense primer C-DraIII (5′-TGACCCCACTCTGTGTCACTTTA-3′) and antisense primer C-AvrII (5′-CTATTCCTAGGGGCTTAATTTCTACCACTT-3′). The resulting PCR products were subcloned into a shuttle vector, pSP72 NLA/S/Av, using the restriction enzyme sites Dra III and AvrII. The pSP72 NLA/S/Av was generated by introducing the envelope gene of pNL4-3 modified with AgeI/SbfI/AvrII by silent mutation into a cloning vector, pSP72 (Promega) and was used for the sequential cloning of patient V1-V5 region of env gene into an Env expression vector, pSRH NL A/S/Av. This vector was generated by modifying the mammalian expression vector pSRH which contains an SV40 promoter and reading frames for NL 4-3 Tat, Rev and Env (kindly provided by Dr. Eric Hunter, Emory University). The chimeric Env expression construct containing patient-derived Env V1-V5 region was generated by substituting EcoRI - XhoI region of pSP72A/S/Av with the corresponding region of pSRH NL A/S/Av. All the patient-derived chimeric Env expression constructs were first screened for biological function using the fusion assay. Briefly, 2 ×104 COS-1 cells per well grown in 24-well plates were transfected with 0.5 μg of the patient-derived Env glycoprotein expression plasmid. Twenty-four hours subsequent to transfection, 5 × 104 of the TZMbl (target) cells were added onto the Env expressing COS-1 (effector) cells. The effector and target cells were allowed to fuse for 24 hours. The cells were stained for β galactosidase expression and the fusion level was determined by the number of blue foci. Between 30% – 70% of the selected clones were biologically functional. Finally, the functional envelope construct was transferred into a proviral expression vector- pNL4-3 A/S/Av, with NL4-3 viral backbone, by substituting EcoRI - XhoI region of pSRH NL A/S/Av with the corresponding region of pNL4-3 A/S/Av, resulting in the infectious molecular clone plasmids. Between 4-13 representative Env clones from each MIP baseline viral population were available for the functional assays as described below.
Virus stocks
NL4-3 or chimeric viruses bearing patient-derived Env V1-V5 region were produced by transfection of 293 T cells with proviral constructs. Nine μg of proviral construct was transfected into 2.2 × 106 293 T cells using Fugene 6 (Roche). The resulting infectious viruses were harvested 48 hrs post-transfection, filtered through 0.45μm filter and stored at −80°C. The tissue culture dose for 50% infectivity (TCID50) was determined for each recombinant virus in TZM-bl cells. Viral titer was defined as TCID50/ml.
Co-receptor usage and cell tropism
Co-receptor usage and cell tropism were defined using Ghost cell lines that express specific co-receptors and MT-2 cells as described previously (Zhang et al., 2005).
Synthesis and processing of Env glycoproteins
The kinetics of glycoprotein synthesis and processing were analyzed by pulse-chase as described previously (West et al., 2002) with some modifications. Briefly, 1.3 × 106 COS-1 cells grown in 10 cm culture dishes were transfected with 9 μg of each Env expression construct. At 24 hrs post-transfection, cells were dissociated from the plate and divided into 6-well plates at a density of 9 ×105 transfected cells per well to allow the cells to grow overnight. COS-1 cells expressing NL4-3 Env protein or chimeric Env proteins bearing patient-derived Env V1-V5 region were starved for 40 min with DMEM Cys/Met-free medium and pulse-labeled with [35S] Methionine for 45 min at 37°C. The pulse labeled cells were then chased in complete medium for 2, 6 and 24 hrs, respectively. At the completion of the chase, the culture supernatant was collected, filtered through a 0.45 μm syringe filter and lysed with lysis buffer to a final concentration of 1% NP40, 0.1% SDS, 0.5% deoxycholic acid sodium salt and 10 μl/ml Halt Protease Inhibitor Cocktail (Pierce). To prepare cell lysates, cells were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed in the buffer described above on ice. The cell lysates were clarified by centrifugation at 20,000 × g for 1 min. The clarified cell lysates and supernatants were immunoprecipitated with pooled subtype C HIV-1 infected patient serum. Viral proteins were resolved on 8% SDS-PAGE and visualized by autoradiography.
Glycoprotein incorporation into virions
To determine the level of viral envelope glycoprotein incorporated into virus particles, 293T cells were transfected with the proviral constructs. Briefly, 2.2 × 106 293T cells grown in 10 cm culture dishes were transfected with 9 μg of each proviral construct. At 48 hrs post-transfection, viral supernatants were harvested and filtered through 0.45 μm filters. A fraction was kept for p24 quantification and infectivity assay, and the remaining virus-containing supernatant was centrifuged to pellet the virus at 65,000 rpm (Beckman, Type 90 Ti) through a 20% (wt/wt) sucrose cushion for 2 hrs at 4°C. The resulting pellet was lysed for SDS-PAGE analysis. Viral proteins were separated by SDS-PAGE, blotted onto PVDF membranes (Amersham Biosciences) and analyzed by Western blotting using monoclonal antibody (mAb) 2F5 (Polymun Scientific Inc, Vienna, Austria) to detect gp41, or anti-p24 mAb (NIH AIDS Research & Reference Reagent Program, Cat. no. 1513) to detect p24. Protein quantification was performed using a Fluor-S™ Multilmager (BIORAD).
Viral infectivity assay
The p24 content of each virus stock from transfected 293T cells was determined using HIV-1 p24 ELISA kit (PerkinElmer Life Sciences, Inc.). Two ng of p24 of each virus was used to infect 2 ×104 TZM-bl target cells in triplicates in the presence of 40 μg/ml of DEAE-dextran in a 96-well plate. At 48 hrs post-infection, cells were lysed and luciferase activity was measured using the Luciferase Assay System (Promega, Madison, WI). Luciferase activity was measured using a LUMIstar Luminometer (BMG Lab technologies Offenburg, Germany). Background luminescence in uninfected wells was subtracted from all experimental wells. Relative infectivity was calculated by comparing the luciferase activity between the patient’s proviral construct and the NL4-3 transfected cells.
Replication kinetics of chimeric viruses
For replication kinetics in TZM-bl cells, equivalent infectious units, 200 TCID50, of each chimeric viruses from transfected 293T cells were added to duplicate wells in a 48-well plate containing 2 ×104 TZM-bl cells per well. After incubation at 37°C overnight, cells were washed 3 times with medium and 500 μl of fresh medium was added. A 250 μl aliquot of each infected culture was sampled on day 0, 2, 4, 6, 8, 10, 12 and 14 and the culture was replenished with an equal volume of fresh medium. In order to determine viral yield at each timepoint, 75 μl of each collected culture supernatant was used to infect 2 ×104 TZM-bl cells in a 96- well plate. At 48 hrs post-infection, cells were lysed and luciferase activity was determined as described above.
Neutralization assays
The susceptibility of the chimeric viruses bearing patient- derived Env V1-V5 region to neutralization by maternal or pooled plasma or mAb IgG1 b12 (Polymun Scientific Inc, Vienna, Austria) was assayed in the TZM-bl cells in 96-well plates as described previously (Montefiori, 2004; Zhang et al., 2005) with some modifications. Briefly, 20 TCID50 of each provirus (in 50 μl) was incubated with equal volumes of threefold serial dilutions of IgG1 b12 or heat-inactivated plasma, or with growth medium alone at 37°C for 1 hr in the presence of 1 μM Indinavir and 40 μg/ml of DEAE-dextran. The virus-plasma or virus-antibody mixture was then added to TZM-bl cells in triplicate for an additional 3 hr at 37°C. Thereafter, 50 ul of medium was added to each well, and the cells were incubated at 37°C for 2 days. After two days, the cells were washed once with PBS, lysed and the luciferase activity of each well was measured as described above. Background luminescence in uninfected wells was subtracted from all experimental wells. Differences between the luciferase activity in the presence of plasma or antibody and growth medium alone were calculated as the percentage of antibody neutralization. The IC50 was defined as the reciprocal dilution of plasma or the concentration of the IgG1 b12 that causes 50% inhibition of virus infection and was calculated using GraphPad Prism software version 5.0 (GraphPad Software, Inc., San Diego, CA). Maternal plasma and pooled plasma from 50 HIV-1 subtype C infected Zambian patients were tested at a starting dilution of 1: 50 and 1:100, respectively. The mAb IgG1 b12 was tested at a starting concentration of 25 μg/ml.
Statistical analysis
All statistical tests were performed with GraphPad Prism software version 5.0. In cases in which the IC50 for maternal plasma was < 50, the midpoint value between 0 and 50, 25, was assigned for statistical analysis. For mAb IgG1 b12, the IC50 value of 25 was used when there was < 50% neutralization at the highest concentration (25 μg/ml) tested, Significance was reported when P ≤ 0.05, and a trend was reported when 0.05 < P ≤ 0.1. Correlation of neutralization IC50 to the Env V1-V5 length and N-glycosylation sites was examined with Spearman’s correlation test.
Results
HIV-1 infected mother-infant pairs
The genetically characterized HIV-1 subtype C Env sequences from six pre-existing mother-infant pairs – MIP 2617, 1449, 1084, 2669, 2873 and 1984 (Zhang et al., 2006) were used as source materials for generation of Env chimeras. These infants were all negative at birth for HIV-1 by both viral isolation and PCR, but were found to be HIV-1 positive at either 2 months (2617, 1449, 2669 and 2873) or 4 months (1084 and 1984) after birth. Thus, HIV-1 transmission most likely occurred either during delivery or through breastfeeding. Because the amount of sample from these children was limited, priority was given to viral isolation in lieu of PCR when necessary (e.g., infant 1084, viral isolation was positive by 4 month but the first PCR was performed 6 months after birth). The first PCR positive time point from each infant is indicated in Table 1 along with the time points studied and the number of clones analyzed for each patient.
Table 1.
Summary of mother and infant transmission pairs
| Patient ID | Subject | Infant’s first HIV-PCR Positive Time a | Time point analyzed b | No. of Clones Analyzed |
|---|---|---|---|---|
| 1449 | Mother | N/A | M00 | 2 |
| Infant | 2 mo | 2 mo | 2 | |
| 2669 | Mother | N/A | M00 | 3 |
| Infant | 2 mo | 2 mo | 5 | |
| 2873 | Mother | N/A | M00 | 4 |
| Infant | 2 mo | 2 mo | 4 | |
| 2617 | Mother | N/A | M00 | 5 |
| Infant | 2 mo | 2 mo | 4 | |
| 1984 | Mother | N/A | M00 | 7 |
| Infant | 4 mo | 4 mo | 6 | |
| 1084 | Mother | N/A | M00 | 4 |
| Infant | 6 mo | 6 mo | 3 |
Months after birth
M00: maternal samples at delivery were defined as baseline N/A means that this category is not applicable to maternal subjects
Co-receptor usage and cell tropism of patient-derived viruses
Previously, we demonstrated that the primary viral isolates from these patients studied here exclusively used CCR5 as co-receptor, exhibited macrophage-tropism, and did not infect T cell lines or form syncytia in vitro (Zhang et al., 2006). To ensure that the characterized Env sequences possessed co-receptor usage properties consistent with those defined for cognate viruses isolated by co-culture, we generated infectious molecular clones in HIV-1 NL4-3 bearing patient-derived Env V1-V5 region. Co-receptor usage of the chimeric infectious molecular clones was tested in Ghost cell lines expressing different co-receptors. Consistent with what we observed for the primary isolates, all chimeric viruses exhibited CCR5 tropism and did not form syncytia with the MT-2 cells (data not shown).
Biosynthesis and processing of patient-derived Env glycoprotein
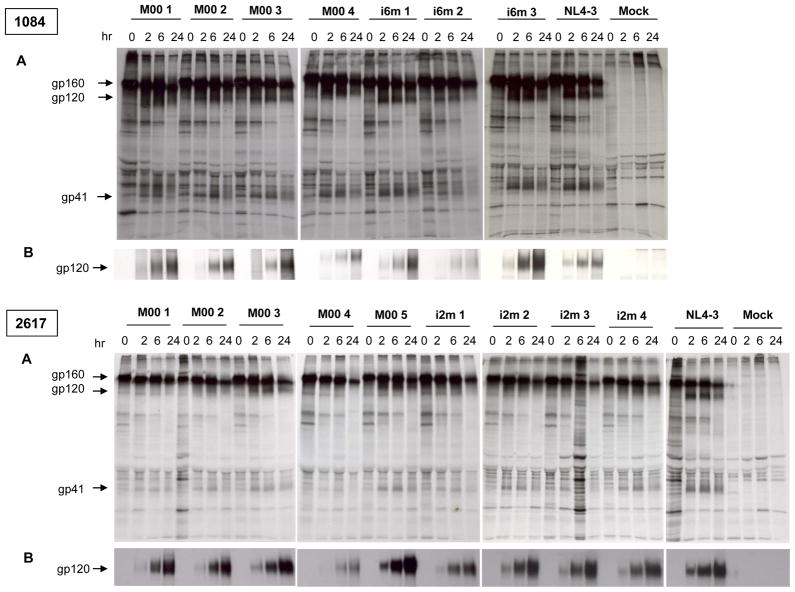
The HIV-1 Env glycoprotein is synthesized as a 160-kDa precursor protein, gp160, that is cleaved by cellular proteases during trafficking to the plasma membrane to generate the mature surface glycoprotein gp120 and the transmembrane glycoprotein gp41. Thus the efficiency of glycoprotein synthesis and cleavage could impact glycoprotein availability for incorporation into particles, and therefore the infectivity of those particles. The kinetics of synthesis and processing of patient-derived Env glycoproteins was analyzed in COS-1 cells by pulse-chase analysis. The results of two representative pairs are shown in Figure 1 and the rest are shown in Supplementary Figure 1. The glycoprotein from maternal and infant variants was synthesized and processed over the course of a 24 hr chase, as evidenced by the decay of labeled gp160 concomitant with the increase of gp120 and gp41 in the cell lysates (Fig. 1A and Supplementary Fig. 1A). However, only a small fraction of the precursor gp160 was processed into the mature gp120. The amount of gp120 detected in the supernatants increased over the chase period indicating shedding from the plasma membrane after glycoprotein processing (Fig. 1B and Supplementary Fig. 1B). As early as at 2 hrs of chase, the gp120 and gp41 appeared in cell lysates, and gp120 was observed in supernatants derived from all the Env expression clones (Fig. 1A and B; Supplementary Fig. 1A and B). Slight differences in electrophoretic mobility of gp120, which could be attributed to differences in the extent of N-glycosylation, were observed for different chimeras in both cell lysates and supernatants (Fig. 1A and B; Supplementary Fig. 1A and B). Nevertheless, no obvious pattern in Env synthesis and processing that could distinguish the transmitted (infant) from non-transmitted (mother) viruses, despite differences in the level of expression and extent of processing among chimeras.
Figure 1. Synthesis and processing of Env glycoproteins gp160 and gp120 of two representative MIPs 1084 and 2617.
COS-1 cells expressing chimeric Env proteins bearing patient-derived Env V1-V5 region were pulse-labeled with [35 S] methionine at 37°C for 40min. Cells were resuspended in complete medium and chased for varying times as indicated (hr). Cell lysates and supernatant were immunoprecipitated with pooled anti-HIV-1 antisera. Viral proteins from cell lysates (A) and supernatant (B) were resolved by SDS-PAGE.
Infectivity of maternal and infant chimeric viruses
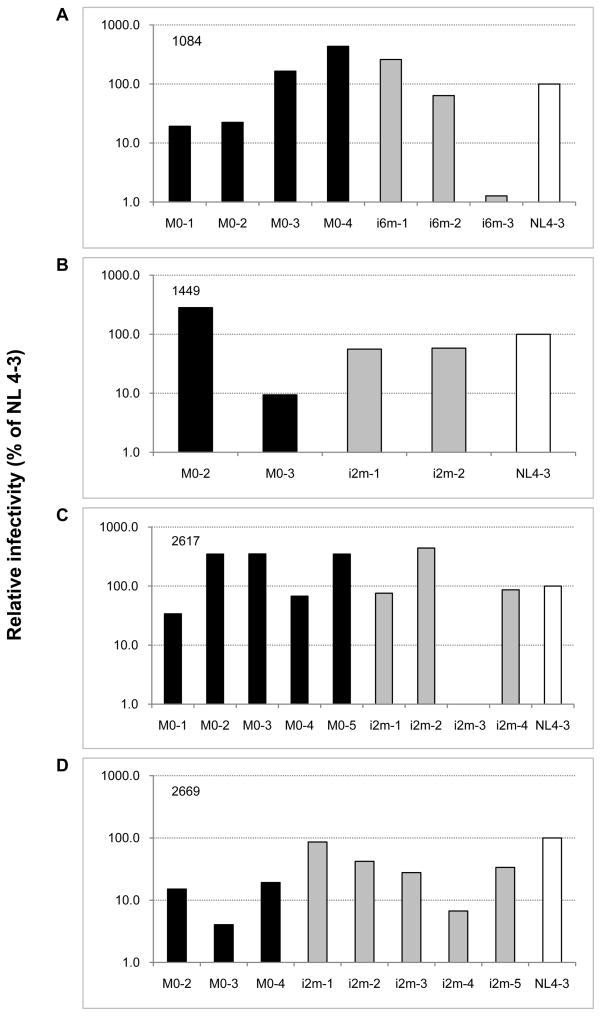
In order to evaluate the ability of each Env glycoprotein to mediate viral infectivity, we generated patient Env V1-V5 region-bearing viruses by transfection of 293T cells. The resulting virus-containing supernatants were normalized for p24 content and were used to infect TZM-bl indicator cells. Differential infectivity phenotypes were observed among transmitted and non-transmitted viruses (Fig. 2). For most patient-derived chimeras, with the exception of MIP 2669, the viral infectivity was either slightly lower (MIP 1449 and 2617 Env) or higher (MIP 1084 and 2617) relative to NL4-3 having wild type Env. There were also some patient-derived viruses that exhibited much lower infectivity (1084 i6m-3 and 2669M0-3) or almost no detectable infectivity (2617 i2m-3) when compared to the NL4-3. These differences in infectivity did not always parallel their fusion capacity, since higher Env-mediated fusogenicity did not always link to efficient viral infectivity (data not shown). Importantly, the differential level of viral infectivity observed among mother and infant viruses did not segregate the transmitted from non-transmitted viruses, suggesting a lack of correlation between the viral infectivity and transmissibility.
Figure 2. Relative infectivity of chimeric viruses from representative pairs.
Chimeric viruses bearing patient-derived Env V1-V5 region or NL4-3 were produced by transfection of 293 T cells with the indicated proviral constructs and normalized to p24. Two ng of p24 of each virus was used to infect TZM-bl target cells in triplicates in the presence of 40 μg/ml of DEAE-dextran as described in material and methods. After 48 hrs post-infection, cells were lysed and luciferase activity was measured. The infectivity of NL 4-3 was set at 100, the infectivity of patient-derived chimeric viruses was calculated relative to this value.
Replication kinetics of maternal and infant chimeric viruses
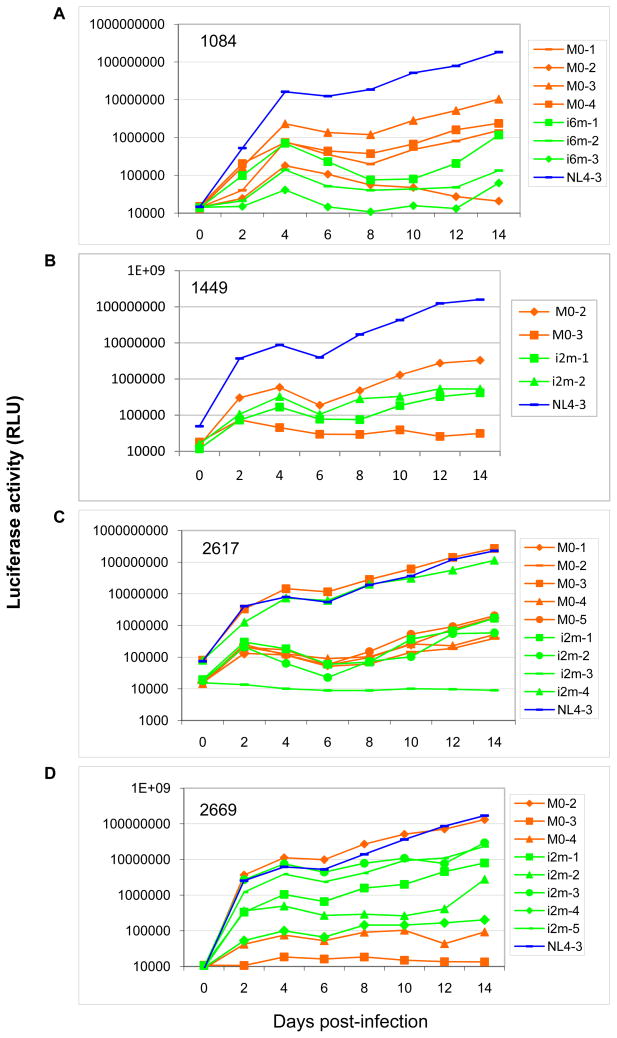
The ability of the chimeric viruses derived from each mother-infant pair to establish a productive infection in TZM-bl cells was compared (Fig. 3). Equivalent infectious units of each chimeric virus were used to infect the cells, and replication levels were determined by measuring the luciferase activity from the infected TZM-bl cells. NL4-3 which was used as control replicated to a higher level relative to most patient-derived chimeric viruses. It also displayed a steady increase in the replication for the duration of the experiment in TZM-bl cells (Fig. 3A, B, C, and D). There were also a few patient-derived viruses that replicated as well as NL4-3 (MIP 2617 M0-3, i2m-4 and 2669 M0-2 and i2m-3). In addition, there were some maternal and infant viruses that were unable to establish a productive infection in TZM-bl cells such as 1084 i6m-3, 2671 i2m-3 and 2669 M0-3. In general, the replication kinetics was variable between different patient isolates and within the MIPs with no distinct patterns being observed except that most patient Env chimera viruses replicated less well than the subtype B NL4-3.
Figure 3. Replication kinetics of chimeric viruses from representative pairs.
Equal amount of infectious unit from each chimeric virus bearing patient-derived Env V1-V5 region was used to infect TZM-bl cells. NL4-3 was used as control. Cell-free supernatant was collected and assayed for replication kinetics at indicated days post-infection. The level of viral replication was determined by infection TZM-bl cells by viruses harvested from day 2, 4, 6, 8, 10, 12 and 14. After 48 hrs post-infection, cells were lysed and luciferase activity was measured.
Incorporation of viral Env into viral particles
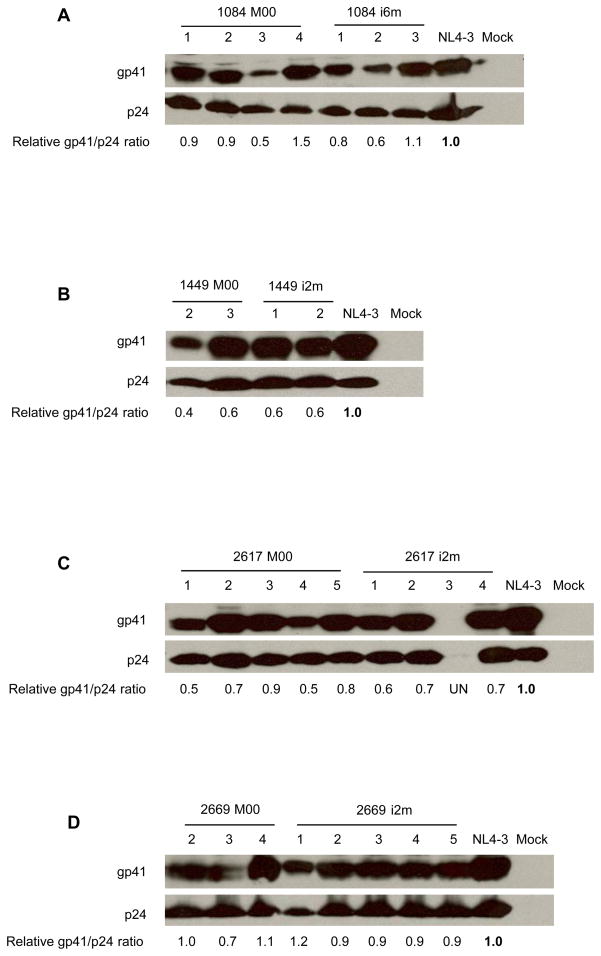
Our investigation of Env synthesis and processing analyses revealed no correlations with transmission, but glycoprotein processing is not always coupled to effective incorporation or function (Dubay et al., 1995). To investigate whether the level of Env incorporation into virions contributed to differences in infectivity or correlated with transmission, we used monoclonal antibody 2F5 to detect the quantity of gp41 incorporated into virions. We chose to quantify Env incorporation using gp41 detection since a specific and sensitive anti-subtype C gp120 antibody was not available and all chimeras share a common gp41 sequence derived from NL4-3. Densitometry analyses of the blots indicated that different levels of gp41 were incorporated into chimeric viral particles from the MIPs, as calculated from gp41/p24 ratio (Fig. 4). For example, with 2617 i2m-3 and 2669 M0-3, where poor Env incorporation was observed (Fig. 4C and D), the virions also exhibited low infectivity and replication deficits resulted (Fig. 2C and D and Fig. 3C and D). For most chimeric viruses, particularly for MIP 2617 such as M0-2, 3, 5 and i2m-2, higher levels of Env in the virions correlated with greater infectivity (Fig. 4 and Fig. 2). However, the differential infectivity cannot be simply explained by the levels of envelope incorporated and is likely also due to differences in inherent infectivity potency, as observed with 1084 i6m-3 and 2669 i2m-4, where poor infection or replication kinetics were observed although substantial levels of Env were incorporated into the particles (Fig. 2A and D; Fig. 3A and D; Fig. 4A and D). For others, only a small fraction of the Env incorporated into the HIV-1 particles was sufficient for infection. This can be seen with 1084 M0-3, 1084 i6m-2 and 1449 M0-2 (Fig. 4A and B; Fig. 2A and B). Despite variability in incorporation of Env into the virions, no identifiable pattern in the level of Env incorporation segregated maternal from infant chimeras.
Figure 4. Incorporation of patient-derived Env glycoprotein into infectious virions.
293T cells were transfected with the indicated proviral expression constructs and the resulting chimeric viruses bearing patient-derived Env V1-V5 region were collected at 48hrs post-transfection. The viruses were pelleted through a 20% (wt/wt) sucrose cushion by ultracentrifugation from the clarified supernatants and the viral proteins were analyzed by Western blot. Gp41 protein was detected by anti-gp41 mAb 2F5, and p24 was detected by using anti-p24 mAb. Bands corresponding to gp41 and p24 are indicated. Env incorporation into virions was calculated as gp41/p24 ratio. For each pair, the NL4-3 value was set at 1, the relative Env incorporation for each virus was calculated relative to this value. UN means that the signal is undetectable.
Sensitivity of maternal and infant envelope variants to neutralization by maternal plasma
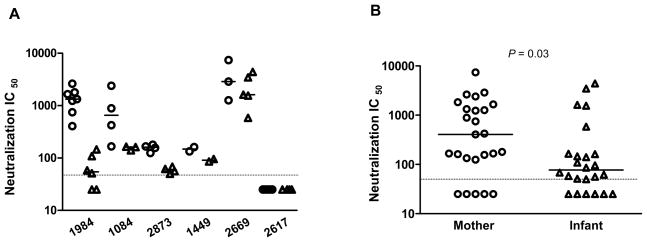
One of the primary selective pressure acting on Env is neutralizing antibodies (nAbs). MTCT is unique in that it occurs in the presence of passively acquired maternal antibody before birth. This passive transfer of maternal antibodies to infants could play a role in the selection of transmitted viruses with an nAb escaped phenotype. Therefore, we determined the sensitivity of mother/infant Env-chimera viruses to neutralization with maternal baseline plasma collected at delivery. A total of 49 functional Env chimeras were tested for their sensitivity to neutralization by maternal baseline plasma. Among the 6 MIPs tested, the maternal variants of 1984, 1084, 2873, 1449 and 2669 were generally sensitive to the maternal plasma; infant viruses, in contrast, were more neutralization-resistant, as indicated by their lower median inhibitory concentrations (IC50) (Fig. 5A). Among the infants tested, infant 2669 harbored relative sensitive variants with higher IC50 compared to those of other infant variants. For MIP 2617, both mother and infant variants displayed very low or undetectable neutralization by the maternal plasma, although it is possible that this is due to the lack of effective neutralization response in the mother (Fig. 5A). When the six pairs were considered in aggregate, the IC 50 value of variants from the infants (median 77) was significantly lower than that from the mothers’ (median 406; P = 0.03; Mann Whitney test), indicating that the transmitted variants were more resistant than the mother’s virus population to maternal time-of-delivery plasma (Fig. 5B). Taken together, our results indicate that neutralization resistant subtype C HIV-1 variants have a higher probability of achieving perinatal transmission, even though variants which are susceptible to nAbs could also be transmitted.
Figure 5. Neutralization sensitivity of envelope variants for maternal plasma.
(A) Comparison of neutralization IC50 of maternal (○) and infant (Δ) variants bearing patient-derived Env V1-V5 region for each transmission pair. The baseline maternal plasma was tested at a starting dilution of 1: 50 as indicated by the dashed horizontal line. The luciferase activity obtained for each virus in the absence of serum was set to 100 %, and the neutralization of the test plasma was calculated relative to this value. The IC50 was defined as the dilution of plasma that causes 50% inhibition of virus infection. The horizontal bars indicate the median IC50 value for each individual. (B) Comparison of IC50 between the mother (○) and infant (Δ) variants. The P value of the comparison is shown. The horizontal bars indicate the median IC50 value. The dashed horizontal line indicates the lowest dilution tested.
Sensitivity of maternal and infant envelope variants to neutralization by heterologous antisera
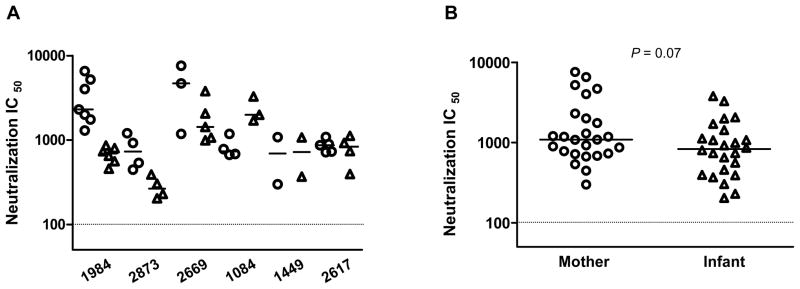
We further assessed the neutralization sensitivity of the MIP viruses using a pooled subtype C HIV-1 infected patient serum to determine if they differed in their sensitivity to neutralization by heterologous antibodies. Comparisons of neutralization sensitivity to pooled HIV-1 plasma indicated that the pooled heterologous plasma was very effective in neutralizing the variants from both mother (median IC50, 1089) and infant (median IC50, 832), suggesting that none of the infant variants were inherently resistant to neutralization. However, the neutralization profile varied among different pairs (Fig. 6A). Some maternal viruses showed higher susceptibility to the heterologous pooled plasma than did their infant variants (MIPs 1984, 2873 and 2669); while some infant viruses were tended to be more susceptible than those in their corresponding mothers (MIP 1084), or viruses from both mother and infant were comparable in their neutralization sensitivity (MIPs 1449 and 2617) (Fig. 6A). Notably, for MIP 2617, all the mother and infant variants were neutralized by pooled plasma, although they all showed very poor neutralization when tested with the maternal plasma, indicating that neutralization resistance is not an intrinsic property of these viruses (Fig. 6A). However, statistical analysis of all the viral variants indicated that there were no significant differences in the sensitivity of the mother and infant variants to neutralization by pooled heterologous plasma although there was a trend toward the infant variants being more resistant to the neutralization by the pooled plasma than the maternal variants (Fig. 6B, P = 0.07; Mann Whitney test). Moreover, for most cases, the pooled plasma neutralized the variants from mother and infant more efficiently than the maternal plasma, indicating high potency and broad specificity of this plasma pool.
Figure 6. Neutralization sensitivity of envelope variants for pooled plasma.
(A) Comparison of neutralization IC50 of maternal (○) and infant (Δ) variants bearing patient-derived Env V1-V5 region for each transmission pair. A pooled HIV-1 plasma was tested at a starting dilution of 1:100 as indicated by the dashed horizontal line. The IC50 from each individual were calculated as in Fig. 5 and compared. The horizontal bars indicate the median IC50 value for each individual. (B) Comparison of IC50 between the mother (○) and infant (Δ) variants. The P value of the comparison is shown. The horizontal bars indicate the median IC50 value. The dashed horizontal line shows the lowest dilution tested.
Susceptibility of maternal and infant envelope variants to neutralization by monoclonal antibody
We also evaluated the susceptibility of the maternal and infant Env-chimera viruses to the monoclonal antibody, IgG1 b12, derived from a subtype B infected individual, and which recognizes an epitope overlapping the CD4-binding site (Burton et al., 1991; Burton et al., 1994; Roben et al., 1994) and was shown to be somewhat effective against the subtype C Env surface subunit as compared to other broadly neutralizing monoclonal antibodies (Binley et al., 2004; Bures et al., 2002; Gray et al., 2006; Kulkarni et al., 2009; Li et al., 2006). Our results indicated that there was a wide range of sensitivity of our subtype C viruses to IgG1 b12. The majority of our panel of viruses (30 of the 49, 61%) were resistant to b12 demonstrating less than 50% neutralization at concentrations up to 25 μg/ml. The most resistant viruses were those from MIPs 2617, 2873 and 2669 (Table 2). The IgG1 b12 neutralized 7 of the 24 infant variants tested, with IC50 ranging from 0.6 to 25 μg/ml; whereas 12 of the 25 maternal variants were neutralized and had IC50 ranging from 0.06 to 25 μg/ml with this mAb (Table 2). In addition, the inter-pair differences in susceptibility to IgG1 b12 were also evident. Notably, for MIP 1984, all the maternal viruses were more sensitive to neutralization by b12 compared to the infant viruses, with IC50 values ranging from 0.06 to 0.6 μg/ml for the maternal viruses whereas 4.3 to > 25 μg/ml for the newly transmitted viruses (Table 2). This is consistent with the observations with autologous and heterologous pooled plasma for this pair. Similarly, the transmitted viruses (IC50 value of 22 to >25 μg/ml) from MIP 1084 were generally less sensitive to IgG1 b12 than the maternal variants (IC50 value of 5.3-7.3 μg/ml) although two exceptions (1084M0-1 and M0-4) were identified among the nontransmitted viruses. The latter two maternal viruses were also more resistant to autologous plasma compared to the rest of the maternal variants. In contrast, variants derived from infant 1449 showed higher susceptibility to this mAb than did the mother variants (Table 2), which is different from the results with autologous plasma. When all the variants from 6 pairs were considered together, there was a marginally significant difference in the susceptibility to IgG1 b12 between the maternal and infant variants, with the infant viruses being more resistant than those of the mothers (P = 0.04; Mann Whitney test). However, this difference could be largely due to MIP 1984, where the mother variants were particularly sensitive (Table 2).
Table 2.
IC50 values of IgG1 b12 with each maternal and infant variant
| Patient ID | Subject | Clone ID | IC50 (μg/ml) |
|---|---|---|---|
| 1984 | Mother | 1, 2, 3, 4, 5, 6, 7 | 0.06–0.6 |
| Infant | 1 | 5.6 | |
| 2, 6 | >25 | ||
| 3, 5 | 25 | ||
| 4 | 4.3 | ||
| 1084 | Mother | 1, 4 | >25 |
| 2 | 5.3 | ||
| 3 | 7.3 | ||
| Infant | 1 | 21.6 | |
| 2, 3 | >25 | ||
| 1449 | Mother | 2 | 2.2 |
| 3 | >25 | ||
| Infant | 1 | 0.6 | |
| 2 | 0.8 | ||
| 2617 | Mother | 1, 2, 3, 4 | >25 |
| 5 | 25 | ||
| Infant | 1, 2, 3, 4 | >25 | |
| 2873 | Mother | 1, 3, 4 | >25 |
| 2 | 1.5 | ||
| Infant | 1, 2, 3, 4 | >25 | |
| 2669 | Mother | 2, 3, 4 | >25 |
| Infant | 1, 2, 3, 4, 5 | >25 | |
Correlation between neutralization sensitivity and Env genetic characteristics
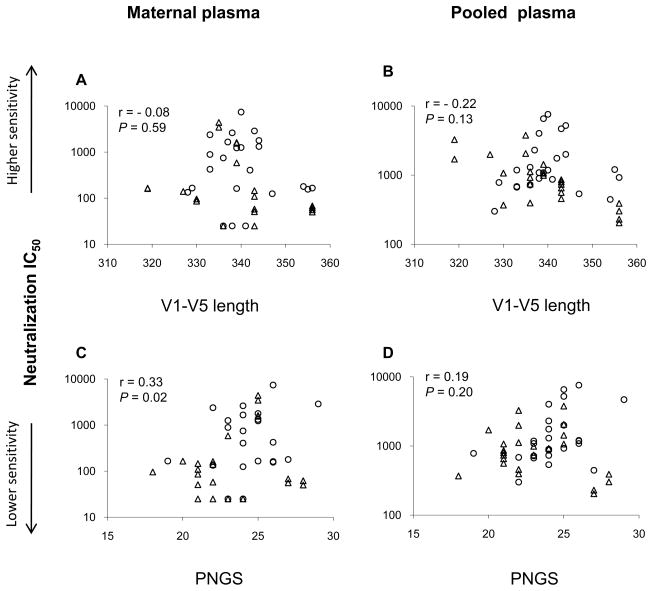
Our genetic analysis indicated that infant Env V1-V5 sequences from five of the six MIPs have lower mean number of N-linked glycosylation sites (PNGS) than those of their mothers, suggesting that viruses harboring Env glycoproteins with fewer potential N-glycan could be selected during the transmission among these MIPs (Zhang et al., submitted for publication). In addition, in this study, we observed that neutralization resistant variants are dominant among transmitted viruses. We, therefore, further evaluated the relationship between neutralization sensitivity and the Env genetic features, such as the length polymorphisms and the number of PNGS in Env. Aggregate analysis of the Env V1-V5 length from six MIPs revealed that the acquisition of the Env V1-V5 length was not significantly associated with neutralization susceptibility of these variants to either the maternal plasma or the pooled plasma (P = 0.59; P = 0.13) (Fig. 7A and B). However, higher number of PNGS in this region was found to correlate with greater neutralization sensitivity to the maternal plasma but not the pooled plasma (P = 0.02; P = 0.20) (Fig. 7C and D).
Figure 7. Correlation between neutralization sensitivity and genetic characteristics of the envelope.
Neutralization of maternal (○) and infant (Δ) variants was performed with either maternal plasma (A and C) or pooled plasma (B and D). The IC50 was defined as the dilution of plasma that causes 50% inhibition of virus infection. Amino acid lengths and PNGS in Env V1-V5 region of the mother and infant variants were calculated. The coefficient r and P values were generated using Spearman’s correlation test.
Discussion
Understanding the properties of viruses capable of establishing infection during perinatal transmission of HIV-1 is critical to guide the design of vaccines or therapeutics attempting to block transmission. In this study, viral Env glycoproteins from six anti-retroviral naïve mother-infant pairs were compared for their biological and immunologic properties to discern any parameters that might correlate with MTCT. To our knowledge, this study represents the first detailed analysis of maternal donor and recipient infant Env biological properties and sensitivity to neutralization by autologous or heterologous antibodies in the context of HIV-1 subtype C perinatal transmission.
We have recently characterized the replicative fitness of these perinatally transmitted viruses in dual infection competitions. Our results indicated that infant viruses possess a growth advantage over those derived from their chronically infected mothers, and the advantage was conferred by Env V1-V5 region (Kong et al., 2008). Higher fitness may thus enable the transmitted virus to establish more efficient propagation at the site of infection in the new host. A number of factors could affect viral fitness and thereby transmission, including more efficient processing and incorporation of the transmitted viral Env. In addition, the ability of the virus to withstand immune selection pressures such as neutralizing antibodies may also affect the transmissibility. We therefore tested whether transmitted viruses were also neutralization resistant or whether a compromise between immune escape and replication robustness in the new host occurs.
Our analysis of the Env processing or incorporation efficiency did not distinguish between maternal (non-transmitted) and infant (transmitted) Env; therefore, the efficiency of Env processing and incorporation do not seem to impact the likelihood of subtype C HIV-1 perinatal transmission in our cohort. The slight differences in the Env’s electrophoretic mobility observed in Env processing assay could indicate the extent of glycosylation among those maternal and infant Env chimeras. Since glycosylation has a profound effect on the proper folding, oligomerization and stability of glycoproteins, further studies will be needed to investigate how the extent of glycosylation affect the biological function of the glycoproteins. Moreover, our study also indicated that the viral replication kinetics did not differ between mother and infant variants tested. This is in agreement with a non-subtype C HIV-1 MTCT, where similar replication profiles of the mother and infant isolates were observed (Kliks et al., 1994). However, our co-infection growth competition assay of these MIPs indicated that the transmitted viruses are more fit than their mother’s (Kong et al., 2008), suggesting that differences in viral fitness during transmission can only be differentiated by direct competition. Indeed, this has been observed in an earlier study, where the differences in viral fitness associated with escape from cytotoxic T lymphocytes between the wild type and mutant viruses can only be detected by direct competition assay (Martinez-Picado et al., 2006).
Unlike other modes of transmission, MTCT occurs in the face of passively acquired maternal antibody before birth. Thus, maternal neutralizing antibody could impose a selective pressure on the transmitted virus. We, therefore, examined whether the maternal nAbs play a role in selecting for the transmissible viruses. Our observations indicated that for most cases, the transmitted viruses were less susceptible to neutralization by their baseline maternal plasma than the contemporaneous maternal viruses. This is consistent with previous studies on subtype A and B perinatal transmission (Dickover et al., 2006; Kliks et al., 1994; Wu et al., 2006). Kliks et al. have found that five of the six infected infants tested harbored viruses which were less susceptible to their mother’s sera (Kliks et al., 1994). More recently, it has been shown that neutralization escape variants were favored during subtype A HIV-1 perinatal transmission (Wu et al., 2006). Similarly, in a study of subtype B MTCT, where in five of the seven transmitting mothers with autologous nAbs, the neutralizing antibody titer to the infant’s virus was lower than that of the maternal isolates (Dickover et al., 2006). Our results indicated that a preferential transmission of neutralizing antibody escape variants also occurred in the context of subtype C HIV-1 MTCT, supporting the notion that maternal nAbs exert a preventive and selective effect in perinatal transmission (Dickover et al., 2006; Kliks et al., 1994; Wu et al., 2006). Among our study cohort, infants 2873 and 1449 are characterized as fast progressors and died within the first year after birth; whereas infants 1084 and 1984 were diagnosed as slow progressors. Our results indicated that sensitivity to neutralization of the infected infants did not appear to correlate with disease progression since variants from these infants were, in general, resistant to the neutralization by their maternal plasma. Collectively, our observations and those of others suggest that neutralization insensitive variants are favored during HIV-1 perinatal transmission, but the transmission of neutralization sensitive variants can still occur in spite of the presence of maternal neutralizing antibodies. In addition, our results also indicate that resistance to maternal neutralizing antibodies is not predictive of disease progression in the infected children.
Understanding the role of nAbs, including heterologous nAbs, in MTCT is important for determining whether passive administration of nAbs will be beneficial. Neutralization analyses employing pooled HIV-1 plasma revealed a higher susceptibility of both maternal and infant variants although the infant variants appeared to be less susceptible than the maternal variants, indicating that heterologous neutralization can be relatively potent. The relatively higher susceptibility of infant viruses to neutralization by pooled HIV-1 plasma compared to maternal plasma indicated that no newly transmitted variants were inherently resistant to HIV-1 antibody neutralization. This finding is in agreement with a previous study on subtype A HIV-1 perinatal transmission (Wu et al., 2006). In addition, our observation supports the proposed combined vaccine/passive monoclonal antibody strategy for prevention of HIV-1 transmission by breast feeding (Dickover et al., 2006).
Our data with IgG1 b12 showed that only 39% (19/49) viruses bearing the maternal or infant Env could be neutralized by IgG1 b12. This is similar to a recent study on subtype C HIV-1 from India, where only 20% of the studied viruses were neutralized by b12 (Kulkarni et al., 2009). However, these results are somewhat different from those of several previous studies on HIV-1 subtype C with relative smaller cohorts, where the IgG1 b12 could neutralized 57–67% of the viruses tested (Binley et al., 2004; Gray et al., 2006; Li et al., 2006). More importantly, our findings indicated that as a group, the newly transmitted viruses were likely to be more resistant than their maternal variants to this mAb although for some pairs both maternal and infant variants showed similar resistance to this mAb. This suggests that there could be a selection for altered IgG1 b12 epitope exposure during the transmission. Viruses harboring Env with under-represented IgG1 b12 epitopes appeared to be favored during HIV-1 subtype C perinatal transmission. The relative insensitivity of the newly transmitted viruses to this mAb implies that IgG1 b12 may have limited benefit in preventing HIV-1 subtype C MTCT. This is supported by a previous subtype C paediatric study with a relative small sample size from South Africa (Gray et al., 2006). Our data, as well as those of others, underscore the importance of evaluating newly transmitted variants in endemic areas when designing neutralizing Ab-based vaccine immunogens.
Our results of selective perinatal HIV-1 transmission of nAbs escape variants differs from heterosexual transmission of subtype C HIV-1 in discordant couples, where viruses with shorter Env V1-V4, and fewer glycans are more susceptible to neutralizing antibodies, but mediate more efficient heterosexual transmission (Derdeyn et al., 2004). These patterns, however, have not been confirmed in subtype B sexual transmission (Chohan et al., 2005; Frost et al., 2005a; Frost et al., 2005b), but viruses with shorter V1-V2 length and reduced sequons have been reported in a subtype A heterosexual transmission study (Chohan et al., 2005). The discrepancy in viral characteristics, including genotypic and phenotypic parameters, leading to preferential transmission of particular Env variants between sexual and perinatal transmission studies could be due to fundamental differences in the mode of transmission or due to subtype-specific virological factors. For sexual transmission, the mucosal barrier is likely to be a major constraint on the transmitting virus, while perinatal transmission is also affected by the presence of passively transferred maternal neutralizing antibodies (Derdeyn and Hunter, 2008; Haaland et al., 2009).
We also investigated the Env genetic characteristics associated with the neutralization profiles of these variants. We found that acquisition of Env N- glycan of the mother and infant’s sequences correlated with the enhanced neutralization susceptibility of these variants to the maternal plasma. This was somewhat unexpected given that neutralization resistance is generally linked to increased PNGS in viruses that evolve during chronic infection (Cheng-Mayer et al., 1999; Wei et al., 2003). Therefore, it is likely that different mechanisms could be involved in determining the neutralization phenotype of the virus in the cases of subtype C perinatal transmission. Further studies will be needed to evaluate how the changes in Env N-glycan affect the neutralization outcome of the viruses during perinatal transmission. Different from a subtype A perinatal transmission study (Wu et al., 2006), where greater sensitivity to maternal plasma neutralization was shown to correlate with shorter variable loops in Env sequence, we did not observed any correlation between the Env length and neutralization sensitivity in our cohort. However, acquisition of length in Env V1-V4 region of donor and recipient’s sequences were linked to enhanced neutralization resistance to the autologous or heterologous antibodies in the cases of subtype C HIV-1 heterosexual transmission (Rong et al., 2007). Therefore, our results, together with those from others, highlight the need to further explore genetic and immunologic correlates of different subtypes and different routes of transmission.
In summary, our data, though limited in sample size, suggests that both viral and immunologic factors, including Env glycosylation status (Zhang et al., submitted for publication), viral replication fitness (Kong et al., 2008), and maternal nAbs play a role in subtype C HIV-1 perinatal transmission. Variants with the characteristics of higher replication fitness, resistance to maternal nAbs and reduced glycosylation in Env were preferentially transmitted through the maternal-infant bottleneck during subtype C HIV-1 transmission. In addition, the newly transmitted variants were relatively sensitive to neutralization by pooled heterologous plasma but most of them were resistant to IgG1 b12. Moreover, neither Env processing nor incorporation efficiency was predictive of viral transmissibility. These findings provide further insights for unraveling selective pressures that occur during HIV-1 perinatal transmission, and may have implications for pediatric vaccine design and prevention of perinatal transmission.
Supplementary Material
Supplementary Figure 1. Synthesis and processing of Env glycoproteins gp160 and gp120 of two MIPs 2669 and 1449. COS-1 cells expressing chimeric Env proteins bearing patient-derived Env V1-V5 region were pulse-labeled with [35S] methionine at 37°C for 40min. Cells were resuspended in complete medium and chased for varying times as indicated (hr). Cell lysates and supernatant were immunoprecipitated with pooled anti-HIV-1 antisera. Viral proteins from cell lysates (A) and supernatant (B) were resolved by SDS-PAGE.
Acknowledgments
This study was supported by PHS grants CA75903, Fogarty Training grant TW001429 and NCRR COBRE grant RR15635 to CW and Layman Award from the University of Nebraska-Lincoln and P20 RR15635 to HZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abebe A, Demissie D, Goudsmit J, Brouwer M, Kuiken CL, Pollakis G, Schuitemaker H, Fontanet AL, Rinke de Wit TF. HIV-1 subtype C syncytium- and non-syncytium-inducing phenotypes and coreceptor usage among Ethiopian patients with AIDS. AIDS. 1999;13(11):1305–11. doi: 10.1097/00002030-199907300-00006. [DOI] [PubMed] [Google Scholar]
- Abraha A, Nankya IL, Gibson R, Demers K, Tebit DM, Johnston E, Katzenstein D, Siddiqui A, Herrera C, Fischetti L, Shattock RJ, Arts EJ. CCR5- and CXCR4-tropic subtype C human immunodeficiency virus type 1 isolates have a lower level of pathogenic fitness than other dominant group M subtypes: implications for the epidemic. J Virol. 2009;83(11):5592–605. doi: 10.1128/JVI.02051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N, Baroudy BM, Baker RC, Chappey C. Genetic analysis of human immunodeficiency virus type 1 envelope V3 region isolates from mothers and infants after perinatal transmission. J Virol. 1995;69(2):1001–12. doi: 10.1128/jvi.69.2.1001-1012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SC, Abraha A, Collins KR, Marozsan AJ, Baird H, Quinones-Mateu ME, Penn-Nicholson A, Murray M, Richard N, Lobritz M, Zimmerman PA, Kawamura T, Blauvelt A, Arts EJ. Comparing the ex vivo fitness of CCR5-tropic human immunodeficiency virus type 1 isolates of subtypes B and C. J Virol. 2003;77(2):1021–38. doi: 10.1128/JVI.77.2.1021-1038.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, Stiegler G, Kunert R, Zolla-Pazner S, Katinger H, Petropoulos CJ, Burton DR. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78(23):13232–52. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorndal A, Sonnerborg A, Tscherning C, Albert J, Fenyo EM. Phenotypic characteristics of human immunodeficiency virus type 1 subtype C isolates of Ethiopian AIDS patients. AIDS Res Hum Retroviruses. 1999;15(7):647–53. doi: 10.1089/088922299310944. [DOI] [PubMed] [Google Scholar]
- Bures R, Morris L, Williamson C, Ramjee G, Deers M, Fiscus SA, Abdool-Karim S, Montefiori DC. Regional clustering of shared neutralization determinants on primary isolates of clade C human immunodeficiency virus type 1 from South Africa. J Virol. 2002;76(5):2233–44. doi: 10.1128/jvi.76.5.2233-2244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Barbas CF, 3rd, Persson MA, Koenig S, Chanock RM, Lerner RA. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci U S A. 1991;88(22):10134–7. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, Sawyer LS, Hendry RM, Dunlop N, Nara PL, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266(5187):1024–7. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C, Brown A, Harouse J, Luciw PA, Mayer AJ. Selection for neutralization resistance of the simian/human immunodeficiency virus SHIVSF33A variant in vivo by virtue of sequence changes in the extracellular envelope glycoprotein that modify N-linked glycosylation. J Virol. 1999;73(7):5294–300. doi: 10.1128/jvi.73.7.5294-5300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chohan B, Lang D, Sagar M, Korber B, Lavreys L, Richardson B, Overbaugh J. Selection for human immunodeficiency virus type 1 envelope glycosylation variants with shorter V1-V2 loop sequences occurs during transmission of certain genetic subtypes and may impact viral RNA levels. J Virol. 2005;79(10):6528–31. doi: 10.1128/JVI.79.10.6528-6531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilliers T, Nhlapo J, Coetzer M, Orlovic D, Ketas T, Olson WC, Moore JP, Trkola A, Morris L. The CCR5 and CXCR4 Coreceptors Are Both Used by Human Immunodeficiency Virus Type 1 Primary Isolates from Subtype C. J Virol. 2003;77(7):4449–4456. doi: 10.1128/JVI.77.7.4449-4456.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdeyn CA, Decker JM, Bibollet-Ruche F, Mokili JL, Muldoon M, Denham SA, Heil ML, Kasolo F, Musonda R, Hahn BH, Shaw GM, Korber BT, Allen S, Hunter E. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;303(5666):2019–22. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- Derdeyn CA, Hunter E. Viral characteristics of transmitted HIV. Curr Opin HIV AIDS. 2008;3(1):16–21. doi: 10.1097/COH.0b013e3282f2982c. [DOI] [PubMed] [Google Scholar]
- Dickover R, Garratty E, Yusim K, Miller C, Korber B, Bryson Y. Role of maternal autologous neutralizing antibody in selective perinatal transmission of human immunodeficiency virus type 1 escape variants. J Virol. 2006;80(13):6525–33. doi: 10.1128/JVI.02658-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickover RE, Garratty EM, Plaeger S, Bryson YJ. Perinatal transmission of major, minor, and multiple maternal human immunodeficiency virus type 1 variants in utero and intrapartum. J Virol. 2001;75(5):2194–203. doi: 10.1128/JVI.75.5.2194-2203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubay JW, Dubay SR, Shin HJ, Hunter E. Analysis of the cleavage site of the human immunodeficiency virus type 1 glycoprotein: requirement of precursor cleavage for glycoprotein incorporation. J Virol. 1995;69(8):4675–82. doi: 10.1128/jvi.69.8.4675-4682.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost SD, Liu Y, Pond SL, Chappey C, Wrin T, Petropoulos CJ, Little SJ, Richman DD. Characterization of human immunodeficiency virus type 1 (HIV-1) envelope variation and neutralizing antibody responses during transmission of HIV-1 subtype B. J Virol. 2005a;79(10):6523–7. doi: 10.1128/JVI.79.10.6523-6527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost SD, Wrin T, Smith DM, Kosakovsky Pond SL, Liu Y, Paxinos E, Chappey C, Galovich J, Beauchaine J, Petropoulos CJ, Little SJ, Richman DD. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc Natl Acad Sci U S A. 2005b;102(51):18514–9. doi: 10.1073/pnas.0504658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray ES, Meyers T, Gray G, Montefiori DC, Morris L. Insensitivity of paediatric HIV-1 subtype C viruses to broadly neutralising monoclonal antibodies raised against subtype B. PLoS Med. 2006;3(7):e255. doi: 10.1371/journal.pmed.0030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaland RE, Hawkins PA, Salazar-Gonzalez J, Johnson A, Tichacek A, Karita E, Manigart O, Mulenga J, Keele BF, Shaw GM, Hahn BH, Allen SA, Derdeyn CA, Hunter E. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog. 2009;5(1):e1000274. doi: 10.1371/journal.ppat.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliks SC, Wara DW, Landers DV, Levy JA. Features of HIV-1 that could influence maternal-child transmission. JAMA. 1994;272(6):467–74. [PubMed] [Google Scholar]
- Kong X, West JT, Zhang H, Shea DM, M’Soka TJ, Wood C. The human immunodeficiency virus type 1 envelope confers higher rates of replicative fitness to perinatally transmitted viruses than to nontransmitted viruses. J Virol. 2008;82(23):11609–18. doi: 10.1128/JVI.00952-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SS, Lapedes A, Tang H, Gnanakaran S, Daniels MG, Zhang M, Bhattacharya T, Li M, Polonis VR, McCutchan FE, Morris L, Ellenberger D, Butera ST, Bollinger RC, Korber BT, Paranjape RS, Montefiori DC. Highly complex neutralization determinants on a monophyletic lineage of newly transmitted subtype C HIV-1 Env clones from India. Virology. 2009;385(2):505–20. doi: 10.1016/j.virol.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Salazar-Gonzalez JF, Derdeyn CA, Morris L, Williamson C, Robinson JE, Decker JM, Li Y, Salazar MG, Polonis VR, Mlisana K, Karim SA, Hong K, Greene KM, Bilska M, Zhou J, Allen S, Chomba E, Mulenga J, Vwalika C, Gao F, Zhang M, Korber BT, Hunter E, Hahn BH, Montefiori DC. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J Virol. 2006;80(23):11776–90. doi: 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzuriaga K, Sullivan JL. Pediatric HIV-1 infection: advances and remaining challenges. AIDS Rev. 2002;4(1):21–6. [PubMed] [Google Scholar]
- Martinez-Picado J, Prado JG, Fry EE, Pfafferott K, Leslie A, Chetty S, Thobakgale C, Honeyborne I, Crawford H, Matthews P, Pillay T, Rousseau C, Mullins JI, Brander C, Walker BD, Stuart DI, Kiepiela P, Goulder P. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J Virol. 2006;80(7):3617–23. doi: 10.1128/JVI.80.7.3617-3623.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, Coico R, editors. Current Protocols in Immunology. John Wiley & Sons; New York, N.Y: 2004. [DOI] [PubMed] [Google Scholar]
- Ndung’u T, Renjifo B, Essex M. Construction and analysis of an infectious human Immunodeficiency virus type 1 subtype C molecular clone. J Virol. 2001;75(11):4964–72. doi: 10.1128/JVI.75.11.4964-4972.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson JR, John GC, Carr JK, Lewis P, Kreiss JK, Jackson S, Nduati RW, Mbori-Ngacha D, Panteleeff DD, Bodrug S, Giachetti C, Bott MA, Richardson BA, Bwayo J, Ndinya-Achola J, Overbaugh J. Subtypes of human immunodeficiency virus type 1 and disease stage among women in Nairobi, Kenya. J Virol. 1999;73(5):4393–403. doi: 10.1128/jvi.73.5.4393-4403.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainwater SM, Wu X, Nduati R, Nedellec R, Mosier D, John-Stewart G, Mbori-Ngacha D, Overbaugh J. Cloning and characterization of functional subtype A HIV-1 envelope variants transmitted through breastfeeding. Curr HIV Res. 2007;5(2):189–97. doi: 10.2174/157016207780076986. [DOI] [PubMed] [Google Scholar]
- Roben P, Moore JP, Thali M, Sodroski J, Barbas CF, 3rd, Burton DR. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J Virol. 1994;68(8):4821–8. doi: 10.1128/jvi.68.8.4821-4828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R, Gnanakaran S, Decker JM, Bibollet-Ruche F, Taylor J, Sfakianos JN, Mokili JL, Muldoon M, Mulenga J, Allen S, Hahn BH, Shaw GM, Blackwell JL, Korber BT, Hunter E, Derdeyn CA. Unique mutational patterns in the envelope alpha 2 amphipathic helix and acquisition of length in gp120 hypervariable domains are associated with resistance to autologous neutralization of subtype C human immunodeficiency virus type 1. J Virol. 2007;81(11):5658–68. doi: 10.1128/JVI.00257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlatti G, Albert J, Rossi P, Hodara V, Biraghi P, Muggiasca L, Fenyo EM. Mother-to-child transmission of human immunodeficiency virus type 1: correlation with neutralizing antibodies against primary isolates. J Infect Dis. 1993a;168(1):207–10. doi: 10.1093/infdis/168.1.207. [DOI] [PubMed] [Google Scholar]
- Scarlatti G, Leitner T, Halapi E, Wahlberg J, Marchisio P, Clerici-Schoeller MA, Wigzell H, Fenyo EM, Albert J, Uhlen M, et al. Comparison of variable region 3 sequences of human immunodeficiency virus type 1 from infected children with the RNA and DNA sequences of the virus populations of their mothers. Proc Natl Acad Sci U S A. 1993b;90(5):1721–5. doi: 10.1073/pnas.90.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422(6929):307–12. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- West JT, Weldon SK, Wyss S, Lin X, Yu Q, Thali M, Hunter E. Mutation of the dominant endocytosis motif in human immunodeficiency virus type 1 gp41 can complement matrix mutations without increasing Env incorporation. J Virol. 2002;76(7):3338–49. doi: 10.1128/JVI.76.7.3338-3349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky SM, Wike CM, Korber BT, Hutto C, Parks WP, Rosenblum LL, Kunstman KJ, Furtado MR, Munoz JL. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992;255(5048):1134–7. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- Wu X, Parast AB, Richardson BA, Nduati R, John-Stewart G, Mbori-Ngacha D, Rainwater SM, Overbaugh J. Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. J Virol. 2006;80(2):835–44. doi: 10.1128/JVI.80.2.835-844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Hoffmann F, He J, He X, Kankasa C, Ruprecht R, West JT, Orti G, Wood C. Evolution of subtype C HIV-1 Env in a slowly progressing Zambian infant. Retrovirology. 2005;2:67. doi: 10.1186/1742-4690-2-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Hoffmann F, He J, He X, Kankasa C, West JT, Mitchell CD, Ruprecht RM, Orti G, Wood C. Characterization of HIV-1 subtype C envelope glycoproteins from perinatally infected children with different courses of disease. Retrovirology. 2006;3:73. doi: 10.1186/1742-4690-3-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Orti G, Du Q, He J, Kankasa C, Bhat G, Wood C. Phylogenetic and phenotypic analysis of HIV type 1 env gp120 in cases of subtype C mother-to-child transmission. AIDS Res Hum Retroviruses. 2002;18(18):1415–23. doi: 10.1089/088922202320935492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Synthesis and processing of Env glycoproteins gp160 and gp120 of two MIPs 2669 and 1449. COS-1 cells expressing chimeric Env proteins bearing patient-derived Env V1-V5 region were pulse-labeled with [35S] methionine at 37°C for 40min. Cells were resuspended in complete medium and chased for varying times as indicated (hr). Cell lysates and supernatant were immunoprecipitated with pooled anti-HIV-1 antisera. Viral proteins from cell lysates (A) and supernatant (B) were resolved by SDS-PAGE.