Abstract
Amino acids are transported into cells by a number of different transport systems, each with their own specific range of substrates. The amino acid transport systems active in preimplantation embryos and the amino acids required by embryos for optimal development have been extensively investigated. Much less is known about amino acid transport systems active in growing and meiotically maturing oocytes or about developmental changes in their activity. As a first step in determining the array of amino acid transporters active in oocytes, the transport characteristics of nine amino acids were measured in small, medium, and large growing oocytes; in fully grown germinal vesicle (GV)-stage oocytes; in metaphase I oocytes; and in metaphase II eggs. Whether each of 11 classically defined amino acid transport systems was likely active in oocytes at each stage was determined using assays based on measuring the transport of radiolabeled amino acids into oocytes and the effect of a limited set of potential competitive inhibitors. Six amino acid transport systems were found to be active during oocyte growth or maturation. L, b0,+, and ASC/asc were active throughout oocyte growth and maturation, increasing during growth. In contrast, GLY, beta, and xc− had little or no activity during growth but became activated during meiotic maturation. Surprisingly, the presence of follicular cells surrounding medium growing oocytes or cumulus cells surrounding GV oocytes did not confer amino acid transport by additional transport systems not present in the oocyte. In some cases, however, follicular cells coupled to the oocyte enhanced uptake of amino acids by the same systems present in the oocyte.
Keywords: amino acid transport, cumulus cells, granulosa cells, meiosis, oocyte development
Three amino acid transport systems are active in growing and maturing mouse oocytes and three more become upregulated during meiotic maturation, while no additional mechanisms of amino acid transport are contributed by coupled follicular cells.
INTRODUCTION
The availability of amino acids substantially enhances mammalian preimplantation (PI) embryo development and developmental potential [1–3], and amino acids are now a key component of most mammalian embryo culture media [4]. Some amino acids and combinations of amino acids have been shown to stimulate PI embryo development from the 1-cell stage in culture, while others were inhibitory [5]. In addition, the effects of amino acids are stage dependent, with different amino acid requirements, for example, in postcompaction vs. cleavage-stage embryos [2, 3].
Virtually all significant effects of amino acids in the external environment on PI embryo development are due to amino acid transport into embryos via selective transport systems [3]. It has long been known that mammalian cells possess an array of specialized transport systems for amino acids [3, 6]. These amino acid transporters generally do not transport only single amino acids but rather accept groups of related compounds as defined by the transporter's substrate binding site structure. Amino acid transport systems were defined classically by the range of their substrate specificities and by the presence or absence of obligate Na+ cotransport [7, 8] (Table 1). Many of these systems exist in several variants with differences in the array of substrates accepted, kinetics, or regulation. In most cases, the more recent identification of the genes encoding the proteins for each transport system revealed that these variants represented products of distinct genes, usually within the same gene family (Table 1).
TABLE 1.
Amino acid transport systems assessed.
The amino acid transporters active in PI embryos, particularly of the mouse (Table 1), have been extensively studied [3]. A striking feature of virtually all of these is that they are developmentally regulated during the PI period and are active only during specific PI embryo stages. Thus, the classic glycine transport system GLY is highly active during the early cleavage stages but is inactive after compaction [16, 17]. In contrast, system B0,+, a transporter that accepts a wide array of cationic and neutral amino acids, is reportedly active only in blastocysts [18]. These expression patterns reflect PI embryo physiology because glycine is required for cell volume regulation at the early cleavage stages but not later [17, 19], while B0,+ activity regulates trophoblast motility during blastocyst implantation in the uterus [20].
There is substantially less information about amino acid transport and transporter activity during the growth and meiotic maturation of oocytes. Several studies [21–26] have shown that amino acids can be taken up by fully grown germinal vesicle (GV)-stage oocytes. However, in most cases, it had not been established that transport was by a saturable transporter, and later work in some of these cases showed that it was not (e.g., proline [27]). The identity of the specific transport system responsible for uptake has been determined in even fewer cases. Colonna et al. [21] reported that leucine uptake by GV oocytes had the characteristics of system L transport, while alanine transport was by system L and by transport resembling ASC activity. Haghighat and Van Winkle [25] similarly identified the glycine transport system in GV oocytes as GLY, while the small amount of cystine and glutamate transport in GV oocytes was identified by Van Winkle et al. [26] as xc−.
More studies [16, 26, 28, 29] of amino acid transport have included unfertilized metaphase II (MII) eggs, with systems β, L, GLY, xc−, and b0,+ shown to be present in eggs. In general, amino acid transporters found in MII eggs were the same as in 1-cell embryos, and their activities were comparable [16, 26, 28, 29]. An exception is the proline and betaine transporter SLC6A20 (system IMINO), which is quiescent in MII eggs but is activated several hours after fertilization [27].
Although there is some information about amino acid transport in GV oocytes, and considerably more in MII eggs, it was obtained separately and under different conditions. Very few reported investigations have systematically assessed amino acid transport characteristics over the course of meiotic maturation, including the demonstration by Colonna et al. [23] that system L activity decreased somewhat from the GV to MII stages and a report of unpublished data by Van Winkle et al. [26] showing that system xc− activity increased by about 5-fold during oocyte maturation. Thus, there is little information available about specific amino acid transport or amino acid transporters in GV oocytes and especially about any changes they undergo during meiotic maturation.
Even less information is available about amino acid transport in growing oocytes. It was shown that leucine, alanine, and glycine uptake, attributed to systems L, ASC, and GLY, respectively, was present in medium (diameter, ∼55–65 μm) growing oocytes [21, 25], and L activity was also reported in small (∼40–50 μm) oocytes [21]. However, whether the array or activity of amino acid transporters changes over the course of oocyte growth has essentially not been determined.
One area that has received more attention is the question of whether follicular granulosa cells provide the enclosed oocyte with amino acids. During growth, the oocyte is coupled to the surrounding granulosa cells via gap junctions, a connection that is required for both oocyte and follicle viability and growth [30]. After ovulation and cumulus expansion, this oocyte-cumulus coupling is lost, and compounds taken up by cumulus cells can no longer be directly transferred into the enclosed oocyte [31, 32]. It is widely accepted that cumulus cells take up a number of compounds that are transported at low rates by oocytes and transfer them to the enclosed oocyte via gap junctions, including uridine, choline, and pyruvate [31–33]. Amino acids whose uptake by oocytes was similarly increased several-fold by the presence of coupled cumulus cells include glycine, alanine, proline, histidine, and serine, with modest stimulation of lysine, glutamate, and tyrosine [22, 24, 25]. However, this effect is selective, with no stimulation of leucine, valine, or phenylalanine uptake by oocytes within the cumulus-oocyte complex (COC) vs. denuded oocytes [22, 24, 25]. Eppig et al. [24] recently found that Slc38a3 mRNA encoding a subtype of the system N transporter is present in cumulus cells but not in oocytes and that substrates accepted by this transporter (histidine and alanine) are accumulated by enclosed oocytes at a higher rate when cumulus is present.
By analogy with advances in PI embryo culture media, growing and maturing oocytes should also benefit from inclusion of stage-appropriate amino acids in culture medium. Also, elucidation of the developmental pattern of amino transport in PI embryos has helped reveal key features of their physiology, including cell volume regulatory systems [19, 27, 34], protective mechanisms against oxidative stress [3, 26], and signaling of trophoblast implantation [20, 35]. Similar insights may be expected with growing and maturing oocytes. However, the current extensive knowledge of amino acid transport in PI embryos has resulted from many years of very detailed work on each system or amino acid substrate. To unequivocally show that a given amino acid transport system is present requires a large number of measurements of kinetic properties, substrate specificities, and inhibition profiles [6], which would be difficult and very time-consuming to carry out on different stages of growing and maturing oocytes with and without surrounding follicular cells.
Fortunately, we now know enough about the transport characteristics of the major classically defined amino acid transport systems and about their molecular underpinnings that simple tests can be devised to indicate the likely activity of each system. Therefore, we have undertaken experiments designed to determine whether major amino acid transport systems (Table 1) are active in growing mouse oocytes at three stages of growth and in fully grown GV oocytes, MI oocytes, and MII eggs. We also assessed the effect of the presence of granulosa cells on amino acid uptake by the enclosed oocyte for growing oocytes and of cumulus cells for GV oocytes. This provides the first complete picture of the likely array of amino acid transporters present in growing and maturing oocytes of any mammalian species and their levels of activity, and the study findings revealed several systems that undergo substantial changes in activity during meiotic maturation.
MATERIALS AND METHODS
Chemicals and Media
All chemicals and enzymes, including amino acids and analogues, were obtained from Sigma (St. Louis, MO) unless otherwise noted. In addition to standard α- and β-amino acids, we used cysteic acid and the amino acid analogues 2-(methylamino)isobutyric acid (MeAIB) and 2-endoamino-bicycloheptane-2-carboxylic acid (BCH) as described herein. All components of culture media were embryo-tested grade or cell culture grade. Collagenase type I was obtained from Worthington Biochemical Corporation (Lakewood, NJ). The specific SLC6A9 (GLYT1) inhibitor ORG23798 was a kind gift of Organon, Cambridge, England. ORG23798 was diluted from a stock in dimethyl sulfoxide to a final concentration of 5 μM in medium previously shown to completely inhibit glycine transport by the GLY transporter (GLYT1, officially known as SLC6A9) in PI embryos [19].
The following radiolabeled amino acids were obtained from Amersham Biosciences (Arlington Heights, IL): l-[2,3-3H]alanine (40–60 Ci/mmol), l-[2,3,4,5-3H]arginine monohydrochloride (35–70 Ci/mmol), l-[2,3-3H]aspartic acid (15–50 Ci/mmol), l-[35S]cystine (40–250 Ci/mmol), l-[G-3H]glutamine (20–50 Ci/mmol [G denotes general labeling with tritiated water]), [3H]glycine (10–30 Ci/mmol), l-[4,5-3H]leucine (45–85 Ci/mmol), l-[4,5-3H]lysine monohydrochloride (75–100 Ci/mmol), and [1,2-3H]taurine (5–30 Ci/mmol). Henceforth, chirality and labeled groups are omitted for brevity (e.g., [3H]alanine and [35S]cystine). All [3H]amino acids were obtained as stocks in 2% ethanol in water and were stored at 4°C until added directly to media. [35S]cystine was obtained as a dry solid and was stored at −80°C until the entire vial (2.09 mg) was dissolved in 1 ml of 0.1 N HCl, diluted to a final concentration of 2.1 mM in water, aliquoted, and stored at −80°C until used. Where the volume of radiolabeled substrate was more than 5% of the final volume, concentrated culture medium made with less water was used so that the final concentration of medium components remained constant within 5%.
Culture and collection media were based on potassium simplex optimized medium (KSOM) and Hepes-KSOM mouse embryo culture media, respectively [36], modified to omit glutamine and bovine serum albumin and containing polyvinyl alcohol (1 mg/ml) as the macromolecular component (termed modified KSOM [mKSOM]). The components of mKSOM were NaCl (95 mM), KCl (2.5 mM), KH2PO4 (0.35 mM), MgSO4.7H2O (0.2 mM), Na lactate (10 mM), glucose (0.2 mM), Na pyruvate (0.2 mM), NaHCO3 (25 mM), CaCl2 (1.7 mM), edetic acid (0.1 mM), K penicillin G (0.16 mM), and streptomycin (0.03 mM). The composition of Hepes-mKSOM was the same except that 21 mM NaHCO3 was replaced by Hepes. NaOH was used to adjust the pH of Hepes-mKSOM to 7.3–7.4. Osmolarity was confirmed using a model 5520 vapor pressure osmometer (Wescor, Logan, UT).
Animals
Female CF1 mice were obtained from Charles River Canada (Saint-Constant, QC, Canada) or from Harlan Sprague Dawley (Indianapolis, IN). Adult CF1 mice from which fully grown oocytes were obtained were approximately 7 wk old. CF1 neonatal female mice, from which growing oocytes were obtained, were ordered from Charles River Canada weekly at specific postnatal ages depending on the timing of experiments and were kept at a ratio of 8:1 per lactating dam. Mice were maintained on a 12L:12D (light, 700-1900 h) and had unrestricted access to food and water. All animal protocols were approved by the Animal Care Committee of the Ottawa Health Research Institute.
Oocyte, Follicle, and COC Isolation
In mice, a coordinated wave of follicular development begins shortly after birth, resulting in oocyte and follicular development being directly related to postnatal age. During Postnatal Days 5–21, a large cohort of oocytes grow to full size, modeling oocyte growth as it happens in sexually mature mice in each reproductive cycle. Female mice at postnatal ages of 5, 10, and 20 days were used to obtain small, medium, and large growing oocytes, respectively. These small, medium, and large growing oocytes had mean ± SEM diameters of 45.5 ± 0.8, 58.0 ± 1.0, and 76.7 ± 0.6 μm, respectively (data not shown). Only the large growing oocytes were meiotically competent (∼90% exit from GV stage [data not shown]). Denuded small growing oocytes (Day 5) were isolated enzymatically by incubating excised ovaries in Ca2+- and Mg2+-free mKSOM with 2 mg/ml of type I collagenase and 0.01 mg/ml of DNase I for 40 min [37, 38]. Denuded medium growing oocytes (Day 10) and large growing oocytes (Day 20) were mechanically isolated following mincing of the ovaries with a razor blade as previously described [39]. The different isolation protocols were used because small oocytes could not be obtained mechanically, while large oocytes could not be obtained enzymatically [39]. It was confirmed in medium growing oocytes, in which both isolation protocols are feasible, that identical amino acid transport measurement results were obtained using both methods (data not shown), thus validating its use with small growing oocytes. The data for medium growing oocytes presented herein, however, were obtained using only the mechanical isolation method because of its greater ease of use.
Intact medium preantral follicles (diameter, 95–115 μm [39]) containing medium growing oocytes were mechanically isolated following mincing of ovaries of Postnatal Day 10 mice. For some experiments, oocytes were removed from Day 10 neonatal follicles by removing a small part of the follicle wall with a short thin flame-pulled Pasteur pipette and then pressing on the follicle to extrude the oocyte from it as previously described [39]. This produces an almost intact follicular cell shell and an intact denuded oocyte from the same follicle.
For fully grown oocytes, adult females were primed with an i.p. injection of 5 IU of equine chorionic gonadotropin (eCG). The COCs containing fully grown GV oocytes were collected from mechanically minced ovaries 44–48 h after eCG administration. For denuded GV oocytes, cumulus cells were removed by repeated pipetting through a narrow-bore pipette. Fully grown GV oocytes had mean ± SEM diameters of 84.6 ± 0.8 μm.
To obtain in vivo-matured oocytes, an i.p. injection of 5 IU of human chorionic gonadotropin (hCG) was administered 47 h after eCG administration. For metaphase I (MI) oocytes, partially expanded COCs were collected from the mechanically minced ovaries 4 h after hCG administration in 0.3 mg/ml of hyaluronidase, and MI oocytes were isolated by repeated pipetting. The MII oocytes were collected from excised oviducts 15 h after hCG administration and were treated with hyaluronidase to disperse the expanded cumulus. All were rinsed four times in collection medium before use.
Transport Measurements
3H and 35S measurements were performed in 4-ml volumes of scintillation fluid using a model 2200CA TriCarb liquid scintillation counter (Packard Instrument Co., Downer's Grove, IL), with each sample counted for 5 min. Conversion of counts per minute to molar amounts of labeled amino acid was performed using a standard curve constructed for each set of experiments by serial dilutions in water of the radiolabeled compounds.
For measurements of amino acid transport in denuded growing (small, medium, and large) or fully grown (GV, MI, and MII) oocytes, isolated oocytes were rinsed three times in equilibrated mKSOM medium in groups of 5–20 and were transferred to pre-equilibrated medium containing the radiolabeled amino acid for the specified period. They were then washed five times in ice-cold medium and transferred to a scintillation vial, and scintillation fluid was added essentially as previously described [19, 34]. A similar volume of the last wash drop was transferred to a separate vial and treated identically to obtain background that was subtracted from each paired measurement. Radiolabeled amino acids were used at a concentration of 1 μM except for taurine, alanine, and aspartic acid, which were used at 10 μM, and cystine at 50 μM. The incubation period was 10 min in all cases except cystine, for which it was 30 min. The increase in concentrations for taurine, alanine, aspartic acid, and cystine and the longer incubation time for cystine were necessary to obtain adequate signal to noise because of their lower transport rates.
For transport measurements on intact follicles from Day 10 neonates, isolated follicles were cultured in mKSOM for 20 min to reduce stickiness, and then groups of five were washed and transferred to medium containing labeled amino acids as already described for denuded oocytes. After the incubation period, oocytes were mechanically removed from the follicle cell shell as already described, and the oocyte and follicle shell were processed separately for scintillation counting. Intact follicles with enclosed oocytes were similarly processed. Labeled amino acid concentrations were as already described for denuded oocytes except when counts per minute were deemed too high (and possibly detrimental), in which case radiolabeled amino acid was mixed with unlabeled amino acid to provide the desired total concentration but at lower specific activity. Incubation times were the same as for denuded oocytes except for one set of experiments in which follicles were incubated for 30, 60, and 90 min as indicated.
Transport measurements on COCs were carried out identically to those on denuded oocytes. At the end of the period of incubation with labeled amino acids, the intact COC or the GV oocyte after mechanical removal of cumulus cells was processed for scintillation counting.
Content of radiolabeled amino acid was expressed as femtomoles per oocyte (after background subtraction). Rates of transport were calculated by dividing the total molar amount of radiolabeled amino acid measured in the sample by the number of oocytes, follicles, or COCs; the incubation time; and the molar amount of labeled amino acid in the incubation medium. Rates were then expressed as femtomoles per oocyte, follicle, or COC per minute. Pilot and previous experiments had confirmed linearity over the incubation periods used and concentrations of labeled amino acid (data not shown).
Data Analysis
Data were expressed as the mean ± SEM. Comparisons between means were performed by ANOVA with Tukey-Kramer post hoc test for multiple (>2) comparisons or two-tailed Student t-test for comparing two means using Instat (GraphPad, San Diego, CA). Differences were assumed to be significant at P < 0.05. Data were plotted using SigmaPlot 8.2 (SPSS, Chicago, IL). For plots of amino acid content vs. time, data were fit by nonlinear least squares regression (SigmaPlot 8.2) to a single exponential of the following form: Amino Acid Content = a(1 − e−bt), where a and b are parameters determined by the fit, and t is time.
Testing for the Presence of Amino Acid Transport Systems
As discussed in the Introduction, unequivocally establishing which amino acid transport systems are responsible for transport of each amino acid in oocytes at each stage of development would be prohibitive. Instead, we utilized a series of simple assays using established substrates of major classic amino acid transport systems to determine whether their activities are likely present. These assays (diagrammed in Fig. 1) are based on first showing that an established substrate for a given transport system is taken up; second, showing that its transport is saturable by competition with excess (10 mM) unlabeled substrate; and third, showing that a characteristic competitive inhibitor or set of inhibitors eliminates the saturable component of transport. In the case of cystine, the substrate was not sufficiently soluble to reach 10 mM. Therefore, we used cysteic acid at 10 mM instead to establish saturable cystine transport.
FIG. 1.
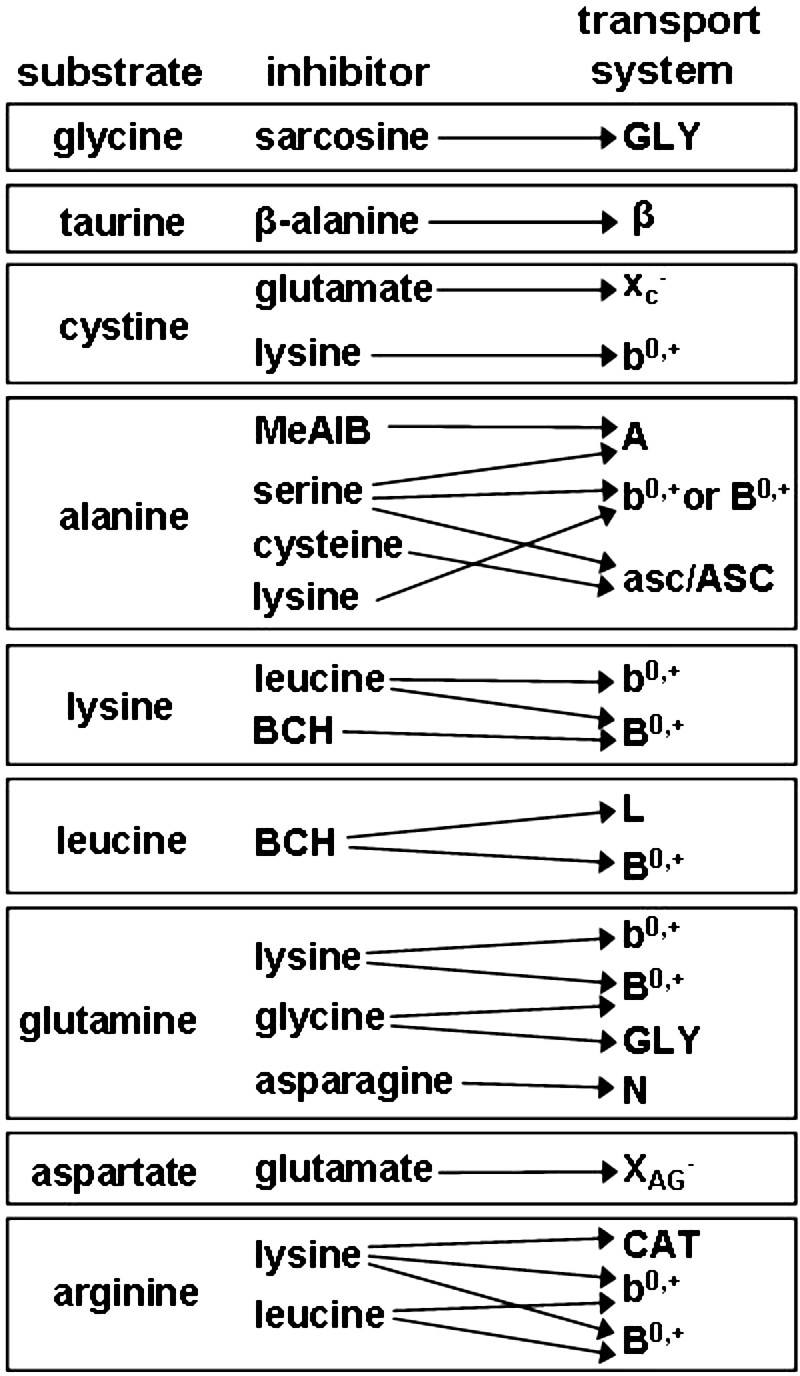
Schematic diagram of tests used to reveal amino acid transport system activities. Measurements of transport rates of the nine radiolabeled substrates were performed in the absence of any addition and in the presence of the putative competitive inhibitors listed. The classic amino acid transport system (at the right) whose substrate transport is blocked by a given inhibitor is indicted by arrows. Details are given in the text.
In some cases, obtaining clear results was straightforward. For example, taurine transport inhibited by excess β-alanine is very likely attributable to the β transport system because it is the only transport mechanism that fits this pattern. In most cases, however, somewhat more complex assessments are needed. Thus, for example, lysine transport inhibited by leucine could indicate activity of either b0,+- or B0,+-mediated transport. A second set of measurements using BCH (which competitively inhibits B0,+ but not b0,+) was then used to differentiate between these two possibilities. We were also able to somewhat reduce the number of measurements needed by utilizing information obtained using one substrate to reduce the possibilities that needed to be assessed for another. Thus, if lysine transport was found to be BCH insensitive at all oocyte stages examined, therefore eliminating the possibility of B0,+ activity, it would be unnecessary to test lysine as a potential competitive inhibitor of leucine to similarly test for B0,+.
We used nine radiolabeled amino acids (Fig. 1) and measured the rate of transport and inhibition profile for each in small, medium, and large growing oocytes; in GV, MI, and MII oocytes; and in medium growing follicles and COCs and oocytes isolated from these after amino acid uptake. Using the resulting information, we assigned transport to the likely classic amino acid transport systems active at each stage of development.
RESULTS
Amino Acid Transport in Growing and Maturing Oocytes
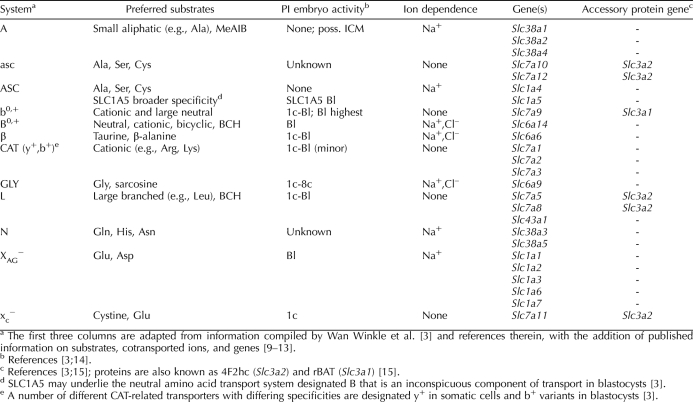
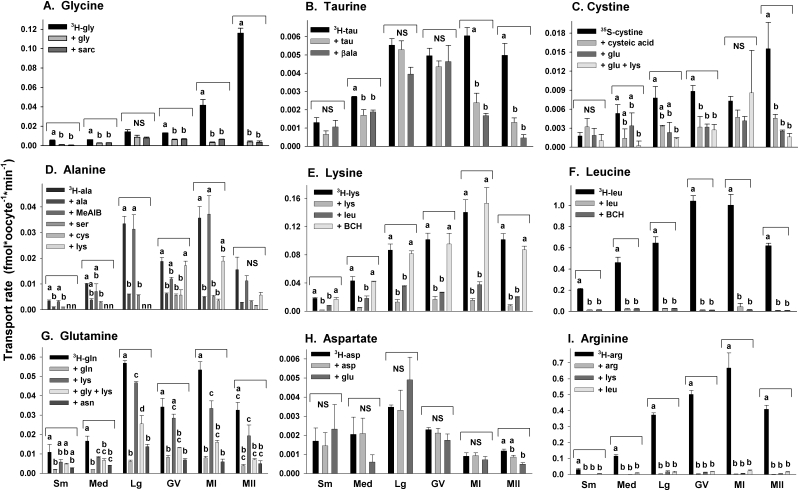
We measured the rate of transport of each of nine radiolabeled amino acid substrates in small, medium, and large growing oocytes and in fully grown GV, MI, and MII oocytes. Saturable transport of each substrate, determined as the difference between the rate of transport in the absence and presence of excess (10 mM) unlabeled substrate, was found to be present during at least some stages of oocyte development for all amino acid substrates tested except aspartate (Figs. 2 and 3).
FIG. 2.
Amino acid transport characteristics in growing and maturing oocytes. The rates of transport of each radiolabeled amino acid as indicated by the labels in A–I were measured for small, medium, and large growing oocytes and for GV, MI, and MII oocytes as indicated at the bottom. Substrate and potential competitive inhibitors used (10 mM each) are indicated by inset keys in each panel. Each bar indicates the mean ± SEM of at least three independent measurements. Means within each stage (i.e., within the same bracket above the bars) were tested for difference by ANOVA with Tukey-Kramer post hoc test. Bars not sharing the same letter within a given stage are significantly different (P < 0.05). NS, no significant difference.
For three amino acids, glycine, taurine, and cystine, saturable transport was very low or absent during all stages of growing oocytes and in fully grown GV oocytes but increased during meiotic maturation (Fig. 3, A–C). For glycine, saturable transport was still increasing in MI oocytes (4 h after hCG administration) and became much greater in MII oocytes (Fig. 3A), while saturable taurine transport was already maximal in MI oocytes (Fig. 3B). An unusual feature of taurine transport was that there was a comparatively large component of nonspecific transport in large growing and GV oocytes, which decreased progressively during meiotic maturation (Fig. 2B) as saturable transport appeared (Fig. 3B). Saturable cystine transport was low in growing and maturing oocytes through the MI stage and then increased significantly at the MII stage (Figs. 2C and 3C).
FIG. 3.
Saturable amino acid transport in growing and maturing oocytes. The data shown in Figure 2 were used to calculate the rate of saturable transport by subtracting the rate in the presence of excess unlabeled substrate from the rate with no addition using the paired measurements within each independent repeat. The amino acid substrates in A–H are as indicated in the panel, and oocyte stages are indicated at the bottom. The mean ± SEM of saturable transport was calculated from at least three independent measurements at each stage and for each amino acid substrate. Bars not sharing the same letter within a given panel are significantly different (P < 0.05 by ANOVA with Tukey-Kramer post hoc test). Asterisks indicate where the amount of saturable transport was not statistically significant as indicated by the lack of significant difference between the rates of transport in the presence or absence of excess unlabeled substrate (as shown in Figure 2).
The remaining five amino acids that exhibited saturable transport in oocytes (alanine, lysine, leucine, glutamine, and arginine) showed similar patterns of progressive increase during oocyte growth and constant transport over the course of meiotic maturation, although saturable alanine transport became nonsignificant at the MII stage (Figs. 2, D–G and I, and 3, D–H).
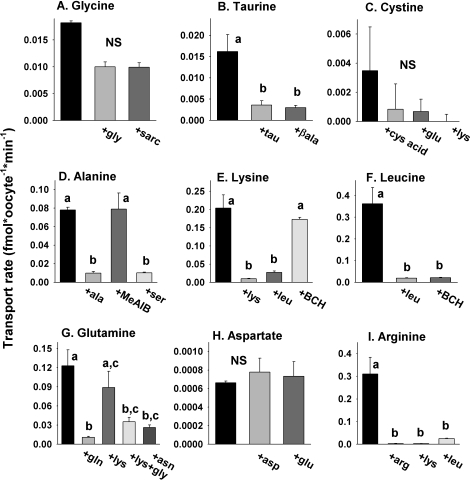
Inhibition Profiles of Amino Acid Transport in Growing and Maturing Oocytes
The likely presence or absence of activities of four classic transport systems could be determined with simple tests (Fig. 1). Glycine transport (Fig. 2A) was entirely inhibited by sarcosine, indicating the presence of appreciable GLY transport system activity beginning at the MI stage. Similarly, the completely β-alanine-inhibitable transport of taurine at the MI and MII stages (Fig. 2B) indicated system β activity. The absence of saturable aspartate transport and the lack of effect of glutamate (Fig. 2H) indicated the absence of any measurable activity of the XAG− transport system at any stage of oocyte development. Cystine transport was entirely inhibited by glutamate, implicating xc− system activity in its transport. A lack of any significant contribution to cystine transport by b0,+ was confirmed by the lack of any further inhibition by adding lysine (Fig. 2C).
Alanine transport was unaffected by the presence of MeAIB, which is a model substrate for system A transport, thus showing a lack of system A activity at any stage (Fig. 2D). Alanine transport was also not significantly inhibited by lysine, indicating that neither b0,+ nor B0,+ was responsible for an appreciable fraction of its transport. However, either serine or cysteine completely inhibited alanine uptake by oocytes, which indicated likely asc- or ASC-type transport activity.
Lysine was completely inhibitable by leucine at all stages (Fig. 2E), a property of either b0,+ or B0,+ activity. However, the insensitivity of lysine transport to BCH eliminated the possibility of appreciable B0,+ activity, indicating the likely presence of b0,+.
Leucine exhibited high transport rates at all stages of oocyte development, which by itself is consistent with the high-capacity transport of system L. This was supported by the complete inhibition of transport by BCH (Fig. 2F), which is a model competitive inhibitor of system L. This also ruled out transport by b0,+, which is insensitive to BCH. However, leucine is also a good substrate for b0,+, whose presence was indicated by the properties of lysine transport (as already described). Thus, there is likely a component of leucine transport by b0,+ that is not detected herein because of the very high rate of leucine transport by L. Having ruled out activity of system B0,+ in oocytes using lysine, we did not test the effect of lysine on leucine transport (Fig. 1).
Asparagine-sensitive glutamine transport was assessed to determine the presence of system N, which has high affinity for glutamine and asparagine. Glutamine, however, is a substrate for a number of different transport systems, including N, b0,+, B0,+, and reportedly GLY (Fig. 1); thus, testing for possible system N activity is complex. We found that glutamine transport in small or medium oocytes was present but minimal. In large growing oocytes or fully grown GV, MI, or MII oocytes, glutamine transport (Fig. 2G) was partially inhibited by lysine, indicating that a portion of its transport was likely via b0,+ (because B0,+ activity already had been shown to be absent). In the presence of excess unlabeled lysine (eliminating the b0,+ component), glycine further reduced glutamine transport essentially to background, which could indicate transport by system GLY or N. Asparagine completely eliminated glutamine transport in the presence of lysine, consistent with system N activity [9]. However, it was also possible that this component was due to system GLY because it has not been established whether a large excess of asparagine inhibits GLY activity and because glutamine has been shown to be a low-affinity inhibitor of glycine transport via GLY in 2-cell mouse embryos [40] and thus is a possible GLY substrate.
Therefore, we tested whether the specific GLY (SLC6A9) transport inhibitor ORG23798 [19] or the presence of the high-affinity GLY substrate sarcosine affected glutamine transport (in the presence of lysine) in GV oocytes. We found that the mean rate of glutamine transport by GV oocytes in the presence of lysine was 0.016 ± 0.003 fmol/oocyte per minute (n = 5) and that this was reduced to 0.0050 ± 0.0005 fmol/oocyte per minute (n = 5) in the presence of excess unlabeled glutamine, similar to results already reported (Fig. 2G). The rate of glutamine transport was not decreased by the presence of 5 μM ORG23798 (0.017 ± 0.002 fmol/oocyte per minute [n = 5]) or by the presence of unlabeled sarcosine (0.020 ± 0.003 fmol/oocyte per minute [n = 5]), neither of which was significantly different from the rate in the presence of lysine only (while all three groups were significantly different from the rate in the presence of excess unlabeled glutamine [P < 0.05 by ANOVA with Tukey-Kramer post hoc test]). This appears to rule out any appreciable transport of glutamine by GLY under these conditions. Thus, transport of glutamine in oocytes is consistent with one component that is carried by b0,+ and another component that is lysine resistant and inhibited by asparagine, which may represent activity of system N.
Finally, arginine transport could be by either cationic amino acid transporter (CAT)-type transport systems, b0,+, or B0,+ (Fig. 1). Arginine transport was found to be entirely inhibited by both lysine and leucine (Fig. 2I), ruling out any significant contribution resembling CAT and implicating one of the systems that accepts both neutral and cationic substrates. Because B0,+ activity was undetectable (as already described), the measured arginine transport likely reflects b0,+ activity.
Amino Acid Transport in Follicle- and Cumulus-Enclosed Oocytes
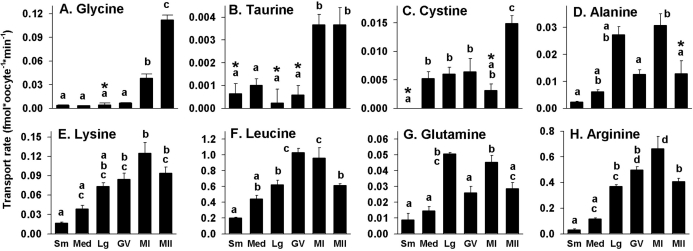
To determine whether the follicular cells surrounding growing oocytes either possess additional amino acid transporters other than those present in oocytes or augment transport of amino acids into enclosed oocytes, we measured amino acid transport by follicles and oocytes obtained from Day 10 neonatal ovaries. For each amino acid tested, four parallel amino acid uptake measurements were made on the following: 1) medium growing oocytes that were denuded before incubation with the radiolabeled amino acid (identical to the measurements already given), 2) oocytes isolated after incubation with radiolabeled amino acid from follicles that were intact during uptake, 3) the isolated follicular shells remaining after these oocytes were removed, and 4) intact follicles with enclosed oocytes.
The highest rates of transport of each amino acid were found in intact follicles with oocytes enclosed (Fig. 4). However, transport of aspartate and cystine was not significantly different when excess unlabeled substrate was present or absent, indicating little or no specific saturable transport by intact follicles. Among the remaining amino acids, all exhibited patterns of inhibition similar to those of denuded oocytes (Figs. 2 and 4).
FIG. 4.
Effect of the presence of follicle cells on amino acid transport characteristics in medium growing oocytes. The rates of transport of each radiolabeled amino acid as indicated by the labels in A–I were measured for medium growing oocytes (Oocyte) that had been removed from follicles before incubation with the labeled substrate (Denuded) and for oocytes that had been within intact follicles during incubation with the labeled substrate (Enclosed), as well as for the follicle cell shells that remained after removing the enclosed oocyte (Shell) and intact follicles that included oocytes (Intact). Substrate and potential competitive inhibitors used (10 mM each) are indicated by inset keys in each panel. Each bar indicates the mean ± SEM of at least three independent measurements. Means within each stage (i.e., within the same bracket above the bars) were tested for difference by ANOVA with Tukey-Kramer post hoc test. Bars not sharing the same letter within a given stage are significantly different (P < 0.05). NS, no significant difference.
For all amino acids exhibiting saturable transport in intact follicles, isolated follicle shells had essentially the same pattern of inhibition by the potential competitive inhibitors tested. In each case, however, the calculated rates of transport into isolated follicle cell shells were lower than those of intact follicles. This was possibly because of the unavoidable loss of a significant portion of the shell upon oocyte removal so that fewer follicle cells remained in shells than were in intact follicles. Finally, oocytes isolated from follicles after the uptake period generally exhibited the lowest accumulation of radiolabeled amino acid. Only alanine, lysine, leucine, and glutamine showed uptake that was significantly different from that in the presence of excess unlabeled substrate (Fig. 4).
The lack of significant saturable uptake of glycine, taurine, and arginine into oocytes that had been enclosed in follicular cells during incubation with the radiolabeled amino acid could indicate that the 10-min incubation period used was insufficient to permit passage of amino acids transported into follicular cells through gap junctions into the oocyte. Therefore, we tested whether increasing the incubation period would result in significant enhancement of transport of these and other amino acids into follicle-enclosed oocytes.
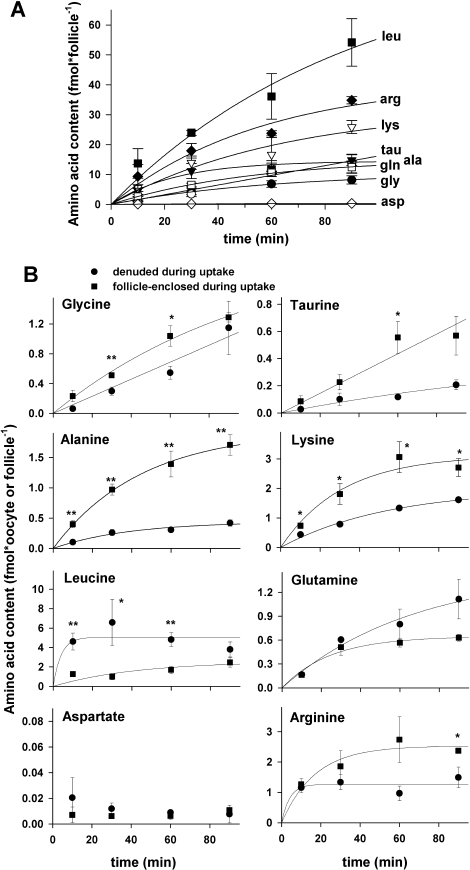
First, the total accumulation of each amino acid substrate was measured into intact follicles after 30-, 60-, and 90-min incubations with the radiolabeled amino acids (Fig. 5A). For all amino acids except aspartate, amino acid content of the follicle-oocyte complex increased with time of incubation, with all except taurine showing evidence of saturation. We next measured the amount of radiolabeled amino acid substrate accumulated by denuded oocytes and by oocytes that had been within intact follicles during incubation after 30, 60, and 90 min (Fig. 5B). These measurements revealed a greater uptake of glycine, taurine, and alanine (but not glutamine or aspartate) by oocytes that had been follicle enclosed vs. denuded during transport. In contrast, leucine uptake was significantly decreased by the presence of follicle cells (Fig. 5B). A slight decrease in arginine uptake was also observed that was significant only at 90 min.
FIG. 5.
Effect of longer periods of uptake on amino acid accumulation by medium growing follicles and oocytes. A) Amino acid accumulation by intact medium growing follicles. The amount of radiolabeled amino acid (indicated at the right) was measured after 30, 60, or 90 min. Data for 10-min incubations (from Fig. 4) performed under identical conditions were also plotted and were used in fitting the single-exponential curves (see text). B) Amino acid accumulation by denuded oocytes and oocytes within follicles. The total accumulation of the radiolabeled amino acids indicated at the upper left of each panel is shown after 30, 60, and 90 min, with the 10-min data (Fig. 4) also plotted. Single-exponential curves were fit to each data set (except aspartate, for which no increased accumulation was found with time). Asterisks indicate significant difference between the amount of amino acid accumulated by denuded oocytes vs. oocytes that were within follicles during the uptake period at a given time point (*P < 0.05 and **P < 0.01 by Student t-test). In A and B, each point is the mean ± SEM of at least three independent measurements.
We then assessed the characteristics of amino acid transport in GV oocytes that were enclosed within COCs during incubation with radiolabeled amino acid substrates. When GV oocytes were isolated from COCs following 10-min periods of amino acid uptake, significant saturable transport components were found for taurine, alanine, lysine, leucine, glutamine, and arginine (Fig. 6). In each case, the inhibition profiles were very similar to those of denuded GV oocytes (Fig. 2).
FIG. 6.
Amino acid transport characteristics in cumulus-enclosed GV oocytes. The rates of transport of each radiolabeled amino acid as indicated by the labels in A–I were measured for GV oocytes that had been within intact COCs during incubation with radiolabeled amino acids for 10 min. Cumulus cells were removed before measurement of radiolabeled amino acid content. The black bars indicate the rate of uptake in the absence of any addition, and potential competitive inhibitors used (10 mM each) are indicated by labels under the gray bars at the bottom of each panel. Each bar indicates the mean ± SEM of at least three independent measurements. Bars not sharing the same letter within a given stage are significantly different (P < 0.05 by ANOVA with Tukey-Kramer post hoc test). NS, no significant difference.
Enhancement of Amino Acid Uptake by Follicle or Cumulus Cells
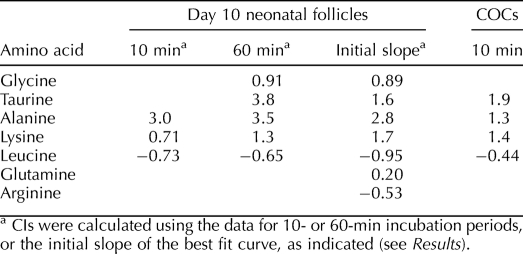
The effect of follicle or cumulus cells on amino acid uptake by enclosed oocytes can be quantified by calculating the coupling index (CI) as follows: CI = (A − B) / B, where A represents the amount of substrate taken up into follicular cell-enclosed oocytes, and B represents the amount taken up by denuded oocytes under the same conditions. A CI greater than 0 indicates enhancement of uptake, while a CI less than 0 indicates inhibition of uptake by the presence of follicle or cumulus cells. The CIs are conventionally considered significant if they lie outside of −0.5 to 0.5 (i.e., >50% enhancement or inhibition) [31, 33].
Because the measurements of amino acid uptake into follicle-enclosed medium growing oocytes indicated a significant effect of the presence of follicle cells for a number of amino acids (Fig. 5), we quantitated the effect by calculating CIs. When using a 10-min incubation period with radiolabeled amino acids, alanine, lysine, and leucine had shown significant effects of the presence of follicle cells during the uptake period (Fig. 5). The calculated CIs were also significant (Table 2), with alanine and lysine uptake increased and leucine uptake conversely decreased by the presence of follicle cells. For 60-min incubations, alanine, lysine, and leucine were again significantly affected by the presence of follicular cells (Fig. 5) and showed CIs (Table 2) similar to those calculated at 10 min. Glycine and taurine also showed significant stimulation of uptake by follicle cells with 60-min incubations (Fig. 5), and the calculated CIs indicated significant stimulation of uptake (Table 2).
TABLE 2.
Metabolic coupling indices (CI) for medium growing follicles and fully-grown cumulus-GV oocyte complexes.
In addition to using data at individual time points to calculate CIs, the entire data sets (Fig. 5) could be fit to single exponentials and the initial slope (first derivative at t = 0) used to determine relative rates of initial uptake for denuded oocytes vs. oocytes that had been follicle-enclosed during uptake. Using these initial rates, CIs were calculated for all amino acids except cystine (for which only 30-min incubations had been carried out and there was no significant saturable transport in follicle-enclosed oocytes), aspartate (for which there was no significant saturable transport [Fig. 4]), and asparagine (for which the saturation of the curve for denuded oocytes [Fig. 5B] caused an anomalous fit). These yielded similar CIs for each amino acid whose CI was calculated at 60 min and showed that glutamine uptake was not significantly affected (Table 2).
Finally, we calculated CIs for cumulus-enclosed fully grown oocytes using the rates of amino acid uptake during 10-min incubation periods for denuded fully grown GV oocytes (Fig. 2) and for GV oocytes removed from COCs after the uptake period (Fig. 6) for those amino acids that exhibited saturable transport into COCs. The results were similar to those for medium growing oocytes. Taurine, alanine, and lysine each exhibited uptake into GV oocytes that was enhanced by the presence of cumulus cells, with similar CIs for taurine and lysine and a somewhat lower CI for alanine (Table 2). Consistent with the data for medium growing oocytes, leucine uptake by GV oocytes was inhibited by the presence of cumulus cells, although the inhibition did not quite reach the −0.5 significance level.
DISCUSSION
Amino Acid Transport Systems GLY, β, and xc− Are Activated in Oocytes During Meiotic Maturation
Three amino acid transport systems were activated during meiotic maturation. The glycine transport system (GLY) was activated in MI oocytes and reached maximal activity in MII oocytes. We have independently confirmed that glycine transport activity by SLC6A9 (also known as GLYT1), which corresponds to the classic GLY system, is essentially quiescent in freshly isolated GV oocytes but becomes activated within a few hours of either triggering ovulation or removing the oocyte from the antral follicle [17].
Similarly, the β-amino acid transporter system β was first activated in MI oocytes. The β system mediates transport of several β-amino acids of physiological significance, including taurine, β-alanine, and hypotaurine. System β (SLC6A6, also known as TAUT) activity has been well characterized in MII eggs and throughout PI embryo development [28]; thus, our identification of β-alanine-inhibitable taurine transport in oocytes as indicating system β activity is likely correct. We also found a comparatively large amount of nonsaturable taurine uptake at the large growing oocyte and GV stages (Fig. 2B). The route of this taurine permeability is unknown, but it may be carried by the swelling-activated highly taurine-permeable channel that has been reported to be more active in GV mouse oocytes than in MII eggs [41, 42]. This is consistent with the similar increase in the component of nonspecific aspartate uptake at the same stages (Fig. 2H) because the swelling-activated channel is also highly permeable to aspartate [42]. Notably, the genes encoding β (Slc6a9) and GLY (Slc6a6) are members of the same family of Na+- and Cl–-dependent transporters [12], and both can function as organic osmolyte transporters mediating cell volume control in PI embryos [19, 43, 44]. That both become activated during meiotic maturation may indicate a key role for volume regulation by organic osmolytes beginning at this point of development [17].
Finally, the substantial increase in glutamate-inhibitable cystine transport at the MII stage indicates an approximately 3-fold increase in xc− transport activity during meiosis. A previous citation of unpublished data [26] noted that xc− transport increased 5-fold between the GV and MII stages, similar to the increase we detect herein. Again, xc− transport has been well characterized in 1-cell embryos [26]; thus, the glutamate-sensitive cystine transport we measure herein is very likely reflective of xc− activity. Inhibition of cystine transport by cysteic acid, which we used herein to establish saturable transport, is also characteristic of xc− transport, supporting this conclusion. Cystine taken up by xc− is utilized by cells to synthesize glutathione for protection against oxidative damage [26]. Thus, the upregulation of xc− in MII oocytes may be in preparation for the increased metabolism in fertilized eggs and early embryos.
The measured transport activities should be reflected by expression of the genes underlying the transport system proteins. A study [45] of mRNA expression during mouse oogenesis using gene arrays has been published, and the data sets (GDS1265 and GDS1266) are deposited in the National Center for Biotechnology Information Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/). These indicate expression of Slc6a9 (GLY), Slc6a6 (β), Slc7a11 (xc−), and Slc3a2 (accessory protein 4F2hc required for xc−) throughout oocyte growth. Using RT-PCR, we have also confirmed that medium growing oocytes, GV oocytes, and MII eggs express Slc6a9 mRNA (Baltz, unpublished results), and it was previously similarly shown that Slc6a6 mRNA is present in GV oocytes and MII eggs [28]. Thus, mRNA encoding the proteins underlying each of these transport systems is likely expressed in growing and fully grown oocytes.
Amino Acid Transport Systems b0,+, L, and asc/ASC Are Active Throughout Oocyte Growth and Maturation
Leucine-inhibitable lysine transport that was insensitive to BCH was present throughout oogenesis and maturation, a strong indication of b0,+ activity. Similarly, transport of arginine, a b0,+ substrate [29], was entirely lysine and leucine inhibitable. Lysine and arginine show almost identical patterns of saturable transport during oocyte development and meiotic maturation (Fig. 3). The higher rate of arginine transport than lysine transport at all oocyte stages is also compatible with b0,+, which has much greater affinity for arginine than lysine [29]. Our identification of this as b0,+ activity is also consistent with the report that b0,+ is by far the most conspicuous transporter of cationic amino acids in MII eggs [29]. Thus, b0,+ appears to be active throughout oocyte growth and maturation.
System L activity was conspicuous as a high rate of leucine transport that was completely inhibited by BCH at each oocyte stage. System L is one of the few amino acid transport systems whose activity had been investigated during oocyte growth and was found to be present in small, medium, and large growing oocytes [21], as well as in MII eggs [29]. Because there are multiple isoforms of L from two gene families (Table 1), further work would be needed to identify which subtypes of L are active at each stage.
We also detected alanine uptake at most stages, consistent with either asc- or ASC-mediated transport. Saturable alanine transport was blocked to the same extent by serine and cysteine, consistent with asc/ASC activity. The lack of effect of either MeAIB or lysine ruled out system A, B0,+, or b0,+ (the latter of which only weakly reacts with alanine [46]). This identification is consistent with the previously reported presence of ASC in medium and large growing oocytes [21]. Further work is needed to determine whether the detected activity corresponds to asc or ASC and to which isoforms, including whether activity can be ascribed to more than one type of transport.
The pattern of increasing activity of b0,+, L, and possibly asc/ASC during oocyte growth may reflect constitutive activity rather than increasing activation during oocyte growth. Fully grown GV oocytes had approximately 6-fold larger volumes than small growing oocytes, which parallels the increases in transport activity (Fig. 3). Activity scaled to volume would facilitate the maintenance of constant intracellular concentrations of amino acid substrates of these systems. Similarly, scaling with surface area (∼3.5-fold increase) would indicate a constant density of active transporters in the plasma membrane during growth.
The mRNA expression data already cited [45] indicated expression of the gene underlying b0,+ activity (Slc7a9) throughout oocyte growth. Similarly, genes encoding asc transporters are expressed, with Slc7a12 present throughout oocyte growth and Slc7a10 present in large oocytes and possibly earlier, although genes encoding ASC isoforms were not detected. Neither gene in the Slc7 family encoding L transporters (Slc7a5 or Slc7a10) was detected, however. It is possible either that this is a false negative or that L transporters of the Slc43 family are expressed, which were not assessed in that data set.
Our results indicate that there may also be system N activity in growing and maturing oocytes based on the detection of a lysine-resistant asparagine-sensitive component of transport that was not due to system GLY. This component increased during oocyte growth and thus may exhibit a developmental pattern similar to that of b0,+, L, and asc/ASC. System N activity has not been investigated in oocytes or early embryos, although the high concentration of glutamine in 4- to 8-cell embryos has led Van Winkle [3] and Van Winkle and Campione [14] to propose its transient activation near those stages. Curiously, despite the demonstrated importance of glutamine in supporting PI embryos' development in vitro and intense study of its effects on embryo development [4], the mechanisms of glutamine transport in oocytes or PI embryos have not been elucidated. Because System N activity has not previously been established by definitive tests at any stage of oogenesis or PI embryogenesis, we do not conclude that the activity we detect herein definitively corresponds to system N. Further study is thus needed to determine the routes of glutamine transport in oocytes and embryos, as well as to establish whether there is system N activity during these stages of development and, if so, which of the isoforms are responsible.
Amino Acid Transport Systems XAG−, B0,+, A, and CAT/y+ Are Not Active in Growing or Meiotically Maturing Oocytes
We were able to rule out the likely activity of several amino acid transport systems in oocytes. The lack of saturable aspartate transport rules out appreciable activity of system XAG−, consistent with the previous report that XAG− transport is not detectable in 1- or 2-cell embryos and is not conspicuous before the blastocyst stage [14].
No activity attributable to B0,+-mediated transport was detected in oocytes. Lysine transport, although completely inhibited by leucine, was insensitive to BCH, which inhibits B0,+. Alanine has higher affinity for B0,+ than lysine [47], and the very low alanine transport compared with lysine transport also argues strongly against any B0,+ activity. Finally, the conclusion that B0,+ is not active during oogenesis and meiotic maturation is consistent with the previously reported lack of detectable activity in MII eggs, extremely low activity at the 2-cell stage, and appearance of robust activity only in blastocysts [48].
The low alanine transport and its insensitivity to MeAIB inhibition indicate that system A is not active during oocyte growth or meiotic maturation. This is consistent with previous reports that system A was not present in growing oocytes [21] and with considerable data showing that it is not active in PI embryos [48] until possibly expressed in the inner cell mass at the blastocyst stage [49].
CAT-family activity was not detected during oogenesis or meiosis. Neither lysine nor arginine exhibited an appreciable leucine-resistant component of transport that would indicate a cationic amino acid-restricted transport system. It has previously been reported that CAT-related activity is present in fertilized eggs but only comprises a trivial approximately 2% of arginine transport [50]. Such a minor component would go undetected herein.
Effect of Follicle Cells on Amino Acid Uptake by Oocytes
We had originally hypothesized that follicle cells surrounding growing oocytes would possess amino acid transporters not active in growing oocytes and that these would supply the enclosed oocytes with amino acids via gap junction-mediated follicle cell-oocyte coupling. Surprisingly, this was not the case. Instead, all amino acids that were specifically transported by intact follicles, into follicle shells, or into follicle-enclosed medium growing oocytes were also transported by denuded medium growing oocytes. Similarly, all amino acids transported into GV oocytes within COCs were also transported by denuded GV oocytes. Furthermore, the pattern of inhibition by putative competitive inhibitors was not altered by the presence of follicle or cumulus cells.
We had also hypothesized that at least some of the amino acid transporters would be missing in growing oocytes but would appear in fully grown oocytes ready to become independent of the follicle at ovulation. Again, this did not prove to be the case. All amino acid transport systems assessed that were present in large growing oocytes or fully grown GV oocytes were also present in small and medium growing oocytes. Furthermore, inhibition patterns did not vary much with oocyte growth, indicating that transport systems for each amino acid did not vary from small to large growing oocytes. We cannot rule out, however, that transport activity may appear at an earlier phase of oocyte growth than we assessed herein.
Although follicle or cumulus cells did not confer additional amino acid transport capabilities on enclosed oocytes, the presence of follicle cells significantly enhanced the rates of transport of glycine, taurine, alanine, and lysine into enclosed oocytes (Table 2). The enhancement of uptake into oocytes when coupled to follicle cells was not specific to a particular transport system because significant positive CIs were found for substrates specific in oocytes for GLY, β, b0,+, and ASC/asc (glycine, taurine, lysine, and alanine, respectively). It has previously been reported that uptake of glycine, alanine, and (to some extent) lysine was enhanced in follicle-enclosed oocytes taken from preantral follicles [25] and in GV oocytes within COCs [22, 24]. Herein, we have confirmed such enhanced uptake of these amino acids by metabolic coupling and have additionally found enhanced uptake of taurine in both medium growing follicles and fully grown COCs.
Conversely, the uptake of leucine was significantly inhibited by the presence of either follicle or cumulus cells (confirmed in independent measurements [data not shown]). Of the amino acids assessed herein, leucine exhibited the highest transport rate. We propose that the presence of follicular cells may represent a diffusional barrier that can decrease the availability of amino acids at the oocyte surface when transport by the oocyte is very rapid. We note that the CIs measured herein are generally negatively correlated with the rate of transport by denuded oocytes, supporting this model. However, this remains to be directly tested, and whether it is physiologically significant would have to be assessed, particularly taking into account the dependence of CI on external concentration of substrate [25].
One set of transport measurements used herein to calculate CIs involved much longer incubation times than other measurements (Fig. 5 and Table 2). For these longer incubations, there may have been significant metabolism or incorporation into proteins of the substrates during the incubation period, which could contribute to the ability of follicle cells to enhance or inhibit the appearance of label from a particular substrate in the oocyte. Thus, the effects of metabolism and conversion of substrates to other compounds will need to be investigated further (e.g., by assessing the effect of inhibition of protein synthesis, or the effect of inhibition of metabolism by decreasing temperature, on measured CIs).
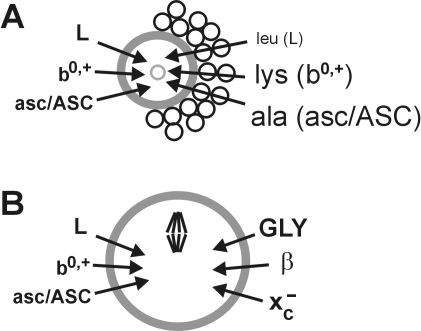
In conclusion, we have found herein that oocytes exhibit at least six of the classic amino acid transport systems (Fig. 7). Three (GLY, β, and xc−) had low activity in growing oocytes and appeared or were strongly upregulated during meiotic maturation (Fig. 7B), while the remaining three (b0,+, L, and asc/ASC) seemed to be constitutively active throughout oocyte growth and maturation. Surprisingly, no additional transport activities were identified in follicles or COCs that were not already present in denuded oocytes, although apparent metabolic coupling to follicular cells enhanced amino acid uptake in several cases (Fig. 7A). We have thus determined the likely array of major amino acid transport systems available to the growing and maturing mouse oocyte. These transport systems likely contribute to the uptake of amino acids needed for protein synthesis during oocyte growth. In addition, the three systems that become activated during meiotic maturation likely provide amino acids for specific physiological functions such as cell volume regulation and protection against oxidative stress. Future work, building on the identification of active transport systems in oocytes and follicles, will be needed to determine the full array of amino acids taken up by oocytes at each stage, their physiological functions, and the role of follicle cells under physiological conditions.
FIG. 7.
Model of major amino acid transport systems in growing and maturing mouse oocytes. A) Major transport systems detected in growing oocytes (left). The effect of the presence of follicle cells on the transport of model substrates (right) for these systems into growing oocytes is indicated by the text size, with smaller text indicating that follicle cells decreased uptake and larger text indicating that follicle cells increased uptake. B) Major transport systems in oocytes during meiotic maturation. Systems detected in growing oocytes remained present through meiosis (left), while three systems whose activity was low or absent during oocyte growth became activated during maturation (right). In both A and B, transport systems with low or uncertain activity (e.g., GLY and β during oocyte growth and N) are not shown.
Footnotes
This work was part of the Program on Oocyte Health funded under the Healthy Gametes and Great Embryos Strategic Initiative of the Canadian Institutes of Health Research (CIHR) Institute of Human Development, Child and Youth Health (IHDCYH), grant number HGG62293.
REFERENCES
- Biggers JD, McGinnis LK, Raffin M.Amino acids and preimplantation development of the mouse in protein-free potassium simplex optimized medium. Biol Reprod 2000; 63: 281–293. [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK.Differential regulation of mouse embryo development and viability by amino acids. J Reprod Fertil 1997; 109: 153–164. [DOI] [PubMed] [Google Scholar]
- Van Winkle LJ.Amino acid transport regulation and early embryo development. Biol Reprod 2001; 64: 1–12. [DOI] [PubMed] [Google Scholar]
- Summers MC, Biggers JD.Chemically defined media and the culture of mammalian preimplantation embryos: historical perspective and current issues. Hum Reprod Update 2003; 9: 557–582. [DOI] [PubMed] [Google Scholar]
- McKiernan SH, Clayton MK, Bavister BD.Analysis of stimulatory and inhibitory amino acids for development of hamster one-cell embryos in vitro. Mol Reprod Dev 1995; 42: 188–199. [DOI] [PubMed] [Google Scholar]
- Van Winkle LJ.Transport kinetics. Van Winkle LJ.Biomembrane Transport San Diego:Academic Press;1999: 65–131. [Google Scholar]
- Christensen HN.Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev 1990; 70: 43–77. [DOI] [PubMed] [Google Scholar]
- Christensen HN.Distinguishing amino acid transport systems of a given cell or tissue. Methods Enzymol 1989; 173: 576–616. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Erickson JD.Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch 2004; 447: 784–795. [DOI] [PubMed] [Google Scholar]
- Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y.CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch 2004; 447: 532–542. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA.The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Arch 2004; 447: 469–479. [DOI] [PubMed] [Google Scholar]
- Chen NH, Reith ME, Quick MW.Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch 2004; 447: 519–531. [DOI] [PubMed] [Google Scholar]
- Babu E, Kanai Y, Chairoungdua A, Kim DK, Iribe Y, Tangtrongsup S, Jutabha P, Li Y, Ahmed N, Sakamoto S, Anzai N, Nagamori S, et al. Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. J Biol Chem 2003; 278: 43838–43845. [DOI] [PubMed] [Google Scholar]
- Van Winkle LJ, Campione AL.Amino acid transport regulation in preimplantation mouse embryos: effects on amino acid content and pre- and peri-implantation development. Theriogenology 1996; 45: 69–80. [Google Scholar]
- Palacin M, Kanai Y.The ancillary proteins of HATs: SLC3 family of amino acid transporters. Pflugers Arch 2004; 447: 490–494. [DOI] [PubMed] [Google Scholar]
- Van Winkle LJ, Haghighat N, Campione AL, Gorman JM.Glycine transport in mouse eggs and preimplantation conceptuses. Biochim Biophys Acta 1988; 941: 241–256. [DOI] [PubMed] [Google Scholar]
- Tartia AP, Rudraraju N, Richards T, Hammer MA, Talbot P, Baltz JM.Cell volume regulation is initiated in mouse oocytes after ovulation. Development 2009; 136: 2247–2254. [DOI] [PubMed] [Google Scholar]
- Van Winkle LJ, Campione AL.Development of amino acid transport system B0,+ in mouse blastocysts. Biochim Biophys Acta 1987; 925: 164–174. [DOI] [PubMed] [Google Scholar]
- Steeves CL, Hammer MA, Walker GB, Rae D, Stewart NA, Baltz JM.The glycine neurotransmitter transporter GLYT1 is an organic osmolyte transporter regulating cell volume in cleavage-stage embryos. Proc Natl Acad Sci U S A 2003; 100: 13982–13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Winkle LJ, Tesch JK, Shah A, Campione AL.System B0,+ amino acid transport regulates the penetration stage of blastocyst implantation with possible long-term developmental consequences through adulthood. Hum Reprod Update 2006; 12: 145–157. [DOI] [PubMed] [Google Scholar]
- Colonna R, Cecconi S, Buccione R, Mangia F.Amino acid transport systems in growing mouse oocytes. Cell Biol Int Rep 1983; 7: 1007–1015. [DOI] [PubMed] [Google Scholar]
- Colonna R, Mangia F.Mechanisms of amino acid uptake in cumulus-enclosed mouse oocytes. Biol Reprod 1983; 28: 797–803. [DOI] [PubMed] [Google Scholar]
- Colonna R, Cecconi S, Buccione R, Mangia F.Stage-dependent modifications of amino acid uptake by antral and metaphase II mouse oocytes. Cell Biol Int Rep 1984; 8: 3–10. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK.Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. Biol Reprod 2005; 73: 351–357. [DOI] [PubMed] [Google Scholar]
- Haghighat N, Van Winkle LJ.Developmental change in follicular cell-enhanced amino acid uptake into mouse oocytes that depends on intact gap junctions and transport system. Gly J Exp Zool 1990; 253: 71–82. [DOI] [PubMed] [Google Scholar]
- Van Winkle LJ, Mann DF, Wasserlauf HG, Patel M.Mediated Na(+)-independent transport of L-glutamate and L-cystine in 1- and 2-cell mouse conceptuses. Biochim Biophys Acta 1992; 1107: 299–304. [DOI] [PubMed] [Google Scholar]
- Anas MK, Lee MB, Zhou C, Hammer MA, Slow S, Karmouch J, Liu XJ, Broer S, Lever M, Baltz JM.SIT1 is a betaine/proline transporter that is activated in mouse eggs after fertilization and functions until the 2-cell stage. Development 2008; 135: 4123–4130. [DOI] [PubMed] [Google Scholar]
- Van Winkle LJ, Patel M, Wasserlauf HG, Dickinson HR, Campione AL.Osmotic regulation of taurine transport via system beta and novel processes in mouse preimplantation conceptuses. Biochim Biophys Acta 1994; 1191: 244–255. [DOI] [PubMed] [Google Scholar]
- Van Winkle LJ, Campione AL, Gorman JM, Weimer BD.Changes in the activities of amino acid transport systems b0,+ and L during development of preimplantation mouse conceptuses. Biochim Biophys Acta 1990; 1021: 77–84. [DOI] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA, Li E, Paul DL.Female infertility in mice lacking connexin 37. Nature 1997; 385: 525–529. [DOI] [PubMed] [Google Scholar]
- Eppig JJ.The relationship between cumulus cell-oocyte coupling, oocyte meiotic maturation, and cumulus expansion. Dev Biol 1982; 89: 268–272. [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Eppig JJ.Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med 2009; 27: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller DT, Cahill DM, Schultz RM.Biochemical studies of mammalian oogenesis: metabolic cooperativity between granulosa cells and growing mouse oocytes. Dev Biol 1981; 84: 455–464. [DOI] [PubMed] [Google Scholar]
- Anas MKI, Hammer MA, Lever M, Stanton JA, Baltz JM.The organic osmolytes betaine and proline are transported by a shared system in early preimplantation mouse embryos. J Cell Physiol 2007; 210: 266–277. [DOI] [PubMed] [Google Scholar]
- Martin PM, Sutherland AE.Exogenous amino acids regulate trophectoderm differentiation in the mouse blastocyst through an mTOR-dependent pathway. Dev Biol 2001; 240: 182–193. [DOI] [PubMed] [Google Scholar]
- Lawitts JA, Biggers JD.Culture of preimplantation embryos. Methods Enzymol 1993; 225: 153–164. [DOI] [PubMed] [Google Scholar]
- Eppig JJ.Analysis of mouse oogenesis in vitro: oocyte isolation and the utilization of exogenous energy sources by growing oocytes. J Exp Zool 1976; 198: 375–382. [DOI] [PubMed] [Google Scholar]
- Erdogan S, FitzHarris G, Tartia AP, Baltz JM.Mechanisms regulating intracellular pH are activated during growth of the mouse oocyte coincident with acquisition of meiotic competence. Dev Biol 2005; 286: 352–360. [DOI] [PubMed] [Google Scholar]
- FitzHarris G, Siyanov V, Baltz JM.Granulosa cells regulate oocyte intracellular pH against acidosis in preantral follicles by multiple mechanisms. Development 2007; 134: 4283–4295. [DOI] [PubMed] [Google Scholar]
- Lewis AM, Kaye PL.Characterization of glutamine uptake in mouse two-cell embryos and blastocysts. J Reprod Fertil 1992; 95: 221–229. [DOI] [PubMed] [Google Scholar]
- Kolajova M, Hammer MA, Collins JL, Baltz JM.Developmentally regulated cell cycle dependence of swelling-activated anion channel activity in the mouse embryo. Development 2001; 128: 3427–3434. [DOI] [PubMed] [Google Scholar]
- Kolajova M, Baltz JM.Volume-regulated anion and organic osmolyte channels in mouse zygotes. Biol Reprod 1999; 60: 964–972. [DOI] [PubMed] [Google Scholar]
- Hammer MA, Baltz JM.Beta-alanine but not taurine can function as an organic osmolyte in preimplantation mouse embryos cultured from fertilized eggs. Mol Reprod Dev 2003; 66: 153–161. [DOI] [PubMed] [Google Scholar]
- Dawson KM, Baltz JM.Organic osmolytes and embryos: substrates of the Gly and beta transport systems protect mouse zygotes against the effects of raised osmolarity. Biol Reprod 1997; 56: 1550–1558. [DOI] [PubMed] [Google Scholar]
- Pan H, O'Brien MJ, Wigglesworth K, Eppig JJ, Schultz RM.Transcript profiling during mouse oocyte development and the effect of gonadotropin priming and development in vitro. Dev Biol 2005; 286: 493–506. [DOI] [PubMed] [Google Scholar]
- Van Winkle LJ, Campione AL, Gorman JM.Na+-independent transport of basic and zwitterionic amino acids in mouse blastocysts by a shared system and by processes which distinguish between these substrates. J Biol Chem 1988; 263: 3150–3163. [PubMed] [Google Scholar]
- Van Winkle LJ, Christensen HN, Campione AL.Na+-dependent transport of basic, zwitterionic, and bicyclic amino acids by a broad-scope system in mouse blastocysts. J Biol Chem 1985; 260: 12118–12123. [PubMed] [Google Scholar]
- Van Winkle LJ, Campione AL, Farrington BH.Development of system B0,+ and a broad-scope Na(+)-dependent transporter of zwitterionic amino acids in preimplantation mouse conceptuses. Biochim Biophys Acta 1990; 1025: 225–233. [DOI] [PubMed] [Google Scholar]
- Jamshidi MB, Kaye PL.Glutamine transport by mouse inner cell masses. J Reprod Fertil 1995; 104: 91–97. [DOI] [PubMed] [Google Scholar]
- Van Winkle LJ, Campione AL.Functional changes in cation-preferring amino acid transport during development of preimplantation mouse conceptuses. Biochim Biophys Acta 1990; 1028: 165–173. [DOI] [PubMed] [Google Scholar]