Abstract
Background
In persons with coronary artery disease (CAD), low body mass index is associated with greater mortality, however it is uncertain if low muscle mass is a risk factor for mortality in this setting.
Methods and Results
903 individuals with CAD provided 24-hour urine collections. We measured urine creatinine and volume, and calculated creatinine excretion rate (CER), a marker of muscle mass. Cox proportional hazards models evaluated the association of CER with mortality risk during follow-up. Two-hundred thirty-two participants (26%) died over a median follow-up of 6.0 years. Compared to the highest sex-specific CER tertile, the lowest tertile (< 1,068 mg/day in men, < 766 mg/day in women) was associated with > 2-fold risk of mortality (hazard ratio [HR] 2.30; 95% confidence interval [CI] 1.51–3.51) in models adjusted for age, sex, race, cystatin C-based eGFR, body mass index, traditional CVD risk factors, and C-reactive protein levels. The association was essentially unaltered with further adjustment for physical fitness, left ventricular (LV) mass, LV ejection fraction, or fasting insulin and glucose levels.
Conclusions
Lower CER is strongly associated with mortality in outpatients with CAD, independent of conventional measures of body composition, kidney function, and traditional CAD risk factors. Future studies should determine whether low CER may be a modifiable risk factor for mortality among persons with CAD, potentially through resistive exercise training or nutrition interventions.
Keywords: Cardiovascular disease, mortality, lean mass, muscle mass, creatinine
INTRODUCTION
While obesity is a risk factor for incident coronary artery disease (CAD), its significance in individuals with established CAD has recently been questioned. In a meta-analysis that included 40 studies and over 250,000 individuals with CAD, there was a reverse J-shaped relationship between body mass index (BMI) and mortality; individuals with the BMI < 20.0 had the greatest mortality risk, while individuals with obesity (BMI 30.0–34.9) or severe obesity (BMI > 35) were not at greater risk than normal weight individuals (BMI 20.0–24.9).1 Because BMI does not discriminate between its relative contributions from adiposity or muscle, this finding has led many to hypothesize that the high death risk associated with low BMI may be due to deficiency in muscle rather than adiposity.2–4 Implicit is the hypothesis that low muscle mass may be a risk factor for mortality in persons with established CAD. Yet, to our knowledge, this association has not been studied in the setting of prevalent CAD.
It is estimated that > 98% of creatinine comes from muscle,5 where it is produced and secreted into serum at a continuous rate.6 Once in serum, creatinine is almost exclusively excreted in the urine in individuals without severe kidney failure.7, 8 Because muscle mass does not change rapidly within individuals, elevations in serum creatinine typically reflect decrements in glomerular filtration rate. When the serum creatinine concentration is in steady state, however, regardless of its serum concentration, creatinine generation must equal creatinine excretion. Thus, the urinary creatinine excretion rate has been recognized as a marker of muscle mass for nearly a century.9 Here, we evaluate the association of CER with all-cause mortality among a cohort of out-patients with stable CAD. Based on the relationship of low BMI with mortality reported elsewhere, we hypothesized that lower CER would be associated with mortality independent of traditional CAD risk factors and kidney function.
METHODS
Study Participants
The Heart and Soul Study is an observational study designed to investigate the influence of psychosocial factors on progression of CAD. Methods have been described previously.10 Briefly, participants were recruited from out-patient clinics in the San Francisco Bay Area if they met one of the following inclusion criteria: (i) history of myocardial infarction; (ii) angiographic evidence of >50% stenosis in one or more coronary vessels; (iii) evidence of exercise-induced ischemia by treadmill or nuclear testing; (iv) history of coronary revascularization or (v) documented diagnosis of coronary artery disease by an internist or cardiologist. Participants were excluded if they were not able to walk one block, had a myocardial infarction within the past 6 months or were likely to move out of the area within three years. The study protocol was approved by the Institutional Review Boards of participating institutions, and all participants provided written informed consent.
Between September 2000 and December 2002, 1,024 participants enrolled and underwent a daylong baseline study appointment that included a medical history, physical examination, and a comprehensive health status questionnaire. Out-patient 24-hour timed urine collections, and fasting (12 h) morning venous blood samples were obtained. For the present analysis, we excluded 107 participants with missing timed urine collections, and 20 individuals without complete covariate data, providing a final analytic sample of 903 participants. Participants were followed for death through December 1, 2008. By this date, 38 (3.7%) participants had been lost to follow-up.
Measurements
Urinary Creatinine Excretion Rate
The protocol used for timed urine collection has been described previously.11 In brief, participants received detailed instructions regarding accurate urine collection and specimen refrigeration. Subjects were asked to void at the end of their study appointment, and to begin the collection from that point forward. Research personnel arrived at the patient's home 24-hours after the timed collection was initiated, to avoid over- or under-collection. If participants reported missing any urine, or collections were < one liter or > three liters, collections were repeated. When participants were unable to collect all urine for any reason, no data were recorded. Urine volume was recorded (ml), and creatinine was measured by the rate Jaffe method. CER was calculated in mg/day (urine volume [ml] * urine creatinine [mg/dL]/100).
Mortality
Between the baseline examination and December 1, 2008, annual telephone interviews were conducted with study participants (or their proxy) for vital status. For any reported event, medical records, death certificates, and coroner's reports were retrieved. Date of death was recorded to provide time-to-event data from the baseline examination.
Other Participant Characteristics and Laboratory Tests
Age, sex, and race/ethnicity were determined by questionnaire. Weight and height were measured in light clothes and without shoes. Body mass index (BMI) was calculated (wt [kg]/height [meters]2). Waist and hip circumferences were measured with a flexible plastic measure to the nearest 0.1 centimeter. Waist circumference was measured midway between the lower rib margin and iliac crest. Hip circumference was measured at the level of the greater trochanters. Waist to hip ratio (WHR) was calculated. Serum cystatin-C concentrations were measured with a particle-enhanced immunonephelometric assay12 (N Latex Cystatin-C, Dade Behring, Inc.) and used to calculate estimated glomerular filtration rate (eGFRcys) using the formula eGFRcys= 76.7 * cystatin C−1.19. This formula has been validated with comparison to iothalamate measured GFR in a pooled cohort of kidney disease studies; showed little bias, and provided a non-creatinine based method to adjust for kidney function for this study.13 History of smoking, hypertension, and diabetes were determined by questionnaire. Systolic and diastolic blood pressures were measured after five minutes rest in the supine position. Total cholesterol, and high-density lipoprotein (HDL) cholesterol were measured using standard clinical chemistry analyzers. High sensitivity C-reactive protein (hsCRP) was measured with the Roche (Indianapolis, Indiana) and the Beckman Extended Range (Galway, Ireland) assays.14 Fasting glucose was measured by a standard clinical analyzer, and fasting insulin was measured by ELISA (Linco Research, St. Charles, MO). Participants provided rest transthorasic echocardiograms that were read by one expert cardiologist who was blinded to all clinical data, as described previously.15 Left ventricular (LV) mass was calculated using the truncated ellipsoid method,16 and was indexed to body surface area. LV ejection fraction was determined by biplane method of disks.17 Thereafter, subjects underwent exercise treadmill test by the modified Bruce protocol. Max number of metabolic equivalents (METs) and maximum heart rate achieved were recorded.
Statistical Analysis
Exploratory analyses demonstrated that the distribution of CER was approximately Gaussian, but differed substantially in men and women. Thus, for Kaplan Meier survival estimates and Cox models, we evaluated sex-specific tertiles of CER as the primary predictor variable. In companion analyses, we evaluated CER as a continuous predictor, defined as one standard deviation lower level in the unadjusted distribution of CER in our dataset. Other secondary predictor variables that were measured on a continuous scale were also evaluated per standard deviation lower level to facilitate comparisons of their strengths of associations with mortality.
We used linear regression to evaluate age and sex adjusted association of key body composition variables, kidney function, and inflammatory markers with CER. Next, we explored the age and sex adjusted functional form of body composition measurements with CER using generalized additive models to fit a cubic B-spline function. The extreme five percent of CER measurements were excluded to avoid implausible extrapolations of the functional form from the extremes of the data distribution. Next, Kaplan Meier survival curves were developed to explore the unadjusted association of CER tertiles with mortality. Subsequently, Cox proportional hazards models were used to evaluate this association while adjusting for potential confounders. Sequential models were developed: model one was unadjusted; model two adjusted for age and sex; and model three adjusted for model two variables plus BMI. Sensitivity analyses were conducted to determine whether results were similar if weight, waist circumference, hip circumference, or WHR replaced BMI in model three. A final model adjusted for the model three variables plus African-American race, eGFR, natural log of C-reactive protein and traditional CAD risk factors (diabetes, hypertension, smoking, systolic blood pressure, diastolic blood pressure, total and HDL cholesterol). The functional forms of each continuous variable with mortality were investigated, and when non-linear associations were observed, appropriate categorizations were employed to capture the observed functional form. Last, we explored the functional form of CER with mortality in the fully adjusted model using a cubic B-spline function. To investigate whether low CER identifies individuals with occult systemic illness which may have led to higher short-term mortality risk, we evaluated a time interaction term, dichotomized at three years after baseline. Last, multiplicative interaction terms were created to evaluate whether results were modified by sex, and analyses were stratified by sex. Results were similar, so only models combining both sexes are presented to maximize statistical power.
To determine whether any association between CER and mortality may be due to incomplete urine collections in patients with low CER, we performed a sensitivity analysis excluding participants whose measured 24 hour urinary creatinine clearance was more than 30% different than their eGFRcys.. This takes advantage of the fact that CER divided by serum creatinine equals creatinine clearance (in ml/min); an estimate of GFR.18 eGFRcys provided another estimate of GFR, yet is independent of the quality of the timed urine collection and of creatinine kinetics. Creatinine clearance was normalized to body surface area (BSA) because eGFRcys is calculated as an estimate of GFR normalized to BSA, to facilitate comparison of the two GFR estimates.
Proportional hazards assumptions were assessed by visually inspecting log-minus-log plots and plots of Schoenfeld residuals vs. survival time. No evidence of violations was observed. Analyses were performed using STATA Statistical Software, version 9.2 (College Station, TX). P values < 0.05 were considered statistically significant for all comparisons including multiplicative interaction terms.
RESULTS
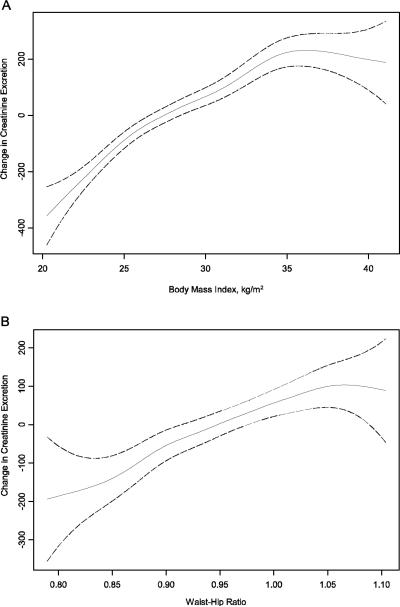
Baseline characteristics of the study participants are shown in Table 1. The mean age of the 903 participants was 67 ± 11 years, 32% were obese, and 31% had moderate CKD determined by cystatin C. The mean CER was 1197 ± 412 mg/day. Table 2 shows the age and sex adjusted associations of demographics, body composition, kidney function, physical fitness variables, and LV mass and LV ejection fraction with CER. As expected, younger participants and men had higher CER, and greater body mass by each measurement was associated with greater CER. When assessed by the relative difference in CER, the association was strongest for weight followed by BMI, whereas WHR had the weakest association with CER. Age and sex adjusted spline functions demonstrated that these associations were fairly linear across the distribution of body composition measures (Figure 1). Individuals achieving greater METs and maximum heart rate on the Bruce treadmill test had greater CER. Individuals with greater CER also had greater LV ejection fraction on average, although the association did not reach statistical significance. Lower eGFRcys was also associated with lower CER, but this association was modest in strength. African American race, C-reactive protein levels, and LV mass were not significantly associated with CER.
Table 1.
Characteristics of the Heart and Soul Cohort at Baseline
| n | 903 |
|---|---|
| Age (years) | 67 ± 11 |
| Male | 82% (741) |
| African American | 15% (137) |
| Diabetes | 26% (237) |
| Hypertension | 71% (639) |
| Current Smoking | 19% (175) |
| History of MI | 54% (483) |
| History of Stroke | 14% (122) |
| History of Coronary Revascularization | 53% (538) |
| LV mass index (g/m2) | 98 ± 26 |
| LV ejection fraction (%) | 62 ± 10 |
| Bruce Treadmill Data | |
| METS Achieved | 7.3 ± 3.3 |
| Maximum Heart Rate Achieved (beats/min) | 130 ± 25 |
| BMI (kg/m2) | 28 ± 5 |
| Obese | 32% (286) |
| WHR | 0.95 ± 0.08 |
| Total Chol. (mg/dL) | 177 ± 42 |
| HDL Chol. (mg/dL) | 45 ± 14 |
| Triglycerides (mg/dL) | 142 ± 130 |
| 24-Hour Urine Creatinine Clearance (ml/min) | 82 ± 29 |
| CKDCrCl (CrCl < 60 ml/min) | 24% (220) |
| eGFRCys (ml/min/1.73m2) | 71 ± 23 |
| CKDCys (eGFRCys < 60ml/min/1.73m2) | 31% (277) |
| CER (mg/day) | 1197 ± 412 |
Data show means ± SD or percent prevalence.
MI = myocardial infarction, LV = left ventricle, BMI = body mass index, WHR = waist hip ratio, Chol. = cholesterol, eGFR = estimated glomerular filtration rate, CKD = chronic kidney disease, CER = creatinine excretion rate.
Table 2.
Age and Sex Adjusted Associations of Variables with Creatinine Excretion Rate†
| Correlate | Difference in CER (mg/day) | (95% CI) | P-value |
|---|---|---|---|
| Age (per 11 years greater)* | −161 | −184 to −138 | < 0.001 |
| Male sex | 421 | 360 to 481 | < 0.001 |
| African American (compared to all other races) | 22 | −44 to 87 | 0.52 |
| Weight (per 18kg greater)* | 169 | 148 to 191 | < 0.001 |
| Height (per 9cm greater)* | 112 | 86 to 138 | < 0.001 |
| BMI (per 5.4kg/m2 greater)* | 139 | 118 to 161 | < 0.001 |
| Waist Circ. (per 15cm greater)* | 129 | 107 to 151 | < 0.001 |
| Hip Circ. (per 14 cm greater)* | 120 | 97 to 142 | < 0.001 |
| Waist Hip Ratio (per 0.8 greater)* | 70 | 44 to 96 | < 0.001 |
| Maximum Heart Rate on Bruce Treadmill Test (per 25 beats/min greater)* | 37 | 12 to 62 | 0.004 |
| METS Achieved on Bruce Treadmill Test (per 3.34 METS greater)* | 32 | 5 to 58 | 0.02 |
| LV Ejection Fraction (per 10% greater)* | 21 | −3 to 44 | 0.08 |
| eGFRCys (per 22ml/min/1.73m2 greater)* | 13 | 10 to 15 | < 0.001 |
| Ln-CRP (per 1.3 greater)* | 8 | −15 to 32 | 0.48 |
| LV Mass Index (per 26.3 g/m2 greater)* | −3 | −26 to 21 | 0.82 |
BMI = Body Mass Index; Circ. = circumference; eGFR = estimated glomerular filtration rate; Ln(CRP) = natural log of c-reactive protein (in mg/L).
Evaluated using linear regression.
Standard deviation difference.
Figure 1. The Association of Body Mass Index (A) and Waist Hip Ratio (B) with Creatinine Excretion Rate are Fairly Linear in Persons with Coronary Artery Disease.
Age and sex adjusted spline functions demonstrating the cross-sectional association of body mass index (A) and waist hip ratio (B) with creatinine excretion rate in persons with coronary artery disease. Solid line represents the hazard ratio, and dotted lines represent the 95% confidence intervals. The extreme 5% of the data distribution was excluded to avoid implausible extrapolation from the extremes of the data.
Association of Creatinine Excretion Rate with Mortality
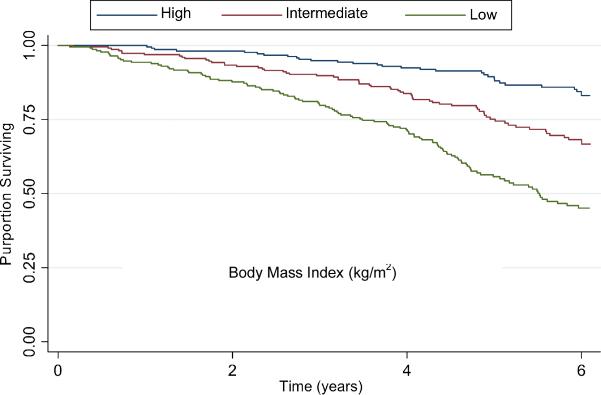
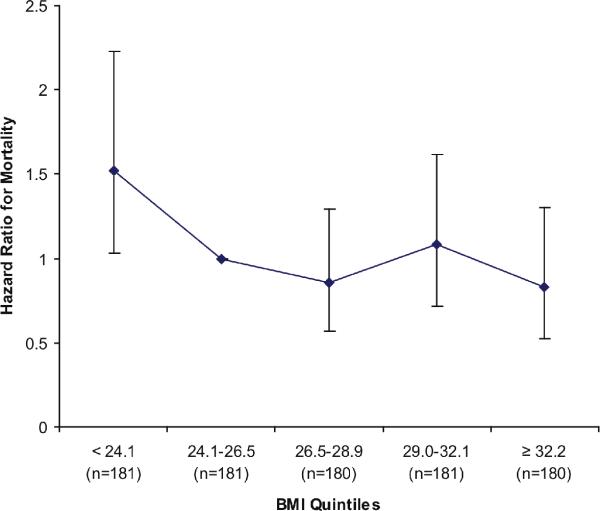
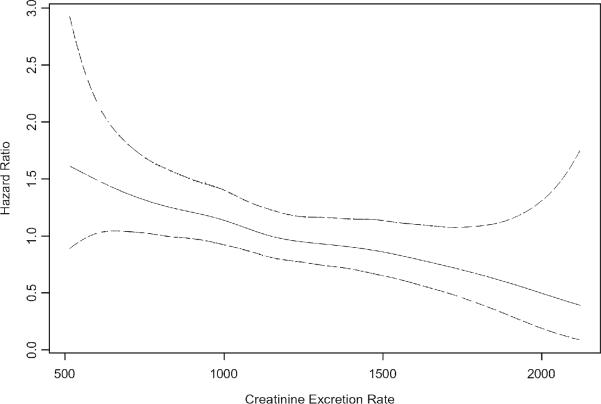
Participants were followed for a median of 6.0 years (interquartile range 4.6 to 6.1 years), during which 232 participants (26%) died. Figure 2 shows Kaplan Meier survival estimates by sex-specific CER tertiles. In the highest CER tertile, 37 (12%) deaths occurred compared with 69 (23%) among persons in the middle tertile, and 126 (42%) among persons in the lowest tertile. Accordingly, compared to the highest CER tertile, subjects in the lowest tertile were at approximately four fold risk of death in unadjusted analysis (Table 3). This association was attenuated to approximately three fold when adjusted for age and sex (mean age ± SD was 61± 10, 67 ± 10, and 71 ± 11 and for highest, middle, and lowest tertiles, respectively). Adjustment for BMI was complex, as the association of BMI with mortality was not linear. In age and sex adjusted models, subjects in the lowest BMI quintile (< 24.1 kg/m2) were at 1.5 fold greater risk for mortality (P=0.03) compared to subjects in the second quintile, and mortality risk was similar across quintiles two to five (Figure 3). Therefore, we categorized BMI by the lowest quartile vs. quartiles two to five. With this adjustment, the lowest CER tertile remained associated with approximately a threefold mortality risk (Table 3). Results were similar when each quintile of BMI was modeled as separate indicator variables, when BMI was modeled continuously, and when BMI plus a quadratic term (BMI2) were modeled concurrently. Results were also similar when weight, height, waist circumference, hip circumference, or WHR replaced BMI (data not shown). When the model was further adjusted for traditional CAD risk factors, kidney function, and CRP, the association was modestly further attenuated, but the lowest CER tertile remained at two fold greater risk of mortality compared to the highest tertile. The functional form of the relationship of CER with mortality was linear throughout the distribution of CER values in the final multivariable model (Figure 4). Results were similar when men and women were evaluated separately (interaction P-value 0.21).
Figure 2. Survival by Creatinine Excretion Tertiles in Persons with Coronary Artery Disease.
Kaplan Meier survival curve demonstrating the unadjusted association of creatinine excretion tertiles with mortality in persons with coronary artery disease.
Table 3.
Association of Creatinine Excretion Rate with Mortality in Persons with Coronary Artery Disease†
| Creatinine Excretion Tertiles | ||||||
|---|---|---|---|---|---|---|
| Highest | Middle | Lowest | Per 412 mg/day§ | |||
| Range (mg/day) | Men (n=758) Women (n=165) | ≥ 1,423 | 1,068 – 1,422 | <1068 | ||
| Women (n=165) | ≥ 1,014 | 766 – 1,013 | < 766 | |||
| Model | Reference | HR (95% CI) | HR (95% CI) | HR (95% CI) | P-value | |
| Events/Number at Risk (%) | 37/301 (12%) | 69/301 (23%) | 126/301 (42%) | |||
| Unadjusted | 1.00 | 2.05 (1.37–3.05) | 4.10 (2.84–5.92) | 1.59 (1.38–1.84) | < 0.001 | |
| Age and sex adjusted | 1.00 | 1.68 (1.12–2.53) | 3.05 (2.06–4.52) | 1.61 (.136–1.91) | < 0.001 | |
| Age, sex, and BMI addjusted* | 1.00 | 1.66 (1.10–2.50) | 2.89 (1.91–4.35) | 1.55 (1.30–1.86) | < 0.001 | |
| Fully adjusted‡ | 1.00 | 1.50 (0.99–2.28) | 2.30 (1.51–3.51) | 1.38 (1.15–1.66) | 0.001 | |
Evaluated using Cox proportional hazards models.
BMI dichotomized at the lowest quintile (< 24.1 kg/m2)
Adjusted for age, sex, race, BMI diabetes, hypertension, current smoking, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL cholesterol, eGFR, and Ln(CRP).
412 mg/day represents one standard deviation lesser creatinine excretion rate.
Figure 3. Age and Sex Adjusted Association of Body Mass Index Quint iles with Mortality in Persons with Coronary Artery Disease.
Models adjusted for age and sex. Second BMI quintile (24.1–26.5 kg/m2) served as the reference category. Error bars reflect 95% confidence intervals.
Figure 4. Adjusted* Association of Creatinine Excretion Rate with Mortality in Persons with Coronary Artery Disease.
Natural piecewise cubic spline function demonstrating that the adjusted association of creatinine excretion rate with mortality was fairly linear in persons with coronary artery disease. Solid line represents the hazard ratio, and dotted lines represent the 95% confidence intervals. The extreme 5% of the data distribution was excluded to avoid implausible extrapolation from the extremes of the data. *The spline function was adjusted for age, sex, race, BMI (lowest quintile vs. greater), diabetes, hypertension, current smoking, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL cholesterol, eGFR, and Ln(CRP).
Table 4 shows hazard ratios for mortality for each predictor variable in the final model. When comparing continuous predictor variables per standard deviation change, only age (per SD, 11 years) was clearly more strongly associated with mortality than CER. The associations of CER, eGFR, and CRP with mortality were of similar strength. Associations of the remainder of the continuously predictors were more modest or altogether absent. Compared to the binary risk factors, one standard deviation lower CER was weaker than male sex and current smoking, similar in strength to diabetes, and stronger than the lowest quintile of BMI, hypertension or African-American race.
Table 4.
Mutually Adjusted Relative Strength of Association of Risk Factors for Mortality in Persons with Coronary Artery Disease
| Risk Factor | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|
| Age (per 11 years greater)* | 1.51 | 1.25–1.83 | < 0.001 |
| eGFR (per 22 ml/min/1.73m2 lower)* | 1.40 | 1.19–1.65 | < 0.001 |
| CER (per 412 mg/day lower)* | 1.38 | 1.15–1.66 | 0.001 |
| Ln CRP (per 1.3 greater)* | 1.33 | 1.16–1.53 | < 0.001 |
| Total Chol. (per 42 mg/dL greater)* | 1.08 | 0.93–1.24 | 0.32 |
| DBP (per 11 mmHg greater)* | 0.93 | 0.77–1.12 | 0.46 |
| HDL Chol. (per 14 mg/dL greater)* | 0.94 | 0.81–1.10 | 0.45 |
| SBP (per 21 mmHg greater)* | 0.99 | 0.82–1.20 | 0.94 |
|
| |||
| Male sex | 1.97 | 1.19–3.19 | 0.008 |
| Current Smoking | 1.88 | 1.31 – 2.69 | 0.001 |
| Diabetes | 1.36 | 1.03 – 1.88 | 0.03 |
| BMI < 24.1kg/m2 | 1.28 | 0.93–1.78 | 0.13 |
| Hypertension | 0.87 | 0.65 – 1.20 | 0.43 |
| African-American race | 0.96 | 0.62 – 1.42 | 0.76 |
Per standard deviation change.
CER = creatinine excretion rate, BMI = body mass index, SBP = systolic blood pressure, DBP = diastolic blood pressure, eGFR = estimated glomerular filtration rate, Chol. = cholesterol, HDL = high density lipoprotein, Ln CRP = natural log (C-reactive protein [in mg/L]), BMI = body mass index.
Evaluation of Potential Mechanisms
Table 5 shows the result of further adjustment of the multivariable model for variables that might mediate the association of CER with mortality. Additional adjustment for maximum heart rate and METs achieved on the Bruce exercise treadmill had little effect on the association of CER with mortality. Results were also similar with adjustment for LV mass index and ejection fraction, prior CVD history, or fasting blood glucose and insulin levels. To evaluate whether low CER may be a marker of occult illness and might therefore be more strongly associated with short-term mortality, we evaluated whether the association differed in individuals who died ≤ 3 vs. > 3 years after their baseline visit. Results were similar. In this model, each standard deviation lower CER was associated with a hazard ratio of 1.39 (95% CI 1.03–1.85) in participants who died in ≤ 3 years (n=80), and 1.35 (95% CI 1.09–1.69) in those who died after 3 years (interaction P-value 0.91).
Table 5.
Adjustment for Potential Mediators on the Association of Creatinine Excretion Rate (per SD decrease§) with Mortality
| Model | HR (95% CI) | P-value |
|---|---|---|
| Fully adjusted model† | 1.38 (1.15–1.66) | 0.001 |
| Fully adjusted model† plus METS and Max Heart Rate | 1.39 (1.14–1.68) | 0.001 |
| Fully adjusted model† plus LV mass index and LV ejection fraction | 1.37 (1.14–1.65) | 0.001 |
| Fully adjusted model† plus history of MI, stroke, or revascularization | 1.39 (1.16–1.68) | < 0.001 |
| Fully adjusted model† plus fasting glucose and insulin | 1.39 (1.16–1.67) | < 0.001 |
Adjusted for age, sex, race, BMI (lowest quintile vs. greater), diabetes, hypertension, current smoking, systolic blood pressure, diastolic blood pressure, total cholesterol, HDL cholesterol, eGFR, and Ln(CRP).
412 mg/day represents one standard deviation lesser creatinine excretion rate.
Sensitivity Analyses
To investigate potential misclassification bias introduced by under- or over-collected urine specimens, we excluded participants whose measured urinary creatinine clearance was discrepant with eGFRcys by > 30% (N=267, 30%). Among those excluded, CrCl overestimated eGFRcys in 136, and underestimated eGFRcys in 131 individuals. The association of CER with mortality was similar in the remaining 636 participants compared to the entire sample. Participants with CER in the lowest tertile were at 3 fold mortality risk compared to the highest tertile (HR 2.99; 95% CI 1.59–5.62), and one standard deviation lower CER was associated with 40% greater mortality risk (HR 1.40; 95% CI 1.03to 1.90) in the fully adjusted model.
DISCUSSION
We demonstrate that lower CER is strongly associated with mortality in out-patients with stable CAD. The association showed a dose-response relationship, and was independent of body mass index, waist hip ratio, kidney function, inflammatory biomarkers, or traditional CAD risk factors. Adjustment for markers of physical fitness, LV structure and function, and insulin resistance rendered the association effectively unaltered. The association was approximately equal in strength to that of kidney function and CRP, which are two of the strongest risk factors for mortality in the secondary prevention setting.19 These findings have important implications for individuals with prevalent CAD.
First, while it is frequently hypothesized that decreased muscle mass is associated with mortality in persons with CAD,1, 20 to our knowledge this hypothesis has not previously been evaluated. Because creatinine is a by-product of muscle, and excreted almost exclusively in the urine, CER is a surrogate for muscle mass, irrespective of serum creatinine concentrations.9 For example, in a sample of 664 community-living individuals (mean urinary creatinine clearance 105 ± 32 ml/min) in Minnesota, depending on log-transformation and correction for body surface area, between 37 and 62% of the variance in CER was accounted for by muscle mass determined by dual energy x-ray absorptiometry (A. Rule, personal communication).21 In another study among 25 young healthy volunteers, mid-arm muscle area by computed tomography accounted for 88% of the variance in CER (r=0.94, R2=0.88).22 In patients with end-stage renal disease, muscle mass measured by bioelectrical impedance accounted for 85% of the variances in total (residual renal plus dialysis) creatinine clearance (r=0.92, r2=0.85).23 Thus, the most likely explanation for the data presented here is that low muscle mass is a strong risk factor for mortality in persons with established CAD.
The mechanisms responsible for this association, however, remain uncertain. Oterdoom and colleagues recently conducted a study evaluating the association of CER with mortality among community-living Europeans predominantly without CVD and demonstrated that low CER was strongly associated with mortality, similar to data presented here.24 Because skeletal muscle is the main site for insulin mediate glucose disposal, these investigators hypothesized that low CER may be associated with insulin resistance. In their study, adjustment for fasting glucose and insulin had little effect on the association of CER with mortality; results that we confirm here, and can extend to populations with CAD. In their discussion, Oterdoom et. al. suggest that CER may be a marker of physical inactivity and/or protein calorie malnutrition, yet they lacked measurement of these factors and suggested that these hypotheses be studied elsewhere. Here, we observed that individuals with lower CER achieved lower maximum METs and lower maximum heart rate on the Bruce protocol, however accounting for these measurements had virtually no effect on the association of CER with mortality. Moreover, adjustment for LV mass and ejection fraction had little effect. Therefore, while the mechanisms responsible for the association of CER with mortality remain unknown, our data suggest that low CER is more than a marker of physical fitness or of cardiovascular structure or function, and might therefore provide complimentary risk information above and beyond traditional markers of disease severity among persons with CAD.
It remains possible that factors other than muscle mass may also influence CER, although their contributions are thought to be smaller.6, 25, 26 Among these, dietary consumption of meat (particularly undercooked meat) may be the most important. We lack diet data, and thus future studies are required to determine the relative contributions of muscle mass vs. diet to CER and mortality in the secondary prevention setting. Infections, fever, trauma, and exercise may also modestly increase CER.27–31 Because each is associated with greater CER, and would more likely also be associated with greater rather than lesser mortality risk, these factors are unlikely to explain the inverse association CER with mortality demonstrated here. These observations suggest, however, that CER may be influenced not only by muscle mass, but may also reflect muscle health and function. Thus, future studies should evaluate the relative contributions of muscle quantity and quality to CER, as well as the independent associations of muscle quantity and quality to health outcomes.
It is common to statistically adjust for BMI as surrogates for body composition in studies evaluating risk factors for CAD. Here, low CER and BMI were each independently associated with mortality. Similarly, adjustment for body weight, height, or waist to hip ratio did not meaningfully attenuate the association of CER with mortality. These data suggest that adjustment for standard measures of body composition will not account for possible confounding by differences in muscle mass. If our findings are confirmed, future studies should consider measuring and adjusting for CER or other measures of muscle mass when evaluating risk factors for CAD and its consequences. Beyond minimizing confounding, such studies may also identify new pathways influencing prognosis in CAD.
This study is the first to our knowledge to evaluate the health consequences of CER in persons with CAD. CER has important advantages and disadvantages in this setting. First, our CER measurements were collected in a research setting with strict quality control. Timed urine collections in clinical practice are frequently over- or under-collected.18 At the outset, it was possible that participants with a greater burden of comorbidity might have differentially under-collected their urine, and that this feature may have biased our results. We took advantage of the fact that dividing CER by serum creatinine concentration calculates creatinine clearance (an estimate of GFR).18 In a sensitivity analysis, we excluded persons whose creatinine clearance differed more than 30% from GFR calculated from a cystatin C-based equation, which is independent of creatinine kinetics and of the accuracy of the timed urine collections. In this analysis, we found that the association of CER with mortality was similar to that observed in the entire cohort. These data provide reassurance that inaccurate timed collections were unlikely to have led to our results. Our study has additional limitations. While prior studies have demonstrated that CER is correlated with muscle mass, we lacked other measures of muscle mass to determine their relative strength with mortality compared to CER. Our study participants were mostly older men, and all had prevalent CVD. Results may differ in other populations.
CER also has important advantages over other measures of muscle mass. Timed urine collection and creatinine measurement is inexpensive, and can be readily obtained even in resource poor healthcare settings, where DXA scans or other measures of muscle mass may not be routinely available or feasible. With the growing burden of CAD world-wide,32 if our findings are confirmed, CER may provide a cost-effective and readily available mechanism to identify individuals at particularly high risk for death in the secondary prevention setting. Moreover, the American Heart Association has recommended prescription of resistive exercise interventions for individuals with established CAD.20, 33 It is possible that CER measurement might ultimately be useful to identify individuals who might benefit most from resistive exercise interventions, and/or to monitor the response to such interventions.
In summary, low CER is strongly associated with mortality in out-patients with prevalent CAD. Adjustment for conventional measures of body composition did not materially attenuate this association. If confirmed, CER may represent a novel, potentially modifiable, readily available, and inexpensively measured risk factor for mortality in the secondary prevention setting.
CLINICAL PERSPECTIVE.
Recent studies have demonstrated that low BMI is associated with mortality in individuals with CAD, while higher BMI is not. Lower BMI may reflect low muscle mass rather than low fat. When serum creatinine is in steady state, urinary creatinine excretion rate (CER) is proportional to muscle mass. In 903 out-patients with stable CAD, we collected 24-hour urine collections and evaluated the relation of CER with mortality over 6 years. Individuals within the lowest tertile of CER were at > 2-fold mortality risk compared to the highest tertile, independent of BMI, waist-hip ratio, traditional CAD risk factors, inflammatory biomarkers, and kidney function. Timed urine collection may provide an inexpensive and readily available method to measure muscle mass in out-patients with CAD and to garner additional information on mortality risk, independent of conventional measures of body composition or traditional CAD risk factors. Future studies are required to determine if resistive exercise and/or nutritional interventions can improve CER and whether such improvements in CER are associated with demonstrable improvements in health outcomes.
Acknowledgements
The authors thank Ms. Clydene Nee for review of the manuscript.
Funding Sources: This study was supported by grants from the American Heart Association (Fellow-to-Faculty Transition) and the National Heart Lung and Blood Institute (R01HL096851) to Dr. Ix. The Heart and Soul Study was supported by the Department of Veterans Epidemiology Merit Review Program; the Department of Veterans Affairs Health Services Research and Development service; the National Heart Lung and Blood Institute (R01 HL079235); the American Federation for Aging Research (Paul Beeson Scholars Program); the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program); and the Ischemia Research and Education Foundation. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Conflict of Interests Disclosures Dr. Ix reports receiving grant support from the National Heart Lung and Blood Institute (NHLBI), the American Heart Association (AHA), and the American Diabetes Association. Dr. de Boer reports no disclosures. Drs. Wassel and Criqui report receipt of grants from the NHLBI. Dr. Shlipak reports receiving grant support from the AHA, NHLBI, National Institutes on Aging, and National Institutes on Diabetes Digestive and Kidney diseases. Dr. Whooley reports receiving grants from the NHLBI, Veterans Affairs, and Department of Defense.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg A. Variability of venom skin tests. Curr Opin Allergy Clin Immunol. 2007;7:342–345. doi: 10.1097/ACI.0b013e3281f828f8. [DOI] [PubMed] [Google Scholar]
- 3.O'Donovan G, Owen A, Kearney EM, Jones DW, Nevill AM, Woolf-May K, Bird SR. Cardiovascular disease risk factors in habitual exercisers, lean sedentary men and abdominally obese sedentary men. Int J Obes (Lond) 2005;29:1063–1069. doi: 10.1038/sj.ijo.0803004. [DOI] [PubMed] [Google Scholar]
- 4.Yataco AR, Busby-Whitehead J, Drinkwater DT, Katzel LI. Relationship of body composition and cardiovascular fitness to lipoprotein lipid profiles in master athletes and sedentary men. Aging (Milano) 1997;9:88–94. doi: 10.1007/BF03340132. [DOI] [PubMed] [Google Scholar]
- 5.Hunter A, editor. Creatine and creatinine, Monographs on Biochemistry. Longmans, Green, and Co., Ltd.; New York: 1928. [Google Scholar]
- 6.Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr. 1983;37:478–494. doi: 10.1093/ajcn/37.3.478. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez R, Pomerene E. Recovery of creatinine after ingestion and after intravenous injection in man. Proceedings of the Society of Experimental Biology and Medicine. 1945;32:26–28. doi: 10.3181/00379727-60-15130. [DOI] [PubMed] [Google Scholar]
- 8.Goldman R. Creatinine excretion in renal failure. Proc Soc Exp Biol Med. 1954;85:446–448. doi: 10.3181/00379727-85-20912. [DOI] [PubMed] [Google Scholar]
- 9.Burger MZ. Die Bedeutung des Kreatininkoefizienten fur die quantitative bewertund der muskulatur als korpergewichtskomponente. Z Ges Exp Med. 1919;9:361–399. [Google Scholar]
- 10.Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, Ali S, Dowray S, Na B, Feldman MD, Schiller NB, Browner WS. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ix JH, Shlipak MG, Liu HH, Schiller NB, Whooley MA. Association between renal insufficiency and inducible ischemia in patients with coronary artery disease: the heart and soul study. J Am Soc Nephrol. 2003;14:3233–3238. doi: 10.1097/01.asn.0000095642.25603.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 13.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314–320. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ix JH, Shlipak MG, Chertow GM, Ali S, Schiller NB, Whooley MA. Cystatin C, left ventricular hypertrophy, and diastolic dysfunction: data from the Heart and Soul Study. J Card Fail. 2006;12:601–607. doi: 10.1016/j.cardfail.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrd BF, 3rd, Wahr D, Wang YS, Bouchard A, Schiller NB. Left ventricular mass and volume/mass ratio determined by two-dimensional echocardiography in normal adults. J Am Coll Cardiol. 1985;6:1021–1025. doi: 10.1016/s0735-1097(85)80304-1. [DOI] [PubMed] [Google Scholar]
- 17.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, Silverman NH, Tajik AJ. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 18.Kassirer JP. Clinical evaluation of kidney function--glomerular function. N Engl J Med. 1971;285:385–389. doi: 10.1056/NEJM197108122850706. [DOI] [PubMed] [Google Scholar]
- 19.Shlipak MG, Ix JH, Bibbins-Domingo K, Lin F, Whooley MA. Biomarkers to predict recurrent cardiovascular disease: the Heart and Soul Study. Am J Med. 2008;121:50–57. doi: 10.1016/j.amjmed.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, Gulanick M, Laing ST, Stewart KJ. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572–584. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 21.Rule AD, Bailey KR, Schwartz GL, Khosla S, Lieske JC, Melton LJ., 3rd For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int. 2009;75:1071–1078. doi: 10.1038/ki.2008.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heymsfield SB, McManus C, Smith J, Stevens V, Nixon DW. Anthropometric measurement of muscle mass: revised equations for calculating bone-free arm muscle area. Am J Clin Nutr. 1982;36:680–690. doi: 10.1093/ajcn/36.4.680. [DOI] [PubMed] [Google Scholar]
- 23.Keshaviah PR, Nolph KD, Moore HL, Prowant B, Emerson PF, Meyer M, Twardowski ZJ, Khanna R, Ponferrada L, Collins A. Lean body mass estimation by creatinine kinetics. J Am Soc Nephrol. 1994;4:1475–1485. doi: 10.1681/ASN.V471475. [DOI] [PubMed] [Google Scholar]
- 24.Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans RO, Bakker SJ. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207:534–540. doi: 10.1016/j.atherosclerosis.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Chattaway FW, Hullin RP, Odds FC. The variability of creatinine excretion in normal subjects, mental patients and pregnant women. Clin Chim Acta. 1969;26:567–576. doi: 10.1016/0009-8981(69)90089-8. [DOI] [PubMed] [Google Scholar]
- 26.Schteingart DE, Conn JW. Characteristics of the increased adrenocortical function observed in many obese patients. Ann N Y Acad Sci. 1965;131:388–403. doi: 10.1111/j.1749-6632.1965.tb34805.x. [DOI] [PubMed] [Google Scholar]
- 27.Waterlow JC, Neale RJ, Rowe L, Palin I. Effects of diet and infection on creatine turnover in the rat. Am J Clin Nutr. 1972;25:371–375. doi: 10.1093/ajcn/25.4.371. [DOI] [PubMed] [Google Scholar]
- 28.Schiller WR, Long CL, Blakemore WS. Creatinine and nitrogen excretion in seriously ill and injured patients. Surg Gynecol Obstet. 1979;149:561–566. [PubMed] [Google Scholar]
- 29.Frawley JP, Artz CP, Howard JM. Muscle metabolism and catabolism in combat casualties; systemic response to injury in combat casualties. AMA Arch Surg. 1955;71:612–616. doi: 10.1001/archsurg.1955.01270160138017. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava SS, Mani KV, Soni CM, Bhati J. Effect of muscular exercises on urinary excretion of creatine and creatinine. Indian J Med Res. 1967;55:953–960. [PubMed] [Google Scholar]
- 31.Hobson W. Urinary output of creatine and creatinine associated with physical exercise, and its relationship to carbohydrate metabolism. Biochem J. 1939;33:1425–1431. doi: 10.1042/bj0331425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Annex Table 2: Deaths by cause, sex, and mortality stratum in WHO regions, estimates for 2002. The world health report 2004-changing history. 2004 [Google Scholar]
- 33.Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B, Limacher M, Pina IL, Stein RA, Williams M, Bazzarre T. AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription: An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation. 2000;101:828–833. doi: 10.1161/01.cir.101.7.828. [DOI] [PubMed] [Google Scholar]