Abstract
Influenza virus leads to acute respiratory disease resulting in seasonal epidemics and periodic pandemics. Little is known about the signaling events that regulate host defense to influenza. One particular pathway, the c-Jun amino-terminal kinase (JNK) cascade is activated following influenza infection and blocking JNK leads to enhanced viral replication. We hypothesize that Mixed Lineage Kinase 3 (MLK3), an upstream regulator of JNK, is involved in the host response to influenza. To test this, wild-type and MLK3−/− mice were infected with pathogenic strain of influenza A virus, A/PR/8/34 (PR8). Although, cellular and humoral immune responses were similar between wild-type and MLK3−/− hosts, the viral load in the lungs was comparatively higher in MLK3−/− mice at day 8 post infection. Consistent with this, MLK3−/− murine lung fibrobalsts had prolonged survival and increased virion production following infection compared to wild-type. These findings support a role for MLK3 in viral production during influenza infection.
Keywords: Mixed Lineage Kinase 3, Influenza virus, viability, JNK, T cell
Introduction
Influenza A virus, a member of the orthomyxoviridae, leads to acute respiratory disease resulting in seasonal epidemics and periodic pandemics. Per year, an estimated 200,000 – 500,000 people worldwide die from seasonal influenza infection and, in 2007, the economic burden was projected to exceed $87 billion annually (Molinari et al., 2007). Influenza infection leads to the onset of an immune response characterized by an increase in the level of chemokine and cytokine production, migration of immune infiltrates to the site of infection and apoptosis of infected cells (Brydon, Morris, and Sweet, 2005). This inflammatory and apoptotic response is important both in containing virus infection and also in the outcome of that infection, since it is directly related to the morbidity and mortality in hosts infected with influenza (Kash et al., 2006; Korteweg and Gu, 2008).
Little is known about the signaling pathways that regulate host defense to influenza virus infection. A potential target for investigation are the mitogen activated protein kinase (MAPK) pathways and their upstream regulators. Influenza A virus infection is known to activate MAPK pathways in the infected cell, including the Jun-N-terminal kinase (JNK) cascade, which is involved in the antiviral, inflammatory and apoptotic responses to influenza (Gallo and Johnson, 2002; Ludwig et al., 2006). Previous studies suggest blockade of JNK activation leads to increased influenza virus titers in vitro, decreased AP-1 transcriptional activity, defective Th cell differentiation and increased cell survival (Dong et al., 2000; Ludwig et al., 2001; Sabapathy et al., 2001). Treatment of human bronchial epithelial cells (BEC) with CEP1347, a pharmacologic inhibitor of MAP3K mixed lineage kinase-3 (MLK3), strongly inhibited JNK activity during in vitro influenza infection (Kujime et al., 2000). Thus, we became interested in the role of MLK3 in influenza infection.
MLK3 is a serine/threonine MAPK kinase kinase (MAP3K) that targets JNK through the activation of its immediate downstream targets MAPK kinase 7 (MKK7) and, to a lesser extent, MKK4 (Handley et al., 2007b). While JNK is thought to be MLK3’s main downstream target, MLK3 is known to activate p38 and ERK as well (Chadee and Kyriakis, 2004; Gallo and Johnson, 2002; Hong and Kim, 2007; Tibbles et al., 1996; Xu et al., 2001). Critical to MLK3 activation are Ras GTPases, which are in turn activated in response to a broad range of extracellular stress stimuli including chemokines and pathogen-associated molecular patterns (PAMP), both of which are present during influenza infection.
A role for the MLK3-JNK cascade in the control of influenza infection has also been suggested on the basis that CEP1347 blockade of the MLK3-JNK cascade resulted in reduced RANTES production by influenza A virus infected human BEC cultures (Kujime et al., 2000). This finding suggests that MLK3 activation may promote immune cell infiltration and inflammation during infection with influenza. MLK3 is also known to regulate cell fate in experimental models of other virus infections, including HIV-1 associated neurologic disease (HAND). For instance, CEP1347 treatment blocked HIV-1 Tat-induced activation of MLK3 and its downstream target JNK, resulting in a substantial reduction in apoptosis in Tat-exposed neurons (Sui et al., 2006). Similar findings have been obtained in other well-studied paradigms of neuronal apoptosis, suggesting that MLK3 may globally regulate cell survival through the action of downstream effectors such as JNK (Mota et al., 2001).
To date, few studies have examined the role of MAPK pathways in virus infection using an animal model system. We therefore employed MLK3 knockout mice to examine the role of MLK3 in host defense to influenza A virus infection. Mice were experimentally infected with a well-characterized, pathogenic strain of influenza A virus, A/PuertoRico/8/34 (PR8). Our results showed that, in the absence of MLK3, there was increased accumulation of influenza virus in the lung. The increased viral load was associated with prolonged survival of MLK3−/− lung cells following in vitro infection with influenza virus, corresponding with increased virus production, relative to cells from wild-type mice. In contrast, MLK3−/− and wild-type mice did not appear to have gross differences in cytokine and chemokine responses to influenza, nor in immune cell infiltration into the lung. Similarly, the magnitude of virus-specific cellular and humoral immune responses that was tested were similar in wild-type and MLK3 deficient mice. These data suggest that MLK3 may regulate the outcome of influenza virus infection by controlling the extent and duration of virus replication in lung cells, resulting in a transient increase in virion production at late time points following infection.
Results
MLK3−/− mice have elevated levels of virus present in the lung
To evaluate the role of MLK3 during influenza infection, we took advantage of an in vivo infection model using MLK3−/− mice backcrossed onto the C57BL/6J strain (Brancho et al., 2005). Previous studies indicated these mice are healthy with no apparent phenotype aside from a slight defect in TNF-stimulated JNK activation (Brancho et al., 2005). In all of our experiments, C57BL/6J (Jackson Laboratory) were used for wild-type controls.
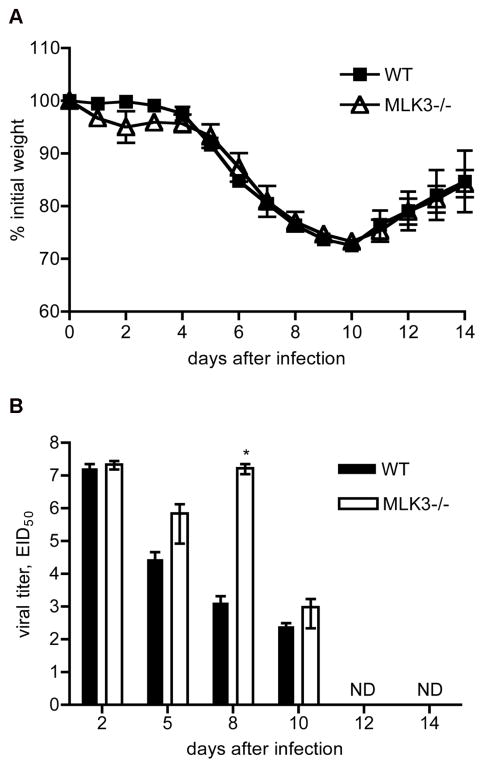
We first determined the LD50 for PR8 intranasal infection in both strains of mice. This analysis did not result in significant quantitative differences between MLK3−/− and wild-type mice and the LD50 was determined to be 505.9 and 482.7 Egg Infectious Doses (EID), respectively. Based on this, we chose to perform subsequent experiments with 400 EID of PR8, a sublethal dose allowing characterization through day 14 post infection. To examine weight loss and general morbidity following infection with PR8 virus, ten-week-old wild-type and MLK3−/− mice were infected intranasally and were monitored daily for 14 days after infection. Weight loss was comparable between MLK3−/− and wild-type mice (Fig. 1A). However, during the course of infection, wild-type mice appeared lethargic with ruffled fur, scrunched posture and stiffness. In contrast, MLK3−/− mice were more active and showed reduced signs of morbidity.
Fig. 1.
Kinetics of weight loss following infection. A) Mice were infected with 400 EID of virus and weight loss was monitored daily for 14 days. B) Kinetics of production and clearance of A/PR/8/34 in the lung of MLK3−/− and wild-type mice infected intransally with 400 EID of virus. On days 2, 5, 8, 10, 12 and 14 post infection lungs were harvested and homogenized. Viral titers in the resulting homogenates were determined by injecting serial dilutions of homogenates into fertilized hen eggs. Hemagglutination assays were performed on allantoic fluid from these eggs. Bars indicate mean values from 3–8 animals; error bars denote the standard error of the mean (SEM). Results shown are representative of 3 total experiments that yielded similar results. *, p < 0.05; Mann-Whitney U test.
Because heightened morbidity is often associated with increased viral titers, we next determined the amount of virus present in the lungs of both groups (Baskin et al., 2009). Lungs from infected mice were isolated at days 2–14 post infection and virus titer in lung homogenates was measured in fertilized hen eggs. Both groups of mice displayed equivalent viral titers in the lung at day 2 post infection. In wild-type mice, there was a progressive decline in the amount of virus present in the lung over time, reaching undetectable levels by day 12. In contrast, sustained high viral titers were detected in the lungs of MLK3−/− mice through day 8 post infection. Titers then declined to undetectable levels by day 12 (Fig. 1B). This suggests that MLK3 ablation resulted in a transient delay in viral clearance in the lung, but that the MLK3 deficient mice were ultimately able to clear the infection.
Immune cell infiltration following influenza infection is similar between wild-type and MLK3−/− mice
Previous work characterizing highly pathogenic strains of influenza virus demonstrate that increased amounts of virus in the lung often correlate with increased cell infiltration to the site of infection (Conenello and Palese, 2007; Kobasa et al., 2004; Perrone et al., 2008; Svitek et al., 2008). Therefore, because an elevated viral load was found in the lungs of MLK3−/− mice, immune cell infiltration into lung tissue was examined by performing H&E staining on sections prepared from lungs of virus-infected animals isolated at days 0, 6 and 8 post infection. Early experiments were performed using an inoculating dose of 400 EID per animal. At this dose, there was very extensive lung pathology in both wild-type and MLK3−/− animals (data not shown). To more clearly address whether or not there were differences between wild-type and MLK3−/− mice, we repeated these experiments at a lower dose of 60 EID per animal. Blind scoring of lung sections revealed that there was no significant difference in immune cell infiltration into the lung between groups at these time points (Fig. 2A).
Fig. 2.
H&E staining of lung sections from influenza-virus infected mice. A) Groups of 5 wild-type (WT) and MLK3−/− mice were infected with 60 EID of PR8 and sacrificed at days 0, 6 and 8 after infection. Lavaged lungs were fixed with 10% formalin prior to isolation. H&E staining was performed on tissue sections. Magnification is ×100. B) Kinetics of the cellular immune response in WT and MLK3−/− mice intranasally infected with 400 EID of PR8. Bronchial alveolar lavage (BAL) fluid and lung tissue was collected at days 2, 4, 6, 8 and 10 after infection. Data points represent pooled cells from 3–5 animals per time point. Total cell counts were calculated by multiplying the frequency of CD4+, CD8+ or CD19+ cells by the total number of cells recovered from the sample. Results shown are representative of three separate experiments.
To further characterize lymphocyte infiltration, mAb staining and analysis was performed to enumerate CD4+, CD8+ and CD19+ cells in bronchial alveolar lavage (BAL) fluid and lung homogenates. Immune infiltrates were analyzed in tissues collected at days 2, 4, 6, 8 and 10 post infection. These experiments revealed equivalent numbers of CD4+, CD8+ and CD19+ cells in both lung and BAL samples isolated from wild-type and MLK3−/− mice (Fig. 2B). Therefore, there was no correlation between lung cell infiltration and virus burden in the lung (Cilloniz et al., 2009).
MLK3−/− mice have similar levels of antigen-specific CD8+ T cells compared to wild-type mice
To characterize T cell priming and differentiation in MLK3-deficient mice, virus-specific CD8+ T cells were quantified at day 8 after infection and the total number of epitope-specific CD8+ T cells in the lung was determined by tetramer staining. Single cell suspensions were labeled with CD4, CD8 and NP366–374 and PA224–233 tetramers and examined by flow cytometry. Figure 3 shows that MLK3−/− and wild-type mice had similar numbers of peptide-specific CD8+ T cells at day 8 in the lung. To further confirm this finding, we also performed IFN-γ ELISPOT analysis on purified CD8+ (Fig. 3B) and CD4+ (Fig. 3C) T cells isolated from the spleen and mediastinal lymph node (MLN) of infected mice at days 4, 6, 8 and 10 after infection. At day 8 after infection, MLK3−/− mice displayed elevated numbers of IFN-γ producing by CD8+ T cells isolated from the spleen and MLN, and by CD4+ T cells isolated from the spleen. IL-2 ELISPOT analysis was similarly performed on days 6, 8 and 10. These experiments revealed no significant difference between groups with regard to the number of IL-2 producing CD4+ T cells specific for selected immunodominant class I (CD8) and class II (CD4) restricted epitopes from the PR8 virus (Supplementary Fig. 1). Collectively, these results suggest that (i) CD8+ T cells can recognize and respond to antigen presentation in the absence of MLK3, (ii) the expansion of the CD8+ T cell population is not significantly impaired by MLK3 deficiency, (iii) that lack of MLK3 does not result in impaired immune cell trafficking to the lung and to secondary lymphoid tissue and (iv) CD4+ and CD8+ T cells isolated from the spleen and MLN of MLK3−/− mice have the capacity to produce IFN-γ and IL-2.
Fig. 3.
Profile of CD8+ T cells specific for immunodominant NP366–374 (NP) and PA224–233 (PA) epitopes at day 8 post-infection in selected tissue compartments. MLK3−/− and wild-type (WT) mice were intranasally infected with 400 EID of PR8. A) Lungs were isolated at day 8 post-infection and processed and assayed by flow cytometry for the presence of NP and PA-specific CD8+ T cells. Data points represent individual mice with 4–5 mice per group; bars denote mean for the group. Results shown are representative of 3 individual experiments. B) ELISPOT analysis of influenza virus-specific CD4 and CD8 IFN-γ producing cells. Spleen and mediastinal lymph node (MLN) were isolated at days 4, 6, 8 and 10 after infection. Cells from the spleen (pooled) and MLN (pooled) were purified using MACS separation. The number of total IFN-γ secreting cells was quantified using ELISPOT analysis with peptides for 2 immunodominant epitopes for CD4 and CD8. Depicted is the average number of spots from triplicate wells. Spot forming units is abbreviated as s.f.u. Results shown are representative of two separate experiments.
MLK3−/− and wild-type mice have similar titers of IgG antibody at day 12 in the serum following influenza virus infection
To further examine the functionality of the T cell response, we next evaluated the antigen-specific IgG antibody response in MLK3−/− mice. It is known that CD4+ T cells contribute to the switch from IgM to IgG antibody isotype and help to develop a high affinity antibody response (Lee et al., 2005; Sangster et al., 2003; Wells, Albrecht, and Ennis, 1981). To evaluate this component of the immune response, we examined levels of the influenza specific IgG antibody in infected mice. Serum was obtained from MLK3−/− and wild-type mice at day 12 following experimental infection with influenza. An IgG specific ELISA assay was employed to determine influenza-specific IgG antibody titers in the serum. These experiments indicated that both strains of mice displayed similar titers of IgG present at day 12 (Fig. 4). These results suggest that MLK3−/− mice are capable of isotype switch from IgM to IgG during infection with influenza, further supporting the conclusion that CD4 T cell responses are intact in MLK3 deficient mice.
Fig. 4.
IgG antibody responses in serum obtained from wild-type and MLK3−/− mice. Tail vein bleeds were performed on both groups at day 12 after intranasal infection with 400 EID of influenza A virus strain PR8. Titers of PR8-specific antibody were determined by ELISA. Lines indicate mean values from 5 animals; error bars denote the standard error of the mean (SEM).
MLK3−/− and wild-type mice have similar levels of inflammatory cytokines and chemokines in the lung following influenza virus infection
In vitro studies using HIV-1 Tat-activated human monocytes have shown that TNF-α release can be regulated by MLK3 (Sui et al., 2006). Similar results have also been reported for RANTES production by influenza virus infected human bronchial epithelial cells (Kujime et al., 2000). Additionally, downstream targets of MLK3, MKK7 and JNK, have been implicated Th cell differentiation during neutral polarizing conditions (Dong et al., 2000). In order to evaluate the role of MLK3 in cytokine and chemokine production during influenza infection in vivo, we examined the levels of MCP-1, IP-10, TNF-α, RANTES, IL-4 and IFN-γ in the lung. ELISA assays performed on whole lung homogenates from wild-type and MLK3−/− mice revealed that the overall production of these cytokines or chemokines in response to PR8 infection was similar in both strains of mice (Fig. 5). Therefore, among the cytokines and chemokines examined in this study, there appears to be no significant or consistent defects in the absence of MLK3.
Fig. 5.
Levels of inflammatory cytokines and chemokines in lungs from influenza virus infected wild-type and MLK3−/− mice. Mice were infected with influenza A virus strain PR8 and lungs were isolated from wild-type (WT) and MLK3−/− mice at various time points after infection. ELISA for the indicated cytokines and chemokines was then performed on lung homogenates. Bars indicate mean values from 3–5 animals; error bars denote the standard error of the mean (SEM).
MLK3−/− murine lung cells have prolonged viability following influenza virus infection and release greater levels of virus progeny, compared to wild-type cells
Evidence in the literature suggests a pro-apoptotic role for the Cdc42 – MLK3 – MKK7 – JNK cascade (Hong and Kim, 2007; Kim et al., 2004; Zhao and Hoffmann, 2006). We therefore hypothesized that the increased level of virus present in the lungs of MLK3−/− mice might be a result of increased survival of infected cells. To test this idea, we examined cellular viability following infection with influenza virus in both murine lung fibroblasts (MLF) and alveolar epithelial cells (AEC) isolated from MLK3−/− and wild-type mice. First, virus-induced cytopathic effects (CPE) such as cell rounding, shrinking and detachment from the plate was evaluated in cells infected with the influenza A virus strain PR8 for 12–72 hours. MLFs from the MLK3−/− host demonstrated significantly reduced CPE beginning at 24h post infection relative to MLFs isolated from wild-type animals (Fig. 6A). Similar to MLFs, MLK3−/− AECs demonstrated reduced CPE during infection with influenza (data not shown). We next quantified the survival of infected MLF and AECs by trypan blue exclusion. Consistent with the CPE data, significantly more MLK3−/− MLFs and AECs were viable at 24 and 48h post infection when compared with the wild-type cells (Fig. 6B). These results suggest the involvement of MLK3 in cellular resistance to influenza infection.
Fig. 6.
MLK3−/− cells are resistant to influenza virus-induced cell killing, and produce larger amounts of progeny virions when compared to cells from wild-type mice. Wild-type (WT) and MLK3−/− MLF or AEC were infected with influenza A virus strain PR8 (MOI = 3) and then analyzed at various time points. A) Virus-infected MLFs (WT and MLK3−/−) were analyzed at the indicated time points by light microscopy. Results are representative of 3 individual experiments. Magnification is ×100. B) Virus-infected MLF and AECs (WT and MLK3−/−) were subjected to trypan blue staining at the indicated time points to determine viability. Bars indicate mean values from three replicates; error bars denote the standard error of the mean (SEM). *, p < 0.05; Mann-Whitney test. C) Progeny virion release in supernatants from virus-infected MLF and AECs (WT and MLK3−/−) was determined at the indicated time points, by TCID50 assay in MDCK cells. Bars indicate mean values from two replicates. Results are representative of at least 2 identical experiments.
Since cytopathic effect is an outcome of virus infection, one possible explanation for these findings is that MLK3−/− cells might be more resistant to influenza virus infection relative to cells from wild-type mice. To determine if this was the case, immunofluorescence staining of cells infected with various doses of PR8 was performed. MLF and AECs from both wildtype and MLK3−/− hosts exhibited similar levels of virus infection, as assessed by immunostaining for viral nucleoprotein and cell nuclei (data not shown).
Finally, we examined whether prolonged survival of influenza virus-infected MLK3−/− cells resulted in increased viral production. To evaluate this, supernatants from infected MLF and AECs were collected at 12, 24, 48 and 72h following infection and the level of virus in the supernatant was titrated on MDCK cells. Results from this experiment showed that both MLK3−/− MLF and AECs exhibited prolonged virus production, relative to wild-type cells (Fig. 6C). Collectively, these data suggest that loss of MLK3 leads to sustained survival of infected target cells allowing for extended shedding of virus.
Discussion
In this study, we suggest a role for MLK3 in regulating the outcome of influenza infection in vivo. Our study revealed that the genetic ablation of MLK3 was associated with elevated virus production at late time points following infection in vivo. Our data also showed that cultured lung cells from MLK3−/− mice were resistant to influenza virus-mediated cell killing, and were able to sustain release of progeny virions for an extended time period, relative to lung cells from wild-type mice. These in vitro findings suggest that during the course of infection infected cells in the MLK3−/− mice will continue to produce virus progeny for longer periods of time leading to more virions produced per infected cell. Over time, in the absence of MLK3, this will lead to a greater accumulation and spread of virus in the lung.
Histological and flow analysis suggest that MLK3 is not required for proliferation of immune cells or the infiltration of these cells into the lung during infection with influenza. Previous reports on MLK3’s role in cell proliferation and migration have been contradictory (Brancho et al., 2005; Chadee and Kyriakis, 2004). MLK3 was suggested to be essential for the proliferation of serum stimulated fibroblasts by Chadee and Kryiakis (Chadee and Kyriakis, 2004), but studies by Brancho and colleagues on the proliferation and migration of MEFs from MLK3−/− mice suggested that MLK has no role in cell proliferation or migration (Brancho et al., 2005). Overall, our results are consistent with those of Brancho and colleagues, but not those of Chadee and Kyriakis, suggesting that MLK3 does not impact either of these processes (Brancho et al., 2005).
MLK3 is known to be expressed in dendritic cells (DC) (Handley et al., 2007b) and previous studies have suggested that MLKs may be involved in DC maturation and activation (Handley et al., 2007a). In our studies, the levels of antigen-specific, cytokine secreting CD8+ T cells were similar in virus-infected MLK3−/− mice versus wild-type mice, suggesting that the function of DC that prime the adaptive immune response is not seriously impaired.
MLK3 is also known to regulate the p38 cascade of the MAPK pathway (Brancho et al., 2005; Chadee and Kyriakis, 2004). Previously, it was shown that CD4+ and CD8+ T cells isolated from MKK6 transgenic mice, in which p38 is constitutively active, have increased IFN-γ production (Merritt et al., 2000; Rincon et al., 1998). We see a similar trend in CD8+ T cells isolated from MLK3−/− mice during infection with influenza. Conze et al. proposed that increased levels of IFN-γ could augment viral clearance (Conze et al., 2000). Consistent with this, MLK3−/− mice show clearance in viral titers by day 10 similar to that detected in the wild-type mice. This ability to clear virus could be promoted by the boost in IFN-γ production by antigen specific CD8+T cells. However, it is important to note that the increased IFN-γ production by T cells is not reflected in the overall IFN-γ levels in the total lung.
CEP1347 treatment of human bronchial epithelial cells has been shown to result in a marked decrease in RANTES release following H3N2 influenza virus infection (Kujime et al., 2000). In contrast, our findings revealed that RANTES production in lungs of influenza virus-infected MLK3−/− mice was similar to that in wild-type mice. This may reflect either (i) in vivo production of RANTES from non-epithelial cell types, such as T cells and/or (ii) differences related to the virus strain used (H3N2 versus H1N1) and/or (iii) the ability of CEP1347 to inhibit not only MLK3, but also other related family members which may be expressed in bronchial epithelial cells (Maroney et al., 2001).
Our in vivo studies revealed a potential role for MLK3 in viral production during infection with influenza. We observed that virus titers in the lung of MLK3−/− mice were increased in comparison to wild-type mice at late time points following infection. In accordance with our in vitro data, the increased level of virus production could be a result of increased virus shedding from surviving infected target cells. Enhanced survival of cells lacking MLK3 has been seen previously in other disease models (Hong and Kim, 2007; Kim et al., 2004; Sui et al., 2006; Zhao et al., 2007).
Overall, our work clarifies the role of MLK3 during influenza infection and reveals that the loss of MLK3 is associated with increased virus titers in the lung at late time points following experimental infection with influenza virus. Finally, our data suggest that elevated virus titers in MLK3−/− mice may be a result of increased virus replication, as a consequence of the prolonged survival of virus-infected MLK3−/− lung cells.
Materials and methods
Mice
MLK3−/− mice were obtained from Dr. R. Davis (Univ. of Massachusetts Medical School, Worchester, MA). C57BL/6 wild-type mice were purchased from Jackson Laboratory (Bar Harbor, ME). All mice were on the C57BL/6J background and were bred and maintained according to The University of Rochester Committee on Animal Resources guidelines.
Virus infection in vivo
All infections were carried out in anesthetized eight to ten week old mice following administration of 75mg/kg Avertin (2,2,2-tribomoethanol). Animals were infected intranasally with PR8 (Allan et al., 1990; Polakos et al., 2007) diluted in cold phosphate buffered saline (PBS) such that a 30 μl volume contained 400 egg infectious doses (EID) of influenza unless otherwise noted. Viral titers in the lungs of infected mice were quantified in fertilized hen eggs. Briefly, lungs were harvested and homogenized in 1 ml cold PBS using a Dunce homogenizer. Samples were centrifuged at 10,000 rpm for 3 minutes and supernatants were aliquot and stored at −80°C. 100 μl of lung homogenate was used to make 10-fold serial dilutions, and 100 μl of each dilution was used to inoculate into eggs in triplicate. Following incubation, allantoic fluid was harvested and used to perform a hemagglutination assay with chicken red blood cells. The viral end point titer was determined using the method of Reed and Muench (Reed and Muench, 1938).
Histology
Bronchial alveolar lavage wash was performed on each mouse by instilling intratracheally 0.6ml PBS three times followed by 1ml of 10% formalin to inflate the lung. The trachea was tied off and the lung was removed and placed in a 50 ml conical tube containing 10 ml 10% formalin and incubated overnight. The following day lungs were then transferred into 70% ethanol for an additional 24h before final transfer into fresh 70% ethanol. Animal Resources, University of Rochester, Rochester, NY had then conducted hematoxylin and eosin staining of serial sections of lung tissues.
Organ harvest
Mice were anesthetized with avertin and exsanguinated via renal artery at the indicated times after infection. Bronchial alveolar lavage (BAL) samples were collected as stated in previous section. Spleen, lung and mediastyinal lymph node (MLN) were isolated. To create single cell suspensions, spleen and MLN samples were homogenized and filtered through nylon mesh. Lungs were mechanically disrupted using a tea strainer, filtered through nylon mesh and lymphocytes were separated using Histopaque 1083 (Sigma, St. Louis, MO). Spleen and lung samples were subjected to RBC lysis in 1X Gey’s solution. Single cell suspensions were counted by hemocytometer.
Flow cytometry
Single cell suspensions from organs were incubated in Hanks Buffered Salt Solution (HBSS) with 1% BSA and 0.0025% sodium azide containing 0.025 μg/ml purified rat anti-mouse CD16/CD32 (Fc block) followed by addition of fluorochrome-conjugated antibodies or MHC class I tetramers. Fluorochrome-conjugated antibodies were obtained from BD Biosciences, San Jose, CA or Biolegend, San Diego, CA. MHC Class I tetramers H-2D containing nucleoprotein (NP)366–374 or polymerase acidic (PA)224–233 were kindly provided through a collaboration with the Trudeau Institute Biochemistry Core (Saranac Lake, NY) as well as obtained from the National Institute of Health (NIH Tetramer Facility, Atlanta, GA). Flow cytometry was performed by using a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ) and the data was analyzed with FlowJo software (Treestar, Ashland, OR).
ELISPOT analysis
ELISPOT assays were performed as previously described (Lazarski et al., 2005; Richards et al., 2007; Richards, Chaves, and Sant, 2009). Briefly, 96-well filter plates (Millipore, Billerica, MA) were coated with 2 μg/ml purified rat-anti-mouse interferon-γ (IFN-γ) or interleukin-2 (IL-2) (BD Biosciences, San Jose, CA) in PBS, washed, and incubated in serum-containing medium to block non-specific binding. CD4+ and CD8+ T cells from the spleen (pooled) and mediastinal lymph node (pooled) were purified by MACS negative selection using a cocktail of biotin-conjugated monoclonal antibodies (Miltenyi Biotech, Auburn, CA). Resulting purified T cells (3x105 cells/well) were co-cultured with naïve splenocytes (5x105 cells/well) with immuno-dominant peptides for CD4 and CD8 at a final concentration of 10 μM each. CD4 peptides were NP261:274 and NP310:325 and CD8 peptides were NP366–374 and PA224–233. Plates were incubated for 18 hours at 37°C in 5% CO2. After incubation, plates were washed and incubated with 2 μg of biotinylated rat-anti-mouse IFN-γ or IL-2 (BD Biosciences, San Jose, CA) for 30 minutes at room temperature. Following this, plates were washed and incubated for 30 minutes with alkaline phosphatase-strepavidin (Jackson Immunoresearch, West Grove, PA; dilution of 1:1000). After a final wash, plates were developed using Vector blue substrate kit III (Vector Laboratories, Burlingame, CA) diluted in 100 mM Tris, pH 8.2. Plates were allowed to dry overnight before counting. Spots were counted using Immunospot 3 software (Cellular Technologies Limited, Shaker Heights, OH).
Serum collection and ELISA
Tail vein bleeding was performed on mice immediately prior to sacrifice. Blood was allowed to clot at room temperature. Blood was pelleted in a microcentrifuge and the serum was collected. Influenza-specific ELISAs were performed by coating plates with purified PR8 viral proteins at 1 μg/ml (a kind gift from T. Randall, Univ. of Rochester, Rochester, NY). Serum samples were diluted in 2-fold serial dilutions in PBS with 10 mg/ml BSA and 0.1% Tween-20 and were added to coated plates and incubated at 37°C. Bound antibody was detected using goat anti-mouse IgG (SouthernBiotech, Birmingham, AL). Following detection, plates were analyzed at OD450.
Quantification of cytokines by ELISA
Lungs were harvested from infected mice at various time points post infection and were homogenized in 1 ml PBS. The concentrations of cytokines and chemokines in lung homogenates were then quantified by conducting experiments with specific ELISA kits (eBioscience, San Diego, CA).
Murine Lung Epithelial Cells
Wild-type and MLK3−/− lung epithelial cells were isolated from the lung as previously described (Wang et al., 2007). Identity was assessed by papanicolaou staining and cultures were typically >92% pure. Cells were maintained in bronchial epithelial cell growth medium (Cambrex) supplemented with 5% charcoal-stripped FBS (Hyclone, Logan, UT) and 10 ng/ml keratinocyte growth factor (Calbiochem) at 37°C in 5% CO2.
Murine Lung Fibroblasts
Wild-type and MLK3−/− lung fibroblasts were prepared from lung tissue explants as previously described (Baglole et al., 2006) and were maintained in minimum essential media (MEM) (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Sigma Aldrich, St. Louis, MO) at 37°C in 5% CO2.
Viability assays
Wild-type and MLK3−/− type II AECs were used by day 7 following isolation. MLFs were seeded at a density of 1 X 105 cells/well one day prior to infection. To infect, both cell types were washed once with PBS and virus in serum-free media was added at a multiplicity of infection of 3 and incubated for 1 hour at 37°C. Following infection, cells were washed three times with PBS and maintained in Dulbecco’s modified Eagles medium (DMEM) with 0.15% bovine serum albumin (BSA). Cell viability was quantified by trypan blue exclusion. Briefly, supernatants from infected MLF were collected and centrifuged. Adherent cells were incubated with trypsin at 37°C followed by the addition of an equal volume of serum containing media. Cell culture pellets and trypsinized monolayers were combined and incubated with trypan blue for five minutes before live/dead counts were performed using a hemocytometer. Additionally, viability was measured by CellTiter-Blue® Cell Viability Assay (Promega, Madison, WI) according to manufacturer’s instruction.
Virus growth in lung fibroblasts and epithelial cells
Wild-type and MLK3−/− MLF and AECs were infected as stated in previous section. Virus titers in supernatants from infected cells were determined using Madin Darby Canine Kidney (MDCK) cells. MDCK cells were grown to confluency in 96-well microtiter plates. Ten-fold serial dilutions of wild-type and MLK3−/− culture supernatants, harvested at various time points following infection, were added to cells. Plates were incubated for 1.5 hours at 37°C before addition of DMEM supplemented with 0.15% BSA and TPCK-treated trypsin. Plates were then incubated for 4 days at 37°C in 5% CO2. Cell supernatants were used at this time to perform a hemagglutination assay with chicken RBCs to determine 50% of tissue culture infective doses (TCID50/ml).
Statistical analysis
Statistical significance was evaluated using the non-parametric Mann-Whitney U test when comparing appropriate groups. A P value of less than 0.05 was considered significant.
Supplementary Material
Profile of T cells specific for immunodominant CD8+ and CD4+ T cell epitopes. at days 6, 8 and 10 post-infection. Mice were infected with 400 EID of influenza A virus strain PR8. Animals were sacrificed at days 6, 8 and 10 post-infection. Cells from the spleen (pooled) and MLN (pooled) were purified using MACS separation. The number of total IFN-γ secreting cells was quantified using ELISPOT analysis with peptides for 2 immuno-dominant epitopes for CD4 and CD8. Depicted is the average number of spots from triplicate wells. Spot forming units is abbreviated as s.f.u. Results shown are representative of two separate experiments.
Acknowledgments
We would like to thank Drs R. Davis, University of Massachusetts Medical School, Worchester, MA, and T. Randall, University of Rochester Medical Center, Rochester, NY for valuable reagents. We are also thankful to Sheila Bello-Irizarry, MS and Drs Jennifer Nayak and Thomas H. Thatcher, University of Rochester Medical Center, NY for excellent technical advice. This work was supported by research grant RO1 HL75432 and HL088325 (to PJS); RO1 HL083761 (to TWW); NS054578 and MH64570 (to SBM); HHSN266200700008C (to AJS, TT, DJT). EAD is supported by NIH Training Grant T32 AI007362.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Emily A. Desmet, Email: Emily_desmet@urmc.rochester.edu.
Joseph A. Hollenbaugh, Email: joseph_hollenbaugh@urmc.rochester.edu.
Patricia J. Sime, Email: patricia_sime@urmc.rochester.edu.
Terry W. Wright, Email: terry_wright@urmc.rochester.edu.
David J. Topham, Email: david_topham@urmc.rochester.edu.
Andrea J. Sant, Email: andrea_sant@urmc.rochester.edu.
Toru Takimoto, Email: toru_takimoto@urmc.rochester.edu.
Stephen Dewhurst, Email: Stephen_dewhurst@urmc.rochester.edu.
Sanjay B. Maggirwar, Email: sanjay_maggirwar@urmc.rochester.edu.
References
- Allan W, Tabi Z, Cleary A, Doherty PC. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990;144(10):3980–6. [PubMed] [Google Scholar]
- Baglole CJ, Bushinsky SM, Garcia TM, Kode A, Rahman I, Sime PJ, Phipps RP. Differential induction of apoptosis by cigarette smoke extract in primary human lung fibroblast strains: implications for emphysema. Am J Physiol Lung Cell Mol Physiol. 2006;291(1):L19–29. doi: 10.1152/ajplung.00306.2005. [DOI] [PubMed] [Google Scholar]
- Baskin CR, Bielefeldt-Ohmann H, Tumpey TM, Sabourin PJ, Long JP, Garcia-Sastre A, Tolnay AE, Albrecht R, Pyles JA, Olson PH, Aicher LD, Rosenzweig ER, Murali-Krishna K, Clark EA, Kotur MS, Fornek JL, Proll S, Palermo RE, Sabourin CL, Katze MG. Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proc Natl Acad Sci U S A. 2009;106(9):3455–60. doi: 10.1073/pnas.0813234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancho D, Ventura JJ, Jaeschke A, Doran B, Flavell RA, Davis RJ. Role of MLK3 in the regulation of mitogen-activated protein kinase signaling cascades. Mol Cell Biol. 2005;25(9):3670–81. doi: 10.1128/MCB.25.9.3670-3681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon EW, Morris SJ, Sweet C. Role of apoptosis and cytokines in influenza virus morbidity. FEMS Microbiol Rev. 2005;29(4):837–50. doi: 10.1016/j.femsre.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Chadee DN, Kyriakis JM. MLK3 is required for mitogen activation of B-Raf, ERK and cell proliferation. Nat Cell Biol. 2004;6(8):770–6. doi: 10.1038/ncb1152. [DOI] [PubMed] [Google Scholar]
- Cilloniz C, Shinya K, Peng X, Korth MJ, Proll SC, Aicher LD, Carter VS, Chang JH, Kobasa D, Feldmann F, Strong JE, Feldmann H, Kawaoka Y, Katze MG. Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes. PLoS Pathog. 2009;5(10):e1000604. doi: 10.1371/journal.ppat.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conenello GM, Palese P. Influenza A virus PB1-F2: a small protein with a big punch. Cell Host Microbe. 2007;2(4):207–9. doi: 10.1016/j.chom.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Conze D, Lumsden J, Enslen H, Davis RJ, Le Gros G, Rincon M. Activation of p38 MAP kinase in T cells facilitates the immune response to the influenza virus. Mol Immunol. 2000;37(9):503–13. doi: 10.1016/s0161-5890(00)00078-x. [DOI] [PubMed] [Google Scholar]
- Dong C, Yang DD, Tournier C, Whitmarsh AJ, Xu J, Davis RJ, Flavell RA. JNK is required for effector T-cell function but not for T-cell activation. Nature. 2000;405(6782):91–4. doi: 10.1038/35011091. [DOI] [PubMed] [Google Scholar]
- Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol. 2002;3(9):663–72. doi: 10.1038/nrm906. [DOI] [PubMed] [Google Scholar]
- Handley ME, Rasaiyaah J, Barnett J, Thakker M, Pollara G, Katz DR, Chain BM. Expression and function of mixed lineage kinases in dendritic cells. Int Immunol. 2007a;19(8):923–33. doi: 10.1093/intimm/dxm050. [DOI] [PubMed] [Google Scholar]
- Handley ME, Rasaiyaah J, Chain BM, Katz DR. Mixed lineage kinases (MLKs): a role in dendritic cells, inflammation and immunity? Int J Exp Pathol. 2007b;88(2):111–26. doi: 10.1111/j.1365-2613.2007.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong HY, Kim BC. Mixed lineage kinase 3 connects reactive oxygen species to c-Jun NH2-terminal kinase-induced mitochondrial apoptosis in genipin-treated PC3 human prostate cancer cells. Biochem Biophys Res Commun. 2007;362(2):307–12. doi: 10.1016/j.bbrc.2007.07.165. [DOI] [PubMed] [Google Scholar]
- Kash JC, Tumpey TM, Proll SC, Carter V, Perwitasari O, Thomas MJ, Basler CF, Palese P, Taubenberger JK, Garcia-Sastre A, Swayne DE, Katze MG. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006;443(7111):578–81. doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Kim BC, Xu Z, Kim SJ. Mixed lineage kinase 3 (MLK3)-activated p38 MAP kinase mediates transforming growth factor-beta-induced apoptosis in hepatoma cells. J Biol Chem. 2004;279(28):29478–84. doi: 10.1074/jbc.M313947200. [DOI] [PubMed] [Google Scholar]
- Kobasa D, Takada A, Shinya K, Hatta M, Halfmann P, Theriault S, Suzuki H, Nishimura H, Mitamura K, Sugaya N, Usui T, Murata T, Maeda Y, Watanabe S, Suresh M, Suzuki T, Suzuki Y, Feldmann H, Kawaoka Y. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature. 2004;431(7009):703–7. doi: 10.1038/nature02951. [DOI] [PubMed] [Google Scholar]
- Korteweg C, Gu J. Pathology, molecular biology, and pathogenesis of avian influenza A (H5N1) infection in humans. Am J Pathol. 2008;172(5):1155–70. doi: 10.2353/ajpath.2008.070791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujime K, Hashimoto S, Gon Y, Shimizu K, Horie T. p38 mitogen-activated protein kinase and c-jun-NH2-terminal kinase regulate RANTES production by influenza virus-infected human bronchial epithelial cells. J Immunol. 2000;164(6):3222–8. doi: 10.4049/jimmunol.164.6.3222. [DOI] [PubMed] [Google Scholar]
- Lazarski CA, Chaves FA, Jenks SA, Wu S, Richards KA, Weaver JM, Sant AJ. The kinetic stability of MHC class II:peptide complexes is a key parameter that dictates immunodominance. Immunity. 2005;23(1):29–40. doi: 10.1016/j.immuni.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Lee BO, Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Makris M, Sprague F, Lund FE, Randall TD. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J Immunol. 2005;175(9):5827–38. doi: 10.4049/jimmunol.175.9.5827. [DOI] [PubMed] [Google Scholar]
- Ludwig S, Ehrhardt C, Neumeier ER, Kracht M, Rapp UR, Pleschka S. Influenza virus-induced AP-1-dependent gene expression requires activation of the JNK signaling pathway. J Biol Chem. 2001;276(24):10990–8. [PubMed] [Google Scholar]
- Ludwig S, Pleschka S, Planz O, Wolff T. Ringing the alarm bells: signalling and apoptosis in influenza virus infected cells. Cell Microbiol. 2006;8(3):375–86. doi: 10.1111/j.1462-5822.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- Maroney AC, Finn JP, Connors TJ, Durkin JT, Angeles T, Gessner G, Xu Z, Meyer SL, Savage MJ, Greene LA, Scott RW, Vaught JL. Cep-1347 (KT7515), a semisynthetic inhibitor of the mixed lineage kinase family. J Biol Chem. 2001;276(27):25302–8. doi: 10.1074/jbc.M011601200. [DOI] [PubMed] [Google Scholar]
- Merritt C, Enslen H, Diehl N, Conze D, Davis RJ, Rincon M. Activation of p38 mitogen-activated protein kinase in vivo selectively induces apoptosis of CD8(+) but not CD4(+) T cells. Mol Cell Biol. 2000;20(3):936–46. doi: 10.1128/mcb.20.3.936-946.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- Mota M, Reeder M, Chernoff J, Bazenet CE. Evidence for a role of mixed lineage kinases in neuronal apoptosis. J Neurosci. 2001;21(14):4949–57. doi: 10.1523/JNEUROSCI.21-14-04949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4(8):e1000115. doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakos NK, Klein I, Richter MV, Zaiss DM, Giannandrea M, Crispe IN, Topham DJ. Early intrahepatic accumulation of CD8+ T cells provides a source of effectors for nonhepatic immune responses. J Immunol. 2007;179(1):201–10. doi: 10.4049/jimmunol.179.1.201. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method for estimating fifty percent endpoints. Amer J Hyg. 1938;27(3):493–497. [Google Scholar]
- Richards KA, Chaves FA, Krafcik FR, Topham DJ, Lazarski CA, Sant AJ. Direct ex vivo analyses of HLA-DR1 transgenic mice reveal an exceptionally broad pattern of immunodominance in the primary HLA-DR1-restricted CD4 T-cell response to influenza virus hemagglutinin. J Virol. 2007;81(14):7608–19. doi: 10.1128/JVI.02834-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards KA, Chaves FA, Sant AJ. Infection of HLA-DR1 transgenic mice with a human isolate of influenza a virus (H1N1) primes a diverse CD4 T-cell repertoire that includes CD4 T cells with heterosubtypic cross-reactivity to avian (H5N1) influenza virus. J Virol. 2009;83(13):6566–77. doi: 10.1128/JVI.00302-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon M, Enslen H, Raingeaud J, Recht M, Zapton T, Su MS, Penix LA, Davis RJ, Flavell RA. Interferon-gamma expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. EMBO J. 1998;17(10):2817–29. doi: 10.1093/emboj/17.10.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabapathy K, Kallunki T, David JP, Graef I, Karin M, Wagner EF. c-Jun NH2-terminal kinase (JNK)1 and JNK2 have similar and stage-dependent roles in regulating T cell apoptosis and proliferation. J Exp Med. 2001;193(3):317–28. doi: 10.1084/jem.193.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster MY, Riberdy JM, Gonzalez M, Topham DJ, Baumgarth N, Doherty PC. An early CD4+ T cell-dependent immunoglobulin A response to influenza infection in the absence of key cognate T-B interactions. J Exp Med. 2003;198(7):1011–21. doi: 10.1084/jem.20021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Z, Fan S, Sniderhan L, Reisinger E, Litzburg A, Schifitto G, Gelbard HA, Dewhurst S, Maggirwar SB. Inhibition of mixed lineage kinase 3 prevents HIV-1 Tat-mediated neurotoxicity and monocyte activation. J Immunol. 2006;177(1):702–11. doi: 10.4049/jimmunol.177.1.702. [DOI] [PubMed] [Google Scholar]
- Svitek N, Rudd PA, Obojes K, Pillet S, von Messling V. Severe seasonal influenza in ferrets correlates with reduced interferon and increased IL-6 induction. Virology. 2008;376(1):53–9. doi: 10.1016/j.virol.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Tibbles LA, Ing YL, Kiefer F, Chan J, Iscove N, Woodgett JR, Lassam NJ. MLK-3 activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6. EMBO J. 1996;15(24):7026–35. [PMC free article] [PubMed] [Google Scholar]
- Wang J, Gigliotti F, Bhagwat SP, Maggirwar SB, Wright TW. Pneumocystis stimulates MCP-1 production by alveolar epithelial cells through a JNK-dependent mechanism. Am J Physiol Lung Cell Mol Physiol. 2007;292(6):L1495–505. doi: 10.1152/ajplung.00452.2006. [DOI] [PubMed] [Google Scholar]
- Wells MA, Albrecht P, Ennis FA. Recovery from a viral respiratory infection. I. Influenza pneumonia in normal and T-deficient mice. J Immunol. 1981;126(3):1036–41. [PubMed] [Google Scholar]
- Xu Z, Maroney AC, Dobrzanski P, Kukekov NV, Greene LA. The MLK family mediates c-Jun N-terminal kinase activation in neuronal apoptosis. Mol Cell Biol. 2001;21(14):4713–24. doi: 10.1128/MCB.21.14.4713-4724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BM, Hoffmann FM. Inhibition of transforming growth factor-beta1-induced signaling and epithelial-to-mesenchymal transition by the Smad-binding peptide aptamer Trx-SARA. Mol Biol Cell. 2006;17(9):3819–31. doi: 10.1091/mbc.E05-10-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Pei DS, Zhang QG, Zhang GY. Down-regulation Cdc42 attenuates neuronal apoptosis through inhibiting MLK3/JNK3 cascade during ischemic reperfusion in rat hippocampus. Cell Signal. 2007;19(4):831–43. doi: 10.1016/j.cellsig.2006.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Profile of T cells specific for immunodominant CD8+ and CD4+ T cell epitopes. at days 6, 8 and 10 post-infection. Mice were infected with 400 EID of influenza A virus strain PR8. Animals were sacrificed at days 6, 8 and 10 post-infection. Cells from the spleen (pooled) and MLN (pooled) were purified using MACS separation. The number of total IFN-γ secreting cells was quantified using ELISPOT analysis with peptides for 2 immuno-dominant epitopes for CD4 and CD8. Depicted is the average number of spots from triplicate wells. Spot forming units is abbreviated as s.f.u. Results shown are representative of two separate experiments.