Abstract
Environmental tobacco smoke (ETS) has been linked to deleterious health effects, particularly pulmonary and cardiac disease; yet, the general public considers ETS benign to brain function in adults. In contrast, epidemiological data have suggested that ETS impacts the brain and potentially modulates neurodegenerative disease. The present study begins to examine yet unknown biochemical effects of ETS on the adult mammalian brain. In the developed animal model, adult male rats were exposed to ETS 3 h a day for 3 weeks. Biochemical data showed altered glial fibrillary acid protein levels as a main treatment effect of ETS, suggestive of reactive astrogliosis. Yet, markers of oxidative and cell stress were unaffected by ETS exposure in the brain regions examined. Increased proteolytic degradation of αII-spectrin by caspase-3 and the dephosphorylation of serine116 on PEA-15 indicated greater apoptotic cell death modulated by the extrinsic pathway in the brains of ETS-exposed animals. Further, β-synuclein was upregulated by ETS, a neuroprotective protein previously reported to exhibit anti-apoptotic and anti-fibrillogenic properties. These findings demonstrate that ETS exposure alters the neuroproteome of the adult rat brain, and suggest modulation of inflammatory and cell death processes.
Keywords: ETS, SHS, Neuroproteomics, Apoptosis, Gliosis, Synuclein
Introduction
Exposure to environmental tobacco smoke (ETS) is a known health risk in adults and children. A recent US Surgeon General Report (2006) reviewed the causal relationship of ETS with disease, particularly pulmonary and cardiac. Neurological effects were deemed inconclusive, with the stated need for more research (US Surgeon General 2006). Yet, ETS is a known risk factor for cerebrovascular disease (Bonita et al. 1999; Garcia-Nunez et al. 2007; Howard et al. 1998). Further, epidemiological studies recently indicated ETS as a risk factor for Alzheimer’s disease (AD) (Barrett 2007; Llewellyn et al. 2009). At the same time, ETS appears to decrease risk for Parkinson’s disease (PD) (Mellick 2006). Despite an apparent clinical impact on the adult brain, the molecular influence of ETS is underexplored, and is largely considered benign.
ETS administered has been shown to alter morphology during primate brain development (Slotkin et al. 2006). ETS exposure caused an increase in smaller glial cells, suggestive of reactive astrogliosis. Astrogliosis was also observed in the developing brain after prenatal nicotine exposure, with increased glial fibrillary acid protein (GFAP) levels in the cerebellum and hippocampus of offspring (Abdel-Rahman et al. 2003). Importantly, GFAP levels remained elevated out to postnatal day 60, and deficiencies in basic sensory motor skills were observed (Abdel-Rahman et al. 2004), a long-term, functional effect. Susceptibility to ETS may differ between the developing and mature mammalian brains, but these data raise the potential of an astrocytic response following adult ETS exposure.
Chemicals from ETS have also been found to influence apoptotic processes in cell cultures. Apoptosis is a cell death processed that can be triggered via internal or external cues. Regulation of extrinsic apoptosis involves PEA-15 (phosphoprotein enriched in astrocytes), an inhibitory protein that binds Fas-associated protein with death domain (FADD) when phosphorylated on Serine 116 (S116) (Renganathan et al. 2005). Dephosphorylation leads to caspase 3 activation and subsequent protein cleavage events. One such cleaved protein is αII-spectrin, the cleavage product of which is a known marker selective for apoptosis in neurons (Martin et al. 1995; Wang et al. 1998). ETS chemical extracts induced apoptosis in cardiac cells, with increased Fas and active forms of caspases 3 and 9 (Kuo et al. 2005a, b). In contrast, nicotine administration alone was neuroprotective in spinal cord neurons challenged by apoptosis inducing arachidonic-acid. Activation of caspases 3, 8, and 9 and release of cytochrome c were all reduced with nicotine relative to vehicle (Garrido et al. 2001, 2003). These data illustrate how the effects of ETS, with a complex chemical formulation (Swan and Lessov-Schlaggar 2007), may not be sufficiently modeled by nicotine administration alone. Different components of ETS may induce competing pro- and anti-apoptotic responses.
Given the aforementioned relationships between ETS exposure and the molecular processes astrogliosis and apoptosis in other systems, we present this initial study examining molecular effects in the adult mammalian brain. The glial selective marker GFAP and the neuronal selective caspase 3 proteolytic fragment of αII-spectrin were employed to characterize these processes across multiple brain regions. Mass spectrometry methods were also employed to test for ETS effects on the neuroproteome. This initial assessment focused on the limbic areas frontal cortex and hippocampus, as well as the cerebellum. Hippocampus and cerebellum were areas shown affected by ETS in the developing brain, and limbic regions, such as frontal cortex and hippocampus, are known to be affected in smokers (Almeida et al. 2008). Further, these limbic areas are affected by increased apoptotic and gliotic pathology in adult-onset neurodegenerative disease (Camins et al. 2008; Pereira et al. 2004; Ross et al. 2003).
Materials and Methods
Animal Procedures and Tissue Collection
Ten-week-old male Sprague Dawley rats (Harlem, Indianapolis, IN, USA) were acclimated to the laboratory for 4 days prior to exposure. Rats were kept under a controlled environment and housed two to a cage. Food and water were provided ad libitum except during exposure, when food was removed. No enrichment was provided to either the control or treated groups.
Following acclimation, rats were placed in a Teague TE-10 smoke exposure system (Teague 1994) for 3 h per day over a 3-week period. The ETS group (n=8) was exposed to a mixture of 15% mainstream (aspirated through filter) and 85% sidestream smoke diluted with air to a concentration of 5 mg/m3 of respirable suspended particulate (RSP). The control group (n=8) was exposed simultaneously to room air. During each exposure, 20 Kentucky 3R4F reference cigarettes (University of Kentucky, Lexington, KY) were smoked at a rate of one puff per minute, 2 s per puff (35 cm3), for eight puffs in 9 min per cigarette. This model provided comparable air-born exposure as experienced in a 50-m3 household room (0.7 air changes per hour), with a smoker consuming two cigarettes per hour over 10 h (per tables in EPA 2004), which was validated by repeated mass measurements of respirable suspended particles.
A day after the last ETS exposure, rats were decapitated under deep anesthesia (5% isoflurane for 5 min). Frontal cortex, hippocampus, and cerebellum were dissected, and snap frozen in liquid nitrogen. All procedures conformed to the US Public Health Service policy with approval of the Institutional Animal Care and Use Committee.
Statistical Analysis
Experiments were performed with n=4 biological replicates per group. Multivariable datasets were analyzed by a two-way repeated measure ANOVA with the Holm–Sidak distribution test and Bonferroni correction. Single-variable datasets were analyzed by a t test with a Kolmogorov–Smirnov distribution test. A Q test was applied to identify outlier values.
Immunoblotting
Lysates were prepared from the brain tissue as described before (Zhang et al. 2007). Protein concentration was determined via Bio-Rad DC Protein Assay (Hercules, CA, USA). Protein-balanced samples were prepared for SDS-PAGE, 4–20% Tris-glycine gel, in a twofold Tris-glycine loading buffer (Invitrogen, Carlsbad, CA, USA). Samples were heated for 90 s at 90°C, and centrifuged for 2 min. Following electrophoresis, separated proteins were transferred to polyvinylidene fluoride membranes by the semi-dry method. Membranes were probed with primary antibodies to: GFAP (Millipore, Billerica, MA, USA) at 1:5,000, αII-spectrin caspase-3 breakdown product (University of Florida, Gainesville, FL, USA) at 1:2,000, β-synuclein (BD Biosciences, San Jose, CA, USA) at 1:20,000, α-synuclein (BD Biosciences) at 1:1,000, heat shock protein 70 (Stressgen, Victoria, British Columbia, Canada) at 1:2,500, inducible nitric oxide synthase (BD Biosciences) at 1:5,000, superoxide dismutase 1 (Millipore) at 1:500, and β-actin (Sigma-Aldrich, St. Louis, MO, USA) at 1:2,000. The blots were then incubated with a biotinylated-conjugated secondary antibody followed by a streptavidin alkaline phosphatase conjugate. Bound antibodies were visualized by colorimetric development with the phosphatase substrate BCIP/NBT (KPL, Gaithersburg, MD, USA). Quantitative evaluation of protein levels was performed via densitometric analysis of 16-bit grayscale images using Image J software (National Institute of Health, v 1.6, Bethesda, MD, USA).
Mass Spectrometry
Fresh-frozen hippocampus tissues were prepared for immobilized metal ion affinity chromatography (IMAC) analysis as described previously (Ficarro et al. 2002). Briefly, Trizol reagent (Invitrogen) was employed for protein extraction as per the manufacturer’s instructions. The protein pellet was resuspended with phosphatase inhibitors (Sigma-Aldrich). Protein concentration was determined by DC protein assay. Protein (50 µg) was then reduced and alkylated with DTT and iodoacetamide, respectively, and digested with endo-Lys-C (Roche, Indianapolis, IN, USA) overnight at 37°C. ETS-exposed and control group samples were reacted for 2 h with heavy and light methanolic HCl, respectively, as described previously (Goodlett et al. 2001). Sample pairs were loaded onto a Poros MC (PerSpective Biosystems, Framingham, MA, USA) packed IMAC column, and separated as described previously (Ficarro et al. 2002). The phosphopeptide-enriched fractions were separately resolved by reversed-phase gradient separation from 0.7% to 28% acetonitrile/0.2% formic acid in 150 min online with a ThermoElectron (San Jose, CA, USA) LTQ Orbitrap XL with electron transfer dissociation source (McAlister et al. 2008). ETD-produced c/z· spectra were searched against a Uniprot Rattus protein database (v14.1) and the reversed image of that database with the OMSSA search engine, and were filtered for a 1% false-detection rate. Integrated peak areas were used to quantify the deuterated and non-deuterated forms of the PEA-15 apoptosis-signaling phosphopeptide.
Results
ETS Exposure Animal Model
The ETS exposure model developed for this study produce no notable stress in the research animals. Exposed and control animals were handled daily. Normal curiosity to a change in environment was observed for all animals. Animals exposed to ETS displayed less spontaneous movement during exposure than controls, but became immediately alert and explorative whenever the ETS exposure ceased (prior to any other change in environment). The pre-exposure mean body mass for the ETS animal group was 5 g less than that of the control group, and finished 8 g less 3 weeks later. Overall, no statistical difference in weight gain rate was observed between the two groups.
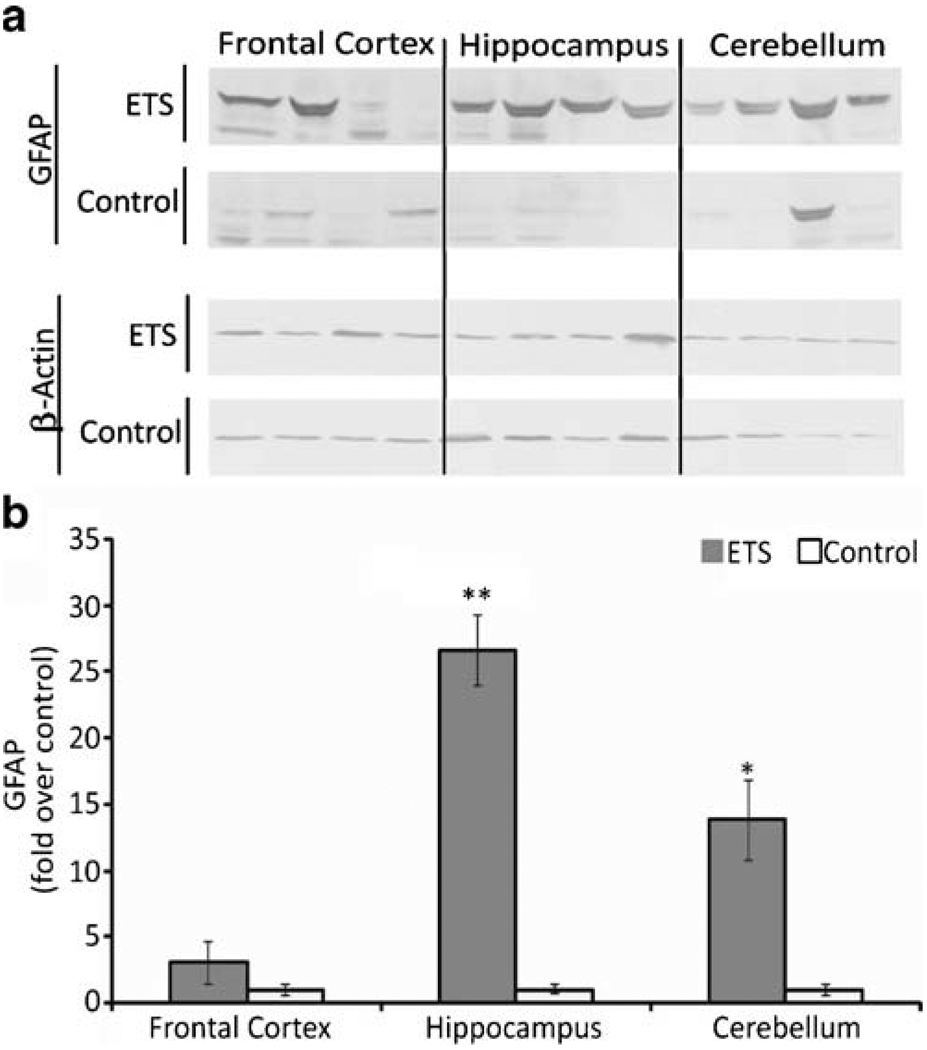
ETS Induces GFAP Expression in the Brain
GFAP was modulated as a main treatment effect of ETS exposure (p=0.003). Multiple comparison analysis also showed an ETS treatment within brain region interaction on GFAP expression (Fig. 1) that was statistically significant in hippocampus (p=0.001) and cerebellum (p=0.02). Frontal cortex exhibited a lower, non-significant (p=0.09) interaction, with the data exhibiting a bimodal distribution of individual values, confirmed by replicate assay to rule out experimental error.
Figure 1.
Effect of ETS on the astrogliosis marker GFAP in the adult rat brain. a GFAP immunoblot analysis as a marker of astrogliosis in three regions of the adult rat brain after a 3-week ETS or room air exposure. b Normalized densitometric quantification of GFAP with β-actin used as a loading control. Symbols indicate significant differences from control (*p≤0.02 and **p<0.001) by a repeated measures ANOVA multiple comparison of treatment × brain area
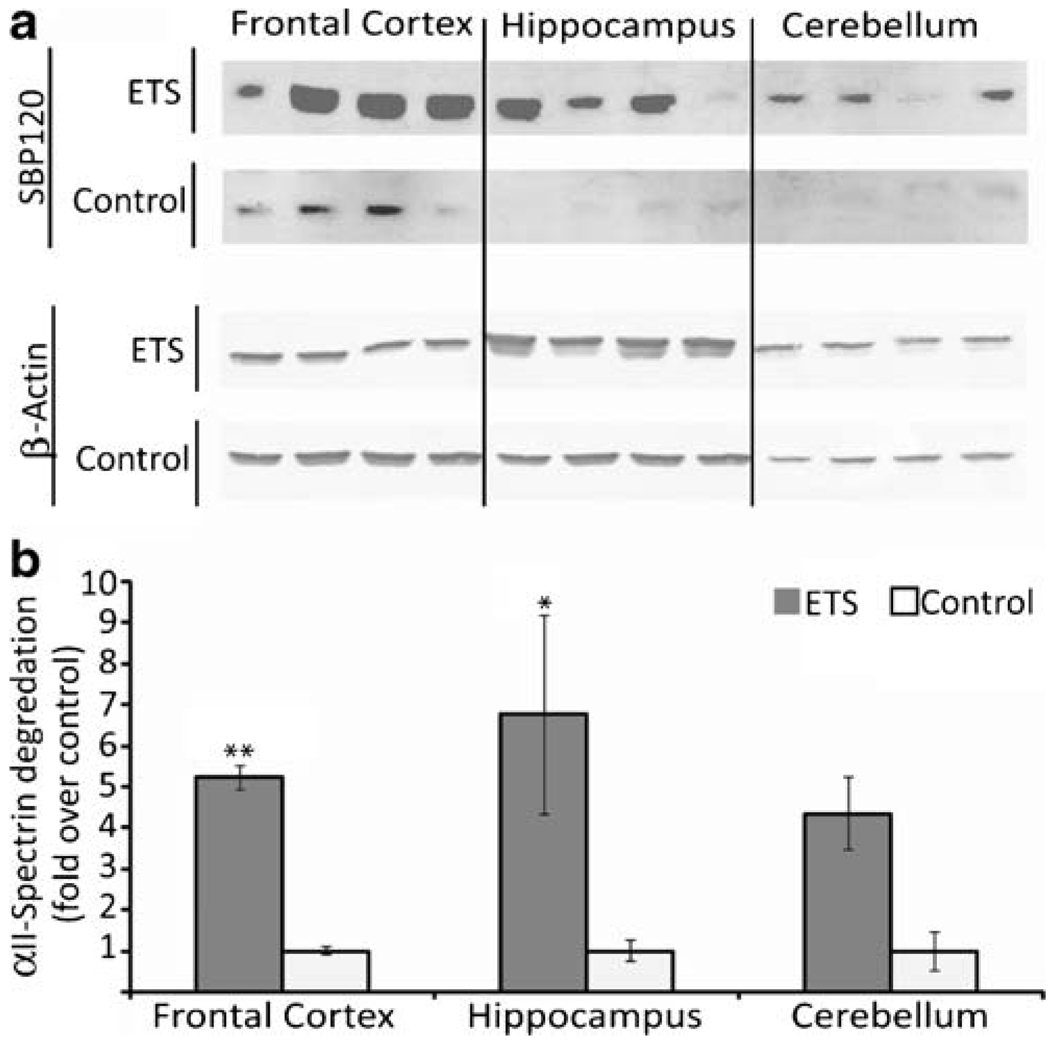
ETS Affects Markers of Apoptosis, not Cell Stress
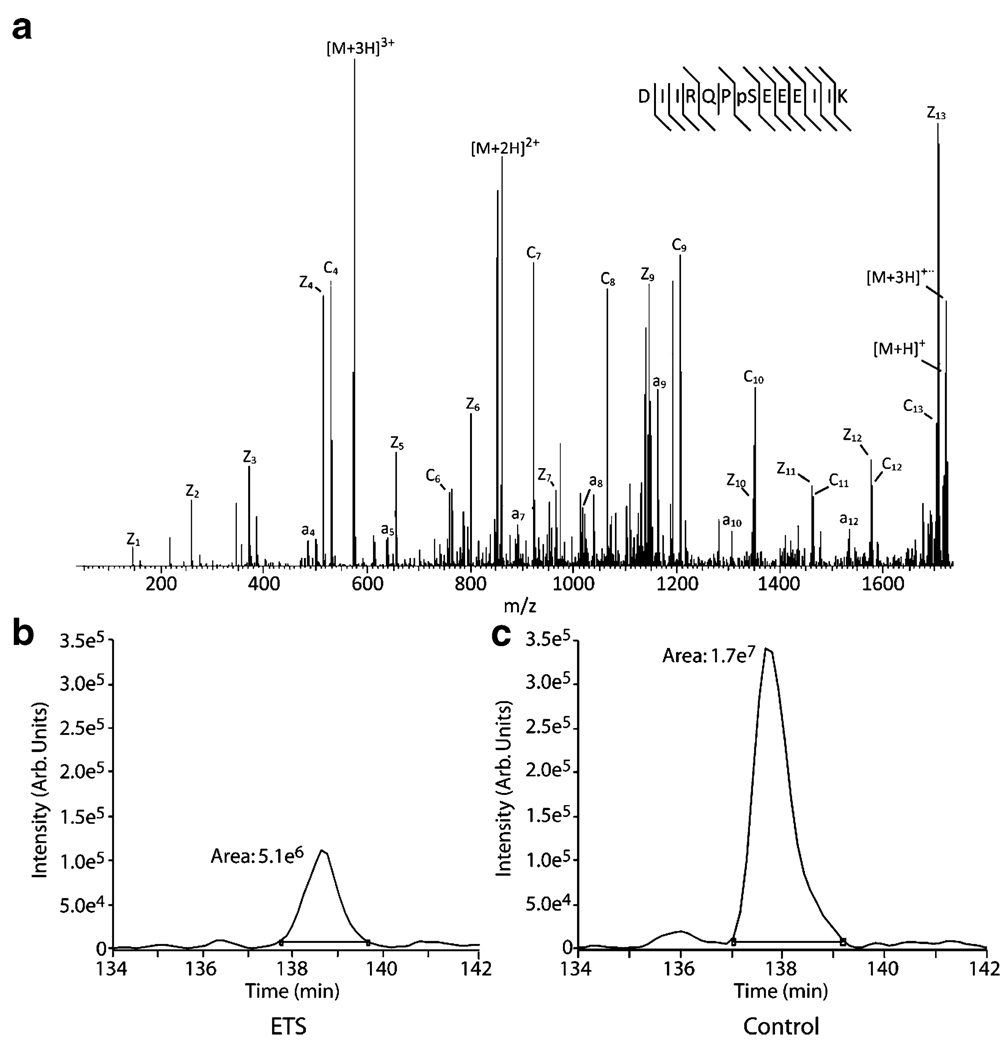
ETS exposure also had a main treatment effect on increased caspase-3 proteolysis of αII-spectrin in the adult rat brain (p<0.001) in exposed animals. There was also an interaction of ETS treatment within brain regions (Fig. 2), with a statistically significant increase in frontal cortex (p=0.001). The breakdown product was up for ETS treatment within hippocampus and cerebellum as well, but the observations were not statistically significant (p=0.03 and p=0.4, respectively). Mass spectrometry analysis revealed dephosphorylation of PEA-15 at S116 (p<0.001) in the hippocampus of ETS exposed animals. Site-specific phosphorylation was confirmed with the selective pattern of c and z· (ETD) fragment ions (Fig. 3). Additional immunochemical studies showed that levels of the oxidative and cell stress-associated proteins (data not shown), inducible nitric oxide synthase (iNOS), superoxide dismutase 1, and heat shock protein 70 kDa (HSP70) were unaffected by ETS exposure across the three brain regions examined in this study.
Figure 2.
Effect of ETS exposure on a neuronal apoptosis marker in the adult rat brain. a Immunoblot analysis of the neuronal αII-spectrin caspase-3 breakdown product as a marker of apoptosis in three regions of the adult rat brain after a 3-week ETS or room air exposure. b Normalized densitometric quantification of the breakdown product with β-actin used as a loading control. Symbols indicate significant differences from control (*p≤0.02 and **p<0.001) by a repeated measures ANOVA multiple comparison of treatment × brain area
Figure 3.
Effect of ETS exposure on DISC inhibiting dephosphorylation of PEA-15. a Example electron transfer dissociation spectrum of c/z· fragment ions selectively identifies the phosphorylated S116 site, with the phosphorylation state assessed for ETS exposed (b) and control (c) groups by chromatographic peak integration
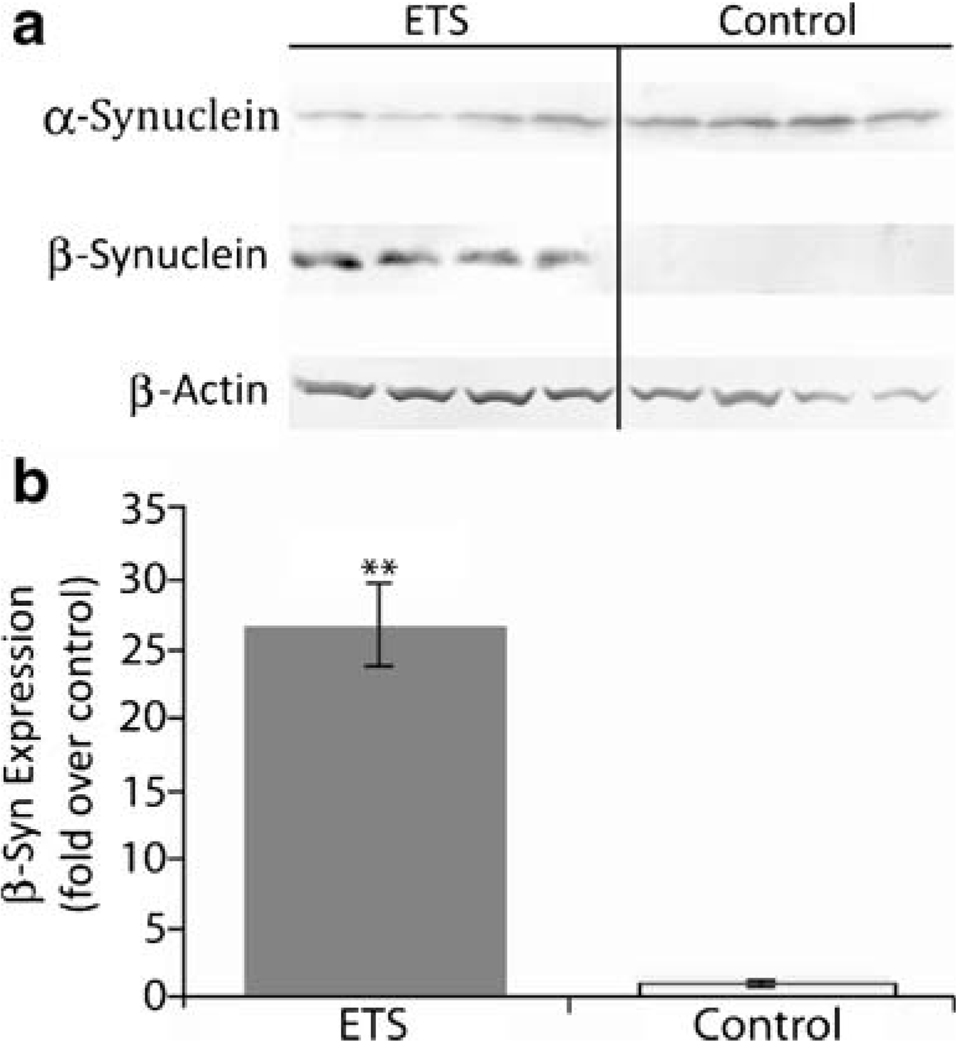
ETS Induces Synuclein Protein Expression
Mass spectrometry analysis also revealed a large quantitative difference in synuclein protein abundance, though the data lacked isoform specificity. Further immunochemical analysis determined that it was the neuroprotective isoform β-synuclein which increased (p<0.001) in the hippocampus of ETS-exposed animals relative to room-air controls (Fig. 4). In contrast, aggregate-forming α-synuclein expression trended slightly lower among ETS-exposed animals, but was not a statistically significant difference.
Figure 4.
Effect of ETS exposure on synuclein proteins in the adult rat hippocampus. a Immunoblot analysis was performed with antibodies against α- and β-synuclein with hippocampal tissue from adult rat brain after a 3-week ETS or room air exposure. b Normalized densitometric quantification of synuclein proteins with β-actin used as a loading control. Symbol indicates a significant difference from control (**p<0.001) by a t test
Discussion
ETS exposure was verified to influence the adult rat brain neuroproteome in this investigative study. Molecular effects were observed across multiple brain areas while animal growth, as a basic physiological measure, was unaffected. Changes in protein markers suggested modulated astrogliosis (GFAP), apoptotic cell death (cytoskeletal degradation of neurons, and DISC complex formation), and the over-expression of β-synuclein as a potential neuroprotective response.
ETS Induces Astrogliosis in Adult Rat Brain
ETS resulted in modulation of GFAP across the three brain areas examined. GFAP levels are a known marker of astrogliosis in the damaged brain (O’Callaghan and Sriram 2005). The ETS-induced difference in GFAP was statistically significant as a main effect and as a regional interactive effect in hippocampus and cerebellum. The adult GFAP results correlate with previous observations of increased GFAP expression consequent to prenatal nicotine exposure (Abdel-Rahman et al. 2003, 2004) and an increase in cell density in the developing brain from ETS (Slotkin et al. 2006).
ETS Induces Apoptosis in Adult Rat Brain
Apoptotic cell death was demarked by greater caspase 3 degradation of neuronal αII-spectrin in the adult rat brain. Increased degradation was a main effect of ETS treatment; although, multiple comparison analysis showed statistically significant treatment within region interactions for frontal cortex. Apoptosis has been reported as a direct result of cigarette smoke-induced cell stress in other organs and cultures. ETD tandem mass spectrometry revealed the dephosphorylation of PEA-15 at S116 in ETS-exposed hippocampus, suggesting the activation of the extrinsic apoptotic pathway, which was previously found activated by ETS in cardiac cells (Kuo et al. 2005a, b).
Neuroinflammation can induce the extrinsic apoptotic pathway through the production of reactive oxygen species (ROS). The ROS nitric oxide is often, though not always (Brown 2007; O’Callaghan et al. 2008), induced from increased iNOS expression. In this study, ETS treatment showed no effect on iNOS expression by immunoblot. While ETS may induce astrogliosis, it separately may be suppressing iNOS expression in glia. Cigarette smoke condensates were previously observed to inhibit iNOS in glial cultures (Mazzio et al. 2005). Superoxide dismutase 1 (SOD 1) is an antioxidant that is also upregulated as a response to brain ROS (Dimayuga et al. 2007). SOD 1 expression, like iNOS, was unaffected by ETS exposure in this study. Rats exposed to direct smoke (as opposed to ETS) have been shown to express significantly greater levels of HSP70 in brain (Anbarasi et al. 2006), pulmonary airways (Doz et al. 2008), and mammalian cell cultures (Vayssier et al. 1998). HSP70 is a neuroprotective protein upregulated during times of cell stress; however, in this study, HSP70 expression was also found to be unaffected by ETS exposure in replicate assays. Together, these data suggest that ROS is not induced by ETS exposure in the brain areas studied with this model and is, therefore, not likely the causal factor promoting apoptosis.
ETS Affects β-Synuclein Protein Expression
β-Synuclein expression was upregulated in ETS exposed animals. α- and β-synuclein are functionally distinct, with the α isoform prone to Lewy body forming aggregation found in the PD brain (Polymeropoulos 1998). In contrast, β-synuclein is immune from aggregation due to its lack of a non-amyloidogenic domain (Ueda et al. 1993). β-synuclein has shown anti-apoptotic properties through down-regulation of p53 expression (da Costa et al. 2003). β-synuclein has also been shown to restore the anti-apoptotic function of α-synuclein (da Costa et al. 2003). The marked increase in β-synuclein levels observed here may be a neuroprotective response to the observed neuronal apoptosis.
Smoking has long been known to reduce PD incidence (Dorn 1959) in a dose-dependent fashion (Allam et al. 2007; Gorell et al. 1999). A recent epidemiological study showed ETS as having a similar effect on PD incidence (Mellick 2006). Many studies have explored a connection between smoke exposure and PD neuroprotection. This is the first study to demonstrate increased expression of neuroprotective β-synuclein by ETS, which could be involved in reduced PD incidence. β-synuclein is known to inhibit α-synuclein aggregation in addition to its anti-apoptotic properties (Hashimoto et al. 2001; da Costa et al. 2003). A recent study pointed to nicotine as an active agent that retards the fibrillogenic activity of α-synuclein (Ono et al. 2007). Future work will examine β-synuclein as an intermediate in PD-relevant brain regions.
In conclusion, the results from this study demonstrate a main treatment effect of ETS on adult rat brain biochemistry, which begins to dispel the notion that ETS exposure is benign to the adult mammalian brain. The data point to modulated apoptosis and astrogliosis via increases in markers of these processes, but without the influence of ROS. The data also suggest differences among individual animals that may signify variable susceptibility to ETS effects. β-synuclein expression is significantly increased by ETS exposure, which may be a neuroprotective response with a potential benefit relative to PD. Future work will investigate the induction of apoptosis by ETS and its connection with the increase in β-synuclein in the adult rat brain.
Acknowledgments
We thank B. McLaurin, Y. Tanimura, and A. Svetlov for surgical and laboratory assistance. We thank D. Swaney and J. Coon for assistance with IMAC and ETD analysis, and for helpful discussions. We thank H. Zhi and G. Shaw for immunohistochemistry assistance and M. Lewis for helpful discussions. K. Wang holds equity and is employed by Banyan Biomarkers. The Flight Attendant Medical Research Institute (FAMRI), grant #052442, provided financial support for this work.
Contributor Information
Brian F. Fuller, Department of Anatomy and Neurobiology, Virginia Commonwealth University, PO Box 980709, Richmond, VA 23298-0709, USA
Mark S. Gold, Department of Psychiatry, McKnight Brain Institute of the University of Florida, Gainesville, FL, USA
Kevin K. W. Wang, Department of Psychiatry, McKnight Brain Institute of the University of Florida, Gainesville, FL, USA Center of Innovative Research, Banyan Biomarkers, Inc, Alachua, FL, USA.
Andrew K. Ottens, Department of Anatomy and Neurobiology, Virginia Commonwealth University, PO Box 980709, Richmond, VA 23298-0709, USA, akottens@vcu.edu
References
- Abdel-Rahman A, Dechkovskaia A, Mehta-Simmons H, Guan X, Khan W, Abou-Donia M. Increased expression of glial fibrillary acidic protein in cerebellum and hippocampus: differential effects on neonatal brain regional acetylcholinesterase following maternal exposure to combined chlorpyrifos and nicotine. J Toxicol Environ Health A. 2003;66:2047–2066. doi: 10.1080/713853982. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman A, Dechkovskaia AM, Mehta-Simmons H, Sutton JM, Guan X, Khan WA, Abou-Donia MB. Maternal exposure to nicotine and chlorpyrifos, alone and in combination, leads to persistently elevated expression of glial fibrillary acidic protein in the cerebellum of the offspring in late puberty. Arch Toxicol. 2004;78:467–476. doi: 10.1007/s00204-004-0560-5. [DOI] [PubMed] [Google Scholar]
- Allam MF, Del Castillo AS, Navajas RF. Parkinson’s disease, smoking, and gender. Mov Disord. 2007;22:1829–1830. doi: 10.1002/mds.21623. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Garrido GJ, Lautenschlager NT, Hulse GK, Jamrozik K, Flicker L. Smoking is associated with reduced cortical regional gray matter density in brain regions associated with incipient Alzheimer disease. Am J Geriatr Psychiatry. 2008;16:92–98. doi: 10.1097/JGP.0b013e318157cad2. [DOI] [PubMed] [Google Scholar]
- Anbarasi K, Kathirvel G, Vani G, Jayaraman G, Shyamala D, Shyamala Devi CS. Cigarette smoking induces heat shock protein 70 kDa expression and apoptosis in rat brain: Modulation by bacoside A. Neuroscience. 2006;138:1127–1135. doi: 10.1016/j.neuroscience.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Barrett JR. Dementia and secondhand smoke. Environ Health Perspect. 2007;115:A401. doi: 10.1289/ehp.115-a401a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonita R, Duncan J, Truelsen T, Jackson RT, Beaglehole R. Passive smoking as well as active smoking increases the risk of acute stroke. Tob Control. 1999;8:156–160. doi: 10.1136/tc.8.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc Trans. 2007;35:1119–1121. doi: 10.1042/BST0351119. [DOI] [PubMed] [Google Scholar]
- Camins A, Pallas M, Silvestre JS. Apoptotic mechanisms involved in neurodegenerative diseases: experimental and therapeutic approaches. Methods Find Exp Clin Pharmacol. 2008;30:43–65. doi: 10.1358/mf.2008.30.1.1090962. [DOI] [PubMed] [Google Scholar]
- da Costa CA, Masliah E, Checler F. Beta-synuclein displays an antiapoptotic p53-dependent phenotype and protects neurons from 6-hydroxydopamine-induced caspase 3 activation: cross-talk with alpha-synuclein and implication for Parkinson’s disease. J Biol Chem. 2003;278:37330–37335. doi: 10.1074/jbc.M306083200. [DOI] [PubMed] [Google Scholar]
- Dimayuga FO, Wang C, Clark JM, Dimayuga ER, Dimayuga VM, Bruce-Keller AJ. SOD1 overexpression alters ROS production and reduces neurotoxic inflammatory signaling in microglial cells. J Neuroimmunol. 2007;182:89–99. doi: 10.1016/j.jneuroim.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn HF. Tobacco consumption and mortality from cancer and other diseases. Public Health Rep. 1959;74:581–593. [PMC free article] [PubMed] [Google Scholar]
- Doz E, Noulin N, Boichot E, Guenon I, Fick L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF, Couillin I. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol. 2008;180:1169–1178. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- EPA US. National Survey on Environmental Management of Asthma and Children’s Exposure to ETS ICR # 1996.0. 2004 [Google Scholar]
- Ficarro SB, McCleland ML, Stukenberg PT, Burke DJ, Ross MM, Shabanowitz J, Hunt DF, White FM. Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat Biotechnol. 2002;20:301–305. doi: 10.1038/nbt0302-301. [DOI] [PubMed] [Google Scholar]
- Garcia-Nunez C, Saez J, Garcia-Nunez JM, Grau J, Molto-Jorda JM, Matias-Guiu J. Passive smoking as a cerebrovascular risk factor. Rev Neurol. 2007;45:577–581. [PubMed] [Google Scholar]
- Garrido R, Mattson MP, Hennig B, Toborek M. Nicotine protects against arachidonic-acid-induced caspase activation, cytochrome c release and apoptosis of cultured spinal cord neurons. J Neurochem. 2001;76:1395–1403. doi: 10.1046/j.1471-4159.2001.00135.x. [DOI] [PubMed] [Google Scholar]
- Garrido R, King-Pospisil K, Son KW, Hennig B, Toborek M. Nicotine upregulates nerve growth factor expression and prevents apoptosis of cultured spinal cord neurons. Neurosci Res. 2003;47:349–355. doi: 10.1016/s0168-0102(03)00222-0. [DOI] [PubMed] [Google Scholar]
- Goodlett DR, Keller A, Watts JD, Newitt R, Yi EC, Purvine S, Eng JK, von Haller P, Aebersold R, Kolker E. Differential stable isotope labeling of peptides for quantitation and de novo sequence derivation. Rapid Commun Mass Spectrom. 2001;15:1214–1221. doi: 10.1002/rcm.362. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Rybicki BA, Johnson CC, Peterson EL. Smoking and Parkinson’s disease: a dose-response relationship. Neurology. 1999;52:115–119. doi: 10.1212/wnl.52.1.115. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Mante M, Mallory M, Masliah E. Beta-Synuclein inhibits alpha-synuclein aggregation: a possible role as an anti-parkinsonian factor. Neuron. 2001;32:213–223. doi: 10.1016/s0896-6273(01)00462-7. [DOI] [PubMed] [Google Scholar]
- Howard DJ, Ota RB, Briggs LA, Hampton M, Pritsos CA. Environmental tobacco smoke in the workplace induces oxidative stress in employees, including increased production of 8-hydroxy-2’-deoxyguanosine. Cancer Epidemiol Biomarkers Prev. 1998;7:141–146. [PubMed] [Google Scholar]
- Kuo WH, Chen JH, Lin HH, Chen BC, Hsu JD, Wang CJ. Induction of apoptosis in the lung tissue from rats exposed to cigarette smoke involves p38/JNK MAPK pathway. Chem Biol Interact. 2005a;155:31–42. doi: 10.1016/j.cbi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Kuo WW, Wu CH, Lee SD, Lin JA, Chu CY, Hwang JM, Ueng KC, Chang MH, Yeh YL, Wang CJ, Liu JY, Huang CY. Second-hand smoke-induced cardiac fibrosis is related to the Fas death receptor apoptotic pathway without mitochondria-dependent pathway involvement in rats. Environ Health Perspect. 2005b;113:1349–1353. doi: 10.1289/ehp.7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn DJ, Lang IA, Langa KM, Naughton F, Matthews FE. Exposure to secondhand smoke and cognitive impairment in nonsmokers: national cross sectional study with cotinine measurement. BMJ. 2009;338:b462. doi: 10.1136/bmj.b462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, O’Brien GA, Nishioka WK, McGahon AJ, Mahboubi A, Saido TC, Green DR. Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J Biol Chem. 1995;270:6425–6428. doi: 10.1074/jbc.270.12.6425. [DOI] [PubMed] [Google Scholar]
- Mazzio EA, Kolta MG, Reams RR, Soliman KF. Inhibitory effects of cigarette smoke on glial inducible nitric oxide synthase and lack of protective properties against oxidative neurotoxins in vitro. Neurotoxicology. 2005;26:49–62. doi: 10.1016/j.neuro.2004.07.005. [DOI] [PubMed] [Google Scholar]
- McAlister GC, Berggren WT, Griep-Raming J, Horning S, Makarov A, Phanstiel D, Stafford G, Swaney DL, Syka JE, Zabrouskov V, Coon JJ. A proteomics grade electron transfer dissociation-enabled hybrid linear ion trap-orbitrap mass spectrometer. J Proteome Res. 2008;7:3127–3136. doi: 10.1021/pr800264t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellick GD. CYP450, genetics and Parkinson’s disease: gene x environment interactions hold the key. J Neural Transm Suppl. 2006;70:159–165. doi: 10.1007/978-3-211-45295-0_25. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Sriram K. Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity. Expert Opin Drug Saf. 2005;4:433–442. doi: 10.1517/14740338.4.3.433. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Sriram K, Miller DB. Defining "neuroinflammation". Ann N Y Acad Sci. 2008;1139:318–330. doi: 10.1196/annals.1432.032. [DOI] [PubMed] [Google Scholar]
- Ono K, Hirohata M, Yamada M. Anti-fibrillogenic and fibril-destabilizing activity of nicotine in vitro: implications for the prevention and therapeutics of Lewy body diseases. Exp Neurol. 2007;205:414–424. doi: 10.1016/j.expneurol.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Pereira C, Ferreiro E, Cardoso SM, de Oliveira CR. Cell degeneration induced by amyloid-beta peptides: implications for Alzheimer’s disease. J Mol Neurosci. 2004;23:97–104. doi: 10.1385/JMN:23:1-2:097. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH. Autosomal dominant Parkinson’s disease and alpha-synuclein. Ann Neurol. 1998;44:S63–S64. doi: 10.1002/ana.410440710. [DOI] [PubMed] [Google Scholar]
- Renganathan H, Vaidyanathan H, Knapinska A, Ramos JW. Phosphorylation of PEA-15 switches its binding specificity from ERK/MAPK to FADD. Biochem J. 2005;390:729–735. doi: 10.1042/BJ20050378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross GW, O’Callaghan JP, Sharp DS, Petrovitch H, Miller DB, Abbott RD, Nelson J, Launer LJ, Foley DJ, Burchfiel CM, Hardman J, White LR. Quantification of regional glial fibrillary acidic protein levels in Alzheimer’s disease. Acta Neurol Scand. 2003;107:318–323. doi: 10.1034/j.1600-0404.2003.02098.x. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Pinkerton KE, Seidler FJ. Perinatal environmental tobacco smoke exposure in rhesus monkeys: critical periods and regional selectivity for effects on brain cell development and lipid peroxidation. Environ Health Perspect. 2006;114:34–39. doi: 10.1289/ehp.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev. 2007;17:259–273. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Public Health Service, Office of the Surgeon General. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Rockville: US Dept. of Health and Human Services, Public Health Service, Office of the Surgeon General; 2006. [Google Scholar]
- Vayssier M, Banzet N, Francois D, Bellmann K, Polla BS. Tobacco smoke induces both apoptosis and necrosis in mammalian cells: differential effects of HSP70. Am J Physiol. 1998;275:L771–L779. doi: 10.1152/ajplung.1998.275.4.L771. [DOI] [PubMed] [Google Scholar]
- Wang KW, Posmantur R, Nath R, McGinnis K, Whitton M, Talanian RV, Glantz SD, Morrow JS. Simultaneous Degradation of αII- and βII-Spectrin by Caspase 3 (CPP32) in Apoptotic Cells. JBC. 1998;273:22490–22497. doi: 10.1074/jbc.273.35.22490. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Ottens AK, Sadasivan S, Kobeissy FH, Fang T, Hayes RL, Wang KW. Calpain-Mediated Collapsin Response Mediator Protein-1, -2, And -4 Proteolysis after Neurotoxic And Traumatic Brain Injury. Journal of Neurotrauma. 2007;24(3):460–472. doi: 10.1089/neu.2006.0078. [DOI] [PubMed] [Google Scholar]