Abstract
We tested the hypotheses that: 1) long-term facilitation (LTF) following acute intermittent hypoxia (AIH) varies among three inbred rat strains: Fischer 344 (F344), Brown Norway (BN) and Lewis rats, and 2) ventral cervical spinal levels of genes important for phrenic LTF (pLTF) vary in association with pLTF magnitude. Lewis and F344, but not BN rats exhibited significant increases in phrenic and hypoglossal burst amplitude 60 min post-AIH that were significantly greater than control experiments without AIH, indicating strain differences in phrenic (98%, 56% and 20%, respectively) and hypoglossal LTF (66%, 77% and 5%, respectively). Ventral spinal 5-HT2A receptor mRNA and protein levels were higher in F344 and Lewis versus BN, suggesting that higher 5-HT2A receptor levels are associated with greater pLTF. More complex relationships were found for 5-HT7, BDNF and TrkB mRNA. BN had higher 5-HT7 and TrkB mRNA versus F344; BN and Lewis had higher BDNF mRNA levels versus F344. Genetic variations in serotonergic function may underlie strain differences in AIH-induced pLTF.
1. Introduction
Acute intermittent hypoxia (AIH) elicits a form of respiratory plasticity known as long-term facilitation (LTF), a serotonin-dependent increase in respiratory motor output that persists long after AIH has ended (Mitchell et al., 2001b; Mahamed and Mitchell, 2007). LTF is observed in many species, including humans (Babcock & Badr, 1998; Pierchala et al., 2008) cats (Millhorn et al, 1980a,b), rats (Bach and Mitchell, 1996), mice (Kline et al., 2002), dogs (Cao et al., 1992), goats (Turner & Mitchell, 1997) and ducks (Mitchell et al., 2001a). In unanesthetized, spontaneously breathing mammals, LTF is prominently expressed as increased breathing frequency, with lesser changes in tidal volume; in contrast, LTF is predominantly expressed as increased respiratory-related nerve burst amplitude (tidal volume equivalent) in anesthetized animals (Powell et al., 1998; Mitchell et al., 2001b; Baker-Herman and Mitchell, 2008).
Although frequency LTF most likely arises from plasticity in brainstem respiratory rhythm generating neurons or in neural pathways to rhythm generating neurons (Powell et al., 1998; Blitz and Ramirez, 2002), amplitude LTF most likely arises predominantly in respiratory motor nuclei (Powell et al., 1998; Mitchell et al., 2001b; Feldman et al., 2003; Mahamed and Mitchell, 2007). Although our understanding of cellular/synaptic mechanisms giving rise to amplitude LTF has advanced considerably in recent years (Feldman et al., 2003; Mahamed and Mitchell, 2007; MacFarlane et al., 2008), mechanisms of frequency LTF remain poorly understood. We hypothesize that a similar mechanism underlies amplitude LTF in phrenic and other respiratory motor pools (e.g., hypoglossal LTF).
pLTF is hypothesized to be initiated by intermittent serotonin release in the cervical spinal cord near phrenic motor neurons, thereby initiating signaling cascades that ultimately strengthen synaptic inputs to respiratory motor neurons (see Baker et al., 2001; Mitchell et al., 2001b; Feldman et al., 2003; Mahamed and Mitchell, 2007; MacFarlane et al., 2008). Our working model is that intermittent 5-HT2 receptor activation on (or near) phrenic motor neurons (Fuller et al., 2001a; Baker-Herman and Mitchell, 2002) or interneurons initiates new BDNF synthesis (Baker-Herman et al., 2004), subsequently activating the high affinity BDNF receptor, TrkB (Baker-Herman et al., 2004). We further hypothesize that TrkB activation strengthens glutamatergic inputs from pre-motor to phrenic motor neurons or interneurons (McGuire et al., 2005, 2008; Bocchairo and Feldman, 2004). AIH may increase the activation of other receptors, including 5-HT7 and/or adenosine A2A receptors, which constrain the 5-HT2 receptor induced pLTF via cross-talk inhibition (Hoffman and Mitchell, 2008a,b; Hoffman et al., 2009).
There is considerable variation in LTF, even in the same species (Fuller et al., 2000b; Mitchell et al., 2001b; Behan et al., 2003). For example, Sprague-Dawley rats from different suppliers (Fuller et al., 2001b), or different colonies within the same supplier (Fuller et al., 2000), exhibit substantial variation in the magnitude of AIH-induced LTF. Sex hormones also influence LTF (Zabka et al., 2001, 2005, 2006), with estrogen proposed to play a key role in this form of neuroplasticity (Behan et al., 2003; Zabka et al., 2006; Behan and Wenninger, 2008). Genetic or epigenetic factors may underlie these differences in the capacity for respiratory plasticity. Indeed, genetic differences in plasticity-related proteins are associated with a differential capacity for plasticity in the hippocampus in different rodent strains (Nguyen et al., 2000; Manahan-Vaughan, 2000; Schimanski et al., 2007).
Here, we tested the hypothesis that variations exist in pLTF, XII LTF and frequency LTF among inbred rat strains, and that these strain differences are associated with variations in gene expression (mRNA) and protein levels of key molecules in the mechanism of pLTF. Thus, we measured AIH-induced LTF in three inbred rat strains that exhibit similar burst amplitude responses in phrenic and XII activity during hypoxia (Golder et al., 2005a): Fisher 344 (F344), Brown Norway (BN) and Lewis rats. We also measured constitutive expression of 5-HT2A and 5-HT7 receptor, BDNF and TrkB receptor mRNA, and 5-HT2A receptor protein levels in ventral cervical segments that encompass the phrenic motor nucleus. Rat strains with greater pLTF exhibit greater ventral cervical spinal levels of 5-HT2A mRNA and protein, whereas the relationships between BDNF, TrkB and 5-HT7 receptor mRNA with pLTF are less clear.
2. Material and Methods
2.1 Animals
Electrophysiological recordings were made from male rats from three inbred strains: Brown Norway (BN, n = 41; 104 ± 9 days old; 285 ± 4 g; Harlan colony 217), Fischer F344 (F344, n = 45; 115 ± 3 days old; 295 ± 9 g; Harlan colonies 202A and 202B, and NIH-NIA Aged Rodent Colony), and Lewis (n = 34; 117 ± 4 days old; 367 ± 7 g; Harlan colonies 202A and 202B). Data were obtained from rats specific to this study (BN, n = 12; F344, n = 18; Lewis, n = 18) and rats used in other published studies from our laboratory using an identical experimental protocol (Golder and Mitchell, 2005; Zabka et al., 2005, 2006; Wilkerson et al., 2005). Although all rats were exposed to the same treatment and experimental protocols, minor differences may exist in surgical preparation. For example, some rats received sham surgeries for gonadectomy (Zabka et al., 2005, 2006) or spinal cord injury (Golder and Mitchell, 2005), or were prepared for bilateral phrenic nerve recordings (Golder and Mitchell, 2005). These additional rats from our other published studies were included to increase our confidence in observed pLTF differences among rat strains. Similar qualitative results were obtained prior to the addition of these rats (compare Bavis et al., 2003 abstract with data presented here).
Separate groups of rats were used for mRNA analyses of 5-HT2A receptors (BN, n = 4; F344, n = 4; Lewis, n = 4), BDNF (BN, n = 4; F344, n = 5; Lewis, n = 5), TrkB (BN, n = 5; F344, n = 5; Lewis, n = 4) and 5-HT7 receptors (BN, n = 4; F344, n = 5; Lewis, n = 4) in the ventral cervical spinal cord. Another separate group of rats was used to assess 5-HT2A receptor protein levels in the ventral cervical spinal cord (BN, n = 3; F344, n = 3; Lewis, n = 3). Rats for the mRNA and protein analyses were obtained from the following colonies: BN, Harlan colony 217; F344, Harlan colony 202A; Lewis, Harlan colony 202A. All procedures were approved by the Institutional Animal Care and Use Committee of the School of Veterinary Medicine at the University of Wisconsin, Madison.
2.2 Electrophysiology
Phrenic and hypoglossal (XII) neurograms were recorded in rats exposed to acute intermittent hypoxia (AIH; phrenic: BN, n = 28; F344, n = 31; Lewis, n = 25; XII: BN, n = 23; F344, n = 36; Lewis, n = 14) or an equivalent duration of recording without AIH (time controls; phrenic: BN, n = 12; F344, n = 4; Lewis, n = 7; XII: BN, n = 11; F344, n = 4; Lewis, n = 4). We were unable to obtain data from both the phrenic and XII nerves in a few rats; thus, sample sizes for phrenic and XII LTF differ slightly.
2.3 Experimental preparation
Rats were induced with isoflurane anesthesia (in 50% O2, balance N2). The trachea was exposed and cannulated to permit mechanical ventilation (2.0–2.5 ml, Rodent respirator model 683; Harvard Apparatus, South Natick, MA). Rats were ventilated with approximately 50% O2, balance N2 throughout surgery and experimental protocols. Bilateral vagotomy was performed to prevent entrainment of respiratory nerve activity with the ventilator. The femoral artery was cannulated to measure arterial blood pressure (model P122, Grass Telefactor, West Warrick, RI) and to sample arterial blood for pH and blood-gas measurements (ABL-500, Radiometer, Copenhagen, Denmark). A femoral or tail vein was cannulated to enable drug and fluid administration (5 ml/kg/h, lactated Ringer with 0.8% sodium bicarbonate). Endtidal PCO2 was measured continuously using a mainstream CO2 monitor (Capnoguard, Novametrix Medical Systems, Wallingford, CT) with sufficient response time to measure endtidal gas composition in anesthetized rats. The left phrenic and hypoglossal nerves were isolated via a dorsal approach, desheathed and submerged in mineral oil. Following surgery, the rats were converted to urethane anesthesia (1.6 g/kg i.v.) and isoflurane was discontinued. Rats received pancuronium bromide (1 mg/kg i.v.) for neuromuscular paralysis.
Phrenic and XII nerves were placed on bipolar silver recording electrodes. Nerve activity was amplified (gain, 10,000; A-M Systems, Everett, WA), band-pass filtered (100 Hz to 10 kHz), rectified, and processed with a moving averager (CWE 821 filter; Paynter, Ardmore, PA; time constant, 50 ms). The signal was then digitized, recorded, and analyzed using the WINDAQ data-acquisition system (DATAQ Instruments, Akron, OH). Inspired CO2 was added and/or ventilation rate was adjusted until fictive respiratory bursts were observed in the phrenic and XII neurogram. This generally occurred at end-tidal PCO2 levels between 40 and 45 mmHg.
2.4 Long-term facilitation protocol
One hour following surgery, the apneic and recruitment CO2 thresholds were determined. Briefly, end-tidal PCO2 was decreased by hyperventilating the rats until phrenic inspiratory activity ceased (apneic threshold), and then slowly increased until phrenic bursting resumed (recruitment threshold). Baseline burst amplitude was set at 2–3 mmHg above the recruitment threshold. End-tidal PCO2 was monitored throughout the protocol and maintained at baseline levels.
Integrated phrenic and hypoglossal neurograms were recorded for 20–30 min following determination of the recruitment threshold to establish baseline respiratory activity. Rats were then given 3, 5 min episodes of isocapnic hypoxia (FIO2 = 0.10–0.12), separated by a 5 min return to baseline conditions (FIO2 = 0.5). After the final hypoxic episode, baseline conditions were again restored and respiratory activity was recorded for at least 60 minutes.
Arterial blood samples (0.3–0.4 ml) were collected for pH and blood-gas measurements during baseline conditions, during the first hypoxic episode, and 15, 30 and 60 minutes following the final hypoxic episode. Peak integrated hypoglossal and phrenic burst amplitude and frequency were measured for 30-s periods immediately before each blood sample. Animals were included in the final analysis if: 1) arterial PO2 (PaO2) during the hypoxic period was between 35 and 45 mmHg, 2) PaO2 during baseline conditions was above 150 mmHg, 3) PaCO2 during hypoxia was within 2 mmHg of the baseline value, and 4) PaCO2 following intermittent hypoxia was within 1 mmHg of the baseline value.
2.5 Quantitative Real time-Reverse Transcriptase-PCR
Ventral cervical spinal sections (C3-C5) were harvested from naïve rats for mRNA analysis. Briefly, rats were initially anesthetized with isoflurane in a closed box, and then were euthanized via an intracardiac injection of sodium pentobarbital (150–200 mg/kg). Cervical spinal segments C3-C5 were then removed quickly. Segments were placed onto a freezing microtome and successive 50-µm sections of dorsal horn were removed and discarded until the ventral aspect of the central canal was visible. Total RNA was extracted from the remaining ventral spinal tissue using TRIzol (1 ml; Invitrogen, Carlsbad, CA). 1 µg RNA was used from each sample for reverse transcriptase using oligo(dT) primers and MMLV reverse transcriptase (Invitrogen). qRT-PCR was performed in duplicate to analyze the expression of 5-HT2A, BDNF and TrkB mRNA using the following primer sequences:
-
BDNF forward 5'-CTGACACTTTTGAGCACGTGATC-3'
reverse 5'-AGGCTCCAAAGGCACTTGACT-3'
-
5-HT2A forward 5'-TGCCTGTGTCCATGTTAACCA-3'
reverse 5'-AAGAGCACATCCAGGTAAATCCA-3'
-
Trk B forward 5'-GGCTGTGGTACCCGATCAGT-3'
reverse 5'-CACTCACCCTTCGCCACCTA-3'
-
18s forward 5'-AACGAGACTCTCGGCATGCTAA-3'
reverse 5'-CCGGACATCTAAGGGCATCA-3'
Primer specificity was confirmed by dissociation (melting) curve analysis and agarose gel electrophoresis.
Data were collected and analyzed using the Comparative CT method. Cycle thresholds (CT) were set in the linear range of amplification for each gene. The gene of interest was normalized as a difference from the amplification cycle at threshold for 18s mRNA. Non-template controls (lacking cDNA) were generated for each gene to control for baseline reagent contamination.
2.6 Immunoblots
Ventral cervical spinal sections (C3-C5) were harvested for 5-HT2A receptor protein measurements. Briefly, a separate group of naïve rats were initially anesthetized with isoflurane in a closed box, and then were euthanized via an intracardiac injection of sodium pentobarbital. Cervical spinal segments C3-C5 were quickly removed and sections were placed onto a freezing microtome. Successive 50-µm sections of dorsal horn were removed and discarded until the ventral aspect of the central canal was visible. The remaining tissue was stored at −80°C until homogenization in lysis buffer [20 mM Tris (pH = 8.0), 137 mM NaCl, 1% Tergitiol (Type NP-40), 10% glycerol, 20 µg/ml aprotinin, 2 µg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride and 1 mM sodium metavanadate]. Samples were centrifuged for 15 min at 12,000×g, and the supernatants collected. Total protein was quantified using a commercially available kit (Pierce Biotechnology Inc., Rockford, IL).
Samples were added to sample buffer (final concentration: 10 mM Tris, 0.75 mM EDTA, 10 mM DTT, 1% SDS, 3% glycerol and 0.1% bromophenol blue), boiled for 4–5 min and 25 µg protein was loaded into each well of a 10% SDS-PAGE gel. Proteins were transferred to Immobilon polyvinylidene difluoride (PVDF) membranes (Millipore Corp., Bedford, MA). Membranes were blocked in 5% non-fat milk/TBST (10 mM Tris-HCL, pH 8.0, 150 mM NaCl, 0.05% Tween 20) at 37°C for 30 min and then probed for 1 hr at 37°C with mouse anti-5-HT2A antibodies (1:1000; BD Biosciences, San Diego, CA) in 5% milk/TBST. Membranes were washed 3×5 min in TBST and subsequently probed with anti-mouse antibodies conjugated to horseradish peroxidase (1:4000; Santa Cruz Biotechnology, Santa Cruz, CA). The immunoreactive bands were visualized using SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL) and the Autochemi detection system (UVP, Inc, Upland, CA). To ensure equal protein loading, blots were probed with antibodies recognizing the cytosolic protein Grb-2 (1:2500; Santa Cruz Biotechnology, Santa Cruz, CA).
2.7 Data Analysis
Variables characterizing phrenic and hypoglossal motor output were averaged in 30s bins (immediately preceding blood sampling) under baseline conditions, during and 60 min following AIH, or an equivalent duration of baseline recording conditions (time controls). Variables measured included peak amplitude of integrated phrenic and hypoglossal burst activity and nerve burst frequency. Changes from baseline in burst amplitude were normalized as a percentage of baseline values (% baseline). Changes in burst frequency are reported as the absolute change in frequency from baseline values. Since there were no obvious strain differences in time-dependent changes in phrenic or XII motor output in time controls, and no a priori expectation of a difference, time controls from each strain were pooled together to increase statistical power.
A one-sample t-test was used to determine if pLTF and XII LTF were significantly different than zero, which represents the baseline value. A one-way ANOVA was used to determine if recruitment thresholds, pLTF, XII LTF or changes in burst frequency 60 min post-AIH differed among treatment groups. Time dependent changes in blood gases, blood pressure and burst frequency were compared among treatment groups using two-way repeated measures ANOVA followed by Student-Newman-Keuls post hoc tests.
For each rat, average CT values for 18s mRNA (control gene) were subtracted from the average CT value for each gene investigated (ΔCT, Table 2). A large ΔCT value indicates the need for more replication cycles to reach amplification threshold; thus ΔCT is inversely related to basal mRNA levels. Strain comparisons were conducted using one-way ANOVA on ΔCT values.
Table 2.
Average delta CT values for Lewis, Fischer (F344) and Brown Norway (BN) rats for 5-HT2A, 5-HT7, BDNF and TrkB mRNA (mean ± SEM). BN rats had significantly higher delta CT values for 5-HT2A mRNA than F344 or Lewis rats, and significantly lower delta Ct values for 5-HT7 mRNA than F344 rats. F344 rats had significantly higher delta CT values for BDNF mRNA than BN or Lewis rats. F344 rats also had significantly higher TrkB mRNA than BN rats. Higher CT values indicate less mRNA in the sample.
| Gene | Strain | Phrenic delta CT |
|---|---|---|
| 5-HT2A | Lewis | 17.2 ± 0.2a |
| F344 | 17.1 ± 0.2a | |
| BN | 19.5 ± 0.3b | |
| 5-HT7 | Lewis | 16.4 ± 0.1a,b |
| F344 | 16.7 ± 0.2b | |
| BN | 16.1 ± 0.1a | |
| BDNF | Lewis | 20.6 ± 0.2a |
| F344 | 21.4 ± 0.2b | |
| BN | 20.5 ± 0.1a | |
| TrkB | Lewis | 13.3 ± 0.1a,b |
| F344 | 13.7 ± 0.2a | |
| BN | 13.1 ± 0.1b |
significant differences between strains are indicated by different letters; (p<0.05).
Immunoblot band density (5-HT2A receptor and Grb-2) was quantified using LabWorks software (UVP, Inc., Upland, CA). 5-HT2A receptor density was normalized to Grb-2, and a one-way ANOVA was used to detect significant differences in 5-HT2A receptor expression among rat strains. Regression analysis was used to determine the relationship between average baseline 5-HT2A receptor levels and average pLTF magnitude.
Statistical tests were conducted using SigmaStat 2.03, SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant at p≤0.05. All data are presented as means ± SEM.
3. Results
3.1. Blood gas variables and mean arterial pressure
CO2 recruitment thresholds and blood gas variables differed among strains (Table 1). F344 rats had significantly lower recruitment thresholds (as measured by ETCO2 levels) than BN or Lewis rats (p<0.001). BN rats had significantly higher PaCO2 and lower PaO2 levels at baseline than F344 and Lewis (each p<0.01), although the PaO2 levels observed were not in a range likely to affect carotid chemoreceptor function. Sixty minutes post-AIH, BN continued to have significantly higher PaCO2 and lower PO2 levels than F344 or Lewis rats (p<0.004). Lewis rats also had significantly higher PaCO2 levels than F344 rats at 60 min post-AIH (p=0.04).
Table 1.
Blood parameters before (baseline) and 60-min following acute intermittent hypoxia (AIH) in Brown Norway (BN), Fisher F344 and Lewis inbred rat strains.
| BN | F344 | LEWIS | |
|---|---|---|---|
| Baseline | |||
| Recruitment threshold (ETCO2, mmHg) | 42.8 +/− 0.7a | 37.8 +/− 0.5b | 43.3 +/− 0.6a |
| PaCO2 (mmHg) | 47.1 +/− 0.6a | 42.8 +/− 0.6b | 44.4 +/− 0.7b |
| PO2 (mmHg) | 213 +/− 5a | 240 +/− 6b | 242 +/− 8b |
| Body temperature (°C) | 37.5 +/− 0.1 | 37.6 +/− 0.1 | 37.5 +/− 0.1 |
| Mean arterial pressure (mmHg) | 86 +/− 2a | 86 +/− 3a | 70 +/− 2b |
| 60 min post-AIH | |||
| PaCO2 (mmHg) | 47.3 +/− 0.6a | 42.8 +/− 0.6b | 44.6 +/− 0.7c |
| PO2 (mmHg) | 177 +/− 5a,* | 228 +/− 8b,* | 238 +/− 7b |
| Mean arterial pressure (mmHg) | 75 +/− 2a,* | 76 +/− 3a,* | 56 +/− 2b,* |
significant differences between strains are indicated by different letters;
significantly different from baseline; (p<0.05).
Within each strain, PaCO2 levels 60 min post-AIH did not differ from baseline PaCO2 values (p>0.05), indicating that time-dependent changes in respiratory neural activity were not due to a time-dependent changes in chemoreceptor stimuli (i.e., PaCO2 levels). PaO2 levels 60 min following AIH were significantly lower than baseline in BN and F344 rats (p<0.03); however, since PO2 remained well above normoxic levels throughout the protocol, we do not believe that this drift is physiologically relevant.
Mean arterial pressure was significantly lower in Lewis rats versus BN or F344 during both baseline and 60 min post-AIH (p<0.001; Table 1). In all strains, mean arterial pressure decreased from baseline values at 60 min post-AIH (p<0.001), but the decrease did not differ among strains.
3.2 pLTF
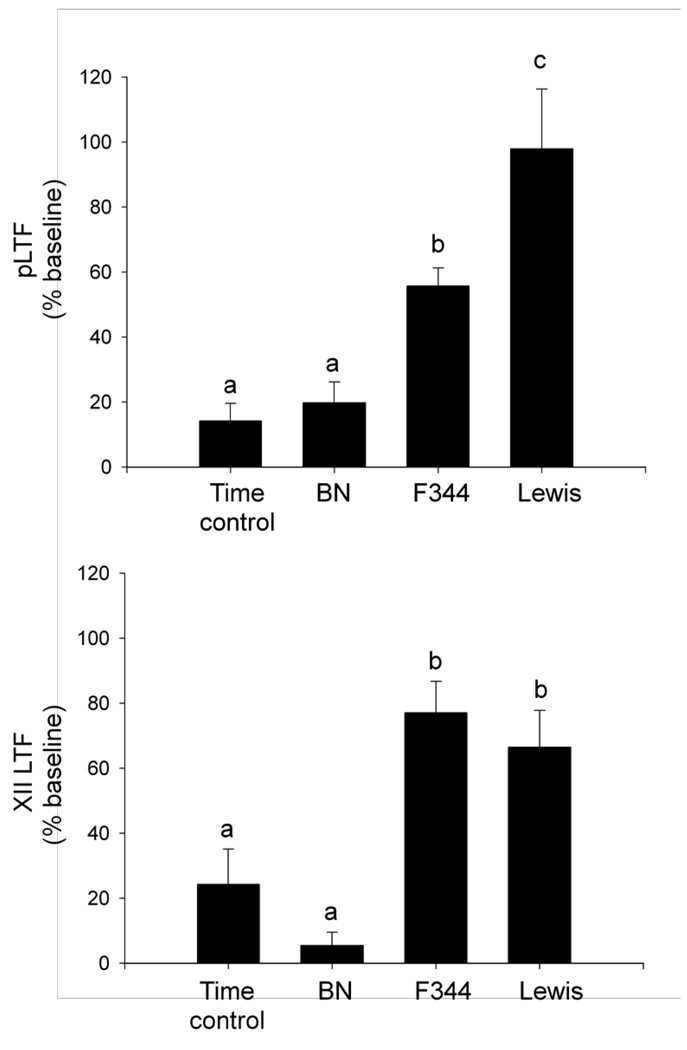
All strains exhibited a significant increase in phrenic burst amplitude from baseline at 60 min post-AIH, indicating pLTF (figure 1a; BN = 20 +/− 6%; F344 = 56 +/− 6%; Lewis = 98 +/− 18% baseline; all p<0.01). The increase in phrenic motor output following AIH differed significantly among rat strains (p<0.01). Time controls (without AIH) were pooled from each strain since there were no time-dependent changes in phrenic motor output among strains over the 90-min recording period (p=0.1). Collectively, time controls had a small, but significant increase in phrenic burst amplitude at 60 min following the sham protocol (control = 14 +/− 5% baseline; p<0.02), indicating that phrenic activity drifts slightly upward in this experimental preparation over a 90-min recording period. F344 and Lewis (p<0.02), but not BN (p>0.7) rats, had a significantly greater increase in phrenic burst amplitude at 60 min post-AIH versus the time controls, indicating that only F344 and Lewis rats exhibit robust pLTF.
Figure 1.
Strain-dependent differences in long-term facilitation (LTF) at 60 min post-AIH in Brown Norway (BN), Fischer (F344) and Lewis rats. A) Lewis rats exhibited the largest phrenic LTF (pLTF) of the three strains, which was significantly greater than pLTF observed in F344, BN, or time control rats. F344 rats exhibited moderate pLTF, which was significantly greater than BN or time control rats not exposed to AIH. BN rats did not exhibit pLTF. B) F344 and Lewis rats exhibited similar XII LTF that was significantly different than BN or time control rats not exposed to AIH. a,b,c bars with different letters are significantly different from one another; p<0.05.
Regression analysis was used to determine if baseline PaCO2 levels or the drift in PaO2, PaCO2 or mean arterial blood pressure from baseline to 60 min post-AIH were significant predictors of pLTF magnitude. No significant relationship was found for any of these variables (all p>0.4; data not shown).
3.3 XII LTF
XII burst amplitude was significantly increased above baseline 60 min post-AIH in F344 and Lewis (figure 1b; F344: 77 +/− 10%; Lewis: 66 +/− 11% baseline; p<0.001), but not BN rats (5 +/− 4%; p=0.2). Although there was no difference between F344 and Lewis rats (p=0.47), both F344 and Lewis rats had a significantly greater XII LTF versus BN rats (p<0.001). Time control rats not exposed to hypoxia also had a small, but significant increase in XII burst amplitude over the duration of a protocol (24 +/− 11% baseline, p=0.04), indicating an upward drift over the 90-min recording period. However, AIH-induced XII LTF in F344 and Lewis rats was significantly greater than corresponding value in time control experiments (p<0.01), indicating that F344 and Lewis (but not BN) rats exhibit robust XII LTF following AIH.
3.4 frequency LTF
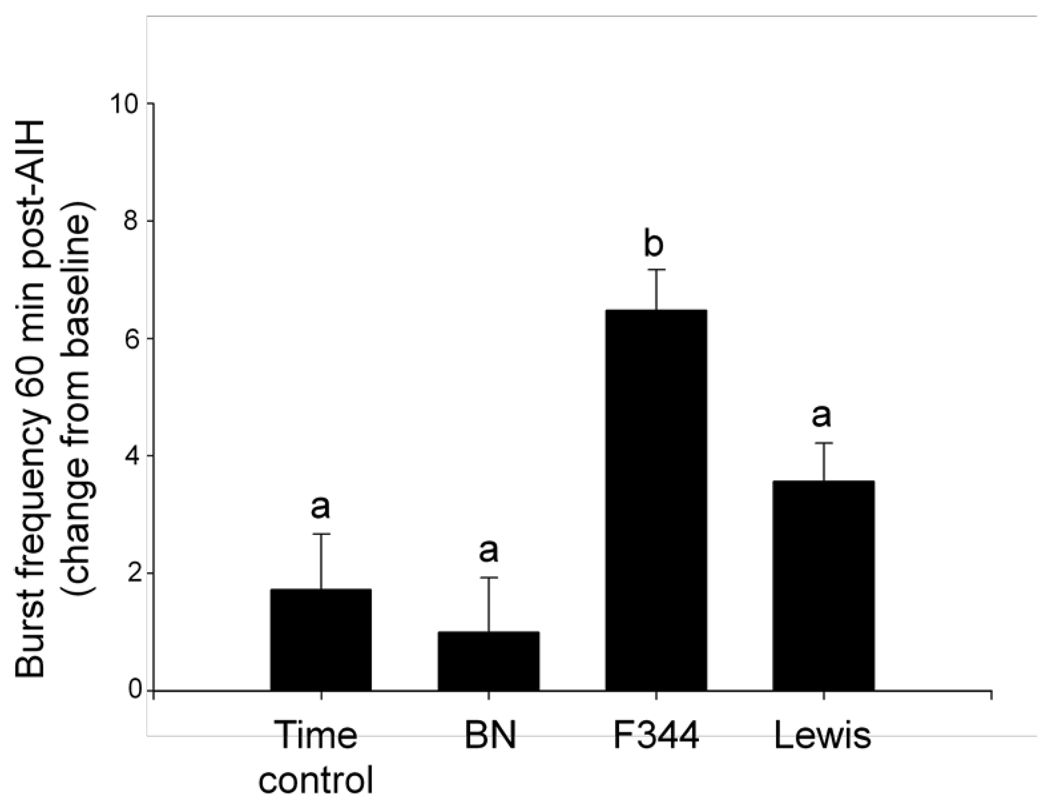
There were strain differences in baseline respiratory-related nerve burst frequency. F344 rats had a significantly higher baseline burst frequency than BN rats (F344: 44 +/− 0.5; BN: 39 +/− 0.5 bursts/min; p<0.001), but not Lewis rats (41 +/− 0.6 bursts/min; p=0.2). There were no significant differences between BN and Lewis rats (p=0.1). Within each rat group, changes in respiratory-related nerve burst frequency were analyzed. Phrenic burst frequency was increased 60 min post-AIH in F344 rats (figure 2; 6.5 +/− 0.7 bursts/min; p<0.001), but not BN or Lewis rats (BN: 1 +/− 0.9; Lewis: 3.6 +/− 0.6 bursts/min; p>0.1) when compared to time controls without hypoxia (1.7 +/− 0.9 bursts/min). Thus, only F344 rats exhibited significant frequency LTF, although it was of a small magnitude. Increased phrenic burst frequency 60-min post-AIH in F344 rats was greater than in BN or Lewis rats (p<0.02), but there were no significant differences between BN and Lewis rats (p=0.07).
Figure 2.
Strain-dependent differences in the change in burst frequency 60 min post-treatment in time controls and AIH-treated rats: Brown Norway (BN), Fischer (F344) and Lewis rats. F344 rats expressed a significantly greater increase in burst frequency from pre-AIH values than BN, Lewis or time control rats 60-min post-AIH. a,b, bars with different letters are significantly different from one another; p<0.05.
3.5 Ventral cervical 5-HT2A, 5-HT7, BDNF and TrkB mRNA levels
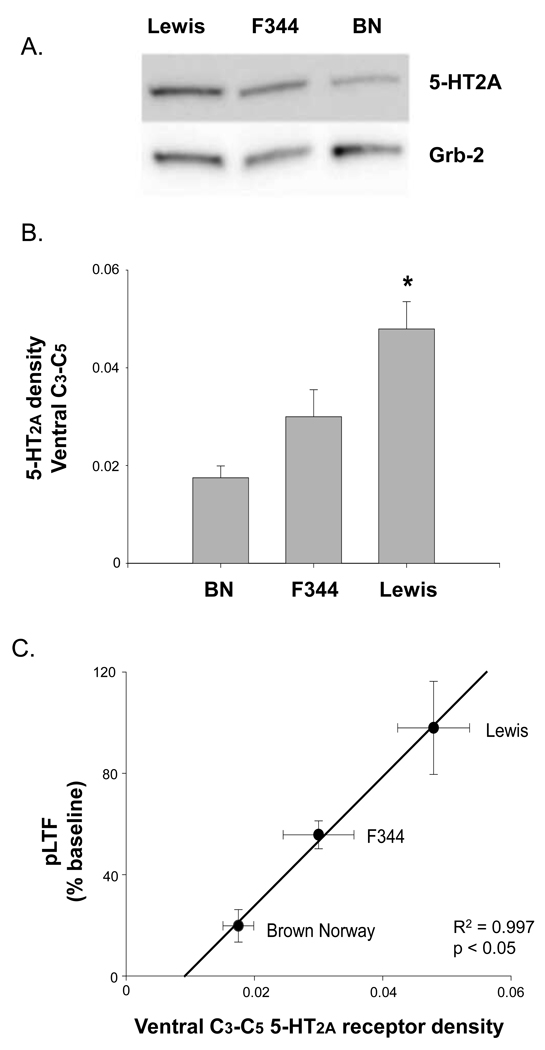
Ventral cervical spinal mRNA levels for 5-HT2A and 5-HT7 serotonin receptors were determined in a subset of naïve rats not exposed to surgical procedures or AIH. Delta CT values for these molecules are included in Table 2. Higher CT values indicate less mRNA. Lewis and F344 rats had significantly higher 5-HT2A mRNA in ventral C3-C5 than BN rats (p<0.003), but there was no difference in 5-HT2A mRNA between F344 and Lewis rats (p=0.8). On average, ventral cervical 5-HT2A mRNA levels in BN were ~80% below levels in F344 and Lewis. 5-HT7 receptor mRNA expression was significantly higher in BN than F344 rats (p=0.03), but there was no difference between BN and Lewis or Lewis and F344 (both, p>0.15). On average, ventral cervical 5-HT7 mRNA levels in BN were 52% higher than in F344 rats. Thus, ventral cervical expression 5-HT2A receptor mRNA varies in association with pLTF, but ventral cervical 5-HT7 receptor mRNA is not clearly correlated with pLTF magnitude.
BN and Lewis had significantly higher BDNF mRNA in ventral C3-C5 than F344 (p<0.03), but there was no difference in BDNF mRNA between BN and Lewis rats (p>0.6). On average, ventral cervical BDNF mRNA levels in F344 were ~45% below levels in BN and Lewis. BN rats had significantly higher levels of TrkB mRNA in ventral C3-C5 than F344 (p<0.03), but no difference was detected between BN and Lewis or F344 and Lewis (p>0.1). On average, ventral cervical TrkB mRNA levels in F344 were 34% below levels in BN. Thus, there was no clear relationship between BDNF or TrkB mRNA and pLTF expression.
3.6 Ventral cervical 5-HT2A receptor protein levels
Consistent with 5-HT2A receptor mRNA results, Lewis rats had significantly higher 5-HT2A protein levels in ventral C3-C5 segments relative to BN rats (p=0.01; Figure 3a and 3b). While there was a trend for F344 rats to have higher 5-HT2A receptor protein expression relative to BN, this trend was not statistically significant (p>0.1). Regression analysis between basal 5-HT2A receptor protein expression and average pLTF in each strain indicated a positive relationship between 5-HT2A receptor density in ventral spinal segments encompassing the phrenic motor nucleus and the capacity for pLTF (R2=0.997, p<0.04; Figure 3c).
Figure 3.
5-HT2A protein levels in spinal regions encompassing the phrenic motor nucleus in Brown Norway (BN), Fischer (F344) and Lewis rats. A) Lewis rats had significantly higher 5-HT2A protein levels in ventral C3-C5 than BN rats. B) Regression analysis indicates a significant relationship between 5-HT2A receptor density near phrenic motoneurons and pLTF magnitude in BN, F344 and Lewis rats. * significantly different than BN rats; P<0.05.
4. Discussion
Here, we report that the magnitudes of AIH-induced phrenic and XII burst amplitude LTF (i.e. pLTF and XII LTF, respectively), as well as frequency LTF, differ among inbred rat strains, suggesting genetic or epigenetic differences in the regulation of respiratory plasticity. We further report that strain differences in pLTF are associated with differential expression of key molecules implicated in the mechanisms of pLTF (Fuller et al., 2001a; Baker-Herman and Mitchell, 2002; Baker-Herman et al., 2004). The most consistent relationships observed were between 5-HT2A receptor mRNA and protein levels in the ventral cervical spinal cord with pLTF. Thus, the strongest molecular candidate underlying pLTF strain differences is differential 5-HT2A receptor gene expression.
The strains chosen for this study are frequently used as models for important physiological principles. For example, F344 rats are frequently studied as a model of aging, including aging effects on respiratory plasticity (Zabka et al., 2001; Behan et al., 2003). Lewis rats are frequently studied as a model of functional recovery following spinal cord injury (Golder et al., 2005), and BN rats are the designated strain for the rat genome project (Gibbs et al., 2004). In addition to their importance as physiological models, BN, F344 and Lewis rats exhibit similar increases in phrenic and XII burst amplitude during hypoxia, although BN and F344 rats differ in the burst frequency response to hypoxia (Golder et al., 2005a). While increased inspiratory activity during hypoxia is not necessary for LTF (Baker et al., 2001; Bocchario and Feldman, 2004; Tadjalli et al., 2007), an association has been found between peak phrenic responses during hypoxia and the magnitude of the subsequent pLTF. Despite similarities in the hypoxic amplitude response, pLTF and XII LTF differed considerably among rat strains (pLTF: Lewis > F344 > BN; XII LTF: F344 ~ Lewis > BN). Overall, Lewis rats tended to have greater capacity for amplitude LTF, with BN exhibiting the lowest amplitude LTF. XII LTF was not significant in BN rats despite a large sample size (23), a finding consistent with our previous report (Wilkerson and Mitchell, 2009). On the other hand, the lack of significant pLTF in BN rats (relative to time controls) may appear to be inconsistent with other recent findings (Wilkerson and Mitchell, 2009). However, the small post-AIH changes in phrenic motor output in BN rats were similar here (20% baseline) versus our earlier study (Wilkerson and Mitchell, 2009; 19% baseline), but time-dependent drift in phrenic nerve activity was greater in the present study (14% versus 8% baseline). Thus, any AIH-induced changes may have been obscured by greater time-dependent drift in the present study.
LTF of respiratory-related burst amplitude may result from intermittent serotonin receptor activation on or near respiratory motor neurons (Mitchell et al., 2001b; Fuller et al., 2001a; Baker-Herman and Mitchell, 2002), a concept consistent with our recent report that intermittent spinal serotonin receptor activation is sufficient to induce long-lasting phrenic motor facilitation (MacFarlane and Mitchell, 2009). pLTF is frequently used as a model to study cellular mechanisms of amplitude LTF and, thus, was the focus of our analysis. We hypothesize that 5-HT2 receptor activation in or near phrenic motor neurons initiates new BDNF synthesis and subsequent TrkB receptor activation, thereby giving rise to pLTF (Baker-Herman et al., 2004). In contrast, the role of 5-HT7 receptor activation in AIH-induced pLTF is more complex. Cervical spinal 5-HT7 receptor activation is sufficient to elicit long-lasting phrenic motor facilitation in Sprague Dawley rats (Hoffman and Mitchell, 2008a), but 5-HT7 receptor activation during AIH constrains (versus contributes to) pLTF via cross-talk inhibition (Hoffman and Mitchell, unpublished observations). Indeed, cervical spinal 5-HT7 receptor inhibition increases AIH-induced pLTF by approximately 100% (Hoffman and Mitchell, unpublished observations). Although mechanisms of 5-HT7 receptor-dependent pLTF constraint remains unclear, our working model is that 5-HT7-induced PKA activation attenuates 5-HT2A-dependent NADPH oxidase activity and ROS formation, thereby constraining pLTF (Hoffman and Mitchell, 2008b; Hoffman et al., 2009; MacFarlane and Mitchell, 2009). Thus, differential 5-HT2A and 5-HT7 receptor expression may contribute to differential pLTF expression in BN vs. Lewis and F344 rats. In specific, lower 5-HT2A (less facilitation) and greater 5-HT7 receptor expression (more cross-talk inhibition) may both contribute to reduced pLTF levels.
More complex relationships between ventral cervical BDNF and TrkB mRNA and pLTF exist, since BN rats have the lowest pLTF but higher BDNF and TrkB mRNA expression levels (versus F344 rats). In contrast, Lewis rats exhibit greater pLTF versus F344 rats, yet have higher BDNF mRNA levels. The finding that BDNF mRNA is greatest in the highest (Lewis) and lowest (BN) pLTF-expressing strains demonstrates that there is no simple relationship between BDNF mRNA and the capacity for pLTF. Similarly, there is no simple relationship between pLTF and TrkB mRNA expression.
Strain differences were also observed in burst frequency LTF. Frequency LTF most likely arises from mechanisms operating at the level of brainstem respiratory rhythm generating neurons (Powell et al., 1998; Blitz and Ramirez, 2002; Baker-Herman and Mitchell, 2008). The mechanisms underlying frequency LTF are poorly understood. Similar to pLTF and XII LTF, serotonin receptor inhibition blocks frequency LTF (Bach and Mitchell, 1996). However, systemic serotonin receptor antagonists tend to alter baseline burst frequency, and a recent meta-analysis suggests that baseline burst frequency influences subsequent frequency LTF magnitude (Baker-Herman and Mitchell, 2008). Thus, the apparent requirement of frequency LTF for serotonin receptor activation may reflect indirect drug effects on baseline burst frequency (Baker-Herman and Mitchell, 2008). More detailed analyses of mechanisms giving rise to frequency LTF are warranted to understand differences among rat strains.
In addition to respiratory plasticity, strain differences were observed in baseline physiological variables, such as baseline burst frequency, CO2 apneic/recruitment threshold and mean arterial pressure. While these differences may have been the result of differential reactions to anesthesia, it also remains possible that genetic differences in key modulatory/signaling pathways give rise to variations in physiological set-points among inbred rat strains. For example, 5-HT receptor activation in brainstem regions responsible for generating respiratory rhythm influences respiratory-related burst frequency (Peña and Ramirez, 2002; Schwarzacher et al., 2002; Viemari and Tryba, 2009); thus, it is possible that differential 5-HT2 receptor levels in respiratory rhythm generating neurons underlie differential baseline burst frequencies in F344 versus BN or Lewis rats.
Taken together, our data suggest that genetic (or epigenetic) factors influence the capacity for respiratory plasticity in phrenic motor output via alterations in constitutive expression of key proteins in the mechanism of pLTF, particularly the expression of serotonin receptors. While we cannot completely rule out different rearing practices as the source of strain differences, we minimized this impact by obtaining most rats from the Harlan Indianapolis facility (with the exception of a group of F344 rats from the NIH-NIA Aged Colony). Nevertheless, our data are consistent with previous studies demonstrating that variations in serotonergic function gives rise to differences in pLTF expression. Increased serotonin terminal density (Kinkead et al., 1998) and increased ventral spinal BDNF protein expression (Johnson et al., 2000) following chronic cervical sensory denervation are associated with enhanced pLTF (Kinkead et al., 1998). Similarly, chronic intermittent hypoxia (days) gives rise to enhanced LTF following AIH (Ling et al., 2001; Zabka et al., 2003; McGuire et al., 2003, 2004; Wilkerson and Mitchell, 2009), an effect that may reflect increased expression of a novel 5-HT receptor subtype (Ling et al., 2001; McGuire et al., 2004) and/or BDNF (Wilkerson and Mitchell, 2009). Lastly, cervical spinal hemisection is associated with 5-HT2A receptor upregulation (Fuller et al., 2005), an effect that correlates with strengthened crossed spinal synaptic pathways to phrenic motor neurons (Zhou and Goshgarian, 2000). Molecular events that underlie such “meta-plasticity” may also contribute to strain differences in pLTF.
Our data highlight the importance of strain selection in studies of pLTF, or any other form of respiratory plasticity. For example, F344 rats would be a useful model for investigations concerning signal transduction mechanisms underlying pLTF since this strain exhibits robust pLTF and high mean arterial pressures. By contrast, the small pLTF in BN rats may represent a useful model for studies of meta-plasticity (Wilkerson and Mitchell, 2009; Ling et al., 2001; Zabka et al., 2003). In some respects, BN rats may be an appropriate model for human respiratory plasticity since humans normally express limited capacity for ventilatory LTF (Babcock & Badr, 1998; Harris et al., 2006; Pierchala et al., 2008), but express robust LTF in sleep apnea patients (Lee et al., 2009).
In summary, inbred rat strains differ in their capacity for AIH-induced respiratory plasticity. Although differences were noted in several manifestations of LTF (pLTF, XII LTF and frequency LTF), we focused on pLTF in this report because more is known regarding the cellular mechanism underlying pLTF. Lewis rats exhibit the greatest pLTF magnitude, whereas F344 rats are intermediate and BN rats exhibit little or no pLTF. Differential pLTF expression is associated with variations in key molecules underlying pLTF, particularly differential expression of serotonin receptors (5-HT2A and/or 5-HT7). We conclude that differences in serotonergic function may account, at least in part, for differences in the capacity for AIH-induced respiratory plasticity among rat strains.
Acknowledgements
This work was supported by grants from the National Institutes of Health (HL080609, HL69064 and HL65383). T.L.B-H. was supported by a Parker B. Francis Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babcock MA, Badr MS. Long-term facilitation of ventilation in humans during NREM sleep. Sleep. 1998;21:709–716. [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker TL, Fuller DD, Zabka AG, Mitchell GS. Respiratory plasticity: differential actions of continuous and episodic hypoxia and hypercapnia. Respir Physiol. 2001;129:25–35. doi: 10.1016/s0034-5687(01)00280-8. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22 doi: 10.1523/JNEUROSCI.22-14-06239.2002. 6239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol. 2008;162:8–17. doi: 10.1016/j.resp.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavis RW, Baker-Herman TL, Zabka AG, Golder FJ, Fuller DD, Behan M, Mitchell GS. Respiratory long-term facilitation differs among inbred rat strains. FASEBJ. 2003 doi: 10.1016/j.resp.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Thomas CF, Mitchell GS. Sex steroid hormones and the neural control of breathing. Respir Physiol Neurobiol. 2003;136:249–263. doi: 10.1016/s1569-9048(03)00086-7. [DOI] [PubMed] [Google Scholar]
- Behan M, Wenninger JM. Sex steroidal hormones and respiratory control. Respir Physiol Neurobiol. 2008 Jun 12; doi: 10.1016/j.resp.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Ramirez JM. Long-term modulation of respiratory network activity following anoxia in vitro. J Neurophysiol. 2002;87:2964–2971. doi: 10.1152/jn.2002.87.6.2964. [DOI] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci USA. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao KY, Zwillich CW, Berthon-Jones M, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J Appl Physiol. 1992;73:2083–2088. doi: 10.1152/jappl.1992.73.5.2083. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol. 2000;121:135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001a;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker TL, Behan M, Mitchell GS. Expression of hypoglossal long-term facilitation differs between substrains of Sprague-Dawley rat. Physiol Genomics. 2001b;4:175–181. doi: 10.1152/physiolgenomics.2001.4.3.175. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker-Herman TL, Golder FJ, Doperalski NJ, Watters JJ, Mitchell GS. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J Neurotrauma. 2005;22:203–213. doi: 10.1089/neu.2005.22.203. [DOI] [PubMed] [Google Scholar]
- Gibbs RA, et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428:493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Zabka AG, Bavis RW, Baker-Herman T, Fuller DD, Mitchell GS. Differences in time-dependent hypoxic phrenic responses among inbred rat strains. J Appl Physiol. 2005;98:838–844. doi: 10.1152/japplphysiol.00984.2004. [DOI] [PubMed] [Google Scholar]
- Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1111–R1119. doi: 10.1152/ajpregu.00896.2005. [DOI] [PubMed] [Google Scholar]
- Hoffman MS, Mitchell GS. Episodic Spinal 5-HT7 Receptor Activation Induces Phrenic Motor Facilitation. FASEB J. 2008a [Google Scholar]
- Hoffman MS, Mitchell GS. Spinal PKA Inhibits Phrenic Long-Term Facilitation Following Acute Intermittent Hypoxia. SFN Abstracts. 2008b [Google Scholar]
- Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal Adenosine 2A Receptor Inhibition Enhances Phrenic Long Term Facilitation Following Acute Intermittent Hypoxia. J Physiol. 2009 Nov 9; doi: 10.1113/jphysiol.2009.180075. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Okragly AJ, Haak-Frendscho M, Mitchell GS. Cervical dorsal rhizotomy increases brain-derived neurotrophic factor and neurotrophin-3 expression in the ventral spinal cord. J Neurosci. 2000;20:RC77. doi: 10.1523/JNEUROSCI.20-10-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonindependent long-term facilitation of respiratory motor output in rats. J Neurosci. 1998;18:8436–8443. doi: 10.1523/JNEUROSCI.18-20-08436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Overholt JL, Prabhakar NR. Mutant mice deficient in NOS-1 exhibit attenuated long-term facilitation and short-term potentiation in breathing. J Physiol. 2002;539:309–315. doi: 10.1113/jphysiol.2001.014571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DS, Badr MS, Mateika JH. Progressive augmentation and ventilatory long-term facilitation are enhanced in sleep apnoea patients and are mitigated by antioxidant administration. J Physiol. 2009;587:5451–5467. doi: 10.1113/jphysiol.2009.178053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Behan M, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21:5381–5388. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. Respiratory long-term facilitation following intermittent hypoxia requires reactive oxygen species formation. Neuroscience. 2008;152:189–197. doi: 10.1016/j.neuroscience.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane PM, Wilkerson JE, Lovett-Barr MR, Mitchell GS. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol. 2008 Jul 23; doi: 10.1016/j.resp.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol. 2009;587:5469–5481. doi: 10.1113/jphysiol.2009.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol. 2007;92:27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Long-term depression in freely moving rats is dependent upon strain variation, induction protocol and behavioral state. Cereb Cortex. 2000;10:482–487. doi: 10.1093/cercor/10.5.482. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Chronic intermittent hypoxia enhances ventilatory long-term facilitation in awake rats. J Appl Physiol. 2003;95:1499–1508. doi: 10.1152/japplphysiol.00044.2003. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R334–R341. doi: 10.1152/ajpregu.00463.2003. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Phrenic long-term facilitation requires NMDA receptors in the phrenic motonucleus in rats. J Physiol. 2005;567:599–611. doi: 10.1113/jphysiol.2005.087650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M, Liu C, Cao Y, Ling L. Formation and maintenance of ventilatory long-term facilitation require NMDA but not non-NMDA receptors in awake rats. J Appl Physiol. 2008;105:942–950. doi: 10.1152/japplphysiol.01274.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980a;41:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980b;42:171–178. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Powell FL, Hopkins SR, Milsom WK. Time domains of the hypoxic ventilatory response in awake ducks: episodic and continuous hypoxia. Respir Physiol. 2001a;124(2):117–128. doi: 10.1016/s0034-5687(00)00197-3. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001b;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn Mem. 2000;7:170–179. doi: 10.1101/lm.7.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierchala LA, Mohammed AS, Grullon K, Mateika JH, Badr MS. Ventilatory long-term facilitation in non-snoring subjects during NREM sleep. Respir Physiol Neurobiol. 2008;160:259–266. doi: 10.1016/j.resp.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Schimanski LA, Ali DW, Baker GB, Nguyen PV. Impaired hippocampal LTP in inbred mouse strains can be rescued by beta-adrenergic receptor activation. Eur J Neurosci. 2007;25:1589–1598. doi: 10.1111/j.1460-9568.2007.05376.x. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Pestean A, Günther S, Ballanyi K. Serotonergic modulation of respiratory motoneurons and interneurons in brainstem slices of perinatal rats. Neuroscience. 2002;115:1247–1259. doi: 10.1016/s0306-4522(02)00540-7. [DOI] [PubMed] [Google Scholar]
- Tadjalli A, Duffin J, Li YM, Hong H, Peever J. Inspiratory activation is not required for episodic hypoxia-induced respiratory long-term facilitation in postnatal rats. J Physiol. 2007;585:593–606. doi: 10.1113/jphysiol.2007.135798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J Physiol. 1997;499:543–550. doi: 10.1113/jphysiol.1997.sp021947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Tryba AK. Bioaminergic neuromodulation of respiratory rhythm in vitro. Respir Physiol Neurobiol. 2009;168:69–75. doi: 10.1016/j.resp.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JER, Molter CM, Mitchell GS. Daily Acute Intermittent Hypoxia Enhances Hypoglossal, But Not Phrenic Long Term Facilitation (LTF) in Brown Norway Rats. FASEB J. 2005 [Google Scholar]
- Wilkerson JER, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp. Neurol. 2009 doi: 10.1016/j.expneurol.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Selected contribution: Time-dependent hypoxic respiratory responses in female rats are influenced by age and by the estrus cycle. J Appl Physiol. 2001;91:2381–2388. doi: 10.1152/jappl.2001.91.6.2831. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Olson EB, Jr, Behan M. Selected contribution: chronic intermittent hypoxia enhances respiratory long-term facilitation in geriatric female rats. J Appl Physiol. 2003;95:2614–2623. doi: 10.1152/japplphysiol.00476.2003. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Behan M. Ageing and gonadectomy have similar effects on hypoglossal long-term facilitation in male Fischer rats. J Physiol. 2005;563:557–568. doi: 10.1113/jphysiol.2004.077511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Conversion from testosterone to oestradiol is required to modulate respiratory long-term facilitation in male rats. J Physiol. 2006;576:903–912. doi: 10.1113/jphysiol.2006.114850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SY, Goshgarian HG. 5-Hydroxytryptophan-induced respiratory recovery after cervical spinal cord hemisection in rats. J Appl Physiol. 2000;89:1528–1536. doi: 10.1152/jappl.2000.89.4.1528. [DOI] [PubMed] [Google Scholar]