Abstract
Human induced pluripotent stem (iPS) cells hold great promise for therapy of a number of degenerative diseases such as ischemic heart failure, Parkinson’s disease, Alzheimer’s disease, diabetes mellitus, sickle cell anemia and Huntington disease. They also have the potential to accelerate drug discovery in 3 ways. The first involves the delineation of chemical components for efficient reprogramming of patient’s blood cells or cells from biopsies, obviating the need for cellular delivery of reprogramming exogenous transgenes, thereby converting hope into reality for patients suffering from degenerative diseases. Patients worldwide stand to benefit from the clinical applicability of iPS cell-based cell replacement therapy for a number of degenerative diseases. The second is the potential for discovering novel drugs in a high throughput manner using patient-specific iPS cell-derived somatic cells possessing the etiology of the specific disease. The third is their suitability for toxicological testing of drugs and environmental factors. This review focuses on these potential applications of iPS cells with special emphasis on recent updates of iPS cell research contributing to the accelerated drug discovery.
INTRODUCTION
The landmark discovery that lineage-restricted somatic cells can be reprogrammed directly to a state of pluripotency has opened a new frontier in the field of regenerative medicine and drug discovery. Induced pluripotent stem (iPS) cells, as they were termed by Shinya Yamanaka, have now been derived from mouse and human somatic cells through the ectopic forced expression of OCT4 and SOX2 with either the combinations of KLF4 and MYC or NANOG and LIN28 [1–3]. iPS cells resemble pluripotent embryonic stem (ES) cells in morphology, proliferation, surface antigens, gene expression, epigenetic status of pluripotent cell-specific genes, telomerase activity and their potential to differentiate into a spectrum of adult somatic cell types. The revolutionary facets of iPS involve their ability to bypass the limitations of immune rejection in existing stem cell therapy approaches unlike the ES cells. The iPS cell discovery is less than 3 years old, yet iPS cell hold great promise for both basic research and therapeutic applications.
A major challenge for experimental research of human disease and drug discovery is the use of biologically relevant methods of investigation. To this end, animal modeling has been a mainstay of the drug development pipeline, with mice frequently used in pharmaceutical research and development (R&D) as a nonclinical efficacy model. There are many potential causes for the failed translation of drug trials from animal models to humans, including species differences in drug penetration of the blood–brain barrier, drug metabolism, and related toxicity, culminating in a variable biological response. In addition, there is the more contentious matter of less than optimal design rigor of testing regimes [4]. Overall, less than 10% of compounds that enter clinical phase testing are approved for market, at an estimated cost of US$1.2–1.7 billion per drug [5,6]. The high failure rate is reflected by the number of new drugs approved for use in the category of neurology by the US Food and Drug Administration (FDA) in 2006, 2007, and 2008 with one, four, and one drugs approved respectively [4].The human organ systems are difficult to study due to its anatomical and functional complexity, compounded by the limitations and/or cost of live animal models, and the constraints for researching human subjects [4]. The human organs include organ/tissue specific cell types which in turn comprise subtypes of cells with specific phenotypes, localizations, and functions. Indeed, different cell-types contribute to different disease states requiring cell-type specific modeling of disease-specific phenotypes and pharmacologically relevant strategies for drug screening. To this end, human iPS cell based models of organ development, function, and disease represent a useful research tool to complement in vivo experimentation to increase productivity and decrease the cost of drug development using strategies that concomitantly bolster innovation and facilitate R&D for early assurance of drug safety and efficacy.
Candidacy of iPS Cells as as a Promising Model for Accelerated Drug Discovery in Pharmaceutical Industry
iPS cells possess 2 important characteristics like any embryonic stem cells- the pluripotency and their capacity to proliferate indefinitely in culture in vitro. iPS cells can give rise to any cell type of human body tissues including cardiomyocytes, neuronal cells and insulin producing beta cells of the islets of Langerhans. Thus iPS cell derived cells are clinically relevant and hold greater potential for cell replacement therapy of heart failure, Parkinson’s and Alzheimer’s disease as well as diabetes. Since any phenotypic cells can be derived from iPS cells and iPS cells can be derived from any patient in unlimited quantities for high throughput assays, iPS cells are considered to be the promising model for accelerated drug discovery. More notably, the iPS cells from the patients serve as a true in vitro model of that particular disease and would reflect the same pathological features as in in vivo conditions reflecting the diseased phenotype obviating the need for any conventional animal model. The unlimited supply, true model of diseased pheno-typic cells of interest from human samples combined with the recent high-throughput technologies will be the added advantage in discovery of novel drugs.
iPS CELL DERIVATION
Reprogramming Factors
iPS cells have now been derived from mouse and human somatic cells through the ectopic forced expression of OCT4 and SOX2 with either the combinations of KLF4 and MYC or NANOG and LIN28 [1–3] (Fig. 1)). Oct4 expression is essential for the development of the inner cell mass (ICM) in vivo, the derivation of ES cells and the maintenance of a pluripotent state [7]. Sox2 has an important role in maintaining ES cell pluripotency and heterodimerizes in a complex with Oct4. In addition to ES cells, Sox2 is expressed in the extra-embryonic ectoderm, trophoblast stem cells and the developing central nervous system. In these cell lineages, Sox2 expression is restricted to cells with stem cell characteristics supporting their self-renewal capability and is no longer expressed in cells with a more restricted developmental potential [8]. c-Myc is a pleiotropic transcription factor that has been linked to several cellular functions, including cell-cycle regulation, proliferation, growth, differentiation and metabolism [9]. c-Myc also functions during both self-renewal and the differentiation of stem and progenitor cells, particularly in interactions between stem cells and the local microenvironment [10]. Krueppel-like factor 4 (Klf4) is a transcription factor expressed in a variety of tissues, including the epithelium of the intestine, kidney and the skin. It has been demonstrated that the forced overexpression of Klf4 in ES cells inhibits differentiation in erythroid progenitors, suggesting a role for this factor in ES-cell function [11]. Nanog is a transcription factor that was involved in maintaining ES-cell self-renewal and pluripotency. Loss of Nanog predisposes ES cells to differentiation but does not mark commitment and is reversible [12]. Lin-28 (also known as zinc finger CCHC domain-containing protein 1) is expressed in ES cells and during early embryogenesis but its expression becomes restricted to several tissues during late embryogenesis and adult life. Several groups have demonstrated that Lin-28 operates as a ‘translational enhancer’ in embryonic and adult cells. It can increase the stability of specific mRNAs and contribute to the identity establishment of the tissue in which it is expressed [13].
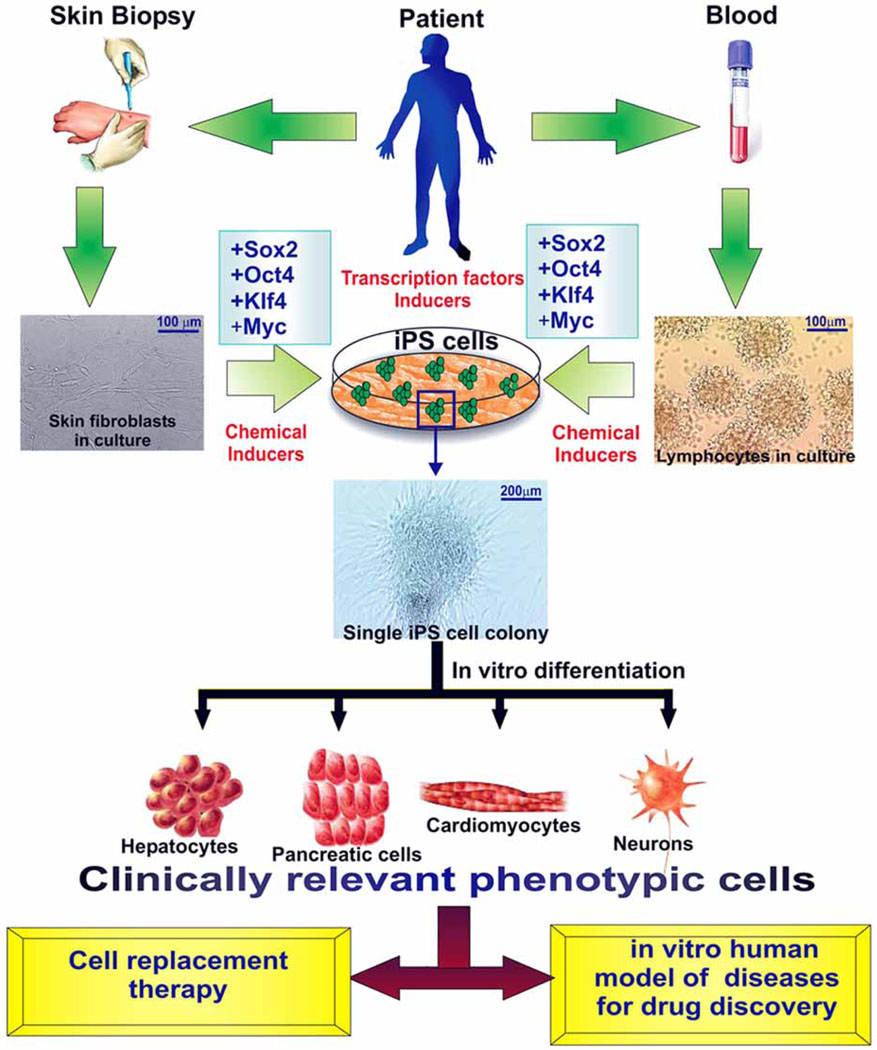
Fig. (1).
Scheme of derivation of iPS cells via reprogramming of adult somatic cell types and possible therapeutically applications.
Source Tissues for Human iPS Derivation and Delivery of Reprogramming Factors
Current reprogramming strategies involve retroviral, lentiviral, adenoviral and plasmid transfection to deliver reprogramming factor transgenes [1,14–16]. In humans, iPS cells are commonly generated from dermal fibroblasts and recently from human keratinocytes isolated from plucked hair and also from mobilized human CD34+ peripheral blood cells [17,18]. However, it remains unclear whether hair cells will be a faithful source for reprogramming since the growth and quality of the hair follicles are dependent on the age, genotype, and the medical conditions of the human donors. Blood cells represent a source of cells that obviate the need for skin biopsies, and require minimal maintenance in culture prior to reprogramming. Reprogramming from human blood cells represents a novel way of establishing iPS cells. The ability to reprogram cells from the human blood will facilitate the development of reliable method to generate patient-specific stem cells. Recently, it has been shown that fusion of a poly-arginine (i.e.,11R) protein transduction domain to the C terminus of four reprogramming factors: Oct4, Sox2, Klf4, and c-Myc enbables derivation of iPS from the somatic cells in combination with with a small molecule called valproic acid (VPA) by DNA-free method [19]. In this context, iPS derivation by small molecules opens a new field in pharmaceutical industry as seen in the recent report that a specific glycogen synthase kinase 3 (GSK-3) inhibitor, CHIR99021, can induce the reprogramming of mouse embryonic fibroblasts (MEFs) transduced by only Oct4 and Klf4 two factors. When combined with Parnate (also named tranylcypromine), an inhibitor of lysine-specific demethylase 1, CHIR99021 can result in the reprogramming of human primary keratinocyte transducted with Oct4 and Klf4 two factors [20].
The technology to generate iPS cells is rapidly evolving and overcoming some of the challenges associated with the first generation iPS cells used in this study. iPS cells generated with adenovirus or plasmid mediated transfections could avoid the potential problems associated with viral integration of transgenes. Small molecules have been used to increase the efficiency of generating iPS cell lines. Given the pace of progress, it seems likely that techniques to generate human iPS cells will continue to rapidly improve. In vitro differentiation studies of various human iPS cell lines have identified derivatives of the 3 primary germ layers-ecto, meso and endoderms [2,21].
THERAPEUTIC CLINICAL APPLICATION OF iPS CELLS
iPS cells have a developmental potency comparable to ES cells in their ability to generate all lineages of the embryo and to contribute to chimera formation. Furthermore, iPS cells have already been differentiated into various functional clinically relevant cell phenotypes such as neurons, cardiomyocytes and hematopoeitic cells [22–24]. Various types of somatic cells derived from pluripotent stem cells could be used in regenerative medicine to repair tissues damaged through disease or injury [25] (Fig. 2).
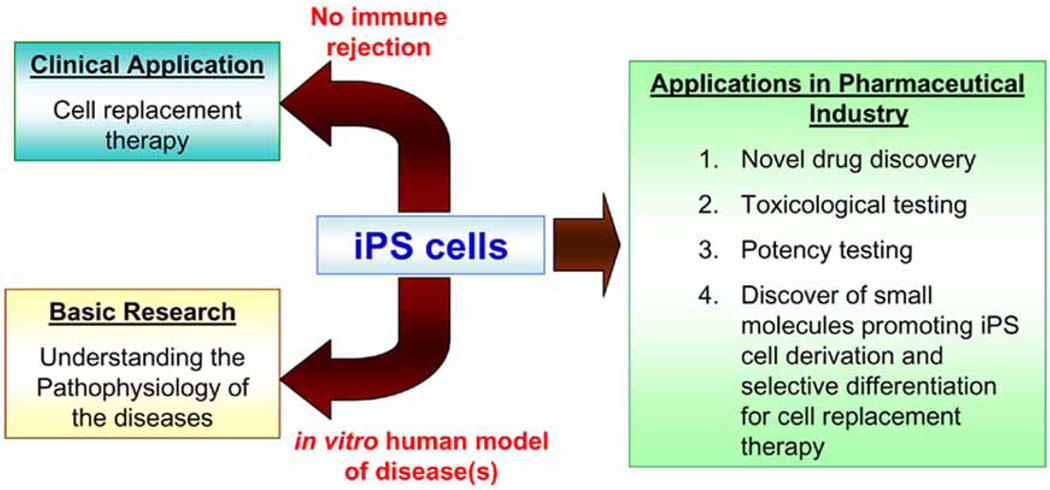
Fig. (2).
Scheme of possible therapeutical application of iPS cells.
Immunological Safety Aspects of iPS Cell-Derived Cells
It has been reported that human ES cell derived cell phenotypes such as cardiomyocytes do express MHC class I molecule although at low levels and expression increased upon differentiation in vitro [26]. Hence, these cells will certainly evoke an immune response in the host upon transplantation. But the iPS cell derived are patient specific and syngeneic, it raises the hope that on one fine day, patient-specific iPS cells will overcome the problem of immune rejection of transplanted cardiomyocytes and this will revolutionize the stem cell -based cell replacement therapy [25].
Monogenic and Polygenic Diseases
Creation of iPS cell lines from patients with single-gene disorders allows experiments on disease phenotypes in vitro, and an opportunity to repair gene defects ex vivo. The resulting cells, by virtue of their immortal growth in culture, can be extensively characterized to ensure that gene repair is precise and specific, thereby reducing the safety concerns of random, viral-mediated gene therapy. Repair of gene defects in pluripotent cells provides a common platform for combined gene repair and cell replacement therapy [25,27] for a variety of genetic disorders, as long as the pluripotent cells can be differentiated into relevant somatic stem cell or tissue populations. The generation of iPS cells from patients with a variety of genetic diseases with either Mendelian or complex inheritance has been described. These diseases include adenosine deaminase deficiency-related severe combined immunodeficiency (ADA-SCID), Shwachman-Bodian-Diamond syndrome (SBDS), Gaucher disease (GD) type III, Duchenne (DMD) and Becker muscular dystrophy (BMD), Parkinson disease (PD), Huntington disease (HD), juvenile-onset, type 1 diabetes mellitus (JDM), Down syndrome (DS)/trisomy 21, and the carrier state of Lesch-Nyhan syndrome. Such disease-specific stem cells offer an unprecedented opportunity to recapitulate both normal and pathologic human tissue formation in vitro, thereby enabling disease investigation and drug development [28].
Neurodegenerative Disorders
Patient-specific fibroblasts offer a unique opportunity for studying and modeling the effects of specific gene defects on human neuronal development in vitro and for testing small molecules or other potential therapies for the relevant neurogenetic disorders. Parkinsons Disease (PD) is the second most common chronic progressive neurodegenerative disorder and is characterized primarily by major loss of nigrostriatal dopaminergic neurons. The majority of cases are sporadic, not linked to a known genetic mutation, and likely the result of complex interactions between genetic and environmental factors [29]. One of the major reasons for the lack of understanding of the underlying pathophysiology of PD is the paucity of reliable experimental models that recapitulate all features of the human disease. The derivation of PD patient-specific iPS cells and subsequent differentiation into dopaminergic neurons would provide patient-specific in vitro models that are otherwise experimentally not accessible. Recently it has been shown that fibroblasts from five patients with sporadic PD could be efficiently reprogrammed and demonstrated that these patient-derived iPS cells could be subsequently differentiated in vitro into dopaminergic neurons [30]. Alzheimer's disease is characterized by degeneration and dysfunction of synapses and neurons in brain regions critical for learning and memory functions. The endogenous generation of new neurons in certain regions of the mature brain, derived from primitive cells termed neural stem cells, has raised hope that neural stem cells may be recruited for structural brain repair. Stem cell therapy has been suggested as a possible strategy for replacing damaged circuitry and restoring learning and memory abilities in patients with Alzheimer's disease [31].
Degenerative Cardiac Diseases
Cardiovascular disease is the number one cause of death globally and is projected to remain the leading cause of death. Since adult cardiomyocytes have a very limited regenerative capacity, their loss permanently compromises myocardial contractile function. Heart failure is characterized by the loss of functional cardiomyocytes and thereby its inability to pump enough blood to maintain physiological functions. Heart transplantation is currently the last resort for end-stage heart failure, but is hampered by a severe shortage of donor organs and immune rejection. Cell replacement therapy is emerging as an innovative approach for the treatment of degenerative cardiac diseases, and pluripotent stem cells appear to be an ideal source of cells in this approach. In particular, human iPS cell-derived cardiomyocytes theoretically fulfill most, if not all, of the properties of an ideal donor cell, but several critical obstacles need to be overcome [25]. iPS cell based cell replacement therapy is currently generating a great deal of interest in the treatment of ischemic heart diseases since iPS cells are capable of differentiating into patient-specific functional cardiomyocytes. The replacement of akinetic scar tissue by viable myocardium should improve cardiac function, impede progressive left ventricular remodeling, and revascularize ischemic areas [32]. But still, much has to be studied about the formation of electrical coupling between endogenous and transplanted cardiomyocytes.
Diabetic Diseases
Type 1 diabetes is characterized by the selective destruction of pancreatic beta-cells caused by an autoimmune attack. Type 2 diabetes is a more complex pathology which, in addition to beta-cell loss caused by apoptotic programs, includes beta-cell dedifferentiation and peripheric insulin resistance. beta-Cells are responsible for insulin production, storage and secretion in accordance to the demanding concentrations of glucose and fatty acids. The absence of insulin results in death and therefore diabetic patients require daily injections of the hormone for survival. However, they cannot avoid the appearance of secondary complications affecting the peripheral nerves as well as the eyes, kidneys and cardiovascular system. These afflictions are caused by the fact that external insulin injection does not mimic the tight control that pancreatic-derived insulin secretion exerts on the body's glycemia. Restoration of damaged beta-cells by transplantation from exogenous sources or by endocrine pancreas re-generation would be ideal therapeutic options [33]. Like the human ES (hES) cells, iPS cells are also shown to differentiate into mature insulin-producing cells in a chemical-defined culture system [34].
iPS CELLS IN PHARMACEUTICAL INDUSTRY
iPS cells augment pharmaceutical industry by making easier or possible the creation of “human disease models” and enabling the high throughput assays such as automated robots controlled cell culture processing systems and large scale microarray profiling making an insurmountable progress in pharmaceutical arena. In recent years, global microarray data analyses of the diseased phenotypic cells versus normal phenotypic cells reveal the disease-perturbed and drug-affected regulatory gene networks in comparison to normal ones thereby serving as an invaluable tool for drug discovery in the pharmaceutical industry [35,36].
Small Molecules Promoting iPS Derivation (Derivation of Chemically Induced Pluripotent Stem Cells)
Ectopic expression of defined transcription factors can reprogram somatic cells to iPS cells, but the utility of iPS cells is hampered by the use of transgenes (Oct4, Sox2, Klf4 and c-Myc) delivery. Small molecules offer an alternative to replace reprogramming transgenes with chemical signaling cues responsible for reprogramming. For example, kenpaullone, in lieu of Klf4 gave rise to iPS cells that are indistinguishable from murine embryonic stem cells [37]. Moving toward the eventual goal of clinical application, it is necessary to overcome limitations such as low reprogramming efficiency and genomic alterations due to viral integration. Since these factors, Oct4/Sox2/Myc/Klf4, represent definable signaling pathways, it is rational to propose a chemical approach to switch on these pathways for iPS reprogramming [38]. To this end, the nascent discipline of chemical biology may adopt iPS as a worthy field and provide small-molecule tools to regulate the iPS process. One can speculate that somatic cells may eventually be reprogrammed chemically to a pluripotent state. These chemically induced pluripotent stem cells (CiPS) cells could be suitable for human therapeutic applications in the future.
Accelerated Novel Drug Discovery
iPS cells could also boost the efficiency of drug discovery efforts if iPS technology can help researchers to identify drugs that are only effective against diseased cells with particular genetic profiles. iPS cells enable the creation of “human disease models” since iPS cells can be derived from the afflicted patient directly with relative easiness. Also, iPS cells provide added advantage that high throughput screening of drugs is possible. Part of the challenge in using iPS cells for drug screens is producing large numbers of identical cells that behave in a consistent way.
Toxicological Testing
A major bottleneck in the drug development process is toxicological testing. In recent years, stem cells have generated much interest as a potential tool for pharmacological and toxicology screening, due to various shortcomings of currently utilized assay models based on established cell lines, primary explanted somatic cells and laboratory animals. The most commonly utilized established cell lines for toxicological testing are of cancerous/tumorigenic origin, that are highly adapted to in vitro culture conditions after countless passages, and which contain chromosomal and genetic aberrations that render them immortal. Such inherent deficiencies make them non-representative of how a normal cell behaves physiologically in vivo. The primary explanted cultures of somatic cells used for toxicology screening are heterogeneous cultures that display a high degree of inter-batch variability, making it challenging to obtain consistent and reproducible results in toxicology screening. Live animal models used for toxicity assays have a number of inherent flaws. 1) An animal model may not compare well with human physiology. 2) The use of live animals in routine toxicology screening of biomedical and cosmetic products may be ethically contentious, and can possibly affect consumer confidence. 3) Live animals are expensive to purchase and maintain compared to in vitro cultured cells. Traditional in vivo tests performed in animals are difficult to automate. Of greater promise in the drug discovery field is the use of iPS cells for toxicology testing. Since iPS cell lines can be isolated from a diverse range of human individuals with well-characterized adult phenotypes and can be used on high throughput assay set-ups, these can potentially provide a valuable tool for characterizing how variation in toxic susceptibility displayed by different individuals correlates to their genetics, disease state and other observable phenotype [39].
POTENTIAL PROBLEMS ASSOCIATED WITH CLINICAL APPLICABILITY OF iPS CELLS
The iPS cell technology potentially could overcome two important obstacles associated with hES cells: immune rejection after transplantation and ethical concerns regarding the use of human embryos. However, the clinical application of iPS cells also faces many obstacles, some shared with ES cells and others that are unique. The first common obstacle is teratoma formation.
Transgene Free iPS Cells
Reprogramming of both mouse and human somatic cells into iPS cells has been achieved by expressing combinations of factors such as OCT4, SOX2, c-Myc, KLF4, NANOG and LIN28. Initial methods used to derive human iPS cells used viral vectors, in which both the vector backbone and transgenes are permanently integrated into the genome. Such vectors can produce insertional mutations that interfere with the normal function of iPS cell derivatives, and residual transgene expression can influence differentiation into specific lineages [1,2] or even result in tumorigenesis [1,2,40]. Vector integration–free mouse iPS cells have been derived from liver cells with adenoviral vectors and from embryonic fibroblasts with repeated plasmid transfections but the low frequencies obtained make it unclear how practical these approaches will be for human cells, which generally require longer exposure to reprogramming factors [15,16]. To this end, recently two alternative approaches were described to remove transgenes from mouse or human iPS cells. In one approach, Cre/LoxP recombination was used to excise integrated transgenes [30,41]. This approach successfully removes transgene sequences, but leaves behind residual vector sequences, which can still create insertional mutations. A second approach used seamless excision of piggyBac transposons to produce vector- and transgene-free mouse iPS cells [42]. Although a promising approach, vector removal from human iPS cells produced by this method has not yet been reported, and removing multiple transposons is labor intensive. More recently, Thomson group has reported another approach where human iPS cells completely free of vector and transgene sequences can be derived from fibroblasts by a single transfection with oriP/EBNA1 (Epstein-Barr nuclear antigen-1)–based episomal vectors [43]. The oriP/EBNA1 vectors are well suited for introducing reprogramming factors into human somatic cells, as these plasmids can be transfected without the need for viral packaging and can be subsequently removed from cells by culturing in the absence of drug selection. However, it will be essential to determine which of these methods most consistently produces iPS cells with the fewest genetic or epigenetic abnormalities, because any abnormalities would affect the application of these cells in basic research, drug development, and transplantation therapies much more than the initial reprogramming frequencies. Substantial challenges also remain in cell-specific differentiation and delivery, but the derivation of vector- and transgene-free human iPS cells is nonetheless an important advance toward the clinical application of these cells [43]. Also, there is a possibility that chemically induced pluripotent stem cells will be ideally the last resort in the near future.
Tumorigenicity
The links between pluripotency and tumorigenicity are exemplified by the fact that many of the genes used to produce iPS cells are either outright established oncogenes such as Myc and KLF4 [44,45] or are in various ways linked to tumorigenesis such as Sox2 [46], Nanog [47] and Oct3/4 [48]. It is remarkable that, to date, one of the most common assays for demonstrating and studying the pluripotency of stem cells, including iPS cells, is the teratoma assay. Often this is referred to as a pluripotency assay, but of course it is also a tumor assay. The fact that a key assay of “stemness” is also a tumor assay illustrates the strong link between stem and tumor cells, a reality too rarely discussed in the field when interpreting results from teratoma assays. Even ignoring for the moment the ability of ES cells and iPS cells to produce malignant tumors in some cases [40,49], the production of benign teratoma as a side effect in humans given a hypothetical regenerative medicine therapy in the future, would be unacceptable. Such tumors could be numerous and would prove highly destructive to surrounding normal or regenerating tissue. Thus, a key concept is that stem cells, even those with potent self-renewal and pluripotency, will almost certainly never be directly used in regenerative medicine if they cannot be proven to lack the ability to cause teratoma in mice [50]. Teratoma is not the only concern as hES cells can also form malignant tumors. A recent study found robust malignant tumor-inducing capacity of hES cell lines H1 and HSF-6 [49]. iPS cells can also form both teratoma as well as malignant tumors such as neuroblastoma and follicular carcinoma [40]. Thus, the potential risk to human patients from both teratoma and malignant tumors is quite real, yet remains difficult to estimate as no human trials of hES cells or iPS cells have been conducted at this time. The stable genetic introduction of a suicide gene such as thymidine kinase (tk) into stem cells has been reported to be effective in combination with Ganciclovir (Gan) treatment [51]. However, in this study, the treatment was not stem cell specific and would have also killed any differentiated progeny from those stem cells in a hypothetical treatment situation causing it to fail. Differentiated teratoma cells were also readily killed by Gan treatment. Nevertheless, relatively simple modifications, such as using oct4 or nanog promoter driven expression of tk, would make the system more stem cell specific to ideally kill only those iPS cells that have escaped differentiation. Of concern is the fact that it remains unknown if all iPS cells express what are thought of as the key stem cell factors such as oct4 and nanog. Although populations of iPS cells do express these seemingly without exception, it is unclear whether small, but functionally relevant subpopulations may not [50]. Also, the major concern with the suicide gene approach is its requirement for genetically modifying the stem cells, which could raise the risk of tumorigenicity from the beginning. The simplest way to slow or even eliminate the tumorigenicity of normal stem cells prior to transplantation may be to take advantage of their natural brakes or pluripotency by partially differentiating them into progenitors. Therefore, a promising proposed method for making stem cell-based regenerative medicine therapies safer may seem paradoxical: to not transplant stem cells at all into patients. This avenue has gained wide acceptance as the most promising approach to regenerative medicine. The idea is to use the stem cells to produce progenitor or precursor cells of the desired lineage and then transplant progenitors purified by sorting or by positive selection with antibiotics [50].
Chromosome Abnormality
Also, the hES cells during long time cultures in vitro develop chromosomal abnormalities. It is anticipated for the same thing to happen with iPS cells during long time culturing. Safety measures need to be formulated to avoid this situation and to abolish the chromosomally aberrant cells, if any, upon transplantation [25].
Need for Xenofree Culture Conditions
The major concern about the use of iPS derived clinically relevant cell phenotypes is the contamination by xenogens. The human iPS cells need to be cultivated on mouse/ human embryonic fibroblasts and with sera obtained from animals like calf and bovine. This poses a major health threat to the human host due the possible exposure to mouse retroviruses and other harmful substances. So, culture of human iPS cells under serum free and feeder (xeno) free conditions is inevitable.
Heterogenous Population
The iPS cells give rise almost all types of tissue specific cells and their subtypes. Refining the protocols to obtain the cell phenotype(s) of interest for cell replacement therapy in purified form will dictate the clinical applicability of iPS cells. This entirely depends on the optimized cell culture conditions for the selective differentiation of the phenotypes of interest and the availability of cell phenotype specific cell surface antigens for FACSorting or magnetic sorting with fluorescent or magnet labeled antibodies.
CONCLUSION AND FUTURE PROSPECTIVE
The potential of iPS cell technology is tremendous, but this technology is still in its infancy. To realize the full application of iPS cells, it will be essential to improve the methodologies for iPS cell generation and to precisely evaluate each clone and subclone of iPS cells for their safety and efficacy [52]. An attractive alternative to iPS cells will be conversion of one somatic cell type directly to another, a process called transdifferentiation [3]. Theoretically fibroblasts which can be easily obtained from the patient’s skin could be converted into cardiomyocytes without passing through the stage of pluripotency. A successful example for transdifferentiation is the conversion of mouse B lymphocytes to macrophages by the transcription factor CEBP [32]. More recently, exocrine pancreas cells were converted into endocrine b-cells in mice in vivo using three pancreatic transcription factors [33]. Thus, transdifferentiation could be used for regeneration in vivo—in contrast to the iPS technology which would represent an uncontrollable risk for the formation of teratomas. However it remains unclear if transdifferentiation is feasible between distantly related cell types (e.g., fibroblasts and cardiomyocytes) or restricted to more related cells. Thus, iPS cells enable the creation of “disease human models” from each of the afflicted patients with almost all diseases, high throughput drug screening, and drug testing thereby enabling acceleration of a “disease” specific drug discovery. In addition, they also offer a great promise to an immunologically safe cell replacement therapy of several degenerative disorders since the patient specific iPS derived transplant cells are syngeneic.
ACKNOWLEDGEMENTS
This work was supported by the grant “Embryonic Stem cell-based Novel Alternative Testing Strategies” (ESNATS), from the European Community within the 7th Framework Program.
REFERENCES
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Vodyanik MA, Smuga-Otto K, ntosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 3.Amabile G, Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends. Mol. Med. 2009;15:59–68. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Crook JM, Kobayashi NR. Human stem cells for modeling neurological disorders: accelerating the drug discovery pipeline. J. Cell Biochem. 2008;105:1361–1366. doi: 10.1002/jcb.21967. [DOI] [PubMed] [Google Scholar]
- 5.Kaitin KI. Obstacles and opportunities in new drug development. Clin. Pharmacol. Ther. 2008;83:210–212. doi: 10.1038/sj.clpt.6100462. [DOI] [PubMed] [Google Scholar]
- 6.Sollano JA, Kirsch JM, Bala MV, Chambers MG, Harpole LH. The economics of drug discovery and the ultimate valuation of pharmacotherapies in the marketplace. Clin. Pharmacol. Ther. 2008;84:263–266. doi: 10.1038/clpt.2008.117. [DOI] [PubMed] [Google Scholar]
- 7.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 8.Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes. Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt EV. The role of c-myc in cellular growth control. Oncogene. 1999;18:2988–2996. doi: 10.1038/sj.onc.1202751. [DOI] [PubMed] [Google Scholar]
- 10.Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov AA, Ko MS, Niwa H. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat. Cell Biol. 2007;9:625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, McClintick J, Zhong L, Edenberg HJ, Yoder MC, Chan RJ. Murine embryonic stem cell differentiation is promoted by SOCS-3 and inhibited by the zinc finger transcription factor Klf4. Blood. 2005;105:635–637. doi: 10.1182/blood-2004-07-2681. [DOI] [PubMed] [Google Scholar]
- 12.Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L, Smith A. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–1234. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- 13.Polesskaya A, Cuvellier S, Naguibneva I, Duquet A, Moss EG, Harel-Bellan A. Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes Dev. 2007;21:1125–1138. doi: 10.1101/gad.415007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brambrink T, Foreman R, Welstead GG, Lengner CJ, Wernig M, Suh H, Jaenisch R. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 17.Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilic J, Pekarik V, Tiscornia G, Edel M, Boue S, Belmonte JC. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 18.Loh YH, Agarwal S, Park IH, Urbach A, Huo H, Heffner GC, Kim K, Miller JD, Daley GQ. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–5479. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, Siuzdak G, Scholer HR, Duan L, Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Zhou H, Abujarour R, Zhu S, Joo JY, Lin T, Hao E, Scholer HR, Hayek A, Ding S. Generation of Human Induced Pluripotent Stem Cells in the Absence of Exogenous Sox2. Stem Cells. 2009 doi: 10.1002/stem.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 23.Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 24.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 25.Doss MX, Sachinidis A, Hescheler J. Human ES cell derived cardiomyocytes for cell replacement therapy: a current update. J. Physiol. 2008;51:226–229. [PubMed] [Google Scholar]
- 26.Drukker M, Katz G, Urbach A, Schuldiner M, Markel G, Itskovitz-Eldor J, Reubinoff B, Mandelboim O, Benvenisty N. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2002;99:9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doss MX, Koehler CI, Gissel C, Hescheler J, Sachinidis A. Embryonic stem cells: a promising tool for cell replacement therapy. J. Cell Mol. Med. 2004;8:465–473. doi: 10.1111/j.1582-4934.2004.tb00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 30.Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, Isacson O, Jaenisch R. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhongling F, Gang Z, Lei Y. Neural stem cells and Alzheimer's disease: challenges and hope. Am. J. Alzheimers Dis. Other Demen. 2009;24:52–57. doi: 10.1177/1533317508327587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lapar DJ, Kron IL, Yang Z. Stem cell therapy for ischemic heart disease: where are we? Curr. Opin. Org. Transplant. 2009;14:79–84. doi: 10.1097/MOT.0b013e328320d2e2. [DOI] [PubMed] [Google Scholar]
- 33.Santana A, Ensenat-Waser R, Arribas MI, Reig JA, Roche E. Insulin-producing cells derived from stem cells: recent progress and future directions. J. Cell Mol. Med. 2006;10:866–883. doi: 10.1111/j.1582-4934.2006.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- 35.Chu LH, Chen BS. Comparisons of robustness and sensitivity between cancer and normal cells by microarray data. Cancer Inform. 2008;6:165–181. doi: 10.4137/cin.s386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li CW, Chu YH, Chen BS. Construction and clarification of dynamic gene regulatory network of cancer cell cycle via microarray data. Cancer Inform. 2007;2:223–241. [PMC free article] [PubMed] [Google Scholar]
- 37.Lyssiotis CA, Foreman RK, Staerk J, Garcia M, Mathur D, Markoulaki S, Hanna J, Lairson LL, Charette BD, Bouchez LC, Bollong M, Kunick C, Brinker A, Cho CY, Schultz PG others. Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4. Proc. Natl. Acad. Sci. USA. 2009;106:8912–8917. doi: 10.1073/pnas.0903860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pei D. The magic continues for the iPS strategy. Cell Res. 2008;18:221–223. doi: 10.1038/cr.2008.21. [DOI] [PubMed] [Google Scholar]
- 39.Heng BC, Richards M, Shu Y, Gribbon P. Induced pluripotent stem cells: a new tool for toxicology screening? Arch. Toxicol. 2009 doi: 10.1007/s00204-009-0414-2. [DOI] [PubMed] [Google Scholar]
- 40.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 41.Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hamalainen R, Cowling R, Wang W, Liu P, Gertsenstein M, Kaji K, Sung HK, Nagy A. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1:39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Knoepfler PS. Why myc? An unexpected ingredient in the stem cell cocktail. Cell Stem Cell. 2008;2:18–21. doi: 10.1016/j.stem.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Shi L, Zhang L, Li R, Liang J, Yu W, Sun L, Yang X, Wang Y, Zhang Y, Shang Y. The molecular mechanism governing the oncogenic potential of SOX2 in breast cancer. J. Biol. Chem. 2008;283:17969–17978. doi: 10.1074/jbc.M802917200. [DOI] [PubMed] [Google Scholar]
- 47.Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, Chou SH, Chien CS, Ku HH, Lo JF. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin. Cancer Res. 2008;14:4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 48.Palma I, Pena RY, Contreras A, Ceballos-Reyes G, Coyote N, Erana L, Kofman-Alfaro S, Queipo G. Participation of OCT3/4 and beta-catenin during dysgenetic gonadal malignant transformation. Cancer Lett. 2008;263:204–211. doi: 10.1016/j.canlet.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 49.Shih CC, Forman SJ, Chu P, Slovak M. Human embryonic stem cells are prone to generate primitive, undifferentiated tumors in engrafted human fetal tissues in severe combined immunodeficient mice. Stem Cells Dev. 2007;16:893–902. doi: 10.1089/scd.2007.0070. [DOI] [PubMed] [Google Scholar]
- 50.Knoepfler PS. Deconstructing stem cell tumorigenicity: a road-map to safe regenerative medicine. Stem Cells. 2009;27:1050–1056. doi: 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuldiner M, Itskovitz-Eldor J, Benvenisty N. Selective ablation of human embryonic stem cells expressing a "suicide" gene. Stem Cells. 2003;21:257–265. doi: 10.1634/stemcells.21-3-257. [DOI] [PubMed] [Google Scholar]
- 52.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]