Abstract
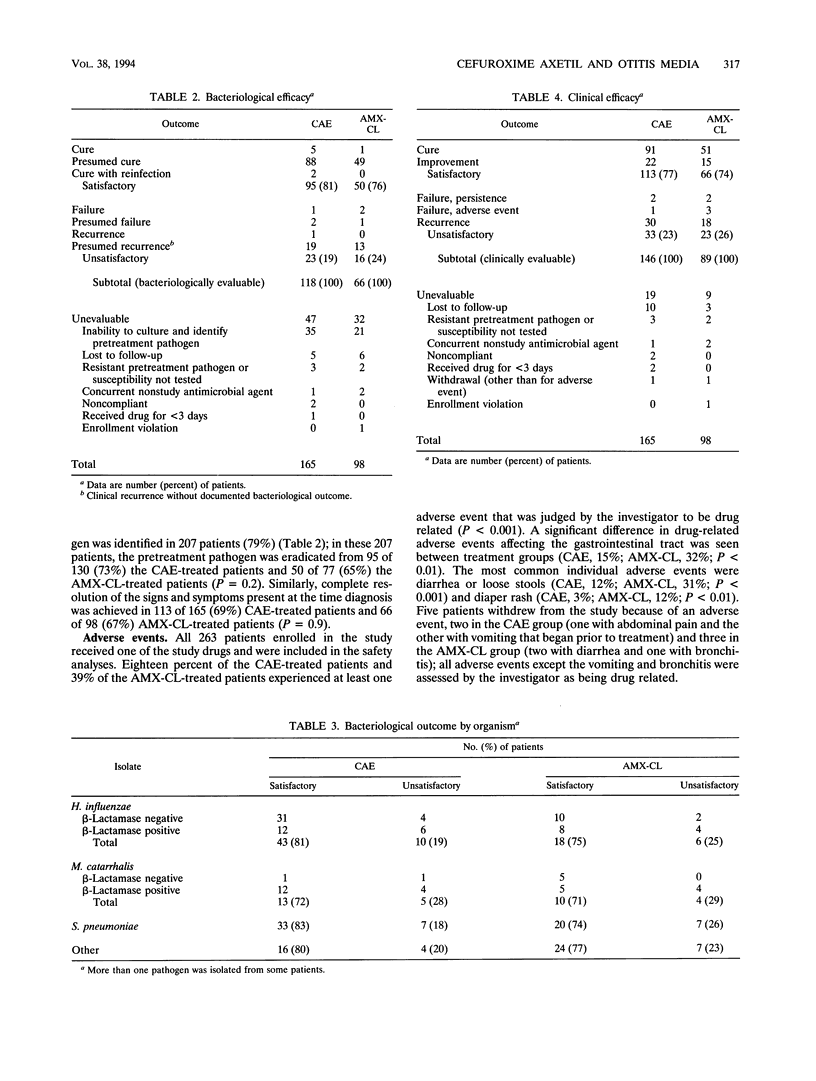
Two hundred sixty-three pediatric patients from the ages of 3 months to 11 years were enrolled in a randomized, investigator-blinded, multicenter study comparing the clinical and bacteriological efficacies and safety of cefuroxime axetil suspension (CAE) with those of amoxicillin-clavulanate suspension (AMX-CL) in the treatment of acute otitis media with effusion. Patients received CAE at 30 mg/kg of body weight per day (n = 165) in two divided doses or AMX-CL at 40 mg/kg/day (n = 98) in three divided doses for 10 days. The primary pathogens among 200 isolates from pretreatment cultures of middle ear fluid were identified as follows: Haemophilus influenzae (39%), over a third of which were beta-lactamase positive; Streptococcus pneumoniae (34%); and Moraxella catarrhalis (16%). Pathogens were eradicated or presumed to be eradicated from 81% (95 of 118) and 76% (50 of 66) of bacteriologically evaluable patients in the CAE and AMX-CL groups, respectively. A satisfactory clinical response (cure or improvement with or without resolution of effusion) occurred in 113 (77%) of 146 clinically evaluable patients in the CAE group and in 66 (74%) of 89 evaluable patients in the AMX-CL group. Clinical failure or recurrence (within 2 weeks following the completion of treatment) occurred in 22 and 26% of CAE- and AMX-CL-treated patients, respectively. Drug-related adverse events occurred in 18% of CAE-treated patients, whereas they occurred in 39% of AMX-CL-treated patients (P < 0.001); diarrhea or loose stools was the most commonly reported adverse event (CAE, 12%; AMX-CL, 31%; P < 0.001). These results indicate that CAE given twice daily is as effective as AMX-CL given three times daily in the treatment of acute otitis media with effusion in pediatric patients, but CAE was associated with significantly fewer drug-related adverse events.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum P. C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992 Jul;15(1):77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- Bluestone C. D. Management of otitis media in infants and children: current role of old and new antimicrobial agents. Pediatr Infect Dis J. 1988 Nov;7(11 Suppl):S129–S136. doi: 10.1097/00006454-198811001-00002. [DOI] [PubMed] [Google Scholar]
- Giebink G. S. The microbiology of otitis media. Pediatr Infect Dis J. 1989 Jan;8(1 Suppl):S18–S20. [PubMed] [Google Scholar]
- Gooch W. M., 3rd, McLinn S. E., Aronovitz G. H., Pichichero M. E., Kumar A., Kaplan E. L., Ossi M. J. Efficacy of cefuroxime axetil suspension compared with that of penicillin V suspension in children with group A streptococcal pharyngitis. Antimicrob Agents Chemother. 1993 Feb;37(2):159–163. doi: 10.1128/aac.37.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussar D. A. Importance of patient compliance in effective antimicrobial therapy. Pediatr Infect Dis J. 1987 Oct;6(10):971–975. doi: 10.1097/00006454-198710000-00036. [DOI] [PubMed] [Google Scholar]
- Kaleida P. H., Bluestone C. D., Rockette H. E., Bass L. W., Wolfson J. H., Breck J. M., Ubinger E. B., Rohn D. D. Amoxicillin-clavulanate potassium compared with cefaclor for acute otitis media in infants and children. Pediatr Infect Dis J. 1987 Mar;6(3):265–271. doi: 10.1097/00006454-198703000-00013. [DOI] [PubMed] [Google Scholar]
- Klugman K. P. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990 Apr;3(2):171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisby-Sutch S. M., Nemec-Dwyer M. A., Deeter R. G., Gaur S. M. Therapy of otitis media. Clin Pharm. 1990 Jan;9(1):15–34. [PubMed] [Google Scholar]
- Marchant C. D., Shurin P. A., Johnson C. E., Murdell-Panek D., Feinstein J. C., Fulton D., Flexon P., Carlin S. A., Van Hare G. F. A randomized controlled trial of amoxicillin plus clavulanate compared with cefaclor for treatment of acute otitis media. J Pediatr. 1986 Nov;109(5):891–896. doi: 10.1016/s0022-3476(86)80721-1. [DOI] [PubMed] [Google Scholar]
- Odio C. M., Kusmiesz H., Shelton S., Nelson J. D. Comparative treatment trial of augmentin versus cefaclor for acute otitis media with effusion. Pediatrics. 1985 May;75(5):819–826. [PubMed] [Google Scholar]
- Odio C. M., Kusmiesz H., Shelton S., Nelson J. D. Comparative treatment trial of augmentin versus cefaclor for acute otitis media with effusion. Pediatrics. 1985 May;75(5):819–826. [PubMed] [Google Scholar]
- Pichichero M., Aronovitz G. H., Gooch W. M., McLinn S. E., Maddern B., Johnson C., Darden P. M. Comparison of cefuroxime axetil, cefaclor, and amoxicillin-clavulanate potassium suspensions in acute otitis media in infants and children. South Med J. 1990 Oct;83(10):1174–1177. doi: 10.1097/00007611-199010000-00013. [DOI] [PubMed] [Google Scholar]



