Abstract
Repetitive behaviors are diagnostic for autism and common in related neurodevelopmental disorders. Despite their clinical importance, underlying mechanisms associated with the expression of these behaviors remain poorly understood. Our lab has previously shown that the rates of spontaneous stereotypy in deer mice (Peromyscus maniculatus) were negatively correlated with enkephalin content, a marker of striatopallidal but not striatonigral neurons. To investigate further the role of the indirect basal ganglia pathway, we examined neuronal activation of the subthalamic nucleus (STN) using cytochrome oxidase (CO) histochemistry in high and low stereotypy mice. CO activity in STN was significantly lower in high stereotypy mice and negatively correlated with the frequency of stereotypy. In addition, exposure to environmental enrichment, which attenuated stereotypy, normalized the activity of STN. Co-administration of the adenosine A2A receptor agonist CGS21680 and the A1 receptor agonist CPA attenuated stereotypy dose-dependently. The significant reduction associated with the lowest dose of the drug combination tested was due to its effects on mice with lower baseline levels of stereotypy seen. Higher doses of the drug combination were required to show robust behavioral effects, and presumably requisite activation of the indirect pathway, in high-stereotypy mice. These findings support that decreased indirect pathway activity is linked to the expression of high levels of stereotypy in deer mice and that striatal A1 and A2A receptors may provide promising therapeutic targets for the treatment of repetitive behaviors in neurodevelopmental disorders.
Six key words: autism, stereotypy, subthalamic nucleus, neurodevelopmental disorders, deer mice, animal models
Introduction
Restricted, repetitive behaviors are typically characterized as inflexible, persistent, and apparently functionless. This categorization captures a wide range of behaviors from sensorimotor (e.g., motor stereotypy, repetitive manipulation of objects, compulsions) to more cognitively driven behaviors (e.g., insistence on sameness, restricted interests) [10, 51]. Restricted, repetitive behaviors constitute one of three diagnostic domains of autism spectrum disorder ([30] for review) and are common features of related neurodevelopmental disorders (e.g., Rett’s syndrome, intellectual and developmental disability) as well as several psychiatric disorders (e.g., obsessive-compulsive disorder (OCD), trichotillomania) and neurological diseases (e.g., Tourette syndrome, frontotemporal dementia). In addition, motor stereotypies have been described in adults and children without diagnosed neurodevelopmental, psychiatric, or neurological disorders [4, 5, 32, 48] and are ubiquitous in normative development [12, 54].
Despite their clinical importance, the specific neurobiological mechanisms associated with the abnormal expression of these behaviors are largely unidentified. Several neuroimaging studies have identified structural and functional basal ganglia differences linked to the expression of repetitive behaviors [21, 33, 35, 46]. Experimental manipulations known to induce stereotypies in animals have provided more direct evidence for the importance of cortico-basal ganglia circuitry in mediating these behaviors (e.g., [57]).
Dysregulation of cortico-striato-thalamo-cortical circuitry associated with motor disorders is thought to be due to an imbalance between the direct and indirect pathways comprising this circuitry [17]. For example, injection of a GABA antagonist to the external aspect of the globus pallidus (GPe) induced stereotypy in non-human primates, which was attenuated by deep brain stimulation (DBS) of the subthalamic nucleus (STN) [3]. Similarly inactivation of STN exacerbated the compulsive lever-pressing observed in rats in the signal attenuation model of OCD [59]. These results point to a potentially important role for the indirect pathway function in the expression of repetitive behavior of unknown etiology.
Our laboratory has employed deer mice (Peromyscus maniculatus) which develop high levels of motor stereotypies (repetitive jumping and/or backward somersaulting) as a consequence of being reared in a standard laboratory environment. These behaviors, which do not require social isolation, specific cues or contexts, pharmacological agents, or specific CNS insult for induction, occur at a high rate, persist across much of the life of the animal and appear relatively early in development. Housing these mice in more complex environments especially early in development attenuates the development and expression of these behaviors [18, 38]. The repetitive hindlimb jumping and backward somersaulting exhibited by deer mice correspond to the repetitive sensorimotor behaviors frequently observed in neurodevelopmental disorders. We have also shown that highly stereotypic deer mice have deficits in a reversal learning task, mirroring inflexibility and resistance to change [53]. These features plus considerable heterogeneity in individual levels of expression, modulation by early experience, and mediation by cortical-basal ganglia circuitry make deer mice a useful model of restricted, repetitive behavior in neurodevelopmental disorders.
We have previously shown that alterations of cortico-basal ganglia circuitry are linked to the expression of spontaneous repetitive behaviors in deer mice [39, 41, 55]. In particular, we found a significant inverse correlation between striatal enkephalin content and stereotypy. Conversely, there was no association between dynorphin and repetitive behavior [40]. This finding lead to the hypothesis that the expression of spontaneous stereotypy is a result of imbalanced activity of striatal pathways driven by reduced activity of the indirect pathway.
In the present study, we conducted two sets of experiments to assess the function of the indirect pathway in the expression of stereotypy. First, we assessed the neuronal metabolic capacity of STN as an index of indirect pathway activity using cytochrome oxidase (CO) histochemistry in adult deer mice (Experiment 1a). We repeated the same analysis in animals which were either housed under standard laboratory or environmentally enriched conditions (Experiment 1b). CO activity reflects oxidative metabolic capacity of neurons and has been shown to be directly related to neuronal functional activity and has been used to identify neuronal pathways activated by experience [44]. Moreover, unlike 2-DG or c-Fos, CO histochemistry reflects sustained alterations in neuronal activity. In addition, Hevner and Wong-Riley [20] have shown that the optical density of histochemically labeled brain sections closely correlates with the amount of CO in CNS tissue.
Second, we pharmacologically manipulated striatopallidal neuronal activity via adenosine A2A receptors which are highly enriched in the striatum and selectively expressed in these neurons [13, 22, 45]. Karcz-Kubicha et al. [23, 24] reported that administration of an A2A agonist alone failed to induce c-Fos in the striatum, whereas co-administration of the same A2A agonist plus an A1 agonist resulted in significant increase in striatal c-Fos expression as well as enkephalin expression. Importantly, this increased expression was observed in striatopallidal but not striatonigral neurons. Thus, we assessed the effect of an adenosine A2A agonist alone (Experiment 2a) as well as the combination of an A2A receptor agonist plus A1 receptor agonist (Experiment 2b, c, and d) on the attenuation of the expression of stereotypy.
General methods
Subjects
All deer mice (Peromyscus maniculatus) were obtained from the breeding colony maintained in our laboratory, and kept on a 16:8-h light/dark cycle with lights off at 10:00 AM. Mice were weaned at 21 days of age. Rodent chow and water were available ad libitum. The room was maintained at 20–25°C and 50–70% humidity. When housed in standard laboratory cages, deer mice exhibit stereotyped behaviors in the form of hindlimb vertical jumping or backward somersaulting with considerable between animal variability. All procedures were performed in accordance with the guidelines set forth in the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Florida Institutional Animal Care and Use Committee.
Stereotypy Assessment
Rates of spontaneous stereotypy were assessed using a modified automated photocell detection apparatus (Columbus Instruments). The session consisted of the eight hours of the dark cycle. Mice were individually placed in testing cages (22 × 28 × 25 cm) made of Plexiglas and habituated for at least one hour prior to the beginning of the dark cycle. Food and water were provided. Stereotypy counts, which equated to the number of vertical jumps or backward somersaults a deer mouse performed during that 8 hours, were automatically scored for each mouse. Photobeams were positioned (13.5cm above the floor) to be interrupted by the vertical motion of jumping and somersaulting but not by rearing. All sessions were digitally video-recorded for identification of behavioral topographies and accuracy of the automated counters. Each animal received a stereotypy score that represented the average stereotypy frequency per hour.
Data Analysis
Differences in CO activity between high- and low-stereotypy mice and between enriched and standard caged mice were assessed by t-test with CO differences in STN being the primary comparison of interest. The association between the frequency of spontaneous stereotypies and the CO activity were analyzed by Pearson correlation (Experiment 1). Behavioral responses to pharmacological manipulations were analyzed by ANOVA and t-test (Experiment 2). All tests were two-tailed and effects were considered significant when p<0.05.
Specific Methods and Results
Experiment 1
Experiment 1a: Neuronal Activation of STN in High- and Low-Stereotypy Mice
Methods
Twenty-one adult mice (>8 weeks post-weaning; 15 males and 6 females) exhibiting repetitive vertical jumping were used. All mice were group-caged (5–6 mice per cage) from weaning in standard rodent cages (48 × 27 × 15 cm). Each mouse was assessed for stereotypy as described previously and separated into two groups (high and low stereotypy) based on a median split of stereotypy scores.
Following behavioral assessment, mice were killed and their brains were quickly removed and frozen by immersing them in cold 2-methylbutane. They were stored at −80°C until cryostat sectioning. The sections were cut sagittally at 20μm at −20°C starting approximately 2.7 mm lateral to the midline and collected every 100μm for both hemispheres. Sections were mounted on pretreated slides (Superfrost plus, Fisher), and stored in the −80°C freezer until assayed.
The CO spectrophotometric analysis and staining assay was carried out according to the Gonzalez-Lima protocol [15]. Standards were cut at five thicknesses (20, 30, 40, 50, and 60μm) and assayed with other brain sections. The CO staining was quantified by densitometric analysis using ImagePro (Media Cybernetics), and standards were used to convert optical density values into enzymatic activity values (μmol/min/g). Multiple optical density readings were made per animal for STN as well as for other areas comprising cortico-basal ganglia circuitry (motor cortex, striatum, substantia nigra pars reticulata (SNpr), substantia nigra pars compacta (SNpc)) and negative controls (hippocampus, somatosensory cortex). Dorsal and ventral aspects of the striatum were defined by horizontal bisection into approximately equivalent halves, whereas medial versus lateral aspects were defined by approximately <2.0mm or >2.0mm from the midline respectively. Under our experimental conditions, the staining intensity was highly correlated to the thickness of the standard sections (r =0.95).
Results
Baseline stereotypy scores varied from 137.9 to 3992.8 counts per hour with a median score of 652.0. The average score was 394.1 for low-stereotypy animals (n=11) and 1393.0 for high-stereotypy animals (n=10).
CO enzymatic activity within selected areas is summarized in Table 1. Significant differences were found in STN (t(19)=2.74, p=0.01) where low-stereotypy mice showed higher CO activity compared to high-stereotypy mice. Similar differences were found in the ventromedial striatum (t(19)=2.21, p=0.04) and SNpr (t(19)=2.25, p=0.04). In addition, individual levels of stereotypy were negatively correlated with CO activities in STN (r=−0.50, p=0.02) (Fig 1) and SNpr (r=−0.53, p=0.01). When the highest stereotypy score was excluded based on its value being more than 2 SDs greater than the mean, the correlations with CO were no longer significant (STN: r=−0.41, p=0.07; SNpr: r=−0.41, p=0.07). In addition, without this outlying score, the differences in the ventromedial striatum and SNpr between high and low stereotypy mice were no longer statistically significant (t(18)=1.96, p=0.07; t(18)=1.91, p=0.07 respectively).
Table 1.
CO activity (μmol/min/g) of STN and the other brain areas in high- and low-stereotypy adult deer mice. Values expressed are group means with SEM in parentheses.
| Low Stereotypy | High Stereotypy | P-value | |
|---|---|---|---|
| Subthalamic Nucleus | 64.60 (1.42) | 59.01 (1.46) | 0.01 |
| Motor Cortex | 48.01 (0.88) | 47.84 (1.12) | 0.89 |
| Somatosensory Cortex | 49.64 (0.74) | 49.67 (1.25) | 0.98 |
| Hippocampus | 44.12 (1.12) | 42.46 (1.79) | 0.80 |
| Caudate/Putamen | |||
| Dorsolateral Striatum | 48.48 (0.81) | 47.87 (1.42) | 0.71 |
| Dorsomedial Striatum | 49.30 (1.02) | 46.76 (1.12) | 0.10 |
| Ventrolateral Striatum | 49.43 (0.74) | 48.25 (1.08) | 0.37 |
| Ventromedial Striatum | 50.28 (1.15) | 46.96 (0.98) | 0.04 |
| Nucleus Accumbens | 50.85 (1.12) | 48.08 (1.02) | 0.09 |
| Substantia Nigra pars Compacta | 45.10 (1.66) | 42.22 (0.91) | 0.16 |
| Substantia Nigra pars Reticulata | 50.72 (1.66) | 46.25 (1.05) | 0.04 |
Fig 1.
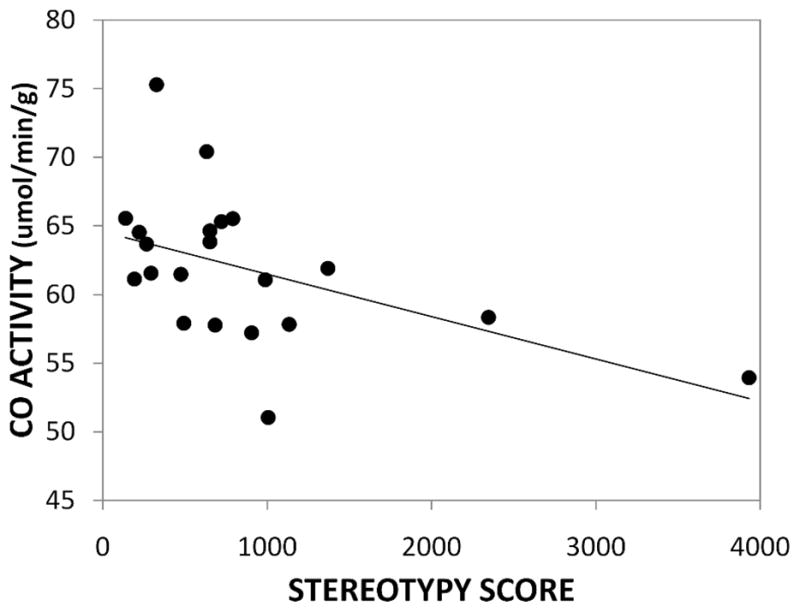
Correlation between the frequency of stereotypy and CO activity in STN.
Experiment 1b: Effects of Enriched Environment on Stereotypy and Neuronal Activation in STN
Methods
Twenty-three mice (14 males and 9 females) were group-caged (5–6 mice per cage) in standard rodent cages (48 × 27 × 15 cm) at weaning for 30 days, and then tested for baseline levels of stereotypy as described in the previous section. After testing, animals were randomly assigned to one of two housing conditions: standard rodent cages (SC) (n=12) or environmental enrichment (EE) (n=11). The EE housing consisted of large dog kennels (122 × 81 × 89 cm) with two extra levels of floors constructed of galvanized wire mesh and connected by ramps of the same material. Bedding, a running wheel, shelters, and various other objects were placed in each kennel. In addition to ad libitum food and water, one oz. of Cockatiel vita seed was scattered throughout the kennel three times each week to encourage foraging behavior. A running wheel remained undisturbed in the kennel, but other objects were removed and replaced with clean novel objects on a weekly basis. The SC housing took place in the same rodent cages described above except that each cage contained 2–3 mice. They received ad libitum food and water as well as Cockatiel vita seed placed at one corner on the same schedule as the EE housing.
Animals were kept in their respective housing conditions for 30 days, during which time handling was kept to a minimum. Each mouse was tested again for stereotypy at the end of the EE or SC housing. Their brains were collected for the CO assessment as described previously.
Results
Mean baseline stereotypy scores assessed 30 days post-weaning were virtually identical for the animals assigned to SC versus EE (t(21)=0.05, p=0.96; see Fig 2). The subsequent 30 days of environmental enrichment significantly attenuated the development of stereotypy, whereas rates of stereotypy continued to increase in SC animals (t(21)=−3.68, p<0.01).
Fig 2.
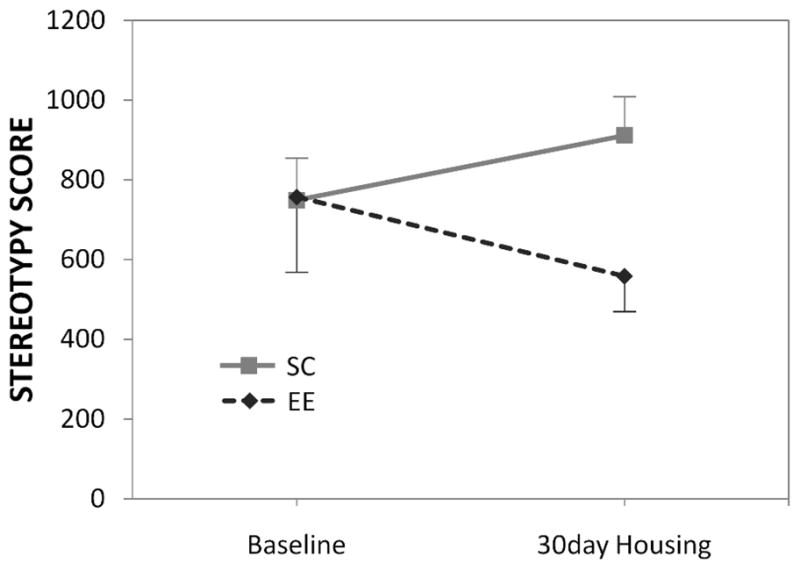
The effect of 30-day EE housing on the stereotypy scores (mean ± SEM).
CO enzymatic activity within selected brain regions is summarized in Table 2. Significant differences were found in STN (t(21)=3.57, p<0.01) as well as SNpc (t(21)=4.16, p<0.01) and SNpr (t(21)=3.74, p<0.01). In these nuclei, EE mice had higher CO activities compared to SC mice.
Table 2.
CO activity (μmol/min/g) of STN and the other brain areas in deer mice housed in the SC and EE conditions. Values expressed are group means with SEM in parentheses.
| SC | EE | P-value | |
|---|---|---|---|
| Subthalamic Nucleus | 64.23 (0.71) | 68.97 (1.15) | <0.01 |
| Motor Cortex | 51.36 (0.58) | 50.62 (0.64) | 0.40 |
| Somatosensory Cortex | 51.36 (0.68) | 51.19 (0.68) | 0.86 |
| Hippocampus | 42.73 (1.12) | 44.83 (0.81) | 0.15 |
| Caudate/Putamen | |||
| Dorsolateral Striatum | 52.31 (0.91) | 52.92 (0.85) | 0.61 |
| Dorsomedial Striatum | 51.23 (1.05) | 53.39 (1.05) | 0.16 |
| Ventrolateral Striatum | 52.65 (0.81) | 52.78 (0.68) | 0.89 |
| Ventromedial Striatum | 50.01 (1.12) | 52.21 (1.02) | 0.16 |
| Nucleus Accumbens | 50.62 (1.29) | 52.28 (0.91) | 0.31 |
| Substantia Nigra pars Compacta | 43.10 (0.88) | 47.30 (0.44) | <0.01 |
| Substantia Nigra pars Reticulata | 47.27 (0.78) | 51.19 (0.71) | <0.01 |
Experiment 2
Experiment 2a: Effects of CGS21680 on Stereotyped Behavior
Methods
Thirty-nine male mice (> 6 weeks post weaning) were randomly assigned to one of four groups and were administered an acute subcutaneous injection of vehicle (n=13), 0.02mg/kg (n=9), 0.03mg/kg (n=9), or 0.05mg/kg (n=8) of 2-p-(2-carboxyethyl)phenethylamino-50-N-ethylcarboxamidoadenosine (CGS21680) (Sigma) dissolved in 5% dimethylsulfoxide (DSMO) in a volume of 10ml/kg of body weight. Drug injections were given two hours before the end of the dark cycle, a time period during which deer mice show higher rates of spontaneous stereotypy (unpublished data). The behavioral response to the drug was assessed starting immediately after drug administration.
Results
Administration of CGS21680 did not significantly alter the frequency of stereotyped behaviors for the 1hr post-injection period at doses up to 0.05mg/kg (F(3,35)=1.45, p=0.25). A preliminary study with a small number of animals indicated that CGS21680 at the 0.1mg/kg level had a non-selective effect on stereotypy as this dose suppressed motor activity in general. Similar effects were also seen during the 30min post-injection period.
Experiment 2b: Effects of Co-administration of CGS21680 and CPA on Stereotyped Behavior
Methods
Eighteen male mice (6 to 8 weeks post-weaning) were administered acute subcutaneous injections of a combination of CGS21680 and the selective A1 receptor agonist N6-cyclopentyladenosine (CPA) (Sigma) dissolved in 5% DSMO. Each mouse received each drug combination (0.03/0.03; 0.05/0.05; and 0.1/0.1mg/kg) and vehicle only, serving as its own control. Each injection was administered one-week apart using successively higher doses and randomizing the order of drug and vehicle. For Experiment 2 (b, c, and d), we employed new testing cages (22 × 28 × 31 cm) made of Plexiglas to replace the old testing cages (22 × 28 × 25 cm).
Results
For all the drug combinations tested (n=18), there was no difference in stereotypy between vehicle and drug groups for the 1hr pre-injection period at 0.03/0.03mg/kg (t(17)=3.28, p<0.01), 0.05/0.05mg/kg (t(17)=−0.60, p=0.56), and 0.1/0.1mg/kg (t(17)=−0.32, p=0.75). For the 1hr post-injection period, drug groups showed significantly lower rates of stereotypy at 0.03/0.03mg/kg (t(17)=3.97, p<0.01), 0.05/0.05mg/kg (t(17)=5.48, p<0.001), and 0.1/0.1mg/kg (t(17)=10.09, p<0.001) when compared to their respective vehicle conditions (Fig 3). Similar drug effects were also seen during the 30min post-injection period.
Fig 3.
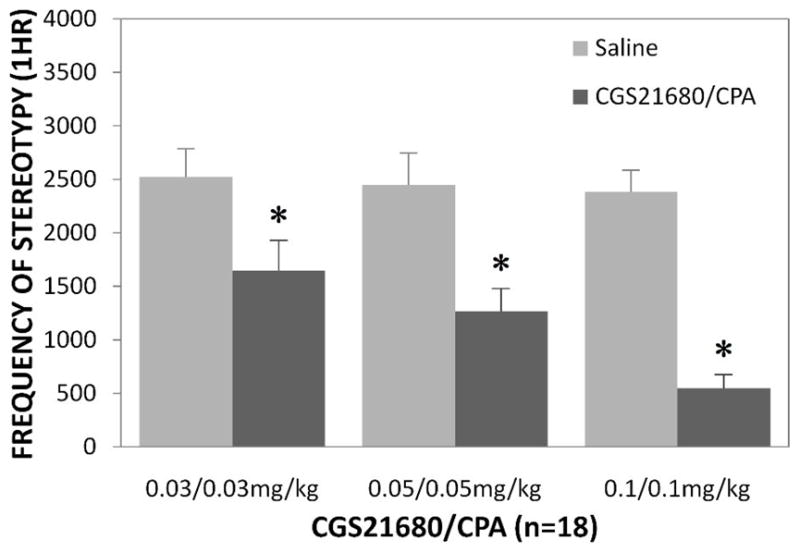
The effects of CGS21680/CPA on stereotypy. The frequency of stereotypy for the 1hr post-injection period (mean ± SEM). * represents statistical significance at p<0.05 as compared to saline group.
To characterize this result further, we used the total pre-injection stereotypy score to categorize mice into high- and low-stereotypy groups based on a median split (n=9 each), and we assessed whether rates of baseline stereotypy predicted the magnitude of the drug response. At the 30min period, there was no difference from pre-injection baseline in high-stereotypy mice (t(8)=0.97, p=0.36) whereas the reduction from baseline for low-stereotypy mice approached significance (t(8)=2.10, p=0.07). This was also seen for the 1hr period in high-stereotypy mice (t(8)=1.00, p=0.35) and in low-stereotypy mice (t(8)=1.91, p=0.09).
The percentage change from baseline also showed that 0.03/0.03mg/kg of CGS21680/CPA was more effective in low-stereotypy mice (63.9% versus 3.5% for vehicle) than in high-stereotypy mice (12.4% reduction versus 0.6% for vehicle). No differential response was seen in high versus low baseline stereotypy mice at 0.05/0.05mg/kg and 0.1/0.1mg/kg.
Experiment 2c: Effects of CGS21680 and CPA in Drug-Naïve Animals
Methods
An independent group of 10 male mice (6 to 8 weeks post-weaning) was used to provide a partial replication of the results described in the prior experiment. In this case, we assessed the effects of 0.05/0.05mg/kg versus vehicle in drug-naïve animals using a cross-over design.
Results
Consistent with what we found previously, there was no difference in the frequency of stereotypy between the drug and vehicle conditions for the 1hr pre-injection periods (t(9)=0.49, p=0.64). Drug-treated animals, however, exhibited significantly less stereotypy for the 1hr post-injection periods compared to vehicle (t(9)=3.40, p=0.01 respectively) (Fig 4). Thus the effect of CGS21680 and CPA within dose combination was replicated in drug-naïve mice. The similar drug effects were also seen during the 30min post-injection period.
Fig 4.
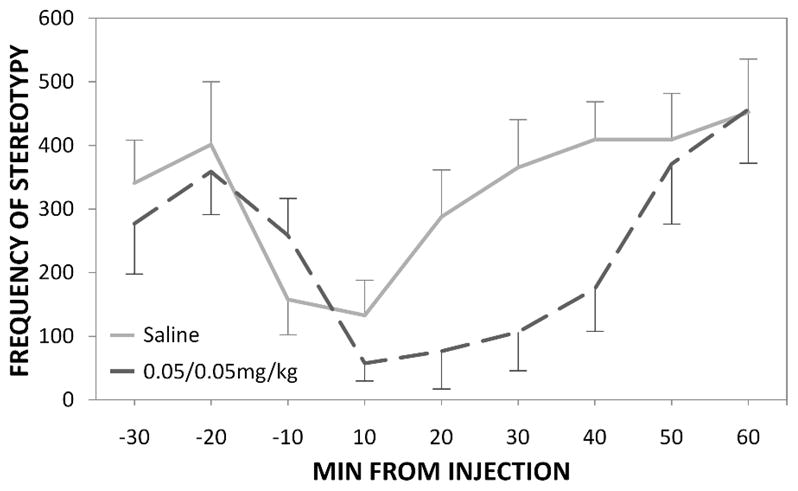
The time-course showing the efficacy of CGS21680/CPA in drug-naïve animals (0.05/0.05mg/kg). Zero at the time of drug or saline injection (mean ± SEM). * represents statistical significance at p<0.05 as compared to saline group.
To determine that the effects of CGS21680/CPA were not solely due to CPA alone, we tested the effect of CPA at 0.05mg/kg using an independent group of animals (n=12) in a crossover design. Administration of CPA alone at 0.05mg/kg induced non-selective motor supression, rendering about a third of animals akinetic.
Experiment 2d: Effects of CGS21680 and CPA on Locomotor Behavior
Methods
To assess how selective the effect of the drug combination was on stereotyped behaviors, the animals from Experiment 2c received an additional administration of vehicle and the CGS21680 and CPA combination (0.05/0.05 and 0.1/0.1mg/kg) in a cross-over design. Their post-injection locomotor activity was tracked for 1 hr using Ethovision (Noldus). One control animal for the 0.05/0.05mg/kg group was excluded from the analysis due to missing data (n=9).
Results
There was a significant difference in distance traveled (cm) for the duration of the 30min post-injection period (t(8)=2.70, p=0.03) but not for the 1hr post-injection period (t(8)=1.88, p=0.10) between vehicle and 0.05/0.05mg/kg groups (Table 3A). There was significant reduction in distance traveled for the 30min post-injection period (t(9)= 4.85, p=0.001) and the 1hr post-injection period between vehicle and 0.1/0.1mg/kg groups (t(9)= 4.58, p=0.001) (Table 3B). There was no difference between 0.05/0.05mg/kg and 0.1/0.1mg/kg groups, however, at either time point. Locomotor activity was significantly correlated with the frequency of stereotypy for the 1hr post-injection period in vehicle-treated mice (r=0.89, p<0.001) but not in drug-treated mice (r=0.51, p=0.13) at 0.05/0.05mg/kg.
Table 3.
Distance traveled immediately after saline or CGS21680/CPA administration (cm). A) 0.05/0.05mg/kg and B) 0.1/0.1mg/kg. Values expressed are group means with SEM in parentheses.
| A | ||
|---|---|---|
| Saline | 0.05/0.05mg/kg CGS21680/CPA | |
| 30min post-injection | 5801.8 (1026.8) | 3479.7 (685.6)* |
| 1hr post-injection | 11554.7 (1730.3) | 8622.6 (1594.6) |
| B | ||
|---|---|---|
| Saline | 0.1/0.1mg/kg CGS21680/CPA | |
| 30min post-injection | 6484.8 (722.9) | 3520.4 (486.3)* |
| 1hr post-injection | 14227.6 (1612.5) | 6649.6 (927.4)* |
represents statistical significance at p<0.01 as compared to saline control group.
Discussion
The present experiments were designed to test the hypothesis that the expression of spontaneous stereotypy in deer mice is linked to decreased activity of the indirect basal ganglia pathway. This hypothesis was based on our previous finding that level of stereotypy in deer mice was negatively correlated with the striatal expression of the neuropeptide enkephalin, whereas no relationship was found between stereotypy and the expression of dynorphin [40].
In support of this hypothesis, CO activity in STN was significantly lower in high-stereotypy mice compared to low-stereotypy mice (Experiment 1a) and in SC mice compared to EE mice (Experiment 1b).
Contrary to our expectation, no difference in CO activity between high- and low-stereotypy mice was found in the dorsolateral striatum, which is typically considered the sensorimotor area of the striatum. The medial aspect of the dorsal striatum, however, showed lower CO activity in high- compared to low-stereotypy mice (Experiment 1a), although a similar difference in the dorsomedial striatum was not found between SC and EE mice (Experiment 1b). The dorsomedial striatum has been implicated in the mediation of behavioral flexibility [43]. We have previously shown that high rates of stereotyped jumping in deer mice were associated with perseverative behavior in a reversal learning task [53], further supporting alterations of the dorsomedial striatum in stereotypic deer mice.
In addition, we also found significantly higher CO activity in SNpc and SNpr in EE versus SC mice (Experiment 1b). For SNpc, this effect is likely due to the monosynaptic excitatory STN-SNpc projection in rodents and non-human primates widely reported in the literature [8, 19, 26, 47]. This difference, however, was not found between high- and low-stereotypy mice (Experiment 1a). For SNpr, increased CO staining was associated with lower stereotypy in both Experiments 1a and 1b. These differences are consistent with higher glutamatergic activation from STN.
If high rates of spontaneous stereotypy are associated with decreases in indirect pathway activation, then stimulation of A2A receptors, which are expressed on striatopallidal neurons and activate Gs/olf proteins upon stimulation, should attenuate stereotypy. When administered alone, the selective A2A receptor agonist CGS21680 failed to reduce stereotypy up to 0.05mg/kg (Experiment 2a). In a preliminary study, 0.1mg/kg of CGS21680 non-selectively reduced motor activity, rendering some mice akinetic. The addition of CPA to CGS21680, however, selectively attenuated stereotypy in a dose-dependent manner without adverse suppression of general motor activity (Experiment 2b, c, and d) even at the 0.1 mg/kg dose. Although the lowest dose of CGS21680/CPA significantly attenuated stereotypy compared to vehicle controls, a subsequent analysis suggested that it was not effective in mice exhibiting higher rates of baseline stereotypy. Higher doses of CGS21680/CPA were required to attenuate stereotypy in this group. These pharmacological results indicate that higher doses of the drug combination may be required to drive the activity of the indirect pathway to levels associated with low stereotypy.
The relative efficacy of the combined stimulation of A2A and A1 receptors compared to A2A alone may be explained by the results reported by Karcz-Kubicha et al. [23, 24]. In this work, administration of an A1 or A2A receptor agonist alone did not induce striatal c-Fos expression. Stimulation of both receptor subtypes, however, did induce striatal c-Fos expression and in a selective fashion with activation seen in striatopallidal, but not striatonigral, neurons. This combined treatment of A1 and A2A receptor agonists also increased striatal enkephalin expression. It should be noted, however, that these effects were seen at higher doses (0.5 mg/kg CGS21680 and 0.3 mg/kg CPA) that were used in the present experiments. Similarly, administration of an A2A receptor antagonist alone produced either no effect or only a slight decrease in c-Fos and preproenkepahlin expression in the striatum [1, 2, 24, 29, 31, 36, 50, 56]. This is consistent with our unpublished data showing that administration of the A2A receptor antagonist SCH58261 failed to induce or exacerbate repetitive behavior.
The mechanisms to account for these effects likely involve functional antagonistic interactions of A2A and D2 receptors on adenynyl cyclase that regulate the cAMP-PKA signaling pathway in striatopallidal neurons. Tonic inhibition of D2 receptors attenuates the ability of an A2A agonist to stimulate this signaling pathway and its downstream effects on c-Fos and preproenkephalin. Moreover, A2A receptor agonist administration has been reported to increase striatal dopamine release, possibly indirectly through A2A receptors on presynaptic glutamate terminals. [14, 24, 42]. Conversely, A1 receptor agonist administration has been reported to decrease striatal dopamine release [34, 42] through A1 receptors on presynaptic dopaminergic terminals where upon stimulation, release of dopamine is directly inhibited via activation of Gi/o protein [6, 24, 61]. Stimulation of presynaptic A1 receptors by CPA allows A2A receptors on striatopallidal neurons to overcome tonic inhibition by D2 receptors to activate the cAMP-PKA pathway. Moreover, the effect of the A1 and A2A receptor heteromeric complex on glutamate release from corticostriatal neurons is dependent on the local concentration of adenosine [9], and so the drug effects reported here may also be due, at least in part, to alterations in glutamate release.
The biochemical and pharmacological findings presented here provide additional, important support for the role of the indirect pathway in mediating repetitive behavior in our model. These findings are consistent with both clinical and animal studies that have found a link between repetitive behavior and the indirect basal ganglia pathway. For example, the uncontrolled motor movements characteristic of Huntington’s disease are attributed to the differential degeneration of striatopallidal neurons [11, 49]. Deep brain stimulation (DBS) applied to STN reduced the severity of symptoms in previously treatment refractory OCD patients [7]. Similarly, DBS of STN and GPe has been found to be effective in ameliorating the L-DOPA induced tardive dyskinesia observed in Parkinson’s disease (e.g., [28]). In animal models, Grabli et al. [16] have reported that stereotyped behavior (e.g., licking and biting of fingers) was induced in monkeys when the GABA antagonist bicuculline was microinjected into the limbic aspect of the GPe, which was reduced by DBS applied to STN without affecting a control motor task [3]. Winter et al. [59] have shown that rats that sustained ibotenic acid lesions to STN exhibited an increase in compulsive lever pressing in the signal attenuation model of OCD. This same research group has also shown that bilateral high frequency stimulation of STN as well as pharmacological inactivation of STN reduced quinpirole-induced compulsive checking in rats [27, 58, 60].
In addition to identifying pathophysiological changes associated with repetitive behavior, our pharmacological findings also point to novel targets for development of drug therapies to treat repetitive behavior in clinical disorders such as autism. Currently, the two drug classes used to treat such behaviors in neurodevelopmental disorders include atypical antipsychotics and selective serotonin re-uptake inhibitors (SSRIs). A recent multi-site study of the SSRI citalopram provided no evidence for its efficacy in treating repetitive behavior in autism [25]. There is limited evidence for the utility of atypical antipsychotics in treating repetitive behavior. Risperidone has recently been FDA approved for use in autism to treat irritability with no approval sought for repetitive behavior. Thus, there is a pressing need for the development of effective drug treatments, based on an understanding of the specific pathophysiological mechanisms of repetitive behaviors.
The present finding suggests that a combination of A2A receptor agonist and A1 receptor agonist to drive indirect pathway activity reduce repetitive behaviors selectively, whereas either drug alone induce no or non-selective effect on behaviors respectively. Although cardiovascular relaxation induced by adenosine receptor agonists could be a possible drawback (e.g., [37, 52]), these findings suggest drug development efforts that may be potentially useful in treating repetitive behaviors in neurodevelopmental disorders.
Acknowledgments
This work was supported by NIH grant MH080055.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aoyama S, Kase H, Borrelli E. Rescue of locomotor impairment in dopamine D2 receptor-deficient mice by an adenosine A2A receptor antagonist. J Neurosci. 2000;20:5848–5852. doi: 10.1523/JNEUROSCI.20-15-05848.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoyama S, Koga K, Mori A, Miyaji H, Sekine S, Kase H, et al. Distribution of adenosine A(2A) receptor antagonist KW-6002 and its effect on gene expression in the rat brain. Brain Res. 2002;953:119–125. doi: 10.1016/s0006-8993(02)03277-8. [DOI] [PubMed] [Google Scholar]
- 3.Baup N, Grabli D, Karachi C, Mounayar S, Francois C, Yelnik J, et al. High-frequency stimulation of the anterior subthalamic nucleus reduces stereotyped behaviors in primates. J Neurosci. 2008;28:8785–8788. doi: 10.1523/JNEUROSCI.2384-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkson G, Andriacchi T, Sherman L. More information on the nature of stereotyped body-rocking. Am J Ment Retard. 2001;106:205–208. doi: 10.1352/0895-8017(2001)106<0205:MIOTNO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Berkson G, Rafaeli-Mor N, Tarnovsky S. Body-rocking and other habits of college students and persons with mental retardation. Am J Ment Retard. 1999;104:107–116. doi: 10.1352/0895-8017(1999)104<0107:BAOHOC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Borycz J, Pereira MF, Melani A, Rodrigues RJ, Kofalvi A, Panlilio L, et al. Differential glutamate-dependent and glutamate-independent adenosine A1 receptor-mediated modulation of dopamine release in different striatal compartments. J Neurochem. 2007;101:355–363. doi: 10.1111/j.1471-4159.2006.04386.x. [DOI] [PubMed] [Google Scholar]
- 7.Burdick A, Goodman WK, Foote KD. Deep brain stimulation for refractory obsessive-compulsive disorder. Front Biosci. 2009;14:1880–1890. doi: 10.2741/3348. [DOI] [PubMed] [Google Scholar]
- 8.Chergui K, Akaoka H, Charlety PJ, Saunier CF, Buda M, Chouvet G. Subthalamic nucleus modulates burst firing of nigral dopamine neurons via NMDA receptors. Neuroreport. 1994;5(10):1185–1188. doi: 10.1097/00001756-199406020-00006. [DOI] [PubMed] [Google Scholar]
- 9.Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, et al. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuccaro ML, Shao Y, Grubber J, Slifer M, Wolpert CM, Donnelly SL, et al. Factor analysis of restricted and repetitive behaviors in autism using the Autism Diagnostic Interview-R. Child Psychiatry Hum Dev. 2003;34:3–17. doi: 10.1023/a:1025321707947. [DOI] [PubMed] [Google Scholar]
- 11.Deng YP, Albin RL, Penney JB, Young AB, Anderson KD, Reiner A. Differential loss of striatal projection systems in Huntington’s disease: a quantitative immunohistochemical study. J Chem Neuroanat. 2004;27:143–164. doi: 10.1016/j.jchemneu.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Evans DW, Leckman JF, Carter A, Reznick JS, Henshaw D, King RA, et al. Ritual, habit, and perfectionism: the prevalence and development of compulsive-like behavior in normal young children. Child Dev. 1997;68:58–68. [PubMed] [Google Scholar]
- 13.Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, et al. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 14.Golembiowska K, Zylewska A. Adenosine receptors--the role in modulation of dopamine and glutamate release in the rat striatum. Pol J Pharmacol. 1997;49:317–322. [PubMed] [Google Scholar]
- 15.Gonzalez-Lima Jones. Cytochrome oxidase in neuronal metabolism and Alzheimer’s disease. New York and London: Plenum Press; 1998. [Google Scholar]
- 16.Grabli D, McCairn K, Hirsch EC, Agid Y, Feger J, Francois C, et al. Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study Brain. 2004;127:2039–2054. doi: 10.1093/brain/awh220. [DOI] [PubMed] [Google Scholar]
- 17.Graybiel AM. The basal ganglia. Curr Biol. 2000;10:R509–511. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- 18.Hadley C, Hadley B, Ephraim S, Yang M, Lewis MH. Spontaneous stereotypy and environmental enrichment in deer mice (Peromyscus maniculatus): Reversibility of experience. Applied Animal Behaviour Science. 2006;97:312–322. [Google Scholar]
- 19.Hammond C, Deniau JM, Rizk A, Feger J. Electrophysiological demonstration of an excitatory subthalamonigral pathway in the rat. Brain Res. 1978;151(2):235–244. doi: 10.1016/0006-8993(78)90881-8. [DOI] [PubMed] [Google Scholar]
- 20.Hevner RF, Wong-Riley MT. Brain cytochrome oxidase: purification, antibody production, and immunohistochemical/histochemical correlations in the CNS. J Neurosci. 1989;9:3884–3898. doi: 10.1523/JNEUROSCI.09-11-03884.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, et al. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biol Psychiatry. 2005;58:226–232. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 22.Jarvis MF, Williams M. Direct autoradiographic localization of adenosine A2 receptors in the rat brain using the A2-selective agonist, [3H]CGS 21680. Eur J Pharmacol. 1989;168:243–246. doi: 10.1016/0014-2999(89)90571-2. [DOI] [PubMed] [Google Scholar]
- 23.Karcz-Kubicha M, Ferre S, Diaz-Ruiz O, Quiroz-Molina C, Goldberg SR, Hope BT, et al. Stimulation of adenosine receptors selectively activates gene expression in striatal enkephalinergic neurons. Neuropsychopharmacology. 2006;31:2173–2179. doi: 10.1038/sj.npp.1301035. [DOI] [PubMed] [Google Scholar]
- 24.Karcz-Kubicha M, Quarta D, Hope BT, Antoniou K, Muller CE, Morales M, et al. Enabling role of adenosine A1 receptors in adenosine A2A receptor-mediated striatal expression of c-fos. Eur J Neurosci. 2003;18:296–302. doi: 10.1046/j.1460-9568.2003.02747.x. [DOI] [PubMed] [Google Scholar]
- 25.King BH, Hollander E, Sikich L, McCracken JT, Scahill L, Bregman JD, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66:583–590. doi: 10.1001/archgenpsychiatry.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kita H, Kitai ST. Efferent projections of the subthalamic nucleus in the rat: light and electron microscopic analysis with the PHA-L method. J Comp Neurol. 1987;260(3):435–452. doi: 10.1002/cne.902600309. [DOI] [PubMed] [Google Scholar]
- 27.Klavir O, Flash S, Winter C, Joel D. High frequency stimulation and pharmacological inactivation of the subthalamic nucleus reduces ‘compulsive’ lever-pressing in rats. Exp Neurol. 2009;215:101–109. doi: 10.1016/j.expneurol.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21 (Suppl 14):S290–304. doi: 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- 29.Le Moine C, Svenningsson P, Fredholm BB, Bloch B. Dopamine-adenosine interactions in the striatum and the globus pallidus: inhibition of striatopallidal neurons through either D2 or A2A receptors enhances D1 receptor-mediated effects on c-fos expression. J Neurosci. 1997;17:8038–8048. doi: 10.1523/JNEUROSCI.17-20-08038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis MH, Bodfish JW. Repetitive behavior diosrders in autism. Mental Retard Dev Disabil. 1998;4:80–89. [Google Scholar]
- 31.Lundblad M, Vaudano E, Cenci MA. Cellular and behavioural effects of the adenosine A2a receptor antagonist KW-6002 in a rat model of l-DOPA-induced dyskinesia. J Neurochem. 2003;84:1398–1410. doi: 10.1046/j.1471-4159.2003.01632.x. [DOI] [PubMed] [Google Scholar]
- 32.Mahone EM, Bridges D, Prahme C, Singer HS. Repetitive arm and hand movements (complex motor stereotypies) in children. J Pediatr. 2004;145:391–395. doi: 10.1016/j.jpeds.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 33.O’Sullivan RL, Rauch SL, Breiter HC, Grachev ID, Baer L, Kennedy DN, et al. Reduced basal ganglia volumes in trichotillomania measured via morphometric magnetic resonance imaging. Biol Psychiatry. 1997;42:39–45. doi: 10.1016/S0006-3223(96)00297-1. [DOI] [PubMed] [Google Scholar]
- 34.Okada M, Mizuno K, Kaneko S. Adenosine A1 and A2 receptors modulate extracellular dopamine levels in rat striatum. Neurosci Lett. 1996;212:53–56. doi: 10.1016/0304-3940(96)12780-4. [DOI] [PubMed] [Google Scholar]
- 35.Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- 36.Pinna A, Wardas J, Cristalli G, Morelli M. Adenosine A2A receptor agonists increase Fos-like immunoreactivity in mesolimbic areas. Brain Res. 1997;759:41–49. doi: 10.1016/s0006-8993(97)00214-x. [DOI] [PubMed] [Google Scholar]
- 37.Ponnoth DS, Sanjani MS, Ledent C, Roush K, Krahn T, Mustafa SJ. Absence of adenosine-mediated aortic relaxiation in A2A adenosine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2009;297(5):1655–1660. doi: 10.1152/ajpheart.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powell SB, Newman HA, Pendergast JF, Lewis MH. A rodent model of spontaneous stereotypy: initial characterization of developmental, environmental, and neurobiological factors. Physiol Behav. 1999;66:355–363. doi: 10.1016/s0031-9384(98)00303-5. [DOI] [PubMed] [Google Scholar]
- 39.Presti MF, Gibney BC, Lewis MH. Effects of intrastriatal administration of selective dopaminergic ligands on spontaneous stereotypy in mice. Physiol Behav. 2004;80:433–439. doi: 10.1016/j.physbeh.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Presti MF, Lewis MH. Striatal opioid peptide content in an animal model of spontaneous stereotypic behavior. Behav Brain Res. 2005;157:363–368. doi: 10.1016/j.bbr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Presti MF, Mikes HM, Lewis MH. Selective blockade of spontaneous motor stereotypy via intrastriatal pharmacological manipulation. Pharmacol Biochem Behav. 2003;74:833–839. doi: 10.1016/s0091-3057(02)01081-x. [DOI] [PubMed] [Google Scholar]
- 42.Quarta D, Borycz J, Solinas M, Patkar K, Hockemeyer J, Ciruela F, et al. Adenosine receptor-mediated modulation of dopamine release in the nucleus accumbens depends on glutamate neurotransmission and N-methyl-D-aspartate receptor stimulation. J Neurochem. 2004;91:873–880. doi: 10.1111/j.1471-4159.2004.02761.x. [DOI] [PubMed] [Google Scholar]
- 43.Ragozzino ME, Mohler EG, Prior M, Palencia CA, Rozman S. Acetylcholine activity in selective striatal regions supports behavioral flexibility. Neurobiol Learn Mem. 2009;91:13–22. doi: 10.1016/j.nlm.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakata JT, Crews D, Gonzalez-Lima F. Behavioral correlates of differences in neural metabolic capacity. Brain Res Brain Res Rev. 2005;48:1–15. doi: 10.1016/j.brainresrev.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferre S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J. An MRI study of the basal ganglia in autism. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:613–624. doi: 10.1016/s0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- 47.Shimo Y, Wichmann T. Neuronal activity in the subthalamic nucleus modulates the release of dopamine in the monkey striatum. Eur J Neurosc. 2009;29:104–113. doi: 10.1111/j.1460-9568.2008.06565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singer HS. Motor stereotypies. Semin Pediatr Neurol. 2009;16:77–81. doi: 10.1016/j.spen.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Starr PA, Kang GA, Heath S, Shimamoto S, Turner RS. Pallidal neuronal discharge in Huntington’s disease: support for selective loss of striatal cells originating the indirect pathway. Exp Neurol. 2008;211:227–233. doi: 10.1016/j.expneurol.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Svenningsson P, Lindskog M, Rognoni F, Fredholm BB, Greengard P, Fisone G. Activation of adenosine A2A and dopamine D1 receptors stimulates cyclic AMP-dependent phosphorylation of DARPP-32 in distinct populations of striatal projection neurons. Neuroscience. 1998;84:223–228. doi: 10.1016/s0306-4522(97)00510-1. [DOI] [PubMed] [Google Scholar]
- 51.Szatmari P, Georgiades S, Bryson S, Zwaigenbaum L, Roberts W, Mahoney W, et al. Investigating the structure of the restricted, repetitive behaviours and interests domain of autism. J Child Psychol Psychiatry. 2006;47:582–590. doi: 10.1111/j.1469-7610.2005.01537.x. [DOI] [PubMed] [Google Scholar]
- 52.Tabrizchi R, Bedi S. Pharmacology of adenosine receptors in the vasculature. Pharmacol Ther. 2001;91(2):133–147. doi: 10.1016/s0163-7258(01)00152-8. [DOI] [PubMed] [Google Scholar]
- 53.Tanimura Y, Yang MC, Lewis MH. Procedural learning and cognitive flexibility in a mouse model of restricted, repetitive behaviour. Behav Brain Res. 2008;189:250–256. doi: 10.1016/j.bbr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Thelen E. Rhythmical stereotypies in normal human infants. Anim Behav. 1979;27:699–715. doi: 10.1016/0003-3472(79)90006-x. [DOI] [PubMed] [Google Scholar]
- 55.Turner CA, Yang MC, Lewis MH. Environmental enrichment: effects on stereotyped behavior and regional neuronal metabolic activity. Brain Res. 2002;938:15–21. doi: 10.1016/s0006-8993(02)02472-1. [DOI] [PubMed] [Google Scholar]
- 56.Wardas J, Pietraszek M, Dziedzicka-Wasylewska M. SCH 58261, a selective adenosine A2A receptor antagonist, decreases the haloperidol-enhanced proenkephalin mRNA expression in the rat striatum. Brain Res. 2003;977:270–277. doi: 10.1016/s0006-8993(03)02759-8. [DOI] [PubMed] [Google Scholar]
- 57.Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winter C, Flash S, Klavir O, Klein J, Sohr R, Joel D. The role of the subthalamic nucleus in “compulsive” behavior in rats. Eur J Neurosci. 2008;27:1902–1911. doi: 10.1111/j.1460-9568.2008.06148.x. [DOI] [PubMed] [Google Scholar]
- 59.Winter C, Lemke C, Sohr R, Meissner W, Harnack D, Juckel G, et al. High frequency stimulation of the subthalamic nucleus modulates neurotransmission in limbic brain regions of the rat. Exp Brain Res. 2008;185:497–507. doi: 10.1007/s00221-007-1171-1. [DOI] [PubMed] [Google Scholar]
- 60.Winter C, Mundt A, Jalali R, Joel D, Harnack D, Morgenstern R, et al. High frequency stimulation and temporary inactivation of the subthalamic nucleus reduce quinpirole-induced compulsive checking behavior in rats. Exp Neurol. 2008;210:217–228. doi: 10.1016/j.expneurol.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 61.Yabuuchi K, Kuroiwa M, Shuto T, Sotogaku N, Snyder GL, Higashi H, et al. Role of adenosine A1 receptors in the modulation of dopamine D1 and adenosine A2A receptor signaling in the neostriatum. Neuroscience. 2006;141:19–25. doi: 10.1016/j.neuroscience.2006.04.047. [DOI] [PubMed] [Google Scholar]


