Abstract
Prenatal testosterone treatment leads to LH excess as well as ovarian follicular and ovulatory defects in the adult. These disruptions may stem from LH excess, abnormal FSH input, compromised ovarian sensitivity to gonadotropins, or intrinsic ovarian defects. To determine if exogenous gonadotropins rescue ovarian and ovulatory function of testosterone-treated sheep, the release of endogenous LH and biopotent FSH in control and prenatal testosterone-treated sheep was blocked with a GnRH antagonist during the first two breeding seasons and with LH/FSH coadministered in a manner approximating natural follicular phase. An acidic mix of FSH was administered the first 36 h at 2-h intervals and a less acidic mix for the next 12 h at 1-h intervals (different FSH preparations were used each year), and ovulation was induced with hCG. Circulating FSH and estradiol responses to gonadotropins measured in 2-h samples differed between treatment groups in Year 1 but not in Year 2. Ovarian follicular distribution and number of corpora lutea (in ewes that ovulated) tracked by ultrasonography and luteal progesterone responses were similar between control and prenatal testosterone-treated females but differed between years. Furthermore, hCG administration induced large cystic and luteinized follicles in both groups of females in Year 2, although the growth rate differed between control and prenatal testosterone-treated females. Our findings provide evidence that 1) ovulatory response in prenatal testosterone-treated females can be rescued with exogenous gonadotropins, 2) resultant follicular response is dependent on the nature of gonadotropic input, and 3) an abnormal follicular milieu may underlie differences in developmental trajectory of cystic follicles in prenatal testosterone-treated females.
Keywords: follicle-stimulating hormone; follicular cysts; follicular development, FSH heterogeneity; gonadotropins; ovarian follicles; ovary; ovulation; ovulatory cycle
Exogenous gonadotropins, following suppression of endogenous gonadotropins with a GnRH antagonist, restores ovulatory function in prenatal testosterone-treated sheep
INTRODUCTION
Prenatal testosterone-treated sheep manifest hypergonadotropism [1–3], polycystic ovarian morphology [4], and progressive deterioration of the reproductive axis [5–7]. Both a reduction in responsiveness to estradiol (E2) negative feedback, which leads to an increase in LH pulse frequency [2], and an increase in pituitary responsiveness to GnRH, which leads to an increase in LH amplitude [8], contribute to the development of hypergonadotropism [1–3, 8]. The increase in the frequency of LH pulses, indicative of an increase in GnRH pulse frequency, is likely to be accompanied by release of less acidic, short-lived, but more biopotent mixes of FSH isoforms [9, 10]. If so, continuous provision of such short-lived, biopotent FSH to the ovary may be detrimental to the progression of the follicle to the preovulatory state. A second possibility is that the ovulatory disruption seen in prenatal testosterone-treated sheep [7, 11] may be the outcome of the elevated LH [1–3, 8] and the ensuing intrafollicular hyperandrogenism [12]. In either case, providing LH and FSH at concentrations approximating those occurring during the natural follicular phase should optimize development of preovulatory follicles and rescue ovulatory function.
A third possibility is that ovarian dysfunction seen in prenatal testosterone-treated females is the result of intrinsic ovarian defects. If these defects are too severe, provision of exogenous gonadotropins will not rescue function. Alternatively, if the defect involves reduced ovarian sensitivity to FSH, ovarian function might be rescued by provision of exogenous FSH. FSH plays a key role in maturation of follicles, prevention of follicular atresia, induction of aromatase, proliferation of granulosa cells, and induction of LH/FSH receptors [13–17]. Treatments that lead to increases in endogenous FSH [18–21] or administration of FSH in various species [22–25] have been shown to overcome ovarian deficits and increase ovulation rates. Administration of highly purified FSH following GnRH analog suppression of gonadotropins stimulated the development of ovulatory-sized follicles in normal sheep that were fully capable of ovulation and formation of normal corpora lutea (CL) [24]. In the present study, we tested the hypothesis that provision of exogenous gonadotropins in a manner closely approximating the follicular phase, after blockade of endogenous LH and biopotent FSH, would improve ovarian function and rescue ovulatory function in prenatal testosterone-treated sheep.
MATERIALS AND METHODS
General
Acyline, a GnRH antagonist [26, 27], was obtained through the Contraception and Reproductive Health Branch, National Institutes of Health. Purified ovine pituitary LH used in the two main studies (same batch used in our earlier study [28]) and purified ovine pituitary FSH (oFSH) used in study 1 were gifts from Dr. Harold Papkoff (University of California, Davis). Because the FSH supply used in study 1 was no longer available, affinity-purified oFSH (NIDDK oFSH-18, AFP5862D; potency 65-fold NIH-FSH-S1 per mg) was purchased for study 2 through Dr. A.F. Parlow (National Hormone and Peptide Program). For both studies, hCG (CG-10), purchased from Sigma-Aldrich, was used to induce ovulation.
Preparation of Less Acidic Mix of FSH Isoforms
Less acidic mix of FSH (N-FSH) was prepared by treating oFSH with neuraminidase, which cleaves terminal sialic acid residues [28]. For study 1, 4 mg of oFSH were dissolved in 8 ml of phosphate buffer (0.1 M Na/KPO4, pH 5.1) containing 1% BSA. Half the stock solution was treated with 0.4 ml of neuraminidase (N-2133; Sigma-Aldrich) and the other half with 0.4 ml of buffer. Both aliquots were incubated at 37°C with mixing for 2.5 h. A 0.2% stock of sodium azide (0.4 ml [w/v] dissolved in water) was added to stop neuraminidase activity. For the second study, 2 mg of oFSH were dissolved in phosphate buffer. Equal aliquots of this FSH stock were treated as described above to produce C-FSH (untreated) and N-FSH (neuraminidase treated).
Chromatofocusing of FSH
The distribution patterns of FSH isoforms were assessed by chromatofocusing as previously described [29]. The C-FSH and N-FSH stocks were dialyzed overnight against 0.025 mol/L of imidazole-HCI (pH 7.4). The solutions were applied to a 15- × 0.9-cm column of PBE 94 resin (Pharmacia) and eluted with polybuffer 74-HCl (pH 4.0; Pharmacia), and 2-ml fractions were collected. When the pH of the eluent reached 4.0, we applied 1 M NaCl to elute any remaining bound FSH. The immunoreactive FSH content of each fraction was determined by RIA. Recovery of FSH for the study 1 and study 2 averaged 85% and 97%, respectively, for C-FSH and 81% and 110%, respectively, for N-FSH.
Breeding, Prenatal Treatment, and Maintenance of Experimental Females
Details of breeding and maintenance of breeder ewes and experimental females have been described in detail elsewhere [6, 30]. Prenatal treatment consisted of twice-weekly injections of 100 mg of testosterone propionate (Sigma-Aldrich Corp.) in cottonseed oil (2 ml) from Days 30 to 90 of gestation (term = 147 d). Control ewes did not receive vehicle, because no differences have been observed in twice-weekly progesterone (P4) patterns or cycle characteristics [30] between those receiving vehicle and those that did not. The same five control and five prenatal testosterone-treated females were used in the two main studies. When twin births were involved, only one offspring from each ewe was included in the study. All procedures were approved by the University Animal Care and Use Committee at the University of Michigan.
Pilot Studies
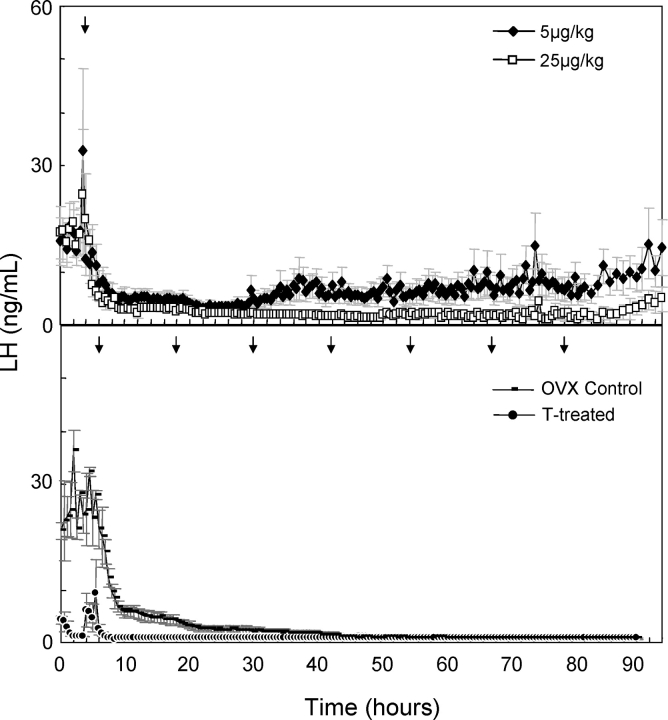
To control LH/FSH input to the ovary of control and prenatal testosterone-treated females (prenatal testosterone treatment leads to LH hypersecretion [1–3, 8]), it is necessary to block endogenous GnRH action. Two pilot studies were conducted to determine the optimal dosage and frequency of administration of the GnRH antagonist. In the first pilot study, eight adult Suffolk sheep, ovariectomized ∼3 mo prior, received one injection of either 5 or 25 μg/kg of GnRH antagonist dissolved in a 5% sterile dextrose (n = 4 sheep for each dosage). Blood samples (5 ml) were collected at 30-min intervals for 4 days beginning 3 h before administration of GnRH antagonist. Suppression of LH with either dose was immediate, but the duration of suppression was longer in the sheep that received the 25 μg/kg dose compared to those that received the 5 μg/kg dose (Fig. 1, Top).
FIG. 1.
Top) Duration of suppression of LH in ovariectomized control females following a single administration of 5 or 25 μg/kg of the GnRH antagonist, acyline. Bottom) Efficacy of 10 μg/kg of GnRH antagonist administered every 12 h to ovariectomized control (OVX control) and ovary-intact prenatal testosterone-treated (T-treated) females in achieving prolonged suppression of LH. Arrows indicate time of administration of the GnRH antagonist.
A second pilot study was performed to determine whether continuous suppression of endogenous LH could be achieved by administering 10 μg/kg of GnRH antagonist every 12 h for 72 h to three adult ewes ovariectomized ∼4 mo prior and three ovary-intact prenatal testosterone-treated females (age, ∼12 mo). Blood samples (3 ml) were taken at 0.5-h intervals beginning 6 h before the first GnRH antagonist injection and ending 12 h after the last injection. Complete and sustained suppression of LH in prenatal testosterone-treated and overiectomized females was achieved beginning at 10 and 45 h, respectively, after the first injection of GnRH antagonist (Fig. 1, Bottom). This dosage and frequency of GnRH antagonist administration (10 μg/kg at 12-h intervals) was chosen for the main studies.
Main Studies
Two studies were conducted to determine if delivery of LH/FSH, in a manner that mimicked the natural follicular phase, after blockade of GnRH action with the GnRH antagonist would normalize ovarian follicular dynamics and restore ovulatory function in prenatal testosterone-treated females. Before initiation of both studies, all control females showed regular progestogenic cycles, whereas only one prenatal testosterone-treated female each year had cycles with some regularity. The other four prenatal testosterone-treated females showed cycle defects that ranged between oligo-ovulatory and anovulatory with prolonged progestogenic cycles suggestive of luteinized follicles, failure of CL to regress, or anovulatory condition [6, 31]. For both studies, collection of blood samples and delivery of LH/FSH were made via an indwelling catheter placed in the jugular vein.
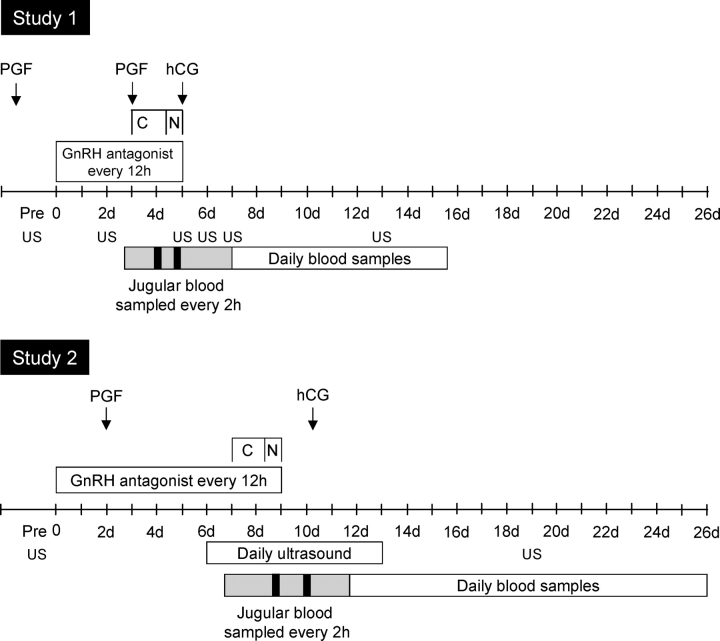
Study 1.
The first study was conducted near the end of the first breeding season. The experimental design is summarized in Figure 2 (Top). Body weights (mean ± SEM) of the control and prenatal testosterone-treated females averaged 46.9 ± 2.4 and 51.0 ± 4.2 kg, respectively, and did not differ. Two 20-mg i.m. injections of prostaglandin F2α (PGF2α; 5 mg/ml of Lutalyse; Pfizer Animal Health) were administered 11 days apart to induce luteolysis and synchronize initiation of the follicular phase in cycling females. Injections of GnRH antagonist (10 μg/kg, 12-h intervals, s.c.) began 3 days before and continued during the 48-h LH/FSH treatment.
FIG. 2.
Schematic detailing the experimental design of the two studies conducted during the first two breeding seasons. The GnRH antagonist was given every 12 h (for 5 days in study 1 and 9 days in study 2) to block endogenous LH and the GnRH-induced release of less acidic FSH. Starting 3 and 7 days after the start of GnRH antagonist treatment, LH/C-FSH was given every 2 h (denoted as C) for 36 h, followed by administration of LH/N-FSH or LH/C-FSH/N-FSH hourly for next 12 h in study 1 and study 2, respectively (denoted as N). Ovulation was induced by administering 1500 IU of hCG. Starting from 4 h before the start of LH/FSH administration, blood samples were collected at 2-h intervals until 96 h (Year 1) or 116 h (Year 2). Black boxes represent the period of frequent sampling (12-min intervals) conducted to characterize the profile of LH and FSH following an injection. Daily blood samples were taken for 15 and 19 days in study 1 and study 2, respectively, for measurement of circulating levels of progesterone to assess function of corpora lutea. Transrectal ultrasonography (US) was performed to characterize ovarian follicular response to the exogenous pulses of LH/FSH and CL development. See text for complete description of dosage and timing of treatments.
Injections of LH/C-FSH (LH, 0.32 μg/kg; C-FSH, 0.17 μg/kg; i.v.) were administered at 2-h intervals for 36 h, followed by injections at 1-h intervals of LH/N-FSH (LH, 0.32 μg/kg; N-FSH, 0.17 μg/kg) for the next 12 h, as detailed previously [4]. This frequency and duration of LH/FSH treatment approximates the GnRH pulse frequency seen during the transition from the early to the late follicular phase [32]. One hour after the last LH/N-FSH injection, 1500 IU of hCG were given i.m. to each ewe to induce ovulation. Blood samples were collected every 2 h starting 4 h before and concluding 96 h after start of the LH/FSH treatment. The delivery pattern of LH and FSH was characterized from blood samples collected every 12 min for 2 h during the LH/C-FSH and LH/N-FSH treatments. Daily blood samples were taken for 14 days after cessation of LH/FSH treatment to determine luteal function after hCG administration. Circulating LH and FSH concentrations were determined in all 2-h and 12-min samples, E2 in alternate 2-h samples (every 4 h), and P4 in daily samples.
Ultrasonography was performed before the start of GnRH antagonist treatment (to assess pretreatment ovarian follicular population), before the start of the LH/FSH treatment (to characterize the effect of GnRH antagonist inhibition of endogenous LH/FSH production on ovarian follicular population), at the end of the LH/FSH treatment (to characterize follicular response to gonadotropin stimulation), 24 and 48 h after hCG administration (to assess ovulatory response), and 10 days after the start of LH/FSH injections (i.e., 8 days after hCG administration; to determine the number of CL developed). Details of the ultrasonographic examination have been described in detail elsewhere [6, 11, 33]. Briefly, ewes were restrained in a crate in the standing position while both ovaries were examined using a rigid-mounted, 7.5-MHz transducer connected to an Aloka SSD-900V.
Study 2.
The second study was conducted near the end of the second breeding season (Fig. 2). The mean body weights at the start of this study for control and testosterone-treated ewes were 53.0 ± 2.6 and 58.0 ± 4.2 kg, respectively, and did not differ. This study began during the luteal phase of a synchronized cycle. All ewes received one 20-mg injection of PGF2α. Based on findings from study 1, the duration of GnRH antagonist treatment was increased from 3 to 7 days to achieve better follicular suppression before starting the LH/FSH injections. The GnRH antagonist treatment continued through the 2 days of LH/FSH injections and ceased just before hCG treatment. The frequency of injections of LH/FSH was similar to that of study 1. Based on the concentrations and profiles of gonadotropins achieved in study 1, adjustments were made in the amount of LH and the amount and mix of FSH injected in study 2. For the first 36 h, 0.11 μg/kg of LH and 0.17 μg of C-FSH were administered at 2-h intervals; for the next 12 h, 0.053 μg/kg of LH (half the 2-h dose), 0.04 μg/kg of C-FSH (one-quarter of the 2-h dose), and 0.17 μg/kg of N-FSH were administered at 1-h intervals. The rationale for mixing C-FSH and N-FSH was to allow delivery of FSH that mimicked the constitutive (acidic) and regulated (less acidic) components of FSH release [34]. To induce ovulation, hCG (1500 IU) was given to each ewe 1 h after the last LH/C-FSH/N-FSH injection. Circulating LH and FSH levels were characterized in blood samples collected every 2 h starting 4 h before and concluding 116 h after the start of the LH/FSH administration as well as in frequent samples taken every 12 min for 2.4 h during the 2-h and 1-h LH/FSH administrations. Circulating E2 was measured in all samples through the end of LH/FSH treatment, and P4 was measured in daily samples.
Transrectal ultrasonography was performed before the start of GnRH antagonist injections (to characterize pretreatment ovarian follicular population), the day before the start of LH/FSH administration (to assess the effect of 7-day treatment with the gonadotropin antagonist on ovarian follicular population), daily for 6 days from the start of LH/FSH administration (to characterize the effects of the LH/FSH and hCG administration on ovarian follicular development and ovulation), and 12 days after the start of LH/FSH administration (to determine the number of CL that developed in response to the treatment paradigm). Because recording involved several sequential days, a Sony DCRTRV33 was used to record the digital video output to monitor changes over time.
Radioimmunoassays
A well-validated assay [35] was used to measure circulating concentrations of LH. Sensitivity of the LH assay averaged 0.8 ± 0.3 ng/ml. The intra-assay coefficients of variations (CVs) based on four quality-control pools measuring 3.2 ± 0.1, 7.9 ± 0.2, 12.8 ± 0.4, and 23.4 ± 0.5 ng/ml averaged 6.2% ± 1.3%, 5.4% ± 1.1%, 4.8% ± 0.4%, and 5.3% ± 0.5%, respectively. Interassay CVs based on the same quality-control pools were 13.3%, 7.0%, 11.4%, and 7.1%, respectively. Circulating concentrations of FSH were measured in duplicate using a validated RIA [34]. Sensitivity of the FSH assay averaged 0.33 ± 0.05 ng/ml. The intra-assay CVs based on two quality-control pools measuring 4.1 ± 0.1 and 12.1 ± 0.2 ng/ml were 7.1% and 6.5%, respectively. Interassay CVs based on the same two quality-control pools were 13.0% and 6.6%, respectively. Circulating concentrations of hCG (study 2 only) were measured with a single two-site chemiluminescent immunoassay on the ADVIA Centaur (Siemens Medical Solutions USA) using constant amounts of two antibodies (an affinity-purified polyclonal goat anti-hCG labeled with acridinium ester [DMAE] and a purified mouse monoclonal antibody covalently coupled to paramagnetic particles). The assay measures analyte concentrations to 1000 mIU/ml, with a minimum detectable concentration of 2.0 mIU/ml. Intra-assay assay CVs for quality-control pools measuring 7.9, 25.6, and 591.7 mIU/ml were 5.8%, 2.9%, and 4.1%, respectively. Circulating concentrations of E2 were measured in duplicate (300 μl of plasma) using a validated assay first developed by Butcher et al. [36] and modified by Tortonese et al. [37]. Assay sensitivity averaged 0.77 ± 0.13 pg/ml (mean ± SEM, n = 12 assays). Intra-assay CVs based on four quality-control pools measuring 2.0 ± 0. 1, 3.5 ± 0.1, 5.6 ± 0.3, and 13.9 ± 0.6 ng/ml averaged 9.9% ± 2.0%, 3.2% ± 0.8%, 13.3% ± 6.2%, and 13.9% ± 1.7%, respectively. The interassay CVs for the same quality-control pools were 20.5%, 14.5%, 16.9%, and 13.7%, respectively. Plasma concentrations of P4 were measured in duplicate using a commercial RIA kit (Coat-A-Count P4; Siemans Medical Solutions/Diagnostic). Validation of this assay for sheep plasma has been described elsewhere [38]. The sensitivity of this assay was 0.06 ± 0.01 ng/ml (n = 20 assays). The intra-assay CV based on two quality-control pools measuring 1.5 ± 0.04 and 14.0 ± 0.4 ng/ml were 3.8% ± 0.8% and 4.3% ± 0.8%, respectively. The interassay CVs for the same quality-control pools were 11.5% and 12.1%, respectively.
Statistical Analyses
For both years, a repeated-measures ANOVA was conducted to compare circulating levels of LH and FSH (2-h and 12-min samples), E2, P4, and hCG. The repeated-measures ANOVA had one between-subjects factor (treatment) and one within-subjects factor (time). The main effects of treatment and time as well as the interactions between treatment and time were examined. The following variables were examined by ANOVA: total P4 produced, peak E2, total amount of E2 produced during the C-FSH and N-FSH pulsing periods, total E2 produced during gonadotropin treatment, and peak hCG. To assess whether prenatal testosterone-treated ewes that were anovulatory (n = 3 each year) responded differently to the LH/FSH treatment, separate analyses were performed. For both years, the proportion of ewes that exhibited a rise in P4 and the number of CL and luteinized follicles after induction of ovulation with hCG was compared by the Fisher exact test and ANOVA, respectively. To assess changes in follicular dynamics during each year, all follicles of 2 mm or larger in diameter were recorded. Follicles were classified into the following size classes: 3 mm or less, greater than 3 mm, 3–4 mm, from greater than 4 to 8 mm, and greater than 8 mm. The size classes of from greater than 3 to 4 mm and of from greater than 4 to 8 mm correspond to gonadotropin-independent recruited follicles and those selected to become ovulatory-sized follicles [16, 17, 39], respectively. Follicular counts of individual sizes and within size classes were examined by ANOVA. Repeated-measures ANOVA was used to characterize the time trajectory of follicle counts for each of the follicle size categories and to compare the prenatal testosterone-treated and control groups. All the counts were square-root transformed before analysis. Time was treated as a discrete variable. The interaction term between time and treatment group was included in each model. When a time × treatment interaction existed, further analysis was carried out by comparing the means of follicle counts on each day of scanning. When no significant interaction was found, the two groups were pooled to test the time effect. A Bonferroni adjustment was used when comparing the means of counts on different days. To assess growth rate of follicles reaching greater than 8 mm after hCG treatment, a mixed model was used to capture the growth curve of follicles and to test the difference between treatment groups. Interaction between time (day) and group was added to the model to allow differences in shape of the curve between groups. All analyses were performed using SAS for Windows 9.1.3 (SAS Institute, Inc.). A P value of less than 0.05 was considered to be significant.
RESULTS
Distribution Profile of FSH Isoform Mixes Used in the Two Studies
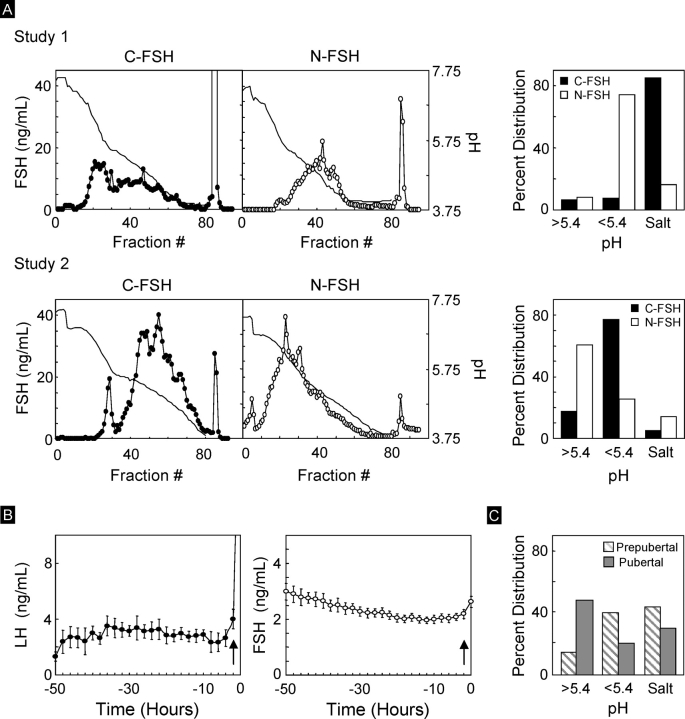
The distribution patterns of FSH isoforms in the C-FSH and N-FSH used in the two studies are shown in Figure 3A. For reference, circulating patterns of LH and FSH during the natural follicular phase (n = 18, composite data from earlier studies) and the FSH isoform distribution pattern before and during the first induced follicular phase of pubertal sheep (closed bars) from a previous publication [40] are provided in the Figure 3, B and C, respectively.
FIG. 3.
A) On the left are elution patterns of FSH isoforms from study 1 (top) and study 2 (bottom) following chromatofocusing separation of C-FSH and N-FSH preparations. Bar graph on the right shows the percentage of FSH eluting in fractions with pH greater than 5.4, pH less than 5.4, and salt peaks. Note the differences in distribution of FSH isoforms used in the two studies. B) Circulating patterns of LH and FSH during the natural follicular phase (n = 18, composite data from earlier studies). Arrows indicate timing of the LH surge. C) FSH eluting in fractions with pH greater than 5.4, pH less than 5.4, and salt peaks following chromatofocusing separation of circulating FSH from prepubertal and pubertal lambs from a previous study [40].
Study 1.
More than 80% of the C-FSH (Papkoff preparation) used in study 1 eluted in the salt peak. Neuraminidase treatment shifted the FSH distribution to a less acidic side, with more than 70% of N-FSH eluting in fractions with pH less than 5.4. Less than 10% of FSH eluted in fractions with pH greater than 5.4 in both C-FSH and N-FSH.
Study 2.
Less than 20% of FSH in C-FSH (Parlow preparation) eluted in the salt peak, as opposed to more than 80% in study 1. In contrast, nearly 80% of FSH isoforms in C-FSH used in study 2 eluted in fractions with pH less than 5.4. Neuraminidase treatment further shifted the FSH distribution to a less acidic side, with more than 60% of N-FSH eluting in fractions with pH greater than 5.4. The distribution of FSH in both C-FSH and N-FSH differed considerably from that in the C-FSH and N-FSH used during study 1.
Reproductive Hormonal Changes
Study 1.
Patterns of gonadotropin release before, during, and after administration of LH/FSH in study 1 are shown in Figure 4. Circulating concentrations of LH (Fig. 4A, top) were near the detection limit in both control and prenatal testosterone-treated females before initiation of LH/FSH treatment, the result of GnRH antagonist treatment (Fig. 4A, top, first three points). Circulating patterns of LH determined at 2-h intervals did not differ between control and prenatal testosterone-treated females during the gonadotropin treatment period. No differences were evident in delivered pulses of LH between control and prenatal testosterone-treated females (Fig. 4A, top, inset). In contrast, circulating FSH as determined in 2-h samples (Fig. 4A, bottom) during the LH/C-FSH administration period was consistently higher (P < 0.05) in the control females compared to the prenatal testosterone-treated females. Circulating FSH patterns during the LH/N-FSH administration at 1-h intervals and corresponding LH/N-FSH delivery profiles were similar (P > 0.05) between control and prenatal testosterone-treated females. In stark contrast to levels of FSH being lower in prenatal testosterone-treated females, the E2 rise (Fig. 4B, left) was higher (P < 0.05). Circulating levels of E2 achieved in the prenatal testosterone-treated females that were anovulatory before initiation of the study did not differ from the group statistics (Fig. 4B, right). Peak and total E2 produced as well as total E2 released during LH/C-FSH and LH/N-FSH pulse periods were higher (P < 0.02) in the prenatal testosterone-treated ewes compared to controls (Fig. 4C).
FIG. 4.

A) Mean circulating patterns of LH and FSH achieved in control (C) and prenatal testosterone-treated (T) females from study 1, conducted during the first breeding season. Delivery patterns of LH/FSH assessed from frequent samples are shown as insets. Period 1 represents the LH/FSH injection period at 2-h intervals and period 2 the injection period at 1-h intervals. B) Mean circulating patterns of estradiol achieved in control and prenatal testosterone-treated females. Circulating patterns of estradiol in the subset of prenatal testosterone-treated females that were anovulatory before the start of study are shown on the right. C) Estradiol summary statistics, amount of estradiol produced during the C-FSH and N-FSH administration, peak levels achieved during gonadotropin treatment, and total estradiol produced during the gonadotropin treatment. Asterisks indicate significant differences from control females (*P < 0.05).
Study 2.
Patterns of gonadotropin release before, during, and after the LH/FSH treatment are shown in Figure 5. No differences were detected in circulating patterns of LH and FSH during the 2-h samples or LH/C-FSH and LH/C-FSH/N-FSH delivery patterns (Fig. 5A, insets) between control and prenatal testosterone-treated females (P > 0.05). Repeated-measures analyses revealed no differences in circulating E2 between control and prenatal testosterone-treated females during the C-FSH treatment period, although significant differences were evident at some time points during the FSH treatment period at 1-h intervals (Fig. 5B). The peak level of E2 achieved at the end of gonadotropin treatment and total amount of E2 released during LH/C-FSH or LH/C-FSH/N-FSH treatment period did not differ between control and prenatal testosterone-treated females (Fig. 5C). When only the three anovulatory prenatal testosterone-treated ewes were considered, all measured variables of E2 were significantly higher in the prenatal testosterone-treated females. This increase was reflected in the E2 release during the LH/C-FSH/N-FSH pulse periods (P < 0.01) as well as peak (P < 0.02) and total (P < 0.01) E2 concentrations achieved. Circulating E2 concentrations during the LH/C-FSH period also tended to be higher (P = 0.06) in anovulatory prenatal testosterone-treated females compared to control females.
FIG. 5.
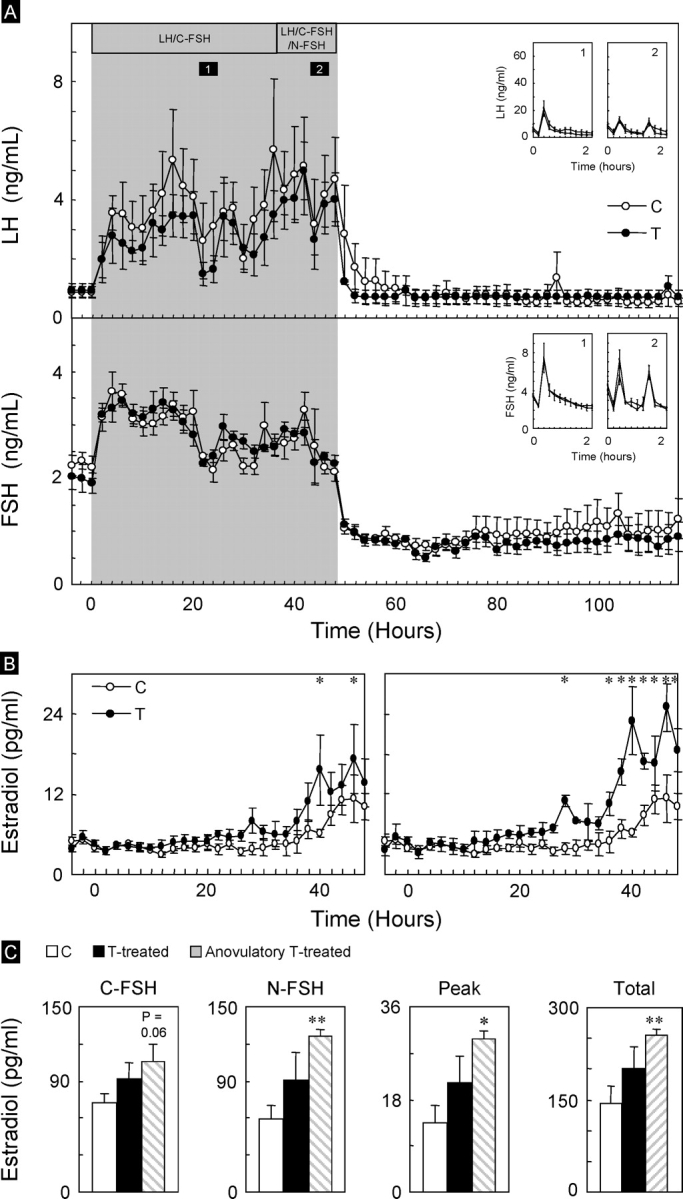
A) Mean circulating patterns of LH and FSH in control (C) and prenatal testosterone-treated (T) females from study 2, conducted during the second breeding season. The LH/FSH delivery patterns from the frequent sampling time periods (period 1, LH/C-FSH administered every 2 h; period 2, LH and C-FSH+N-FSH administered every 1 h) are shown as insets. B) Mean circulating patterns of estradiol achieved in control and prenatal testosterone-treated females. Results for the subset of prenatal testosterone-treated females that were anovulatory before the start of study are shown on the right. C) Estradiol summary statistics (amount of estradiol produced during the C-FSH and C-FSH+N-FSH administration, peak levels achieved during gonadotropin treatment, and total estradiol produced during the gonadotropin treatment). Asterisks indicate significant differences from control females (*P < 0.05, **P < 0.01).
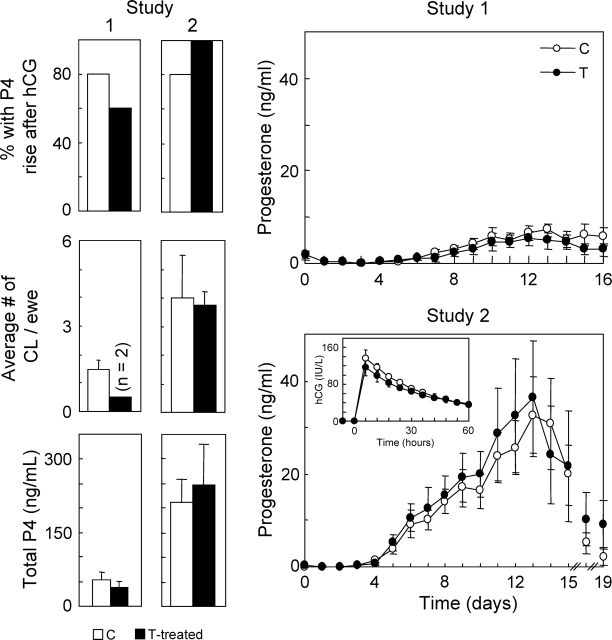
hCG Stimulation and Luteal Response (Study 1 vs. Study 2)
Patterns of hCG achieved in study 2 (not determined in study 1) and luteal response that followed hCG administration at the end of LH and FSH treatment for both study 1 and study 2 are presented in Figure 6. Patterns of hCG achieved in control and prenatal testosterone-treated females were similar (Fig. 6, inset, study 2). Peak circulating concentrations of hCG averaged approximately 130 IU/ml. Circulating concentrations of hCG did not return to baseline at the end of 60 h. In study 1, four of five control (80%) and three of five prenatal testosterone-treated (60%) females had a luteal P4 increase in response to hCG administration. In study 2, four of five control (80%) and five of five prenatal testosterone-treated (100%) females had a P4 rise following hCG stimulation. Although the average number of CL and total P4 released were similar between control and prenatal testosterone-treated females in both years, the number of CL and total P4 released were greater in study 2 compared to study 1. Multiple luteinized follicles were detected on the ovaries of ewes from both treatment groups in study 2 (5 ± 1.1 and 6 ± 3.3 in control and prenatal testosterone-treated females, respectively) on Day 19 after PGF2α.
FIG. 6.
Left) Percentage of ewes that showed a rise in P4 following hCG administration, number of CL per ewe, and total progesterone production from both study 1 and study 2. Right) Mean circulating patterns of progesterone in control (C) and prenatal testosterone-treated (T) females following ovulation induction with hCG at the end of LH/FSH treatment from study 1 (top) and study 2 (bottom). Inset shows circulating patterns of hCG achieved following administration of 1500 IU of hCG (not determined in study 1).
Follicular Dynamics (Study 1 vs. Study 2)
Study 1.
Distribution of the total number of follicles within each size class before and after GnRH antagonist treatment, during LH and FSH treatment, and after hCG treatment is shown in Figure 7 (left). Three days of treatment with the GnRH antagonist was not sufficient to alter the distribution pattern of any follicular size classes in control or prenatal testosterone-treated females. The LH and FSH treatment tended to increase the number of follicles with a diameter of less than 3 mm (P = 0.08). The numerical increase in follicles of >3 mm, 3–4 mm, and from greater than 4 to 8 mm in diameter in both control and prenatal testosterone-treated females did not achieve significance.
FIG. 7.
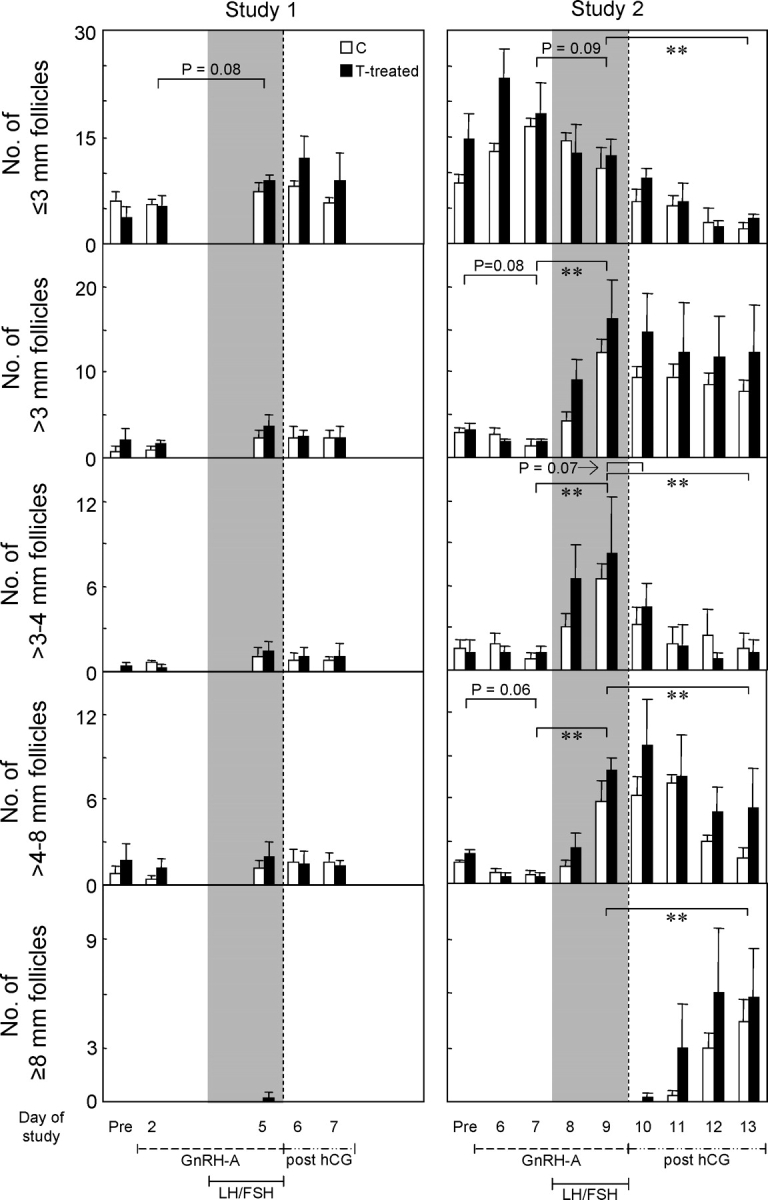
Total number of follicles in size classes of ≤3 mm, greater than 3 to 4 mm (gonadotropin-dependent recruited follicles), greater than 4 to 8 mm (follicles selected to become ovulatory sized), and greater than 8 mm as detected by ultrasonography in both ovaries of control (C) and prenatal testosterone-treated (T-treated) sheep from study 1 and study 2. Ultrasonography was performed before initiation of the study, after 2 days of GnRH antagonist treatment, after 48 h of LH/FSH injections, and 24 and 48 h after induction of ovulation with hCG in study 1 and before initiation of the study, after 6 and 7 days of GnRH antagonist treatment, after 24 and 48 h of LH/FSH injections, and 24, 48, 72, and 96 h after hCG administration in study 2. Gray-shaded areas correspond with LH/FSH injections. Double asterisks indicate significant differences (**P < 0.01).
Study 2.
Ultrasonographic images of follicles before and after GnRH antagonist treatment, during and end of LH and FSH treatment, and after hCG administration from both ovaries of one control and one prenatal testosterone-treated female are shown in Figure 8. Only small-sized follicles were evident after GnRH antagonist treatment in either control or prenatal testosterone-treated females (Fig. 8, Post GnRH-A) but before LH/FSH administration. The LH and FSH treatment increased follicular size. Follicles larger than 8 mm were evident after hCG treatment, persisted, and continued to grow. To facilitate comparison with study 1, summary statistics of distribution of follicles within each diameter class from study 2 are presented in the Figure 7 (right). Unlike the 3-day treatment in study 1, the 7-day GnRH antagonist treatment tended to decrease the number of follicles of larger than 3 mm (P = 0.08) and, specifically, follicles in the size class of 4–8 mm (P = 0.06). Treatment with LH/FSH increased the number of follicles larger than 3 mm (P < 0.05). This increase was reflected in both the 3–4 mm and 4–8 mm follicular classes. Follicles larger than 8 mm were not present in control or prenatal testosterone-treated females. Administration of hCG resulted in a progressive decline of follicular number in all the above classes (≤3 mm, 3–4 mm, and 4–8 mm; P < 0.05; pre-hCG vs. 96-h post-hCG). Follicles larger than 8 mm were evident after hCG administration in both control and prenatal testosterone-treated females. Analyses of growth rate of follicles during the 48 h of LH/FSH treatment found a significant effect of day (P < 0.0001) but not of prenatal testosterone treatment. Considering all follicles together, the average growth rate was approximately 1 mm/day. Growth curve analyses after hCG administration of follicles that reached larger than 8 mm found that the shape of the growth curves differed between the control and prenatal testosterone-treated groups (P < 0.01). The growth curve of follicles from the control group followed a quadratic function, whereas follicles from the prenatal testosterone-treated group exhibited a cubic function. Growth rates of follicles in prenatal testosterone-treated females averaged 2.1, 2.9, 2.1, and 0 mm for prenatal testosterone-treated group at 24, 48, 72, and 96 h post-hCG, respectively. Corresponding follicular growth rates for controls averaged 1.2, 2.3, 2.8, and 2.9 mm, respectively. The fastest growth rate was evident 48 h post-hCG in prenatal testosterone-treated females and 96 h in control females.
FIG. 8.
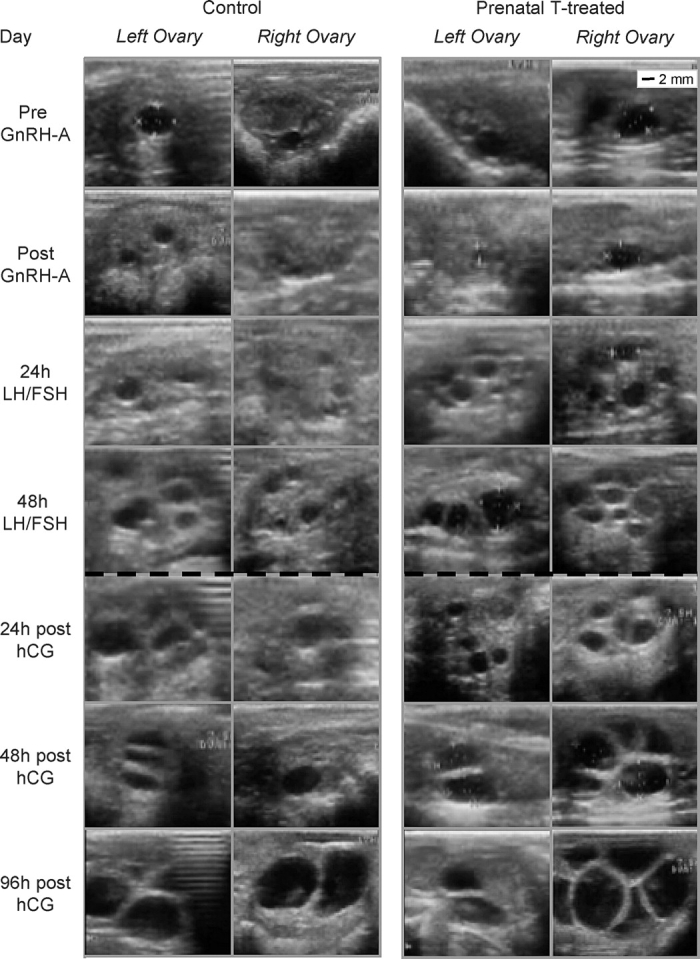
Ultrasonographic images of ovary from one control and one prenatal testosterone-treated (Prenatal T-treated) ewe before and after GnRH antagonist (GnRH-A) administration, 24 and 48 h after start of LH/FSH treatment, and 24, 48, and 96 h after hCG administration. Scale shown at top right applies to all images.
DISCUSSION
The findings of the present study provide evidence that ovarian function, as determined on the basis of ovulatory/luteal response in prenatal testosterone-treated females, can be restored by blockade of endogenous GnRH action and provision of exogenous gonadotropins. From the standpoint of assisted reproductive technology, the present study also stresses the importance of providing an appropriate mix of FSH isoforms in achieving optimal ovarian follicular development. In addition, because hCG treatment induced cystic follicular development not only in prenatal testosterone-treated sheep but also in control ewes, the intrafollicular milieu following exogenous gonadotropin drive is likely to have been abnormal, contributing to development of large, persistent follicles.
Rescue of Ovarian Function in Prenatal Testosterone-Treated Females
The main goal of the present study was to determine if we can restore ovulatory function in prenatal testosterone-treated females. Consistent with our earlier studies, which documented that prenatal testosterone-treated females manifest oligo-ovulation or anovulation [7, 11], four of five prenatal testosterone-treated females manifested absent or prolonged progestogenic cycles (suggestive of existence of luteinized follicles or failure of CL to regress) before the start of the study. In study 1 and study 2, ovarian stimulation with exogenous gonadotropins after GnRH antagonist treatment resulted in comparable ovulatory and luteal response in control and prenatal testosterone-treated females. Lack of differences in the percentage of control and prenatal testosterone-treated females ovulating, number of CL formed, and pattern and amplitude of P4 rise achieved between control and prenatal testosterone-treated females supports the idea that exogenous gonadotropins can, indeed, restore ovulatory/luteal function in prenatal testosterone-treated females.
In earlier studies, we found the prenatal testosterone-treated females exhibit defects at both the ovarian and neuroendocrine levels [41, 42]. The ability to rescue ovulatory function in prenatal testosterone-treated sheep with exogenous LH/FSH indicates that the primary defect responsible for ovulatory failure resides at the neuroendocrine level. Restoration of ovulatory function, however, does not imply that the ovary is completely normalized. The finding of temporal differences in the development of cystic follicles following hCG stimulation supports this premise. Earlier studies in rhesus monkeys found that prenatal testosterone treatment compromises oocyte quality [43]. Our earlier studies using sheep treated prenatally with testosterone from Days 60 to 90 of gestation, which manifested a less severe reproductive phenotype than the females treated with testosterone from Days 30–90 of gestation (present study), found a significant decrease in pregnancy outcomes [44], suggestive of compromised oocyte quality. Future studies need to address if oocyte quality is normalized in follicles that develop following exogenous gonadotropin stimulation.
Importance of Nature of Gonadotropin for Rescuing Ovulatory Function
Our findings emphasize the importance of using appropriate gonadotropic stimuli to achieve desired assisted reproductive technology outcomes and avoidance of deleterious effects, such as ovarian hyperstimulation syndrome. These differences were reflected in number of CL formed (one to two in study 1 and multiple in study 2). From the standpoint of overcoming human infertility with exogenous FSH, the outcome achieved with the gonadotropin treatment provided in study 2 may be optimal for IVF protocols but not for gonadotropin stimulation protocols, in which the end goal is to facilitate a monovular condition.
The fact that a higher percentage of prenatal testosterone-treated animals ovulated during study 2 compared with study 1 may reflect differences in gonadotropin stimulation or in age of the treated animals. The number of prenatal testosterone-treated females available precluded follow-up studies comparing the two FSH preparations in parallel, thus limiting the nature of the conclusions being drawn. To what extent do the gonadotropic delivery patterns in these two studies parallel what is seen during the follicular phase in vivo? No differences were observed in frequency of gonadotropin administration between the two studies, but clear differences were found in the amount of gonadotropins delivered and in the distribution pattern of FSH. The delivery of gonadotropins at 2-h intervals for the first 36 h and then at 1-h intervals for the next 12 h for the most part mimicked the low-frequency LH pulses seen during the early follicular phase and the high-frequency pulses seen during the late follicular phase [34]. The circulating concentrations of LH achieved during gonadotropin administration at 1-h intervals in study 1 were higher than those occurring during a natural follicular phase, but the mean circulating concentrations of LH achieved in study 2 paralleled levels in control ewes during a natural follicular phase (Fig. 3B). The circulating levels of FSH achieved in studies 1 and 2 were within the range seen during a normal follicular phase; however, the isoform distribution profile of C-FSH used in the two studies differed. The lower circulating concentrations of FSH achieved in prenatal testosterone-treated females compared with control females in study 1 during the C-FSH administration period, in spite of similar administration of FSH from the same stock preparations, indicate differences in the rate of clearance. This suggests the presence of higher concentrations of enzymes involved in the conversion of FSH isoforms eluting at pH less than 4 (salt peak) to pH 4–5.4 in prenatal testosterone-treated females compared to control females. In contrast to study 1, the circulating patterns of FSH achieved in control and prenatal testosterone-treated females in study 2 were identical during both C-FSH and N-FSH administration. Such differences between these two studies likely relate to differences in mixes of FSH administered (see Fig. 3).
Ovarian response reflects not only the amount of FSH delivered but also the FSH isoform mix provided. This is highlighted by the fact that the FSH treatment in study 2, in spite of achieving circulating amounts of FSH nearly comparable to those in study 1, produced more follicles larger than 3 mm. To what extent the higher LH achieved in study 1 contributed to such differences is unclear. Paradoxically, the higher levels of LH achieved did not facilitate growth of follicles larger than 8 mm in study 1 during the 2 days following hCG stimulation (such follicles were evident in study 2 at 2 days post-hCG). Furthermore, the fact that circulating patterns of FSH were within the range seen during the follicular phase, but that the FSH isoform distribution patterns were not, highlights the complexity in achieving the desired delivery pattern of FSH using available FSH preparations. Until designer FSH, comprised of the FSH isoform mixes seen during the early and later follicular phases, is available, it will not be possible to replicate the delivery pattern of FSH seen in natural cycles.
From the standpoint of ovarian response, higher peak concentrations of E2 were achieved in study 2 compared to study 1 in the face of similar circulating concentrations of FSH. This may stem from higher numbers follicles in the size classes of 3–4 and 4–8 mm that developed in response to exogenous gonadotropin stimulation in study 2 compared to study 1. On the other hand, the absence of differences in follicular diameter classes between control and prenatal testosterone-treated females in study 1, in spite of lower circulating FSH in prenatal testosterone-treated females, indicates that the higher E2 rise in prenatal testosterone-treated females reflects increased follicular sensitivity to gonadotropins.
Follicular Persistence
The development of large follicles (>8 mm) by 48 h post-hCG and their persistence in both control and prenatal testosterone-treated females in study 2 indicate that the prolonged half-life of hCG may have contributed to follicular persistence. Many other studies, however, have found that elevated LH is not associated development of cystic follicles [45, 46]. In support of this, large cystic follicles were not evident in study 1 (studied only on Day 2 post-hCG), despite elevated LH concentrations achieved during gonadotropin administration at 1-h intervals and a similar hCG trigger for ovulation induction. Therefore, differences between study 1 and study 2 indicate that the FSH isoform mix used in study 2 may be conducive to an abnormal follicular environment and development of follicular cysts. For example, increased incidences of aberrant follicular development and of unovulated follicles frequently are detected when eCG, as opposed to other FSH preparations, is used in superovulatory regimens (for review, see [46]). Although large follicles and follicular persistence are noted in both prenatal testosterone-treated and control females, the observation that their growth dynamics differ is indicative of persisting differences in the intrafollicular milieu between the two groups.
Overall, the present study documents that ovulatory function can be rescued in prenatal testosterone-treated females with exogenous gonadotropins. The present results, however, do not address the root cause of the reproductive defects. To what extent the ovulatory defects in prenatal testosterone-treated females are the result of altered ovarian gonadotropin receptor expression, are caused by defects in steroidogenic potential of ovarian follicles, and involve differences in gonadotropic drive of control and prenatal testosterone-treated females remains to be determined.
In summary, our findings provide evidence that ovulatory response in prenatal testosterone-treated females can be restored with exogenous gonadotropins, that optimal ovarian follicular development is dependent on the nature of gonadotropin input, and that an abnormal follicular milieu may contribute to the temporal differences in development of cystic follicles between prenatal testosterone-treated and control females.
Acknowledgments
We are grateful to Douglas Doop for help with breeding and lambing, expert animal care, and facility management; Dr. Mohan Manikkam, Carol Herkimer, Olga Astapova, Jonathan Flak, and Pamela Olton for assistance with prenatal testosterone treatment, animal experimentation, and/or performance of gonadotropin/P4 assays; and Dr. Almudena Veiga-Lopez for proofreading help.
Footnotes
1This study was supported by USPHS grants R01-HD 41098 and P01-HD44232 to V.P.
REFERENCES
- Sharma TP, Herkimer C, West C, Ye W, Birch R, Robinson JE, Foster DL, Padmanabhan V. Fetal programming: prenatal androgen disrupts positive feedback actions of estradiol but does not affect timing of puberty in female sheep. Biol Reprod 2002; 66: 924 933 [DOI] [PubMed] [Google Scholar]
- Sarma HN, Manikkam M, Herkimer C, Dell'Orco J, Welch KB, Foster DL, Padmanabhan V. Fetal programming: excess prenatal testosterone reduces postnatal LH, but not FSH, responsiveness to estradiol negative feedback in the female. Endocrinology 2005; 146: 4281 4291 [DOI] [PubMed] [Google Scholar]
- Wood RI, Foster DL. Sexual differentiation of reproductive neuroendocrine function in sheep. Rev Reprod 1998; 3: 130 140 [DOI] [PubMed] [Google Scholar]
- West C, Foster DL, Evans NP, Robinson J, Padmanabhan V. Intrafollicular activin availability is altered in prenatally androgenized lambs. Mol Cell Endocrinol 2001; 185: 51 59 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Scaramuzzi RJ, Short RV. Ovulation in prenatally androgenized ewes. J Endocrinol 1977; 73: 385 389 [DOI] [PubMed] [Google Scholar]
- Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects: partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology 2006; 147: 1997 2007 [DOI] [PubMed] [Google Scholar]
- Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology 2003; 144: 1426 1434 [DOI] [PubMed] [Google Scholar]
- Manikkam M, Thompson RC, Herkimer C, Flak J, Padmanabhan V. Developmental programming: effects of prenatal testosterone excess on pre- and postnatal gonadotropin regulation. Biol Reprod 2008; 78: 648 660 [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Midgley AR, Jr, Beitins IZ, Padmanabhan V. Follicle stimulating isohormones: biological characterization and physiological relevance. Endocr Rev 1995; 16: 765 787 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Lee JS, Beitins IZ. Follicle-stimulating isohormones: regulation and biological significance. Thatcher WW, Inskeep EK, Niswender GD, Doberska C. Reproduction in Domestic Ruminants IV. The Journals of Reproduction and Fertility Ltd., Cambridge, UK; 1998: 87 99 [PubMed] [Google Scholar]
- Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology 2007; 148: 3532 3540 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Manikkam, Recabarren S, Foster DL. Prenatal testosterone programs: reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol 2006; 246: 165 174 [DOI] [PubMed] [Google Scholar]
- Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev 1994; 15: 725 751 [DOI] [PubMed] [Google Scholar]
- Adashi EY. The ovarian follicular apparatus. Adashi EY, Rock JA, Rosenwaks Z. Reproductive Endocrinology, Surgery, and Technology. New York: Lippincott-Raven; 1995: 17 40 [Google Scholar]
- Findlay JK, Drummond AE. Control of follicular growth. Filicori M, Flamingi C. The Ovary: Regulation, Dysfunction, and Treatment. Amsterdam: Elsevier; 1996: 13 21 [Google Scholar]
- Campbell BK, Scaramuzzi RJ, Webb R. Control of antral follicle development and selection in sheep and cattle. J Reprod Fertil Suppl 1995; 49: 335 350 [PubMed] [Google Scholar]
- Hunter MG, Robinson RS, Mann GE, Webb R. Endocrine and paracrine control of follicular development and ovulation rate in farm species. Anim Reprod Sci 2004; 82–83: 461 477 [DOI] [PubMed] [Google Scholar]
- Wallace JM, McNeilly AS. Increase in ovulation rate after treatment of ewes with bovine follicular fluid in the luteal phase of the estrous cycle. J Reprod Fertil 1985; 73: 505 515 [DOI] [PubMed] [Google Scholar]
- Amaya-Montoya C, Matsui M, Kawashima C, Hayashi KG, Matsuda G, Kaneko E, Kida K, Miyamoto A, Miyake Y. Induction of ovulation with GnRH and PGF2α at two different stages during the early postpartum period in dairy cows: ovarian response and changes in hormone concentrations. J Reprod Dev 2007; 53: 867 875 [DOI] [PubMed] [Google Scholar]
- Takedomi T, Kaneko H, Aoyagi Y, Konishi M, Kishi H, Watanabe G, Taya K. Effects of passive immunization against inhibin on ovulation rate and embryo recovery in Holstein heifers. Theriogenology 1997; 47: 1507 1518 [DOI] [PubMed] [Google Scholar]
- Nambo Y, Kaneko H, Nagata S, Oikawa M, Yoshihara T, Nagamine N, Watanabe G, Taya K. Effect of passive immunization against inhibin on FSH secretion, folliculogenesis, and ovulation rate during the follicular phase of the estrous cycle in mares. Theriogenology 1998; 50: 545 557 [DOI] [PubMed] [Google Scholar]
- Balen A, Platteau P, Amdersen AN, Devroey P, Helmgaard L, Arce JC; for the Bravelle Ovulation Induction (BOI) Study Group Highly purified FSH is as efficacious as recombinant FSH for ovulation induction in women with WHO group II anovulatory infertility: a randomized controlled noninferiority trial. Hum Reprod 2007; 22: 1816 1823 [DOI] [PubMed] [Google Scholar]
- McNatty KP, Hudson N, Gibb M, Ball K, Henderson KM, Heath DA, Lun S, Kieboom LE. FSH influences follicle viability, estradiol biosynthesis, and ovulation rate in Romney ewes. J Reprod Fertil 1985; 75: 121 131 [DOI] [PubMed] [Google Scholar]
- Picton HM, Tsonis CG, McNeilly AS. FSH causes a time-dependent stimulation of preovulatory follicle growth in the absence of pulsatile LH secretion in ewes chronically treated with gonadotrophin-releasing hormone agonist. J Endocrinol 1990; 126: 297 307 [DOI] [PubMed] [Google Scholar]
- Gong JG, Wilmut I, Bramley TA, Webb RG. Pretreatment with recombinant bovine somatotropin enhances the superovulatory response to FSH in heifers. Theriogenology 1996; 45: 611 622 [DOI] [PubMed] [Google Scholar]
- Rivier JE, Jiang G, Porter J, Hoeger CA, Craig AG, Corrigan A, Vale W, Rivier CL. Gonadotropin-releasing hormone antagonists: novel members of the azaline B family. J Med Chem 1995; 38: 2649 2662 [DOI] [PubMed] [Google Scholar]
- Herbst KL, Anawalt BD, Amory JK, Bremner WJ. Acyline: the first study in humans of a potent, new gonadotropin-releasing hormone antagonist. J Clin Endocrinol Metab 2002; 87: 3215 3220 [DOI] [PubMed] [Google Scholar]
- West CR, Carlson NE, Lee JS, McNeilly A, Sharma TP, Ye W, Padmanabhan V. Acidic mix of FSH isoforms are better facilitators of ovarian follicular maturation and estradiol production than the less acidic. Endocrinology 2002; 143: 107 116 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Lang LL, Sonstein J, Kelch RP, Beitins IZ. Modulation of serum follicle-stimulating hormone bioactivity and isoform distribution by estrogenic steroids in normal women and in gonadal dysgenesis. J Clin Endocrinol Metab 1988; 67: 465 473 [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Ye W, Phillips DJ, Herkimer C, Knight PG, Padmanabhan V. Developmental programming: reproductive hormone dynamics and ovulatory outcomes in prenatal testosterone-treated sheep. Biol Reprod 2008; 78: 636 647 [DOI] [PubMed] [Google Scholar]
- Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology 2006; 148: 3532 3540 [DOI] [PubMed] [Google Scholar]
- Goodman RL. Neuroendocrine control of the ovine estrous cycle. Knobil E, Neill JD. The Physiology of Reproduction, vol. 2. Raven Press, New York, 1994: 659 709 [Google Scholar]
- Lopez-Sebastian A, Gonzalez de Bulnes A, Santiago Moreno J, Gomez-Brunet A, Townsend EC, Inskeep EK. Patterns of follicular development during the estrous cycle in monovular Merino del Paris ewes. Anim Reprod Sci 1997; 48: 279 291 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, McFadden K, Mauger DT, Karsch FJ, Midgley AR., Jr Neuroendocrine control of follicle-stimulating hormone (FSH) secretion. I. Direct evidence for separate episodic and basal components of FSH secretion. Endocrinology 1997; 138: 424 432 [DOI] [PubMed] [Google Scholar]
- Niswender GD, Reichert LE, Jr, Midgley AR, Jr, Nalbandov AV. Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology 1969; 84: 1166 1173 [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone, and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology 1974; 94: 1704 1708 [DOI] [PubMed] [Google Scholar]
- Tortonese DJ, Lewis PE, Papkoff H, Inskeep EK. Roles of the dominant follicle and the pattern of estradiol in induction of preovulatory surges of LH and FSH in prepubertal heifers by pulsatile low doses of LH. J Reprod Fertil 1990; 90: 127 135 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Evans NP, Dahl GE, McFadden KL, Mauger DT, Karsch FJ. Evidence for short or ultrashort loop negative feedback of GnRH secretion. Neuroendocrinology 1995; 62: 248 258 [DOI] [PubMed] [Google Scholar]
- Driancourt MA. Regulation of ovarian follicular dynamics in farm animals. Implications for manipulation of reproduction. Theriogenology 2001; 55: 1211 1239 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Mieher CD, Borondy M, I'Anson H, Wood RI, Landefeld TD, Foster DL, Beitins IZ. Circulating bioactive follicle-stimulating hormone and less acidic follicle-stimulating hormone isoforms increase during experimental induction of puberty in the female lamb. Endocrinology 1992; 131: 213 220 [DOI] [PubMed] [Google Scholar]
- Foster DL, Jackson LM, Padmanabhan V. Novel concepts about normal sexual differentiation of reproductive neuroendocrine function and the developmental origins of female reproductive dysfunction: the sheep model. Soc Reprod Fertil Suppl 2007; 64: 83 107 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A, Abbott DH, Dumesic DA. Developmental programming of ovarian disruption. Gonzalez-Bulnes A. Novel Concepts in Ovarian Endocrinology. Research Signpost, Kerala, India, 2007: 329 352
- Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH. Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocrinol Metab 2002; 87: 1111 1119 [DOI] [PubMed] [Google Scholar]
- Steckler TL, Roberts EK, Doop DD, Lee TM, Padmanabhan V. Developmental programming in sheep: administration of testosterone during 60–90 days of pregnancy reduces breeding success and pregnancy outcome. Theriogenology 2007; 67: 459 467 [DOI] [PubMed] [Google Scholar]
- Christman SA, Bailey MT, Head WA, Wheaton JE. Induction of ovarian cystic follicles in sheep. Domest Anim Endocrinol 2000; 19: 133 146 [DOI] [PubMed] [Google Scholar]
- Kafi M, McGowan MR. Factors associated with variation in the superovulatory response of cattle. Anim Reprod Sci 1997; 48: 137 157 [DOI] [PubMed] [Google Scholar]