Abstract
Kinesins are enzymes that use the energy of ATP to perform mechanical work. There are approximately 14 families of kinesins within the kinesin superfamily. Family classification is derived primarily from alignments of the sequences of the core motor domain. For this reason, the enzymatic behavior and motility of each motor generally reflects its family. At the cellular level, kinesin motors perform a variety of functions during cell division and within the mitotic spindle to ensure that chromosomes are segregated with the highest fidelity possible. The cellular functions of these motors are intimately related to their mechanical and enzymatic properties at the single molecule level. For this reason, motility studies designed to evaluate the activity of purified molecular motors are a requirement in order to understand, mechanistically, how these motors make the mitotic spindle work and what can cause the spindle to fail. This review will focus on a selection of illustrative kinesins, which have been studied at the molecular level in order to inform our understanding of their function in cells. In addition, the review will endeavor to point out some kinesins that have been studied extensively but which still lack sufficient molecular underpinnings to fully predict their contribution to spindle function.
Keywords: Mitosis, Kinesin, Motor, Microtubule, Mitotic Spindle
1. Introduction: The discovery of motors in the mitotic spindle
“The venerable and provocative speculation that all forms of movement in biological systems have a common molecular basis can, at present, be subjected to few kinds of experimental test, for all of which muscle contraction provides the paradigms. One test is the search for proteins corresponding to actin and myosin in their physical properties, a second is the preparation of working “models,”’ and a third is the demonstration that the molecules composing the working structure not only interact with but split ATP.”(1)
Ever since the surprising discovery that myosin(2) was capable of hydrolyzing ATP(3), the energy source for muscle contraction, students of mitosis have exhibited a keen interest in molecular motors. This might derive from the appearance of mitotic chromosomes which, even in fixed preparations, seemed to be experiencing motive force. It did not escape the eye of David Mottier (1903)(4) that chromosomes attached to the mitotic spindle appeared to be experiencing a pulling force exerted by the spindle fibers (Figure 1). The isolation of an ATPase from Tetrahymena cilia in 1965(5) only served to intensify the resolve among cell biologists to link these motors to mitotic spindle function. Especially compelling was the fact that this ciliar enzyme, dynein, resembled an ATPase isolated from mitotic spindles(1) more closely than it resembled myosin. Two decades later another microtubule motor called kinesin was purified from squid axoplasm and implicated as the motor powering fast axonal transport in neurons(6). Kinesin, which ironically, bears considerable structural similarity to myosin (6, 7), seemed like a valuable molecule for use within the spindle if it could only be identified in non-neuronal cells. Kinesin and dynein suggested a palette of force generators potentially available to the spindle. Dynein, which travels toward microtubule minus ends in combination with kinesin, whose founding member travels unidirectionally toward microtubule plus-ends seemed like a perfect complement of motors that could be employed for all the mechanical requirements of cell division. Unfortunately, neither of these molecules could be identified, unambiguously, with the mitotic spindle for a number of years using the biochemical techniques available at the time. The 1990’s, however, were a comparatively rich decade for motors in the spindle in that several genetic studies simultaneously galvanized and revolutionized the mitosis field by identifying mitotic kinesins that strongly resembled but were not identical to previously identified neuronal kinesin(8–12). At the same time and after much hard work, cytoplasmic dynein was also unambiguously identified in the mitotic spindle(13, 14). The mitotic spindle can now flex its muscles.
Figure 1.
Chromosome segregation in the Lillium pollen shore mother cell from Mottier (1903)(4).
Kinesins, which now number over 45 unique genes in mammals, fall into approximately 14 subfamilies based on sequence homology within the catalytic core motor domain(15). It would not be an overstatement to say that almost all identified kinesin families participate to some extent in cell division. The purpose of this review will be to examine a subset of kinesins, which have been carefully evaluated for activity in isolation and then attempt to place this activity in the context of spindle function. Space constraints prevent me from a comprehensive review of all mitotic motors, which would include kinesins, dyneins and myosins. Finally, I hope to emphasize that there are a number of interesting, heavily investigated, mitotic kinesins which still lack thorough evaluation of enzymatic function in vitro and that without these data a complete understanding of cellular function is impossible.
2. Kinesin: The Movement Protein
2.1 Introduction
For the majority of enzymes it is difficult to “see” them work. One can measure the accumulation of product or the decrease in substrate. Detailed structural studies can reveal mechanistic snapshots of the protein in action. But generally speaking it is often difficult to watch an enzyme working in real time. In contrast, many details of motor protein mechanism can be viewed in the light microscope in real time. The early biochemical purification of neuronal kinesin made use of this property in the form of a “motility assay”(6). Motors can be purified, affixed to a substrate, a bead, or a fluorescent tag and fed with a little ATP. Then their directionality, processivity, on-rate and off-rate can be measured directly as they travel along the surface of the microtubule lattice.
To date approximately 45 unique kinesin superfamily genes have been identified in mammals falling into 14 families(15, 16). These motors participate in a wide variety of often cell- and tissue-specific functions and the majority possess one or more members that assist during mitosis or meiosis. The 14 families of kinesin genes are classified based on a combination of sequence (and therefore structural) similarity and functional grouping(16, 17). The functional grouping of kinesins must be, by necessity, relatively broad because kinesins exhibit tissue-specific functions. An example is the microtubule-depolymerizing kinesin, MCAK, a member of the kinesin-13 family of which there are 3 unique members in mammals. MCAK depolymerizes microtubules(18). In mitotic cells, MCAK promotes the turnover of kinetochore fiber microtubules during cell division, which leads to improvements in error-correction, congression and spindle assembly(19–22). MCAK is not, thus far, found in post-mitotic or terminally differentiated cells(23). However, postmitotic neurons do express a kinesin-13 family member, Kif2A, which limits collateral branch extension in developing neurons(24). What is the functional link between these two jobs? In both cases they are accomplished by disassembling microtubules. Thus, the functional classification of kinesins ends up being dependent on how they move on, and what they do to, microtubules.
2.2 Kinesin Families
Kinesin families fall into three broad functional categories based on what they do in conjunction with microtubules: (i) kinesins that translocate to the plus ends of microtubules (kinesins-1 through 7, kinesin-12), (ii) kinesins that translocate to the minus ends of microtubules (kinesin-14), (iii) kinesins that depolymerize microtubules (kinesin-13, kinesin-8). As always, there are interesting exceptions to these classifications (kinesin-10) and a few not rigorously tested (kinesin-9, kinesin-11). Within these broad functional classifications detailed analyses of motility reveal themes consistent or likely to be consistent within each family. As a cautionary note, it is usually the case that individuals within a subfamily have not been tested for motile properties relative to each other. Perhaps this is because researchers are afraid such an analysis will be boring or repetitive. I suspect, however, that comparative studies within subfamilies will discover interesting, functionally relevant, specializations. Nevertheless, for most kinesin families there is at least one member who has been run through its paces (Table 1).
Table 1.
Motility and Function of Kinesin Families
| Family | Common Names | Structure | Motility in vitro | Mitotic Function |
|---|---|---|---|---|
| Kinesin-1 | UKHC, Kif5, UNC-116 | Heterotetramer: 2 HC and 2 LC | Plus-end directed, processive, hand-over-hand motility (68). | No known function in mitotic spindles but mediates translocation of meiotic spindle to the oocyte cortex in C. elegans meiosis (98). |
| Kinesin-2 | Kif3A/B/C | Hetero- and Homodimer | Plus-end directed, fast, processive, variable based on composition of heterodimer(99). | Dominant-negative mutants result in aneuploidy and multipolar spindles(98, 100). |
| Kinesin-3 | Kif14; Kif13B/Gakin | Dimer | Plus-end directed rapid motility (101). | Interacts with PRC1, implicated in late stage cytokinesis (102–104). |
| Kinesin-4 | Kif4, Xklp1, Klp38B | Not confirmed, Dimer? | Plus-end directed motility, inhibits dynamics(55, 105). | Congression, spindle assembly, cytokinesis.(87, 106) |
| Kinesin-5 | Eg5, BimC, Cin8 | Bipolar tetramer | Bundling, parallel and antiparallel microtubule sliding(29, 107). | Spindle elongation(108), spindle assembly(109), congression(110). |
| Kinesin-6 | MKLP1, MKLP2, Pavarotti, Subito, Klp9p, Cho1, Kif12, Rab6Kinesin, Kif20, Kif23 | Dimer or Tetramer | Plus-end directed, antiparallel microtubule sliding (97). | Spindle assembly(111), spindle elongation(86), cleavage furrow positioning(96), regulation of midzone assembly(112), cytokinesis (113). |
| Kinesin-7 | Cenp-E | Dimer | Plus-end directed processive motility(114–116). | Congression(117, 118) |
| Kinesin-8 | Kip3, Kif18A, Klp5/6, Klp67A | Not confirmed, Dimer(119) | Length-dependent depolymerization(81), increase catastrophe and rescue, decreased dynamicity (84). | Congression(80, 83), kinetochore fiber dynamics(83, 120), central spindle dynamics(121). |
| Kinesin-9 | Kif6, Kif9, Klp1 | Unknown | Unknown | Tumor suppressor(122), flagellar(123). |
| Kinesin-10 | Kif22, Kid, Nod | Monomer(50) | Weak plus-end directed motility(51) or no motility (52, 54). | Congression(124–126). chromosome compaction (127), meiotic chromosome positioning (128). |
| Kinesin-11 | Smy1, Kif26A, Vab-8 | Unknown | Unknown | None identified |
| Kinesin-12 | Krp180, Klp-10, Xklp2, Hklp2, Kif12, Kif15 | Unknown | Slow plus-end directed | Centrosome separation(129). Spindle positioning(130), Ki-67 interaction (131). |
| Kinesin-13 | Kif2A,B,C, MCAK, Klp10A, Klp57C, XKCM1, Dsk1 | Homodimer | Depolymerizer(18, 62), promote catastrophes(132). | Congression(22), error correction(76, 78), increase K-fiber turnover(21). |
| Kinesin-14 | Ncd, CHO2, Xctk2, Kar3,KlpA, KifC2, KifC2, Kata | Dimer | Nonprocessive minus-end directed motility(10, 133), sliding of anti-parallel microtubules, bundling(134), depolymerizer(47, 135). | Bipolar spindle assembly (43, 136, 137), pole focusing(138), regulate microtubule length and number(139). |
2.3 Kinesin Motility
Initial studies of representative family members in vitro revealed basic features that can easily be related to function. Using the energy of ATP, motile motors will translocate unidirectionally along the surface of a microtubule relative to the microtubule’s inherent structural polarity (minus ends anchored at the spindle pole, plus ends extending distally to the cell membrane, kinetochores and spindle midzone). The direction a motor will “walk” will then define its suitability for certain tasks during cell division. The majority of kinesins walk toward the plus end of the microtubule putting them in an excellent position to transport chromosomes to the end of kinetochore fibers during congression, or to slide two half spindles apart during spindle elongation. Some kinesins (and all dyneins) walk toward the minus ends of microtubules. This predicts a potential utility in anaphase chromosome movement, focusing anastral spindle microtubules and positioning the spindle within the cell via minus-end directed motors anchored to the submembranous cytoskeleton. Two families of kinesins (kinesin-8 and kinesin-13) have been implicated in modulating the kinetics of microtubule assembly and disassembly from free tubulin dimers. These kinesins are suspiciously enriched near the centrosomes and centromeres, areas rich with microtubule ends, where assembly and disassembly occur. It continues to be useful for researchers to apply the characteristics that individual motors display on microtubules in vitro into the context of the mitotic spindle in order to evaluate the role a particular motor will play during cell division (Figure 2). In addition to motility and directionality, it is important to consider carefully the potential “attachment points”, the structures, either identified or speculative, against which the motor will exert force during movement. These “attachment points” constitute one of the most poorly understood aspects of motor function in the spindle. For example, if a motor is proposed to use its plus end directed motor activity to slide a microtubule relative to another microtubule is the motor anchored statically to one microtubule and sliding the other? Or, is the motor anchored to a “matrix” of structural proteins surrounding the microtubules? Is a motor anchored to a chromosome arm able to exert force on the entire chromosome given the inherent flexibility of the chromatin to which it is attached? These are the sorts of questions that still flourish, generally unanswered, in the mitotic motor field.
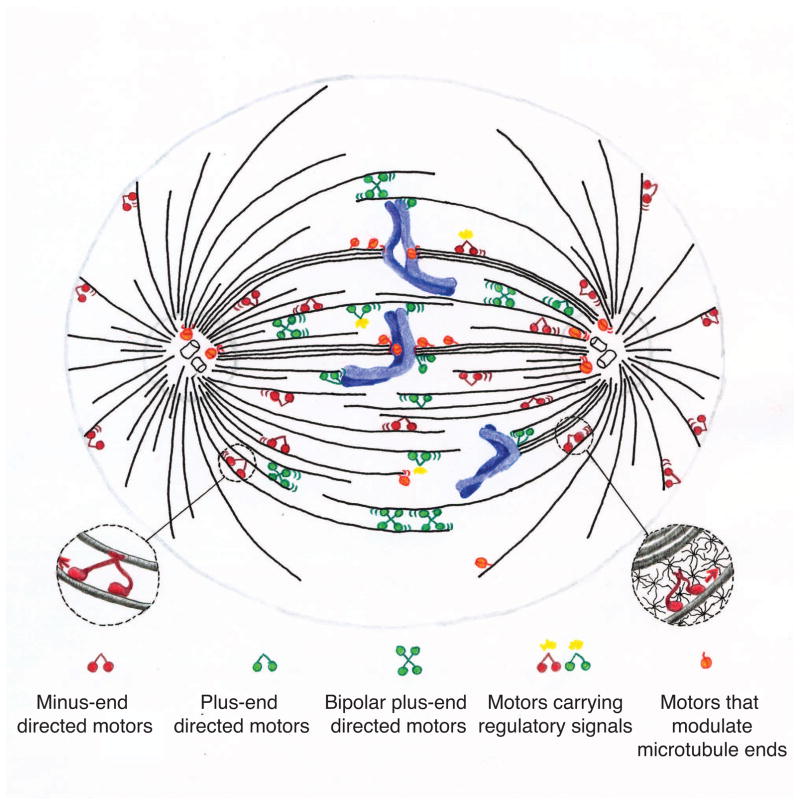
Figure 2.
Motors contribute diverse mechanical activities to the mitotic spindle. Minus-end directed motility (examples are NCD and dynein), plus-end directed motility (Kid, MKLP1, MKLP2, and others); bipolar plus-end directed motility (Eg5, presently no bipolar minus-end directed motors have been reported); plus- and minus-end directed motility transporting signaling molecules (MKLP1, dynein); depolymerizing and end modulating activity (MCAK, Kif18A, Xklp1, Nod). Motors may couple to microtubules (left inset), spindle matrix components such as NuMa(140, 141) (right inset) or other cargo.
2.3.1 Eg5
Kinesin families have broad structural divisions, which, in turn, reflect their particular motility style. Kinesin-5 family members (Eg5/BimC, for example, are bipolar tetramers with two plus-end directed motors at each end(25). Such a molecule is admirably suited to slide two parallel microtubules relative to each other, minus end leading if it has a separate attachment site (such as a spindle matrix) against which to exert force(26). Alternatively, the molecule may push two anti-parallel microtubules relative to each other without attachment ot other structures. Not surprisingly, in vitro the molecule exhibits a clear preference to slide antiparallel microtubules relative to each other(27, 28).
What does it mean for Eg5 to exhibit a “preference” for anti-parallel microtubules? This is where the fine details of motility in vitro become potentially important. Fine details include: processivity, the number of cycles the enzyme undergoes before detaching from the microtubule substrate; and diffusion, a non-energy requiring, gliding that motors do for short distances along the microtubule which is generally unbiased in directionality. Processivity, in the case of walking kinesins, refers to the number of steps that the motor will take before detaching. A motor that is highly processive can do a lot of work all on its own without the assistance of other motors. A non-processive motor, in contrast, may function most effectively in groups. Diffusion tends to be an extremely rapid method of scanning short distances along the microtubule but falls off in importance when efforts over a long distance are required. The bipolar tetramer, Eg5, is a rather poor motor at physiological salt conditions, dithering back and forth on the microtubule in ATP-independent diffusive mode. However, once the motor encounters another microtubule that it can crosslink and, presumably, push against, then it becomes an active, ATP-hydrolyzing directional motor(29). Furthermore, the motor domains appear to be oriented in a manner that favors the second bound microtubule to be in an antiparallel orientation(28). There would be no way to predict this without careful, detailed in vitro assays of purified motor and microtubules in isolation. Yet, the information fits, mechanistically, very well with the proposed functions of kinesin-5s in driving spindle elongation and establishing bipolarity(30–32). However, motors by virtue of their propensity to cycle through strong and weakly microtubule-bound states during ATP hydrolysis, have the potential capability to crosslink microtubules and oppose movement as well as promote it. Eg5 is no exception and has also been reported to limit spindle elongation in some systems(33, 34). Many excellent studies and essential tools, such as Eg5-specific small molecule inhibitors, have made Eg5 one of the mechanistically better understood motors contributing to mitotic spindle function. The motor has a clear role in “establishing spindle bipolarity”. Yet, the complete manner in which it does so is still maddeningly elusive.
2.3.2 NCD
NCD (Non-Claret Disjunctional) is a minus-end directed kinesin originally identified as essential for proper chromosome segregation in Drosophila female meiosis and early embryos(8). The motor domain has been crystallized and shows striking similarity to plus-end directed kinesins (35). In fact, electron microscopy in conjunction with 3D reconstruction shows that motors kinesin motors strongly bound to microtubules will always bind in one orientation(36). Thus, directionality, as has been shown in numerous studies, relies on domains outside of the motor domain. NCD can be released from minus-end directionality by a single point mutation in either of two, highly conserved, interacting residues, one in the “neck” outside the motor domain and one in the core motor domain(37). The resultant motors can translocate in either direction in an ATP-dependent manner. This would be an excellent point at which to regulate directionality, the control of which has yet to be demonstrated, in any motor in a cellular setting.
Motility studies of NCD have long shown that it is relatively non-processive, suggesting that it is a motor that is most effective in groups(38). Kinesin-14 family members have long been known to be prodigious bundlers of microtubules in addition to their minus-end directed motility. Far from being a nuisance for motility assays, recent in vitro studies of full-length NCD and its orthologue from S. pombe have demonstrated that these molecules preferentially slide anti-parallel microtubules relative to each other in various bound motor orientations. In contrast, microtubules with uniform parallel orientation become locked in place by inter-microtubule-bound motors working against each other(39, 40). HSET, the kinesin-14 orthologue in human cells, is non-essential for cell division in normal cells yet is helpful at rescuing multipolar spindles via centrosome clustering in cells with multiple centrosomes(41). Thus, its force-locked microtubule bundling activity may be more functionally important than sliding and may be especially important in female meiosis in Drosophila where the spindles lack the focusing activites of centrosomes or in situations in which too many centrosomes are present and need to be coalesced into one pole-focusing entity.
Minus-end directed directional sliding should implicate kinesin-14s in promoting spindle collapse and opposing the activity of Eg5(42). Yet recent studies have shown that overexpression of HSET, lengthens spindles while depletion modestly shortens them without interfering with cell division. Mutational analysis in which sliding ability was eliminated indicated that both sliding and crosslinking contribute to spindle elongation(43). How sliding of parallel microtubules in the half-spindle accomplishes this task when kinesin-14 molecules tend to become force-locked in these conditions is mysterious. It may be that minus-end directed motility and crosslinking are not fully separable activities. For example, in ATP, a microtubule may be immovable because the collective motors operating on it are equivalently balanced in opposition to each other. They may be able to progress partially through the cross-bridge cycle until a strongly bound or force-locked state is reached. This would result in strong crosslinking without a lot of overt microtubule sliding. Regardless, it is necessary to know the orientation and anchor points (both static and dynamic) of the motors to the half-spindle microtubules are essential in order to understand how HSET functions in the spindle. This is best understood by extension from in vitro studies of purified protein supplemented by computational modeling(44).
Kar3p is a kinesin-14 important for mitosis and karyogamy in S. cerevesiae. Up to this point we have been discussing kinesin-14 motors that are two-headed homodimers connected by a long coiled-coil stalk. The orthologous protein in yeast, Kar3, preferentially heterodimerizes with either Vik1p or Cik1p, although it can homodimerize as well(45). Structural studies have revealed these proteins to be kinesin motor domains that lack an ATP-binding site yet still bind microtubules(46). Dimerization with these “dead heads” significantly enhances Kar3p minus-end directed motility(45) and confers the ability to destabilize the microtubule plus-end as well(45, 47). Nothing analogous to Vik1p or Cik1p has been identified in mammalian cells, but it is well within the realm of possibility that similar non-motor motors will be identified as a means to structurally and functionally diversify kinesin activity. More precise motility assays of these two heterodimers in order to mechanistically evaluate how they operate in promoting spindle positioning, spindle integrity, chromosome maintenance, and synaptogenesis(48, 49). A key unanswered question is whether Kar3p and its partners behave similarly to other kinesin-14s with respect to microtubule crosslinking.
2.3.3 NOD
NOD is a kinesin-10 family member, which includes the mitotic chromokinesin, KID. Both KID and NOD are found on chromosome arms and both are implicated in chromosome positioning during congression. NOD is required for the proper positioning of achiasmate chromosomes during meiosis in Drosophila. KID appears to be monomeric(50) and exhibited plus-end directed motility only when surveyed using a bead assay (motors affixed to a bead rather than a glass coverslip)(51). NOD is widely considered to be non-motile(52) and has been reported to bind preferentially to microtubule ends and stimulate microtubule polymerization(53). Structural studies suggest that NOD binds and releases microtubules concurrent with ATP hydrolysis but that ATP turnover is inhibited when the motor binds the microtubule end until the end elongates(54). This would be an excellent means by which to help coax chromosomes to the plus-ends of microtubules without relying on hand-over-hand motility.
It must be said, however, that the disparity between the motility described for NOD and for KID feels incomplete for two motors in the same general family. There is still much work to be done with these motors. Furthermore, there is another family of chromokinesins (kinesin-4), which is characterized by Kif4/XKLP1. An extensive study of XKLP1 shows that it is a plus-end directed motor that also modulates microtubule ends by inhibiting both microtubule growth and shrinkage(55). This activity and also those ascribed to the kinesin-10s, KID and NOD, are all compatible with their proposed role in promoting the congression of chromosomes to the metaphase plate. Previously, it was though that microtubules polymerizing from the spindle pole produced a “polar ejection force” that tended to assist with the job of moving chromosomes away from the pole and toward the spindle midzone(56). This force promotes the ejection of chromosomes from microtubule-dense areas near the spindle pole, toward the plus-ends of microtubules at the spindle midzone even when the chromosome arm is physically detached from the kinetochore(57). Now, with the discovery of chromokinesins (plus-end directed motors on chromosome arms), it is widely thought that motor-dependent plus-end directed motility associated with chromosome arms corresponds to the polar ejection force. Ejection force arising from polymerizing microtubules and microtubule motor-dependent polar ejection force are not mutually exclusive and it is not presently known which of these activities is acting on chromosome arms to produce the polar ejection force. Nevertheless, chromosome arm-associated microtubule-stabilizing activity would be a useful way of promoting congression either by facilitating the maintenance of a track for plus-end directed motility, or by promoting a preferential association with polymerizing microtubule ends (as is proposed for NOD), or by facilitating microtubule polymerization, an activity which is likely to be capable of producing a polar ejection force in isolation. These activities are extremely difficult to isolate within the context of the spindle so further assays of the chromokinesins in vitro are likely to be informative with respect to the motor activities possessed by chromosome arms.
2.3.4 MCAK
MCAK was originally identified as a kinesin-related protein that was enriched in the inner centromere region of mitotic chromosomes(58). Rapid depletion of this kinesin from cellular extracts led to explosive microtubule growth(59) and a classic study subsequently confirmed that the motor was capable of completely disassembling microtubules stabilized against disassembly by either paclitaxel or non-hydrolyzable GTP analogs(18). Subsequent studies confirmed that MCAK and other members of its kinesin-13 family are capable of disassembling microtubules(18, 60, 61) in a catalytic manner and that it is most likely that one motor can remove approximately twenty tubulin dimers prior to dissociating from the microtubule(62). The process involves the motor preferentially stabilizing a curved (rather than straight) protofilament conformation that weakens the association of tubulin with the microtubule(63, 64). Tubulin dimer removal from the microtubule is tightly coupled to ATP hydrolysis and can be isolated from tubulin dimer release by the bound motor by introducing a point mutation into the switch II region motor domain(65). Such mutations have been commonly used to isolate the pre-and post-power stroke structures of myosin and other motile kinesins(66–68). In the case of MCAK, the process of ATP-binding and hydrolysis triggers the motor to sequentially bend, remove and release a tubulin dimer during one ATP hydrolysis cycle(65). Thus, MCAK and its family members are potent, catalytic microtubule depolymerizers and it is likely that this is the activity that they contribute to the centromeric, centrosomal, and spindle midzone regions of the mitotic spindle.
Evaluating the role of these depolymerizers in cells is challenging because the activity of MCAK in the cell appears to be intimately associated with the ratio of tubulin dimer to polymer. This is not surprising as it has been known for quite some time that cells adjust their level of tubulin expression to the level of free tubulin dimer in the cell(69, 70). However, slow (12–36 hour) depletion of MCAK such as occurs during siRNA treatment, gives the cell ample time to adjust microtubule polymer dynamics and results in a rather subtle mitotic phenotype. Cells experience a modest increase in lagging chromosomes and longer astral microtubules(20, 71). Defects in congression due to improper kinetochore microtubule attachment(72) and antagonism of bipolar spindle assembly have also been reported(20, 72–75). MCAK’s activity and centromere localization are regulated by Aurora B kinase, further implicating the motor in kinetochore function and error correction(76–79). Depletion of centromere-associated MCAK leads to decreased speed of chromosome movement and also to decreased kinetochore fiber microtubule turnover(21). In contrast, addition of more MCAK to the centromere resulted in fewer lagging chromosomes (suggesting increased error-correction activity), increased microtubule turnover in the kinetochore fiber and greater overall speed of chromosome movement and fewer erroneous microtubule connections(21). This suggests, commensurate with its potent microtubule depolymerizing activity, that MCAK facilitates kinetochore fiber microtubule turnover and error correction by gently antagonizing microtubule attachment at the kinetochore.
2.3.5 Kif18A
The kinesin-8 family possesses unique attributes that significantly inform our perception of their role during cell division. Members of this family were described in Drosophila as plus-end directed motors whose elimination led to unexpectedly long microtubules. This conundrum was partially solved with the discovery that the kinesin-8, Kip3p, from S. cerevesiae is a highly processive plus-end directed motor that disassembles microtubules from the plus-end in a length dependent manner(80, 81). This length dependence is related to the high processivity of the motor in that, once it is translocating along the microtubule, it is unlikely to detach. This leads to an accumulation of the motors at the plus end of the microtubule if the motors do not walk off the end of the microtubule. This ability of kinesin-8s to stay attached at the end of the microtubule is an interesting phenomenon in and of itself. Not all motile kinesins do this. Once the concentration of Kip3p is high enough the microtubule begins to shorten in a length dependent manner. This is because the high processivity of the motor results in longer microtubules accumulating more motor over time, thus leading to a positive relationship between the length of the microtubule and the rate of disassembly. The importance of a high concentration of motor is underscored by the observation that one molecule of Kip3 will not remove a tubulin dimer until another motor kicks it off(82). Thus, the motor will only remove tubulin dimers when the lattice near the plus-end is essentially saturated.
The mammalian orthologue of Kip3p, Kif18A, is also capable of disassembling microtubules(80) and distributes in a gradient along kinetochore fibers suggesting it may provide positional information to congressing chromosomes(83). However the activity that Kif18A supplies to the gradient may not be as simple to interpret as the depolymerase activity of MCAK. Based on the length-dependent disassembly of microtubules by Kip3p(81), the molecule has been implicated in using its depolymerase activity to limit microtubule length within the spindle. Furthermore, loss of Kif18A leads to a modest lengthening in spindles and longer microtubules. So far so good. However, there is more than one way to shorten a microtubule. Suppressing microtubule dynamics can shift the steady state length of microtubules to a narrow distribution around a shorter length. One can accomplish this by either simultaneously suppressing rate of assembly and disassembly or by simultaneously increasing both catastrophe and rescue (the transitions between disassembly and assembly). Kip3p in living yeast appears to have its principal effect in vivo by increasing the transitions between growth and shortening(84). Thus a microtubule has less time to grow and less time to shorten in the presence of Kip3p. Overall this is going to shift the distribution of microtubule lengths in the cell to a narrow distribution of shorter lengths. Interestingly, the expression of excess Kif18A protein in mitotic cells has similar effects on chromosome oscillations(83). Bioriented chromosomes exhibit oscillatory movement during metaphase. This movement is related to the growth and shrinkage of microtubule ends bound by kinetochores in that it can be suppressed by microtubule drugs that suppress microtubule dynamics. Similarly, expression of Kif18A suppresses chromosome movement without appreciably affecting their ability to congress. Conversely, depletion of Kif18A increases the speed at which chromosomes travel and decreases the frequency with which they switch directions, leading to an inability to congress because they sail past the metaphase plate without stopping(83). By extension, this resembles the situation in yeast whereby microtubule transition frequencies decrease with the loss of Kip3p(84). This suggests that further evaluation of the effect of purified Kif18A protein on dynamic microtubule in vitro is definitely warranted in order to reconcile the microtubule shortening effect of kinesin-8s with their cellular role in suppressing microtubule dynamics.
2.3.6 MKLP1
Both MKLP1 and MKLP2 are kinesin-6 family members. Previous evidence indicates that they are dimers in metazoans(85) but recent crosslinking data suggests that klp9p, the kinesin-6 family member in S. pombe, may form a tetramer(86). This is consistent with the recent discovery that klp9p may slide bipolar mitotic spindles apart in S. pombe during anaphase B (spindle elongation)(86). Klp9p accomplishes this in conjunction with a microtubule bundling protein Ase1 (Prc1 in metazoans). In a perplexing twist, prc1 in mammals also bundles microtubules and interacts with three plus-end directed kinesins, the chromokinesin, Kif4A (kinesin-4 family), the kinesin-6, MKLP1, and the kinesin-7, CENP-E(87).
In Drosophila, which possesses all three representatives implicated in spindle midzone function: Kif4A (Klp3A; kinesin-4), MKLP1 (Pavarotti; kinesin-6) and MKLP2 (Subito; kinesin-6), the kinesin-4 Klp3A, is proposed to operate in spindle elongation by promoting midzone bundling and suppressing flux(88). As expected for a chromokinesin there is also a mild congression defect associated with Klp3A loss (89). In mammalian cells, depletion of Kif4A lead to long anaphase spindles implying a role in opposing spindle elongation(90). This is interesting as both kinesin-6s and kinesin-4s are plus-end directed. Most studies implicating a balance of forces involved antagonism between motors of opposite directionality. A detailed in vitro study of the Xenopus kinesin-4, Xklp1, may provide some answers. Xklp1 was shown to be a rapid plus-end directed motor with the ability to suppress both depolymerization and polymerization in an ATP-independent manner once it reached the end of the microtubule (55). This suggests how kinesin-4s might antagonize the sliding activities of kinesin-6s, by limiting the polymerization of interzonal microtubules and thus limiting the extent of the zone of anti-parallel microtubule overlap.
Kinesin-6s such as MKLP1/2 have not been as clearly implicated in pole separation by sliding of antiparallel microtubules in cells other than S. pombe. This may be because anaphase B spindle elongation is less pronounced in other systems. Furthermore, the role of kinesin-6s in “organizing” the central spindle can lead to mechanistically difficult to interpret phenotypes such as broken, splayed or collapsed spindles. Instead, a much larger body of work from C. elegans, Drosophila and mammalian cells (reviewed in (91)) demonstrates that a dimer of the kinein-6 family member, MKLP1, forms a complex with a dimer of RhoGAP to form the centralspindlin complex, which is required for Rho-dependent cleavage furrow ingression(85). MKLP2 (found in Drosophila and mammals but not C. elegans) relocalizes from the inner centromere at mitosis to the spindle midzone, interacts with polo kinase, INCENP and Aurora kinase and is required to localize Aurora kinase to the midzone, which then phosphorylates MKLP1(92). Phosphorylation of MKLP1 is, in turn, required for specification of the cleavage furrow (93). Less well understood is the more phylogenetically restricted kinesin-6, MPP1, although it is also implicated in the completion of cytokinesis in human cultured cells(94). Regardless, the take-home message is that, in contrast to S. pombe, the kinesin-6 family in other organisms seems to be primarily involved in positioning and facilitating the function of the cleavage furrow, rather than physically pushing the spindle poles apart. This is curious in that pushing the spindle poles apart might be an excellent way to locally reduce the number of cortically associated astral microtubules and to redundantly localize furrowing(95). Perhaps the redundancy is not as evolutionarily robust when imparted by the same motor?
In organisms that possess more than one kinesin-6, they appear to interact with different signaling molecule complexes. For example, in contrast to MKLP2 which complexes with midzone passenger proteins, MKLP1 forms a complex with RhoGAP and RhoGEF and is required for Rho-dependent cleavage furrow ingression(96). Both of these kinesin-6s are strongly associated with anti-parallel microtubules in the spindle midzone. MKLP1 is a plus-end directed motile kinesin that can crosslink and appears to be able to slide anti-parallel microtubules relative to each other(97). The activity of MKLP2 in vitro is likely to be similar but has not been tested. It would be interesting to understand whether kinesin-6s can elongate mitotic spindles by exerting plus-end directed force on each half spindle and whether this contributes to their role in cleavage furrow specification or represents a separate function.
Our understanding of the role of MKLP1 in directing the establishment of the cleavage furrow has benefited from informative biochemical and genetic studies. However, from the perspective of MKLP1, the motor, the ability of the kinesin-6 family proteins to slide anti-parallel microtubules relative to each other is consistent with their localization to the interdigitating anti-parallel microtubules of the midzone but superficially inconsistent with their role as transporters of signaling complexes. Such tasks could be accomplished by any plus-end directed motor. Thus, a true mechanistic evaluation of their function remains to be determined. How, precisely does MKLP1 direct the accumulation of RhoA to the forming cleavage furrow and is the ability to slide antiparallel microtubules required for this process? How is its actin-binding activity useful for this process? Does MKLP1 transport RhoA regulators to the cortex or does it localize them at the midzone and rely on diffusion to signal cortical actin? More detailed analysis of the activity kinesin-6 family motors on single and bundled microtubules coupled with live imaging of the dynamic behavior of these motors during cell division would help answer some of these questions.
3. Conclusions
A comprehensive survey of kinesins in cell division is not possible within the constraints of this review. However, I have endeavored to touch on a few kinesins for which detailed analysis in vitro has been or is likely to be informative with respect to cellular function. This is especially the case for mitosis, as compared to other microtubule-based processes, because the organization of microtubules during mitosis is complex and dynamic. Mitotic spindle microtubules form parallel arrays, anti-parallel arrays, asters and bundles. They undergo dynamic instability, flux, sliding and exhibit high rates of nucleation and turnover during cell division. Future studies in vitro should include experiments designed to mimic these substrates.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Mazia D, Chaffee RR, Iverson RM. Adenosine triphosphatase in the mitotic apparatus. Proceedings of the National Academy of Sciences of the United States of America. 1961 Jun 15;47:788–90. doi: 10.1073/pnas.47.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhne W. Untersuchungen fiber das Protoplasma und die Contractilitat. Leipzig: von Wilhelm Engelmann; 1864. [Google Scholar]
- 3.Engelhardt WA, Ljubimowa MN. Myosine and adenosinetriphosphatase. Nature. 1939;144:668–69. [Google Scholar]
- 4.Mottier DM. The behavior of the chromosomes in the spore mother-cells of higher plants and the homology of the pollen and embryo-sac mother cells. Bot Gazette. 1903;35:250–85. [Google Scholar]
- 5.Gibbons IR, Rowe AJ. Dynein: A Protein with Adenosine Triphosphatase Activity from Cilia. Science (New York, NY) 1965 Jul 23;149(3682):424–6. doi: 10.1126/science.149.3682.424. [DOI] [PubMed] [Google Scholar]
- 6.Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kull FJ, Sablin EP, Lau R, Fletterick RJ, Vale RD. Crystal structure of the kinesin motor domain reveals a structural similarity to myosin. Nature. 1996 Apr 11;380(6574):550–5. doi: 10.1038/380550a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endow SA, Henikoff S, Soler-Niedziela L. Mediation of meiotic and early mitotic chromosome segregation in Drosophila by a protein related to kinesin. Nature. 1990 May 3;345(6270):81–3. doi: 10.1038/345081a0. [DOI] [PubMed] [Google Scholar]
- 9.Enos AP, Morris NR. Mutation of a gene that encodes a kinesin-like protein blocks nuclear division in A. nidulans. Cell. 1990 Mar 23;60(6):1019–27. doi: 10.1016/0092-8674(90)90350-n. [DOI] [PubMed] [Google Scholar]
- 10.McDonald HB, Stewart RJ, Goldstein LS. The kinesin-like ncd protein of Drosophila is a minus end-directed microtubule motor. Cell. 1990 Dec 21;63(6):1159–65. doi: 10.1016/0092-8674(90)90412-8. [DOI] [PubMed] [Google Scholar]
- 11.Paschal BM, Shpetner HS, Vallee RB. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. The Journal of cell biology. 1987 Sep;105(3):1273–82. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang P, Knowles BA, Goldstein LS, Hawley RS. A kinesin-like protein required for distributive chromosome segregation in Drosophila. Cell. 1990 Sep 21;62(6):1053–62. doi: 10.1016/0092-8674(90)90383-p. [DOI] [PubMed] [Google Scholar]
- 13.Pfarr CM, Coue M, Grissom PM, Hays TS, Porter ME, McIntosh JR. Cytoplasmic dynein is localized to kinetochores during mitosis. Nature. 1990 May 17;345(6272):263–5. doi: 10.1038/345263a0. [DOI] [PubMed] [Google Scholar]
- 14.Steuer ER, Wordeman L, Schroer TA, Sheetz MP. Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature. 1990 May 17;345(6272):266–8. doi: 10.1038/345266a0. [DOI] [PubMed] [Google Scholar]
- 15.Miki H, Okada Y, Hirokawa N. Analysis of the kinesin superfamily: insights into structure and function. Trends in cell biology. 2005 Sep;15(9):467–76. doi: 10.1016/j.tcb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence CJ, Dawe RK, Christie KR, Cleveland DW, Dawson SC, Endow SA, et al. A standardized kinesin nomenclature. The Journal of cell biology. 2004 Oct 11;167(1):19–22. doi: 10.1083/jcb.200408113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawrence CJ, Malmberg RL, Muszynski MG, Dawe RK. Maximum likelihood methods reveal conservation of function among closely related kinesin families. Journal of molecular evolution. 2002 Jan;54(1):42–53. doi: 10.1007/s00239-001-0016-y. [DOI] [PubMed] [Google Scholar]
- 18.Desai A, Verma S, Mitchison TJ, Walczak CE. Kin I kinesins are microtubule-destabilizing enzymes. Cell. 1999 Jan 8;96(1):69–78. doi: 10.1016/s0092-8674(00)80960-5. [DOI] [PubMed] [Google Scholar]
- 19.Ems-McClung SC, Hertzer KM, Zhang X, Miller MW, Walczak CE. The interplay of the N- and C-terminal domains of MCAK control microtubule depolymerization activity and spindle assembly. Molecular biology of the cell. 2007 Jan;18(1):282–94. doi: 10.1091/mbc.E06-08-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganem NJ, Compton DA. The KinI kinesin Kif2a is required for bipolar spindle assembly through a functional relationship with MCAK. The Journal of cell biology. 2004 Aug 16;166(4):473–8. doi: 10.1083/jcb.200404012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wordeman L, Wagenbach M, von Dassow G. MCAK facilitates chromosome movement by promoting kinetochore microtubule turnover. The Journal of cell biology. 2007 Dec 3;179(5):869–79. doi: 10.1083/jcb.200707120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walczak CE, Gan EC, Desai A, Mitchison TJ, Kline-Smith SL. The microtubule-destabilizing kinesin XKCM1 is required for chromosome positioning during spindle assembly. Curr Biol. 2002 Oct 29;12(21):1885–9. doi: 10.1016/s0960-9822(02)01227-7. [DOI] [PubMed] [Google Scholar]
- 23.Ginkel LM, Wordeman L. Expression and partial characterization of kinesin-related proteins in differentiating and adult skeletal muscle. Molecular biology of the cell. 2000 Dec;11(12):4143–58. doi: 10.1091/mbc.11.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Homma N, Takei Y, Tanaka Y, Nakata T, Terada S, Kikkawa M, et al. Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell. 2003 Jul 25;114(2):229–39. doi: 10.1016/s0092-8674(03)00522-1. [DOI] [PubMed] [Google Scholar]
- 25.Kashina AS, Baskin RJ, Cole DG, Wedaman KP, Saxton WM, Scholey JM. A bipolar kinesin. Nature. 1996 Jan 18;379(6562):270–2. doi: 10.1038/379270a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapoor TM, Mitchison TJ. Eg5 is static in bipolar spindles relative to tubulin: evidence for a static spindle matrix. The Journal of cell biology. 2001 Sep 17;154(6):1125–33. doi: 10.1083/jcb.200106011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapitein LC, Peterman EJ, Kwok BH, Kim JH, Kapoor TM, Schmidt CF. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature. 2005 May 5;435(7038):114–8. doi: 10.1038/nature03503. [DOI] [PubMed] [Google Scholar]
- 28.van den Wildenberg SM, Tao L, Kapitein LC, Schmidt CF, Scholey JM, Peterman EJ. The homotetrameric kinesin-5 KLP61F preferentially crosslinks microtubules into antiparallel orientations. Curr Biol. 2008 Dec 9;18(23):1860–4. doi: 10.1016/j.cub.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapitein LC, Kwok BH, Weinger JS, Schmidt CF, Kapoor TM, Peterman EJ. Microtubule cross-linking triggers the directional motility of kinesin-5. The Journal of cell biology. 2008 Aug 11;182(3):421–8. doi: 10.1083/jcb.200801145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brust-Mascher I, Sommi P, Cheerambathur DK, Scholey JM. Kinesin-5-dependent poleward flux and spindle length control in Drosophila embryo mitosis. Molecular biology of the cell. 2009 Mar;20(6):1749–62. doi: 10.1091/mbc.E08-10-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirasu-Hiza M, Perlman ZE, Wittmann T, Karsenti E, Mitchison TJ. Eg 5 causes elongation of meiotic spindles when flux-associated microtubule depolymerization is blocked. Curr Biol. 2004 Nov 9;14(21):1941–5. doi: 10.1016/j.cub.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 32.Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science (New York, NY) 1999 Oct 29;286(5441):971–4. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- 33.Tikhonenko I, Nag DK, Martin N, Koonce MP. Kinesin-5 is not essential for mitotic spindle elongation in Dictyostelium. Cell Motil Cytoskeleton. 2008 Nov;65(11):853–62. doi: 10.1002/cm.20307. [DOI] [PubMed] [Google Scholar]
- 34.Saunders AM, Powers J, Strome S, Saxton WM. Kinesin-5 acts as a brake in anaphase spindle elongation. Curr Biol. 2007 Jun 19;17(12):R453–4. doi: 10.1016/j.cub.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sablin EP, Kull FJ, Cooke R, Vale RD, Fletterick RJ. Crystal structure of the motor domain of the kinesin-related motor ncd. Nature. 1996 Apr 11;380(6574):555–9. doi: 10.1038/380555a0. [DOI] [PubMed] [Google Scholar]
- 36.Hoenger A, Milligan RA. Motor domains of kinesin and ncd interact with microtubule protofilaments with the same binding geometry. Journal of molecular biology. 1997 Feb 7;265(5):553–64. doi: 10.1006/jmbi.1996.0757. [DOI] [PubMed] [Google Scholar]
- 37.Endow SA, Higuchi H. A mutant of the motor protein kinesin that moves in both directions on microtubules. Nature. 2000 Aug 24;406(6798):913–6. doi: 10.1038/35022617. [DOI] [PubMed] [Google Scholar]
- 38.Crevel IM, Lockhart A, Cross RA. Kinetic evidence for low chemical processivity in ncd and Eg5. Journal of molecular biology. 1997 Oct 17;273(1):160–70. doi: 10.1006/jmbi.1997.1319. [DOI] [PubMed] [Google Scholar]
- 39.Braun M, Drummond DR, Cross RA, McAinsh AD. The kinesin-14 Klp2 organizes microtubules into parallel bundles by an ATP-dependent sorting mechanism. Nature cell biology. 2009 Jun;11(6):724–30. doi: 10.1038/ncb1878. [DOI] [PubMed] [Google Scholar]
- 40.Fink G, Hajdo L, Skowronek KJ, Reuther C, Kasprzak AA, Diez S. The mitotic kinesin-14 Ncd drives directional microtubule-microtubule sliding. Nature cell biology. 2009 Jun;11(6):717–23. doi: 10.1038/ncb1877. [DOI] [PubMed] [Google Scholar]
- 41.Kwon M, Godinho SA, Chandhok NS, Ganem NJ, Azioune A, Thery M, et al. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 2008 Aug 15;22(16):2189–203. doi: 10.1101/gad.1700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mountain V, Simerly C, Howard L, Ando A, Schatten G, Compton DA. The kinesin-related protein, HSET, opposes the activity of Eg5 and cross-links microtubules in the mammalian mitotic spindle. The Journal of cell biology. 1999 Oct 18;147(2):351–66. doi: 10.1083/jcb.147.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai S, Weaver LN, Ems-McClung SC, Walczak CE. Kinesin-14 family proteins HSET/XCTK2 control spindle length by cross-linking and sliding microtubules. Molecular biology of the cell. 2009 Mar;20(5):1348–59. doi: 10.1091/mbc.E08-09-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goshima G, Nedelec F, Vale RD. Mechanisms for focusing mitotic spindle poles by minus end-directed motor proteins. The Journal of cell biology. 2005 Oct 24;171(2):229–40. doi: 10.1083/jcb.200505107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chu HM, Yun M, Anderson DE, Sage H, Park HW, Endow SA. Kar3 interaction with Cik1 alters motor structure and function. The EMBO journal. 2005 Sep 21;24(18):3214–23. doi: 10.1038/sj.emboj.7600790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allingham JS, Sproul LR, Rayment I, Gilbert SP. Vik1 modulates microtubule-Kar3 interactions through a motor domain that lacks an active site. Cell. 2007 Mar 23;128(6):1161–72. doi: 10.1016/j.cell.2006.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sproul LR, Anderson DJ, Mackey AT, Saunders WS, Gilbert SP. Cik1 targets the minus-end kinesin depolymerase kar3 to microtubule plus ends. Curr Biol. 2005 Aug 9;15(15):1420–7. doi: 10.1016/j.cub.2005.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cottingham FR, Gheber L, Miller DL, Hoyt MA. Novel roles for saccharomyces cerevisiae mitotic spindle motors. The Journal of cell biology. 1999 Oct 18;147(2):335–50. doi: 10.1083/jcb.147.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shanks RM, Kamieniecki RJ, Dawson DS. The Kar3-interacting protein Cik1p plays a critical role in passage through meiosis I in Saccharomyces cerevisiae. Genetics. 2001 Nov;159(3):939–51. doi: 10.1093/genetics/159.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shiroguchi K, Ohsugi M, Edamatsu M, Yamamoto T, Toyoshima YY. The second microtubule-binding site of monomeric kid enhances the microtubule affinity. The Journal of biological chemistry. 2003 Jun 20;278(25):22460–5. doi: 10.1074/jbc.M212274200. [DOI] [PubMed] [Google Scholar]
- 51.Yajima J, Edamatsu M, Watai-Nishii J, Tokai-Nishizumi N, Yamamoto T, Toyoshima YY. The human chromokinesin Kid is a plus end-directed microtubule-based motor. The EMBO journal. 2003 Mar 3;22(5):1067–74. doi: 10.1093/emboj/cdg102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matthies HJ, Baskin RJ, Hawley RS. Orphan kinesin NOD lacks motile properties but does possess a microtubule-stimulated ATPase activity. Molecular biology of the cell. 2001 Dec;12(12):4000–12. doi: 10.1091/mbc.12.12.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui W, Sproul LR, Gustafson SM, Matthies HJ, Gilbert SP, Hawley RS. Drosophila Nod protein binds preferentially to the plus ends of microtubules and promotes microtubule polymerization in vitro. Molecular biology of the cell. 2005 Nov;16(11):5400–9. doi: 10.1091/mbc.E05-06-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cochran JC, Sindelar CV, Mulko NK, Collins KA, Kong SE, Hawley RS, et al. ATPase cycle of the nonmotile kinesin NOD allows microtubule end tracking and drives chromosome movement. Cell. 2009 Jan 9;136(1):110–22. doi: 10.1016/j.cell.2008.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bringmann H, Skiniotis G, Spilker A, Kandels-Lewis S, Vernos I, Surrey T. A kinesin-like motor inhibits microtubule dynamic instability. Science (New York, NY) 2004 Mar 5;303(5663):1519–22. doi: 10.1126/science.1094838. [DOI] [PubMed] [Google Scholar]
- 56.Rieder CL, Salmon ED. Motile kinetochores and polar ejection forces dictate chromosome position on the vertebrate mitotic spindle. The Journal of cell biology. 1994 Feb;124(3):223–33. doi: 10.1083/jcb.124.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ault JG, DeMarco AJ, Salmon ED, Rieder CL. Studies on the ejection properties of asters: astral microtubule turnover influences the oscillatory behavior and positioning of mono-oriented chromosomes. Journal of cell science. 1991 Aug;99( Pt 4):701–10. doi: 10.1242/jcs.99.4.701. [DOI] [PubMed] [Google Scholar]
- 58.Wordeman L, Mitchison TJ. Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. The Journal of cell biology. 1995 Jan;128(1–2):95–104. doi: 10.1083/jcb.128.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walczak CE, Mitchison TJ, Desai A. XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell. 1996 Jan 12;84(1):37–47. doi: 10.1016/s0092-8674(00)80991-5. [DOI] [PubMed] [Google Scholar]
- 60.Manning AL, Ganem NJ, Bakhoum SF, Wagenbach M, Wordeman L, Compton DA. The kinesin-13 proteins Kif2a, Kif2b, and Kif2c/MCAK have distinct roles during mitosis in human cells. Molecular biology of the cell. 2007 Aug;18(8):2970–9. doi: 10.1091/mbc.E07-02-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore AT, Rankin KE, von Dassow G, Peris L, Wagenbach M, Ovechkina Y, et al. MCAK associates with the tips of polymerizing microtubules. The Journal of cell biology. 2005 May 9;169(3):391–7. doi: 10.1083/jcb.200411089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hunter AW, Caplow M, Coy DL, Hancock WO, Diez S, Wordeman L, et al. The kinesin-related protein MCAK is a microtubule depolymerase that forms an ATP-hydrolyzing complex at microtubule ends. Mol Cell. 2003 Feb;11(2):445–57. doi: 10.1016/s1097-2765(03)00049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moores CA, Yu M, Guo J, Beraud C, Sakowicz R, Milligan RA. A mechanism for microtubule depolymerization by KinI kinesins. Mol Cell. 2002 Apr;9(4):903–9. doi: 10.1016/s1097-2765(02)00503-8. [DOI] [PubMed] [Google Scholar]
- 64.Ogawa T, Nitta R, Okada Y, Hirokawa N. A common mechanism for microtubule destabilizers-M type kinesins stabilize curling of the protofilament using the class-specific neck and loops. Cell. 2004 Feb 20;116(4):591–602. doi: 10.1016/s0092-8674(04)00129-1. [DOI] [PubMed] [Google Scholar]
- 65.Wagenbach M, Domnitz S, Wordeman L, Cooper J. A kinesin-13 mutant catalytically depolymerizes microtubules in ADP. The Journal of cell biology. 2008 Nov 17;183(4):617–23. doi: 10.1083/jcb.200805145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rice S, Lin AW, Safer D, Hart CL, Naber N, Carragher BO, et al. A structural change in the kinesin motor protein that drives motility. Nature. 1999 Dec 16;402(6763):778–84. doi: 10.1038/45483. [DOI] [PubMed] [Google Scholar]
- 67.Sasaki N, Shimada T, Sutoh K. Mutational analysis of the switch II loop of Dictyostelium myosin II. The Journal of biological chemistry. 1998 Aug 7;273(32):20334–40. doi: 10.1074/jbc.273.32.20334. [DOI] [PubMed] [Google Scholar]
- 68.Tomishige M, Stuurman N, Vale RD. Single-molecule observations of neck linker conformational changes in the kinesin motor protein. Nat Struct Mol Biol. 2006 Oct;13(10):887–94. doi: 10.1038/nsmb1151. [DOI] [PubMed] [Google Scholar]
- 69.Gonzalez-Garay ML, Cabral F. alpha-Tubulin limits its own synthesis: evidence for a mechanism involving translational repression. The Journal of cell biology. 1996 Dec;135(6 Pt 1):1525–34. doi: 10.1083/jcb.135.6.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pachter JS, Yen TJ, Cleveland DW. Autoregulation of tubulin expression is achieved through specific degradation of polysomal tubulin mRNAs. Cell. 1987 Oct 23;51(2):283–92. doi: 10.1016/0092-8674(87)90155-3. [DOI] [PubMed] [Google Scholar]
- 71.Stout JR, Rizk RS, Kline SL, Walczak CE. Deciphering protein function during mitosis in PtK cells using RNAi. BMC Cell Biol. 2006;7:26. doi: 10.1186/1471-2121-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kline-Smith SL, Khodjakov A, Hergert P, Walczak CE. Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Molecular biology of the cell. 2004 Mar;15(3):1146–59. doi: 10.1091/mbc.E03-08-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holmfeldt P, Zhang X, Stenmark S, Walczak CE, Gullberg M. CaMKIIgamma-mediated inactivation of the Kin I kinesin MCAK is essential for bipolar spindle formation. The EMBO journal. 2005 Mar 23;24(6):1256–66. doi: 10.1038/sj.emboj.7600601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sampath SC, Ohi R, Leismann O, Salic A, Pozniakovski A, Funabiki H. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004 Jul 23;118(2):187–202. doi: 10.1016/j.cell.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X, Ems-McClung SC, Walczak CE. Aurora A phosphorylates MCAK to control ran-dependent spindle bipolarity. Molecular biology of the cell. 2008 Jul;19(7):2752–65. doi: 10.1091/mbc.E08-02-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, et al. Aurora B regulates MCAK at the mitotic centromere. Dev Cell. 2004 Feb;6(2):253–68. doi: 10.1016/s1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- 77.Knowlton AL, Lan W, Stukenberg PT. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr Biol. 2006 Sep 5;16(17):1705–10. doi: 10.1016/j.cub.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 78.Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, et al. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr Biol. 2004 Feb 17;14(4):273–86. doi: 10.1016/j.cub.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 79.Ohi R, Sapra T, Howard J, Mitchison TJ. Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Molecular biology of the cell. 2004 Jun;15(6):2895–906. doi: 10.1091/mbc.E04-02-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mayr MI, Hummer S, Bormann J, Gruner T, Adio S, Woehlke G, et al. The human kinesin Kif 18A is a motile microtubule depolymerase essential for chromosome congression. Curr Biol. 2007 Mar 20;17(6):488–98. doi: 10.1016/j.cub.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 81.Varga V, Helenius J, Tanaka K, Hyman AA, Tanaka TU, Howard J. Yeast kinesin-8 depolymerizes microtubules in a length-dependent manner. Nature cell biology. 2006 Sep;8(9):957–62. doi: 10.1038/ncb1462. [DOI] [PubMed] [Google Scholar]
- 82.Varga V, Leduc C, Bormuth V, Diez S, Howard J. Cooperative removal of the terminal tubulin dimer underlies length-dependent depolymerization by yeast kinesin-8. Dev Cell. 2009 doi: 10.1016/j.cell.2009.07.032. In press. [DOI] [PubMed] [Google Scholar]
- 83.Stumpff J, von Dassow G, Wagenbach M, Asbury C, Wordeman L. The kinesin-8 motor Kif 18A suppresses kinetochore movements to control mitotic chromosome alignment. Dev Cell. 2008 Feb;14(2):252–62. doi: 10.1016/j.devcel.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gupta ML, Jr, Carvalho P, Roof DM, Pellman D. Plus end-specific depolymerase activity of Kip3, a kinesin-8 protein, explains its role in positioning the yeast mitotic spindle. Nature cell biology. 2006 Sep;8(9):913–23. doi: 10.1038/ncb1457. [DOI] [PubMed] [Google Scholar]
- 85.Pavicic-Kaltenbrunner V, Mishima M, Glotzer M. Cooperative assembly of CYK-4/MgcRacGAP and ZEN-4/MKLP1 to form the centralspindlin complex. Molecular biology of the cell. 2007 Dec;18(12):4992–5003. doi: 10.1091/mbc.E07-05-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fu C, Ward JJ, Loiodice I, Velve-Casquillas G, Nedelec FJ, Tran PT. Phospho-regulated interaction between kinesin-6 Klp9p and microtubule bundler Ase1p promotes spindle elongation. Dev Cell. 2009 Aug;17(2):257–67. doi: 10.1016/j.devcel.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kurasawa Y, Earnshaw WC, Mochizuki Y, Dohmae N, Todokoro K. Essential roles of KIF4 and its binding partner PRC1 in organized central spindle midzone formation. The EMBO journal. 2004 Aug 18;23(16):3237–48. doi: 10.1038/sj.emboj.7600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brust-Mascher I, Civelekoglu-Scholey G, Kwon M, Mogilner A, Scholey JM. Model for anaphase B: role of three mitotic motors in a switch from poleward flux to spindle elongation. Proceedings of the National Academy of Sciences of the United States of America. 2004 Nov 9;101(45):15938–43. doi: 10.1073/pnas.0407044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goshima G, Vale RD. The roles of microtubule-based motor proteins in mitosis: comprehensive RNAi analysis in the Drosophila S2 cell line. The Journal of cell biology. 2003 Sep 15;162(6):1003–16. doi: 10.1083/jcb.200303022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu C, Zhao J, Bibikova M, Leverson JD, Bossy-Wetzel E, Fan JB, et al. Functional analysis of human microtubule-based motor proteins, the kinesins and dyneins, in mitosis/cytokinesis using RNA interference. Molecular biology of the cell. 2005 Jul;16(7):3187–99. doi: 10.1091/mbc.E05-02-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Glotzer M. The 3Ms of central spindle assembly: microtubules, motors and MAPs. Nat Rev Mol Cell Biol. 2009 Jan;10(1):9–20. doi: 10.1038/nrm2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gruneberg U, Neef R, Honda R, Nigg EA, Barr FA. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. The Journal of cell biology. 2004 Jul 19;166(2):167–72. doi: 10.1083/jcb.200403084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neef R, Klein UR, Kopajtich R, Barr FA. Cooperation between mitotic kinesins controls the late stages of cytokinesis. Curr Biol. 2006 Feb 7;16(3):301–7. doi: 10.1016/j.cub.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 94.Abaza A, Soleilhac JM, Westendorf J, Piel M, Crevel I, Roux A, et al. M phase phosphoprotein 1 is a human plus-end-directed kinesin-related protein required for cytokinesis. The Journal of biological chemistry. 2003 Jul 25;278(30):27844–52. doi: 10.1074/jbc.M304522200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dechant R, Glotzer M. Centrosome separation and central spindle assembly act in redundant pathways that regulate microtubule density and trigger cleavage furrow formation. Dev Cell. 2003 Mar;4(3):333–44. doi: 10.1016/s1534-5807(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 96.Jantsch-Plunger V, Gonczy P, Romano A, Schnabel H, Hamill D, Schnabel R, et al. CYK-4: A Rho family gtpase activating protein (GAP) required for central spindle formation and cytokinesis. The Journal of cell biology. 2000 Jun 26;149(7):1391–404. doi: 10.1083/jcb.149.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nislow C, Lombillo VA, Kuriyama R, McIntosh JR. A plus-end-directed motor enzyme that moves antiparallel microtubules in vitro localizes to the interzone of mitotic spindles. Nature. 1992 Oct 8;359(6395):543–7. doi: 10.1038/359543a0. [DOI] [PubMed] [Google Scholar]
- 98.Ellefson ML, McNally FJ. Kinesin-1 and cytoplasmic dynein act sequentially to move the meiotic spindle to the oocyte cortex in Caenorhabditis elegans. Molecular biology of the cell. 2009 Jun;20(11):2722–30. doi: 10.1091/mbc.E08-12-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y, Hancock WO. The two motor domains of KIF3A/B coordinate for processive motility and move at different speeds. Biophys J. 2004 Sep;87(3):1795–804. doi: 10.1529/biophysj.104.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haraguchi K, Hayashi T, Jimbo T, Yamamoto T, Akiyama T. Role of the kinesin-2 family protein, KIF3, during mitosis. The Journal of biological chemistry. 2006 Feb 17;281(7):4094–9. doi: 10.1074/jbc.M507028200. [DOI] [PubMed] [Google Scholar]
- 101.Hammond JW, Cai D, Blasius TL, Li Z, Jiang Y, Jih GT, et al. Mammalian Kinesin-3 motors are dimeric in vivo and move by processive motility upon release of autoinhibition. PLoS Biol. 2009 Mar 31;7(3):e72. doi: 10.1371/journal.pbio.1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Unno K, Hanada T, Chishti AH. Functional involvement of human discs large tumor suppressor in cytokinesis. Exp Cell Res. 2008 Oct 15;314(17):3118–29. doi: 10.1016/j.yexcr.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carleton M, Mao M, Biery M, Warrener P, Kim S, Buser C, et al. RNA interference-mediated silencing of mitotic kinesin KIF14 disrupts cell cycle progression and induces cytokinesis failure. Mol Cell Biol. 2006 May;26(10):3853–63. doi: 10.1128/MCB.26.10.3853-3863.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gruneberg U, Neef R, Li X, Chan EH, Chalamalasetty RB, Nigg EA, et al. KIF14 and citron kinase act together to promote efficient cytokinesis. The Journal of cell biology. 2006 Jan 30;172(3):363–72. doi: 10.1083/jcb.200511061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Castoldi M, Vernos I. Chromokinesin Xklp1 contributes to the regulation of microtubule density and organization during spindle assembly. Molecular biology of the cell. 2006 Mar;17(3):1451–60. doi: 10.1091/mbc.E05-04-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vernos I, Raats J, Hirano T, Heasman J, Karsenti E, Wylie C. Xklp1, a chromosomal Xenopus kinesin-like protein essential for spindle organization and chromosome positioning. Cell. 1995 Apr 7;81(1):117–27. doi: 10.1016/0092-8674(95)90376-3. [DOI] [PubMed] [Google Scholar]
- 107.Kwok BH, Kapitein LC, Kim JH, Peterman EJ, Schmidt CF, Kapoor TM. Allosteric inhibition of kinesin-5 modulates its processive directional motility. Nat Chem Biol. 2006 Sep;2(9):480–5. doi: 10.1038/nchembio812. [DOI] [PubMed] [Google Scholar]
- 108.Saunders WS, Koshland D, Eshel D, Gibbons IR, Hoyt MA. Saccharomyces cerevisiae kinesin- and dynein-related proteins required for anaphase chromosome segregation. The Journal of cell biology. 1995 Feb;128(4):617–24. doi: 10.1083/jcb.128.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kapoor TM, Mayer TU, Coughlin ML, Mitchison TJ. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. The Journal of cell biology. 2000 Sep 4;150(5):975–88. doi: 10.1083/jcb.150.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gardner MK, Bouck DC, Paliulis LV, Meehl JB, O’Toole ET, Haase J, et al. Chromosome congression by Kinesin-5 motor-mediated disassembly of longer kinetochore microtubules. Cell. 2008 Nov 28;135(5):894–906. doi: 10.1016/j.cell.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cesario JM, Jang JK, Redding B, Shah N, Rahman T, McKim KS. Kinesin 6 family member Subito participates in mitotic spindle assembly and interacts with mitotic regulators. Journal of cell science. 2006 Nov 15;119(Pt 22):4770–80. doi: 10.1242/jcs.03235. [DOI] [PubMed] [Google Scholar]
- 112.Raich WB, Moran AN, Rothman JH, Hardin J. Cytokinesis and midzone microtubule organization in Caenorhabditis elegans require the kinesin-like protein ZEN-4. Molecular biology of the cell. 1998 Aug;9(8):2037–49. doi: 10.1091/mbc.9.8.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kuriyama R, Gustus C, Terada Y, Uetake Y, Matuliene J. CHO1, a mammalian kinesin-like protein, interacts with F-actin and is involved in the terminal phase of cytokinesis. The Journal of cell biology. 2002 Mar 4;156(5):783–90. doi: 10.1083/jcb.200109090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Espeut J, Gaussen A, Bieling P, Morin V, Prieto S, Fesquet D, et al. Phosphorylation relieves autoinhibition of the kinetochore motor Cenp-E. Mol Cell. 2008 Mar 14;29(5):637–43. doi: 10.1016/j.molcel.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 115.Kim Y, Heuser JE, Waterman CM, Cleveland DW. CENP-E combines a slow, processive motor and a flexible coiled coil to produce an essential motile kinetochore tether. The Journal of cell biology. 2008 May 5;181(3):411–9. doi: 10.1083/jcb.200802189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yardimci H, van Duffelen M, Mao Y, Rosenfeld SS, Selvin PR. The mitotic kinesin CENP-E is a processive transport motor. Proceedings of the National Academy of Sciences of the United States of America. 2008 Apr 22;105(16):6016–21. doi: 10.1073/pnas.0711314105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schaar BT, Chan GK, Maddox P, Salmon ED, Yen TJ. CENP-E function at kinetochores is essential for chromosome alignment. The Journal of cell biology. 1997 Dec 15;139(6):1373–82. doi: 10.1083/jcb.139.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kapoor TM, Lampson MA, Hergert P, Cameron L, Cimini D, Salmon ED, et al. Chromosomes can congress to the metaphase plate before biorientation. Science (New York, NY) 2006 Jan 20;311(5759):388–91. doi: 10.1126/science.1122142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Unsworth A, Masuda H, Dhut S, Toda T. Fission yeast kinesin-8 Klp5 and Klp6 are interdependent for mitotic nuclear retention and required for proper microtubule dynamics. Molecular biology of the cell. 2008 Dec;19(12):5104–15. doi: 10.1091/mbc.E08-02-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garcia MA, Koonrugsa N, Toda T. Spindle-kinetochore attachment requires the combined action of Kin I-like Klp5/6 and Alp14/Dis1-MAPs in fission yeast. The EMBO journal. 2002 Nov 15;21(22):6015–24. doi: 10.1093/emboj/cdf611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gatt MK, Savoian MS, Riparbelli MG, Massarelli C, Callaini G, Glover DM. Klp67A destabilises pre-anaphase microtubules but subsequently is required to stabilise the central spindle. Journal of cell science. 2005 Jun 15;118(Pt 12):2671–82. doi: 10.1242/jcs.02410. [DOI] [PubMed] [Google Scholar]
- 122.Al Sarakbi W, Sasi W, Jiang WG, Roberts T, Newbold RF, Mokbel K. The mRNA expression of SETD2 in human breast cancer: correlation with clinico-pathological parameters. BMC Cancer. 2009;9:290. doi: 10.1186/1471-2407-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bernstein M, Beech PL, Katz SG, Rosenbaum JL. A new kinesin-like protein (Klp1) localized to a single microtubule of the Chlamydomonas flagellum. The Journal of cell biology. 1994 Jun;125(6):1313–26. doi: 10.1083/jcb.125.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Levesque AA, Howard L, Gordon MB, Compton DA. A functional relationship between NuMA and kid is involved in both spindle organization and chromosome alignment in vertebrate cells. Molecular biology of the cell. 2003 Sep;14(9):3541–52. doi: 10.1091/mbc.E03-02-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Antonio C, Ferby I, Wilhelm H, Jones M, Karsenti E, Nebreda AR, et al. Xkid, a chromokinesin required for chromosome alignment on the metaphase plate. Cell. 2000 Aug 18;102(4):425–35. doi: 10.1016/s0092-8674(00)00048-9. [DOI] [PubMed] [Google Scholar]
- 126.Funabiki H, Murray AW. The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell. 2000 Aug 18;102(4):411–24. doi: 10.1016/s0092-8674(00)00047-7. [DOI] [PubMed] [Google Scholar]
- 127.Ohsugi M, Adachi K, Horai R, Kakuta S, Sudo K, Kotaki H, et al. Kid-mediated chromosome compaction ensures proper nuclear envelope formation. Cell. 2008 Mar 7;132(5):771–82. doi: 10.1016/j.cell.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 128.Theurkauf WE, Hawley RS. Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. The Journal of cell biology. 1992 Mar;116(5):1167–80. doi: 10.1083/jcb.116.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Boleti H, Karsenti E, Vernos I. Xklp2, a novel Xenopus centrosomal kinesin-like protein required for centrosome separation during mitosis. Cell. 1996 Jan 12;84(1):49–59. doi: 10.1016/s0092-8674(00)80992-7. [DOI] [PubMed] [Google Scholar]
- 130.Rogers GC, Chui KK, Lee EW, Wedaman KP, Sharp DJ, Holland G, et al. A kinesin-related protein, KRP(180), positions prometaphase spindle poles during early sea urchin embryonic cell division. The Journal of cell biology. 2000 Aug 7;150(3):499–512. doi: 10.1083/jcb.150.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sueishi M, Takagi M, Yoneda Y. The forkhead-associated domain of Ki-67 antigen interacts with the novel kinesin-like protein Hklp2. The Journal of biological chemistry. 2000 Sep 15;275(37):28888–92. doi: 10.1074/jbc.M003879200. [DOI] [PubMed] [Google Scholar]
- 132.Newton CN, Wagenbach M, Ovechkina Y, Wordeman L, Wilson L. MCAK, a Kin I kinesin, increases the catastrophe frequency of steady-state HeLa cell microtubules in an ATP-dependent manner in vitro. FEBS Lett. 2004 Aug 13;572(1–3):80–4. doi: 10.1016/j.febslet.2004.06.093. [DOI] [PubMed] [Google Scholar]
- 133.Walker RA, Salmon ED, Endow SA. The Drosophila claret segregation protein is a minus-end directed motor molecule. Nature. 1990 Oct 25;347(6295):780–2. doi: 10.1038/347780a0. [DOI] [PubMed] [Google Scholar]
- 134.Matuliene J, Essner R, Ryu J, Hamaguchi Y, Baas PW, Haraguchi T, et al. Function of a minus-end-directed kinesin-like motor protein in mammalian cells. Journal of cell science. 1999 Nov;112( Pt 22):4041–50. doi: 10.1242/jcs.112.22.4041. [DOI] [PubMed] [Google Scholar]
- 135.Endow SA, Kang SJ, Satterwhite LL, Rose MD, Skeen VP, Salmon ED. Yeast Kar3 is a minus-end microtubule motor protein that destabilizes microtubules preferentially at the minus ends. The EMBO journal. 1994 Jun 1;13(11):2708–13. doi: 10.1002/j.1460-2075.1994.tb06561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Walczak CE, Verma S, Mitchison TJ. XCTK2: a kinesin-related protein that promotes mitotic spindle assembly in Xenopus laevis egg extracts. The Journal of cell biology. 1997 Feb 24;136(4):859–70. doi: 10.1083/jcb.136.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Prigozhina NL, Walker RA, Oakley CE, Oakley BR. Gamma-tubulin and the C-terminal motor domain kinesin-like protein, KLPA, function in the establishment of spindle bipolarity in Aspergillus nidulans. Molecular biology of the cell. 2001 Oct;12(10):3161–74. doi: 10.1091/mbc.12.10.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Endow SA, Chandra R, Komma DJ, Yamamoto AH, Salmon ED. Mutants of the Drosophila ncd microtubule motor protein cause centrosomal and spindle pole defects in mitosis. Journal of cell science. 1994 Apr;107( Pt 4):859–67. doi: 10.1242/jcs.107.4.859. [DOI] [PubMed] [Google Scholar]
- 139.Saunders W, Hornack D, Lengyel V, Deng C. The Saccharomyces cerevisiae kinesin-related motor Kar3p acts at preanaphase spindle poles to limit the number and length of cytoplasmic microtubules. The Journal of cell biology. 1997 Apr 21;137(2):417–31. doi: 10.1083/jcb.137.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cassimeris L, Morabito J. TOGp, the human homolog of XMAP215/Dis1, is required for centrosome integrity, spindle pole organization, and bipolar spindle assembly. Molecular biology of the cell. 2004 Apr;15(4):1580–90. doi: 10.1091/mbc.E03-07-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gaetz J, Kapoor TM. Dynein/dynactin regulate metaphase spindle length by targeting depolymerizing activities to spindle poles. The Journal of cell biology. 2004 Aug 16;166(4):465–71. doi: 10.1083/jcb.200404015. [DOI] [PMC free article] [PubMed] [Google Scholar]