Abstract
BSP proteins and their homologs are a family of structurally related proteins characterized by the presence of tandem fibronectin type II domains. In the bovine species, BSP proteins were shown to be involved in sperm capacitation, a posttesticular maturation event necessary for sperm to acquire the ability to fertilize an oocyte. Recently, many new genes from this family have been discovered in numerous mammalian species. However, inconsistency in the nomenclature is creating much confusion. In light of the rapid growth of the BSP superfamily of proteins, we propose a new nomenclature in collaboration with the HUGO Gene Nomenclature Committee.
Keywords: epididymis, male reproductive tract, seminal vesicles, sperm capacitation, sperm motility and transport
New nomenclature for mammalian BSP genes.
INTRODUCTION
Mammalian sperm undergo a series of maturation events before acquiring the ability to fertilize an oocyte. Over the past few decades, special attention has been paid to proteins present in seminal plasma and to their potential roles in sperm maturation events. In the bovine species, a family of closely related proteins (bovine seminal plasma, or BSP proteins) constituting approximately 60% of the total seminal plasma proteins was discovered and shown to be essential for bovine sperm capacitation, a maturation event taking place inside the female reproductive tract [1–3]. These proteins also have been shown to play a role in sperm binding to the bovine oviductal epithelium and formation of the oviductal sperm reservoir [4, 5]. Since the discovery of BSP proteins in bovine seminal plasma, homologs of these proteins have been identified in the seminal plasma of numerous other mammals, such as boar, stallion, goat, ram, and bison, indicating that these proteins would be ubiquitous in mammalian seminal plasma [6]. In addition, the characterized cDNA sequences of the bovine proteins [7] allowed for identification of homologous DNA sequences in the genomes of many other species, such as human, horse, mouse, rat, chimpanzee, dog, and rabbit [8]. The conservation of BSP-encoding genes across many mammalian species implies that these proteins constitute a family with an important role in reproduction.
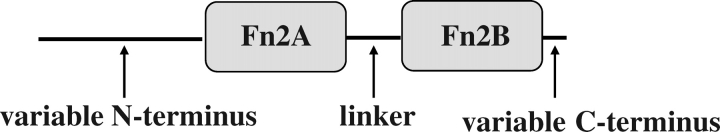
Proteins of the BSP family are all of relatively low molecular mass (12–30 kDa) and can be glyscosylated or not [6]. These proteins were originally thought to be inhibins because they could inhibit the secretion of gonadotropins from cultured pituitary cells [1]. They share a common secondary structure composed of a variable N-terminal domain followed by two tandemly arranged fibronectin type II domains (∼40 amino acids each) separated by a seven-amino acid linker, and a short, variable C-terminal domain (Fig. 1). In bovine, the type II domains confer many binding properties to BSP proteins, such as binding to glycosaminoglycans [9], choline phospholipids [10], high-density lipoproteins [11], gelatin [12], and sperm binding [13]. The bovine BSP proteins also bind to components of semen extenders (egg yolk low-density lipoproteins and milk caseins) used for semen preservation, and these interactions play a key role in protecting sperm during storage in liquid or frozen state [6, 14]. In boar, stallion, goat, ram, and bison, BSP proteins were isolated from the seminal plasma or seminal vesicle secretions because of the secretion of these proteins by seminal vesicles. However, important differences exist between these BSP family proteins and those found in mouse and human. First, the expression of mouse and human BSP homologs is detected in the epididymis rather than the seminal vesicles, which could suggest other functions [15]. In addition, the concentrations of BSP proteins or homologs found in the seminal plasma of bulls vary greatly compared with mice and humans [14]. Approximately 60% of the total seminal plasma proteins in bull are BSPs, compared with less than 0.01% in mice and humans [16].
FIG. 1.
General structure of BSP proteins. Fn2A, first Fn2 domain; Fn2B, second Fn2 domain.
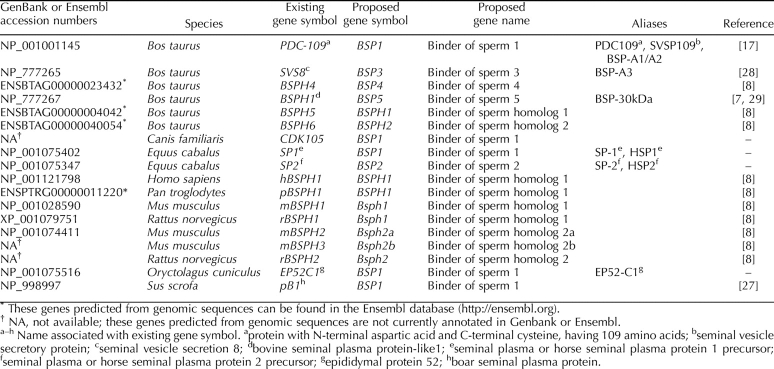
Over the years, BSP family proteins and genes have been named by the groups that discovered them without much concern for a naming consensus and/or guidelines. For example, the bovine protein PDC-109 was first named according to its N- and C-terminal amino acids followed by the total number of amino acid residues [17]. PDC-109 is actually composed of two proteins, which differ only in their degree of glycosylation and which were later named BSP-A1 and BSP-A2, the “A” referring to their acidic nature [1]. BSP-A3, the third member of the bovine protein family, also was named in this way. However, BSP-30kDa was named according to its molecular mass [12, 18]. Reference to molecular mass also was employed when naming homologous proteins detected in goat [19], ram [20], stallion (HSP-12kDa) [21], and bison [22] seminal plasma. These names also indicate the species and biological fluid from which each protein was detected and/or isolated; for example, in the name BiSV-16kDa, “Bi” stands for bison, whereas “SV” designates seminal vesicle fluid. Stallion seminal plasma also contains two other homologs of the BSP proteins that are named horse seminal plasma proteins 1 and 2 (HSP-1 and HSP-2), where the numbers simply indicate the order in which they were discovered [23, 24]. For the above-mentioned species, the genes encoding the BSP homologs have yet to be characterized. In the case of the porcine homolog, pB1, the name simply represents porcine seminal plasma protein 1 [25]. Another porcine seminal plasma protein, DQH, named after the first three amino acids at the amino terminus, appears to be a homolog of pB1 [26]. The cDNA encoding the porcine homolog DQH has been cloned [27].
Fibronectin type II domains, also called Fn2 domains, are really the signature of BSP proteins (Fig. 1). Recently, an extensive bioinformatics analysis of all proteins containing Fn2 domains in fully or partially sequenced genomes of several mammalian species was conducted, identifying many new potential homologs of BSP proteins [8]. In mouse and human, these genes were named according to their homology to the bovine BSPs (hBSPH1 for human BSP homolog 1; and mBSPH1, mBSPH2, and mBSPH3 representing mouse BSP homologs 1, 2, and 3, respectively). The complete cDNA sequences of these genes have been cloned and their mRNA expression patterns investigated, which indicated that the mBSPH3 gene was not actively expressed [15]. Similar naming approaches were used for the homologous DNA sequences identified in rat and chimpanzee [8]. The complete list of BSP homologs discovered to date is catalogued in Table 1.
TABLE 1.
Proposed nomenclature for the BSP gene family.
The heterogeneous naming style used to designate genes of the BSP family is beginning to create confusion, especially with the expansion of this emerging superfamily. It is becoming necessary to create a standardized manner for naming these genes. In this paper, we propose a unified approach to naming BSP family members, which includes reviewing and renaming. Table 1 groups all the BSP genes discovered thus far, along with their proposed new names, in accordance with the HUGO Gene Nomenclature Committee (http://www.genenames.org). In addition, there may be many more homologs belonging to this family that have yet to be discovered. Upon their identification, we propose that these genes be named according to the following nomenclature guidelines.
GUIDELINES
First, the signification of the name BSP, which presently stands for bovine seminal plasma, will be changed to binder of sperm. This avoids mentioning the species in the gene name and no longer alludes to the protein source. Although sperm binding has not been demonstrated for all BSP family proteins, we propose that the symbol BSP be conserved for historical reasons. The proposed new gene symbols and names for existing BSP genes based on Figure 2 are enumerated in Table 1. In addition, the following guidelines are proposed for naming newly discovered BSP genes.
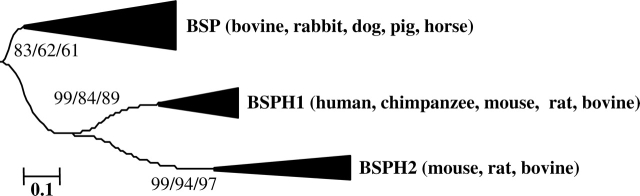
FIG. 2.
The BSP clade is composed of three subfamilies. Neighbor-joining trees of the two Fn2 domains of BSP-related proteins, showing that BSP proteins and their relatives are separated into three subfamilies: BSP, BSPH1, and BSPH2. Bootstrap values from 1000 replicates for neighbor-joining (first number), 1000 replicates for maximum parsimony (second number), and 100 replicates for maximum likelihood (third number) are indicated at the nodes and were used to assess the robustness of the trees. Genetic distance is indicated as the number of substitutions per amino acid site (see details in Fan et al. [8]).
1. Only proteins containing two Fn2 domains, which are defined within the lineages as described previously [8], will be considered members of the BSP superfamily. The name BSP should not be attributed to proteins that are homologous to only one of the Fn2 domains or to proteins that contain more than two of these domains.
2. If and when new genes and gene families are discovered within this superfamily in mammalian species, we propose that they be given the names BSP, BSPH1, or BSPH2, depending on their phylogenetic relationships, or named BSPH3, BSPH4, etc. in the case of new gene families.
3. We propose that the symbol BSPL [no.] (BSP-like group [no.]) may be used to designate genes that fulfill these criteria and:
are from organisms other than mammals
are homologous to BSPs (i.e., homology to both Fn2 domains)
do not fall within the established family.
In consensus with the proposed gene names, the proteins encoded by these genes would be designated in the same manner but without italics. Interestingly, in ungulates like ram (Ovis aries), bison (Bison bonasus), and goat (Capra hircus), three to four BSP-homologous proteins have been purified from either seminal plasma or seminal vesicle secretions [19, 20, 22]. However, the genes encoding these proteins have not yet been characterized. In addition, one BSP gene is predicted from genomic sequences in the chimpanzee (Pan troglodytes) as well as two in the rat (Rattus norvegicus) [8]. We hope that these naming guidelines will aid in eliminating the confusion existing with the current names attributed to BSP genes. In addition, these guidelines will allow a standardized way to name newly discovered genes of the BSP superfamily of proteins.
Acknowledgments
The following scientists have agreed to follow this nomenclature, and we hope that other scientists working on BSP genes/proteins will adhere to these recommendations: Dr. Susan S. Suarez, Department of Biomedical Sciences, College of Veterinary Medicine, Cornell University, Ithaca, NY; Dr. Alexander J. Travis, Baker Institute for Animal Health, Cornell University College of Veterinary Medicine; Dr. Brett Nixon, School of Environmental and Life Sciences, University of Newcastle, Callaghan, Australia; Dr. Michel Lafleur, Department of Chemistry, Université de Montréal, Montréal, Canada; Dr. Claude Lazure, Clinical Research Institute, Montréal, Canada; Dr. Robert Sullivan, Centre de Recherche en Biologie de la Reproduction, Université Laval, Ste-Foy, Canada; Vera Jonakova, Institute of Biotechnology, Academy of Sciences of the Czech Republic, Prague, Czech Republic; and Prof. Jerzy Strzeżek, Department of Animal Biochemistry and Biotechnology, University of Warmia and Mazury, Olsztyn-Kortowo, Poland. Dr. Lois Maltais, Mouse Genome Nomenclature Committee, has approved this new nomenclature.
Footnotes
1Supported by the Canadian Institutes of Health Research and Natural Sciences and Engineering Research Council of Canada.
REFERENCES
- Manjunath P.Gonadotropin release stimulatory and inhibitory proteins in bull seminal plasma. Sairam MR, Atkinson LE.Gonadal Proteins and Peptides and Their Biological Significance Singapore:World Science Publishing;1984: 49–61. [Google Scholar]
- Therien I, Bleau G, Manjunath P.Phosphatidylcholine-binding proteins of bovine seminal plasma modulate capacitation of spermatozoa by heparin. Biol Reprod 1995; 52: 1372–1379. [DOI] [PubMed] [Google Scholar]
- Therien I, Soubeyrand S, Manjunath P.Major proteins of bovine seminal plasma modulate sperm capacitation by high-density lipoprotein. Biol Reprod 1997; 57: 1080–1088. [DOI] [PubMed] [Google Scholar]
- Gwathmey TM, Ignotz GG, Suarez SS.PDC-109 (BSP-A1/A2) promotes bull sperm binding to oviductal epithelium in vitro and may be involved in forming the oviductal sperm reservoir. Biol Reprod 2003; 69: 809–815. [DOI] [PubMed] [Google Scholar]
- Gwathmey TM, Ignotz GG, Mueller JL, Manjunath P, Suarez SS.Bovine seminal plasma proteins PDC-109, BSP-A3, and BSP-30-kDa share functional roles in storing sperm in the oviduct. Biol Reprod 2006; 74: 501–507. [DOI] [PubMed] [Google Scholar]
- Manjunath P, Bergeron A, Lefebvre J, Fan J.Seminal plasma proteins: functions and interaction with protective agents during semen preservation. Roldan ER, Gomendio M.Society for Reproduction and Fertility Supplement, vol. 65 Thrumpton, UK:Nottingham University Press;2007: 217–228. [PubMed] [Google Scholar]
- Salois D, Menard M, Paquette Y, Manjunath P.Complementary deoxyribonucleic acid cloning and tissue expression of BSP-A3 and BSP-30-kDa: phosphatidylcholine and heparin-binding proteins of bovine seminal plasma. Biol Reprod 1999; 61: 288–297. [DOI] [PubMed] [Google Scholar]
- Fan J, Lefebvre J, Manjunath P.Bovine seminal plasma proteins and their relatives: a new expanding superfamily in mammals. Gene 2006; 375: 63–74. [DOI] [PubMed] [Google Scholar]
- Therien I, Bergeron A, Bousquet D, Manjunath P.Isolation and characterization of glycosaminoglycans from bovine follicular fluid and their effect on sperm capacitation. Mol Reprod Dev 2005; 71: 97–106. [DOI] [PubMed] [Google Scholar]
- Desnoyers L, Manjunath P.Major proteins of bovine seminal plasma exhibit novel interactions with phospholipid. J Biol Chem 1992; 267: 10149–10155. [PubMed] [Google Scholar]
- Manjunath P, Marcel YL, Uma J, Seidah NG, Chretien M, Chapdelaine A.Apolipoprotein A-I binds to a family of bovine seminal plasma proteins. J Biol Chem 1989; 264: 16853–16857. [PubMed] [Google Scholar]
- Manjunath P, Sairam MR, Uma J.Purification of four gelatin-binding proteins from bovine seminal plasma by affinity chromatography. Biosci Rep 1987; 7: 231–238. [DOI] [PubMed] [Google Scholar]
- Manjunath P, Chandonnet L, Leblond E, Desnoyers L.Major proteins of bovine seminal vesicles bind to spermatozoa. Biol Reprod 1994; 50: 27–37.[published erratum appears in Biol Reprod 1994; 50:977]. [DOI] [PubMed] [Google Scholar]
- Bergeron A, Manjunath P.New insights towards understanding the mechanism of sperm protection by egg yolk and milk. Mol Reprod Dev 2006; 71: 1338–1344. [DOI] [PubMed] [Google Scholar]
- Lefebvre J, Fan J, Chevalier S, Sullivan R, Carmona E, Manjunath P.Genomic structure and tissue-specific expression of human and mouse genes encoding homologs of the major bovine seminal plasma proteins. Mol Hum Reprod 2007; 13: 45–53. [DOI] [PubMed] [Google Scholar]
- Manjunath P, Therien I.Role of seminal plasma phospholipid-binding proteins in sperm membrane lipid modification that occurs during capacitation. J Reprod Immunol 2002; 53: 109–119. [DOI] [PubMed] [Google Scholar]
- Esch FS, Ling NC, Bohlen P, Ying SY, Guillemin R.Primary structure of PDC-109, a major protein constituent of bovine seminal plasma. Biochem Biophys Res Commun 1983; 113: 861–867. [DOI] [PubMed] [Google Scholar]
- Manjunath P, Baillargeon L, Marcel YL, Seidah NG, Chrétien M, Chapdelaine A.Diversity of novel proteins in gonadal fluids. McKerns KW, Chrétien M.Molecular Biology of Brain and Endocrine Peptidergic Systems New York:Plenum Press;1988: 259–273. [Google Scholar]
- Villemure M, Lazure C, Manjunath P.Isolation and characterization of gelatin-binding proteins from goat seminal plasma. Reprod Biol Endocrinol 2003; 1: 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron A, Villemure M, Lazure C, Manjunath P.Isolation and characterization of the major proteins of ram seminal plasma. Mol Reprod Dev 2005; 71: 461–470. [DOI] [PubMed] [Google Scholar]
- Menard M, Nauc V, Lazure C, Vaillancourt D, Manjunath P.Novel purification method for mammalian seminal plasma phospholipid-binding proteins reveals the presence of a novel member of this family of protein in stallion seminal fluid. Mol Reprod Dev 2003; 66: 349–357. [DOI] [PubMed] [Google Scholar]
- Boisvert M, Bergeron A, Lazure C, Manjunath P.Isolation and characterization of gelatin-binding bison seminal vesicle secretory proteins. Biol Reprod 2004; 70: 656–661. [DOI] [PubMed] [Google Scholar]
- Calvete JJ, Mann K, Schafer W, Sanz L, Reinert M, Nessau S, Raida M, Topfer-Petersen E.Amino acid sequence of HSP-1, a major protein of stallion seminal plasma: effect of glycosylation on its heparin- and gelatin-binding capabilities. Biochem J 1995; 310: 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete JJ, Raida M, Gentzel M, Urbanke C, Sanz L, Topfer-Petersen E.Isolation and characterization of heparin- and phosphorylcholine-binding proteins of boar and stallion seminal plasma. Primary structure of porcine pB1. FEBS Lett 1997; 407: 201–206. [DOI] [PubMed] [Google Scholar]
- Sanz L, Calvete JJ, Mann K, Gabius HJ, Topfer-Petersen E.Isolation and biochemical characterization of heparin-binding proteins from boar seminal plasma: a dual role for spermadhesins in fertilization. Mol Reprod Dev 1993; 35: 37–43. [DOI] [PubMed] [Google Scholar]
- Bezouska K, Sklenar J, Novak P, Halada P, Havlicek V, Kraus M, Ticha M, Jonakova V.Determination of the complete covalent structure of the major glycoform of DQH sperm surface protein, a novel trypsin-resistant boar seminal plasma O-glycoprotein related to pB1 protein. Protein Sci 1999; 8: 1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plucienniczak G, Jagiello A, Plucienniczak A, Holody D, Strzezek J.Cloning of complementary DNA encoding the pB1 component of the 54-kilodalton glycoprotein of boar seminal plasma. Mol Reprod Dev 1999; 52: 303–309. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Manjunath P, Rochemont J, Sairam MR, Chretien M.Complete amino acid sequence of BSP-A3 from bovine seminal plasma. Homology to PDC-109 and to the collagen-binding domain of fibronectin. Biochem J 1987; 243: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete JJ, Mann K, Sanz L, Raida M, Topfer-Petersen E.The primary structure of BSP-30K, a major lipid-, gelatin-, and heparin-binding glycoprotein of bovine seminal plasma. FEBS Lett 1996; 399: 147–152. [DOI] [PubMed] [Google Scholar]