Abstract
Cannabinoid CB2 receptors represent a therapeutic target that circumvents unwanted central side effects (e.g., psychoactivity and/or addiction) associated with activation of CB1 receptors. One of the primary investigative tools used to study functions of the CB2 receptor is the aminoalkylindole (R,S)-AM1241. However, (R,S)-AM1241 has been described as an atypical CB2 agonist which produces antinociception mediated indirectly by opioid receptors. (R,S)-AM1241 and its enantiomers, (R)-AM1241 and (S)-AM1241, were evaluated for antinociception in response to thermal (Hargreaves) and mechanical (von Frey) stimulation. Pharmacological specificity was established using antagonists for CB1 (rimonabant [SR141716]) and CB2 (SR144528). The opioid antagonist naloxone was administered locally in the paw or systemically to evaluate the contribution of opioid receptors to CB2-mediated antinociception produced by (R,S)-AM1241, (R)-AM1241, and (S)-AM1241. Comparisons were made with the opioid analgesic morphine. (R,S)-AM1241, (R)-AM1241, and (S)-AM1241 (0.033–10 mg/kg i.p.) produced antinociception to thermal, but not mechanical, stimulation of the hindpaw in naive rats. Antinociception produced by (R,S)-AM1241 and (S)-AM1241 exhibited an inverted U-shaped dose response curve. (R)-AM1241 produced greater antinociception than either (S)-AM1241 or (R,S)-AM1241 at the lowest (0.033 and 0.1 mg/kg i.p.) and highest (10 mg/kg i.p.) doses. Similar levels of antinociception were observed at intermediate doses. (R,S)-AM1241, (R)-AM1241, and (S)-AM1241 each produced CB2-mediated antinociception that was blocked by SR144528 but not by rimonabant. Local and systemic naloxone blocked morphine-induced antinociception but did not block antinociceptive effects of (R,S)-AM1241, (R)-AM1241, or (S)-AM1241. The antinociceptive effects of the CB2-selective cannabinoid (R,S)-AM1241 and its enantiomers, (R)-AM1241 and (S)-AM1241, are not dependent upon opioid receptors.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-009-9170-8) contains supplementary material, which is available to authorized users.
Key words: antinociception, cannabinoid, CB2, endogenous opioid, naloxone, pain
INTRODUCTION
Cannabis sativa has been used for both medicinal and recreational purposes throughout recorded history. The discovery by Gaoni and Mechoulam (1) of ∆9-tetrahydrocannabinol, the major psychoactive ingredient in marijuana, ushered in a new era of research focused on understanding the functional roles of cannabinoid receptors in the nervous system. The cloning of cannabinoid CB1 and CB2 receptors and isolation of their endogenous ligands (endocannabinoids) marked a transition in the cannabinoid field. Cannabinoids could no longer be thought of merely as illicit drugs of abuse, but rather represented pharmacological tools for studying the functional roles of CB1 and CB2 receptors in the nervous system. Activation of cannabinoid CB1 and CB2 receptors suppresses pathological pain in animal models (for review, see 2–4). CB1 receptors are localized primarily within the central nervous system (CNS) (5) and are associated with the rewarding aspects of several addictive compounds including nicotine, alcohol, and cocaine (6). Activation of CB1 receptors produces hypothermia, motor ataxia, catalepsy, and hypoactivity (for review, see 7). The discovery of the CB2 receptor opened the door to exploring the role of this receptor as a therapeutic target for pain and inflammation. CB2 receptors are localized preferentially, but not exclusively (8,9), to immune cells in the periphery (10,11) and are upregulated in the CNS in pathological pain states (12–15). CB2 agonists lack centrally mediated side effects (16,17), suggesting that they represent a promising therapeutic target for producing antinociception in the absence of unwanted side effects such as psychoactivity or addiction. Thus, the CB2 receptor offers the potential to separate analgesic properties of cannabinoids and drug abuse liability. A key pharmacological tool for studying the functional roles of the CB2 receptor has been the aminoalkylindole (R,S)-AM1241 (for review, see 3). Because this compound has been widely used as a research tool, it is important to fully characterize the pharmacological properties of (R,S)-AM1241 and its two enantiomers (R)-AM1241 and (S)-AM1241.
(R,S)-AM1241, the CB2 agonist that has most penetrated the literature, has proven an important research tool for investigating CB2-mediated antinociception. (R,S)-AM1241 produces antinociception following local (intrapaw, i.paw) and systemic administration in naive rats (16). Behavioral, neurochemical, and electrophysiological studies suggest that (R,S)-AM1241 suppresses persistent pain through a CB2-specific mechanism (see 3 for review). (R,S)-AM1241 behaves as a CB2 agonist in vivo and a protean agonist in vitro (18). In cAMP inhibition assays, (R,S)- and (R)-AM1241 are inverse agonists, whereas (S)-AM1241 is an agonist (19). Antinociception produced by (R,S)-AM1241 has been attributed to an indirect modulation of the endogenous opioid system (16,20); in naive rats, (R,S)-AM1241-induced antinociception is blocked by local injection of naloxone in the paw (20). The report on (R,S)-AM1241’s purported mechanism of action has motivated testing of novel CB2 agonists for modulation of the endogenous opioid system. Several compounds have recently been described which differ from (R,S)-AM1241 on this basis (21–23). (S)-AM1241, which exhibits lower affinity for CB2 than (R)-AM1241, shows greater efficacy than (R)-AM1241 in suppressing visceral and inflammatory pain (19). It remains unknown whether preferential efficacy of (S)-AM1241 is observed in naive rats or is attributable to altered CB2 receptor levels in persistent pain states. Moreover, it remains unclear whether naloxone sensitivity is a feature of racemic (R,S)-AM1241 or could be restricted to either of its enantiomers.
We evaluated antinociceptive properties of (R,S)-AM1241 (Ki: CB1vs. CB2: 239.4 vs. 3.41 nM) and its enantiomers (R)-AM1241 (Ki: CB1vs. CB2: 139.7 vs. 1.4 nM) and (S)-AM1241 (Ki: CB1vs. CB2: 2.03 μM vs. 160.5 nM) (24) in tests of thermal and mechanical sensitivity in naive rats. Pharmacological specificity was evaluated using selective antagonists for CB1 (rimonabant [SR141716]), CB2 (SR144528), and opioid (naloxone) receptors. (R,S)-AM1241, (R)-AM1241, and (S)-AM1241 (Fig. 1) were evaluated for naloxone sensitivity and compared with morphine.
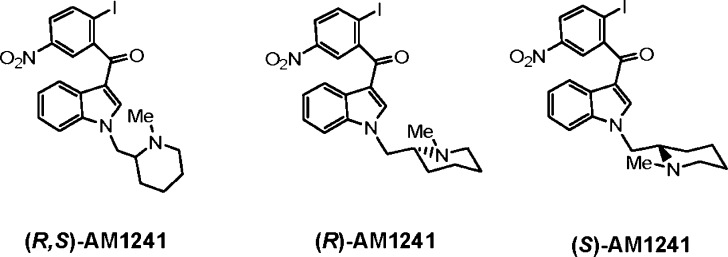
Fig. 1.
Chemical structures of the aminoalkylindoles (R,S)-AM1241, (R)-AM1241, and (S)-AM1241
MATERIALS AND METHODS
Subjects
Three hundred and sixty adult male Sprague Dawley rats (300–400 g; Harlan, Indianapolis, IN, USA) were used in these experiments. All animals were maintained on a 12-h light/12-h dark cycle (0700–1900) in a temperature-controlled facility. Animals were single housed and had access to food and water ad libitum. All procedures were approved by the University of Georgia Animal Care and Use Committee and followed the guidelines for the treatment of animals of the International Association for the Study of Pain (25). Animal experiments were conducted in full compliance with local, national, ethical, and regulatory principles and local licensing regulations of the Association for Assessment and Accreditation of Laboratory Animal Care International’s expectations for animal care and use/ethics committees.
Drugs and Chemicals
(R,S)-AM1241, ((R,S)-(2-iodo-5-nitrophenyl)-[1-((1-methyl-piperidin-2-yl)methyl)-1H-indol-3-yl]-methanone), (R)-AM1241, and (S)-AM1241 were synthesized starting from racemic N-methyl-2-hydroxymethyl piperidine which was resolved by fractional crystallization of the diastereoisomeric dibenzoyltartaric acid salts, and this material was used for synthesis of the respective enantiomeric products. The enantiomeric purity of the chiral products was determined using chiral HPLC analysis on CHIRALPAC® AD-H analytical column. Rimonabant (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide) and SR144528 (5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-N-(1,3,3-rimethylbicyclo[2.2.1]heptan-2-yl)-1H-pyrazole-3-carboxamide) were provided by the National Institute on Drug Abuse. Naloxone hydrochloride dihydrate, morphine sulfate, and dimethyl sulfoxide (DMSO) were purchased from Sigma Aldrich (St. Louis, MO, USA). All drugs delivered intraperitoneally (i.p.) were dissolved in a vehicle of 100% DMSO. This is the same vehicle that has been employed in previous work (16,20,26,27). Cannabinoids were dissolved in a volume of 1 ml/kg bodyweight with the following exceptions. Morphine was dissolved in DMSO and administered subcutaneously (s.c.) in a volume of 1 ml/kg. Thus, the volume of DMSO administered was uniform between animals in all studies involving systemically administered agonists. Naloxone was dissolved in saline and administered locally into the dorsal surface of the paw (intrapaw; 50-µl volume) as described previously (20) or intraperitoneally in a volume of 1 ml/kg.
General Experimental Methods
Baseline responses to mechanical stimulation to the hindpaw were evaluated at least 1 h prior to evaluation of baseline responses to thermal stimulation. In a subset of experiments (approximately 25%), the order of baseline testing was reversed (i.e., baseline responses to thermal stimulation were assessed at least 1 h prior to evaluation of baseline responses to mechanical stimulation). This modification enabled us to confirm that hypersensitivity to thermal or mechanical stimulation was not produced by the order of testing mechanical and thermal responses (data not shown). Following completion of baseline testing, all rats were returned to their home cages for approximately 2 h prior to administration of drug or vehicle. All studies were conducted by a single experimenter who was blinded to the drug conditions. Animals were randomly assigned to drug or vehicle treatments.
Assessment of Mechanical Withdrawal Thresholds and Thermal Paw Withdrawal Latencies
Mechanical withdrawal thresholds were assessed using a digital Electrovonfrey Anesthesiometer (IITC model Alemo 2290-4; Woodland Hills, CA, USA) equipped with a rigid tip. Rats were placed underneath inverted plastic cages and positioned on an elevated mesh platform. Rats were allowed 10–15 min to habituate to the chamber prior to testing. Stimulation was applied to the midplantar region of the hindpaw through the floor of a mesh platform. Mechanical stimulation was terminated upon paw withdrawal; consequently, there was no upper threshold limit set for termination of a trial. Mechanical paw withdrawal thresholds are reported as the mean of duplicate determinations averaged across paws.
Paw withdrawal latencies to radiant heat were measured in duplicate for each paw using the Hargreaves test (28) and a commercially available plantar stimulation unit (IITC model 336; Woodland Hills, CA, USA). Rats were placed underneath inverted plastic cages positioned on an elevated glass platform. Rats were allowed 10–15 min to habituate to the chamber prior to testing. Radiant heat was presented to the midplantar region of the hindpaw through the floor of the glass platform. The intensity of the heat source was adjusted such that an average baseline latency of approximately 20 s was achieved (16). Stimulation was terminated upon paw withdrawal or after 40 s to prevent tissue damage. Thermal paw withdrawal latencies are reported as the mean of duplicate determinations averaged across paws, with the exception of studies where i.paw injections were administered.
Baseline mechanical withdrawal thresholds and thermal paw withdrawal latencies were assessed prior to pharmacological manipulations. Mechanical paw withdrawal thresholds were assessed at 15 min following injection of drug or vehicle. The 15-min time point was selected because the antinociceptive dose–response profile of (R,S)-AM1241 to thermal stimulation in the Hargreaves test has been previously characterized at this time point following systemic administration (16). Thermal paw withdrawal latencies were subsequently measured in the same animals at 30, 60, and 120 min postinjection to assess the time course of CB2 agonist actions.
The antinociceptive effects of aminoalkylindole CB2 agonists were evaluated for responsiveness to mechanical (electrovonfrey) and thermal (in the Hargreaves test) stimulation. Separate groups of animals received either racemic (R,S)-AM1241 (0.033, 0.1, 0.330, 1, 5, or 10 mg/kg i.p.; n = 7–8 per group), chiral (R)-AM1241 (0.033, 0.1, 0.33, 1, 5, or 10 mg/kg i.p.; n = 8 per group), chiral (S)-AM1241 (0.033, 0.1, 0.33, 1, 5, or 10 mg/kg i.p.; n = 8 per group), or vehicle (n = 19). Separate groups received the opioid agonist morphine (2 mg/kg s.c.; n = 8).
To determine pharmacological specificity, either the CB1 antagonist rimonabant (6 mg/kg i.p.) or the CB2 antagonist SR144528 (6 mg/kg i.p.) was coadministered with either (R,S)-AM1241 (1 mg/kg i.p., n = 7–8 per group), (R)-AM1241 (1 mg/kg i.p., n = 8 per group), or (S)-AM1241 (1 mg/kg i.p., n = 8 per group). Rimonabant (6 mg/kg i.p., n = 8 per group) and SR144528 (6 mg/kg i.p.; n = 8) were administered to separate groups of animals to evaluate possible antagonist-induced changes in basal nociceptive thresholds.
To evaluate whether opioid receptors contributed to the antinociceptive effects of CB2 agonists from the aminoalkylindole class, (R)-AM1241 (1 mg/kg), (S)-AM1241 (1 mg/kg), (R,S)-AM1241 (1 mg/kg and 0.33 mg/kg i.p.), or morphine (2 mg/kg s.c.) was administered in tandem with a local injection of naloxone in the dorsal surface of the paw (10 μg or 50 μg i.paw; n = 6–8 per group). Additional groups received dorsal paw injections of either naloxone (10 μg or 50 μg i.paw; n = 8 per group) or saline (n = 8). Right or left paw injections were counterbalanced between subjects. Paw withdrawal thresholds and latencies were measured in both the injected and noninjected paw for all animals at baseline and all postinjection time points.
In a separate study, groups of animals received naloxone (10 mg/kg i.p.) 20 min prior to injection of either (R,S)-AM1241 (1 mg/kg i.p.; n = 8), (R)-AM1241 (1 mg/kg i.p.; n = 8), (S)-AM1241 (1 mg/kg i.p.; n = 8), or morphine (2 mg/kg s.c.; n = 8). A separate group of animals received naloxone alone (10 mg/kg i.p.; n = 8).
Statistical Analyses
Data were analyzed using analysis of variance (ANOVA) for repeated-measures, one-way ANOVA or planned comparison Student t tests, as appropriate. SPSS 16.0 (SPSS Incorporated, Chicago, IL, USA) statistical software was employed. The Greenhouse–Geissser correction was applied to the interaction term of all repeated factors. Degrees of freedom reported for interaction terms of repeated factors are the uncorrected values. Post hoc comparisons between control groups and other experimental groups were performed using the Dunnett test. Post hoc comparisons between different experimental groups were performed to assess dose–response relationships and pharmacological specificity using the Tukey test. P ≤ 0.05 was considered statistically significant.
RESULTS
General Results
Thermal paw withdrawal latencies and mechanical withdrawal thresholds did not differ between right and left paw for any group with the exception of studies in which i.paw injections were administered unilaterally. Therefore, withdrawal thresholds are presented as the mean of duplicate measurements, averaged across paws, in all studies not employing i.paw injections. In all studies, baseline paw withdrawal latencies or mechanical withdrawal thresholds were similar between groups prior to administration of drug or vehicle. Baseline thermal paw withdrawal latencies did not differ between groups; therefore, baselines in the log dose–response plots (Fig. 2) were averaged across all doses of the same drug for statistical analyses. Moreover, thermal paw withdrawal latencies and mechanical withdrawal thresholds did not differ based upon the order of thermal and mechanical testing at baseline; therefore, the two vehicle groups are combined for all studies presented.
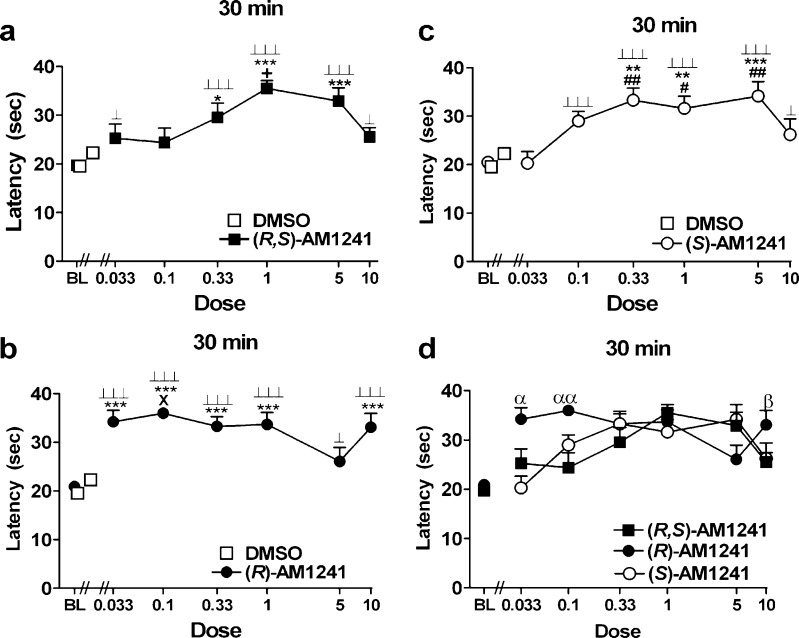
Fig. 2.
a–c Log dose response of a (R,S)-AM1241, b (R)-AM1241, and c (S)-AM1241 shows withdrawal latencies to thermal stimulation in the plantar test at 30 min postinjection. Doses are in milligram per kilogram (mg/kg). d Comparison of log dose response plots for (R,S)-AM1241, (R)-AM1241, and (S)-AM1241 at 30 min postinjection. *P < 0.05, **P < 0.01, ***P < 0.001 vs. DMSO control condition, ⊥ P < 0.05, ⊥⊥⊥ P < 0.001 vs. baseline, + P < 0.05 vs. (R,S)-AM1241 (0.033, 0.1, and 10 mg/kg i.p.), X P < 0.05, vs. (R)-AM1241 (5 mg/kg), # P < 0.05, ## P < 0.01 vs. (S)-AM1241 (0.033 mg/kg i.p.; ANOVA; Dunnett and Tukey post hoc tests). α P < 0.05, αα P < 0.01 vs. all groups at the same dose, β P < 0.05 vs. (R,S)-AM1241 at the same dose (Student t test). N = 7–19 per group
(S)-AM1241 (10 mg/kg i.p.) induced seizure-like activity (e.g., wet dog shakes, muscle spasms, foaming at the mouth, etc.) in two animals tested. No other animals tested with (S)-AM1241 at this or lower doses showed evidence of similar symptoms.
Responses to Mechanical Stimulation
Systemic administration of morphine (2 mg/kg s.c.) increased paw withdrawal thresholds to von Frey stimulation relative to baseline preinjection thresholds (preinjection vs. postinjection: 65.7 ± 3.1 vs. 79.0 ± 4.0 g; P < 0.05 planned comparison t test; Supplementary Table I). By contrast, neither (R,S)-AM1241 nor (R)-AM1241 nor (S)-AM1241 altered mechanical withdrawal thresholds relative to either baseline or vehicle treatment at the same postinjection time point (see Supplementary Table I). Naloxone treatment completely blocked morphine-induced antinociception to mechanical stimulation (Supplementary Table II). However, naloxone, administered either locally or systemically, did not alter paw withdrawal thresholds when administered either alone or in combination with CB2-specific agonists relative to either baseline (preinjection) thresholds or vehicle treatment (data not shown). Cannabinoid antagonist coadministration did not alter mechanical withdrawal thresholds in any study (see Supplementary Table II), with one exception. Coadministration of rimonabant (6 mg/kg i.p.) with (R,S)-AM1241 (1 mg/kg i.p.) increased paw withdrawal thresholds relative to the vehicle condition (preinjection vs. postinjection 67.6 ± 4.1 vs. 107.6 ± 9.5 g; F9, 81 = 2.93, P < 0.01; P < 0.001 for relevant comparison), all other drug conditions (P < 0.05 planned comparison t tests), and baseline (preinjection) thresholds (F9, 81 = 2.90, P < 0.01; P < 0.01 planned comparison t test; Supplementary Table II).
The Aminoalkylindole (R,S)-AM1241 and its Enantiomers Produce Antinociception to Thermal but not Mechanical Stimulation
(R,S)-AM1241 (0.33, 1, and 5 mg/kg i.p.) increased thermal paw withdrawal latencies relative to vehicle treatment at 30 min postinjection (F6, 59 = 5.71, P < 0.001; P < 0.05 for each comparison). (R,S)-AM1241 (0.033, 0.33, 1, 5, and 10 mg/kg i.p.) also increased paw withdrawal latencies relative to baseline at this time point (F6, 87 = 13.64, P < 0.001; P < 0.05 for each comparison; Fig. 2a). An inverted U-shaped dose–response curve was observed at the time point of maximal antinociception (30 min postdrug); (R,S)-AM1241 (1 mg/kg i.p.) produced greater antinociception than either the two lowest (0.033 and 0.1 mg/kg i.p.) or the highest (10 mg/kg i.p.) doses (P < 0.05 for each comparison).
The entire dose range of (R)-AM1241 (0.033, 0.1, 0.33, 1, and 10 mg/kg i.p.) increased thermal paw withdrawal latencies relative to the vehicle condition at 30 min postinjection (F6, 60 = 8.71, P < 0.001; P < 0.001 for each comparison). All doses of (R)-AM1241 also produced antinociception relative to baseline measurements (F6, 89 = 24.74, P < 0.001; P < 0.001 for each comparison, Fig. 2b).
(S)-AM1241 (0.1, 0.33, 1, and 5 mg/kg i.p.) increased thermal paw withdrawal latencies relative to vehicle at 30 min postinjection (F6, 60 = 4.40, P < 0.001; P < 0.01 for each comparison). (S)-AM1241 (0.1, 0.33, 1, 5, and 10 mg/kg i.p.) also produced thermal antinociception relative to baseline at this time point (F6, 89 = 16.43, P < 0.001; P < 0.05 for each comparison; Fig. 2c).
Comparison of Antinociceptive Effects of Racemic (R,S)-AM1241 and Its Enantiomers
Comparisons were made between the antinociceptive effects of racemic (R,S)-AM1241 and the enantiomers (R)- and (S)-AM1241 across the entire dose range. At the time point of maximal antinociception (30 min postinjection), differences in the magnitude of antinociception, relative to baseline, were noted between groups (F17, 125 = 2.81, P < 0.001). Planned comparisons at this time point revealed that the lowest doses of (R)-AM1241 (0.033 and 0.1 mg/kg i.p.) produced greater antinociception than either (S)-AM1241 (P < 0.05; Fig. 2d) or (R,S)-AM1241 (P < 0.05) at the same doses. The highest dose of (R)-AM1241 (10 mg/kg i.p.) also produced greater antinociception relative to the same dose of (R,S)-AM1241 (P < 0.05, planned comparison t test).
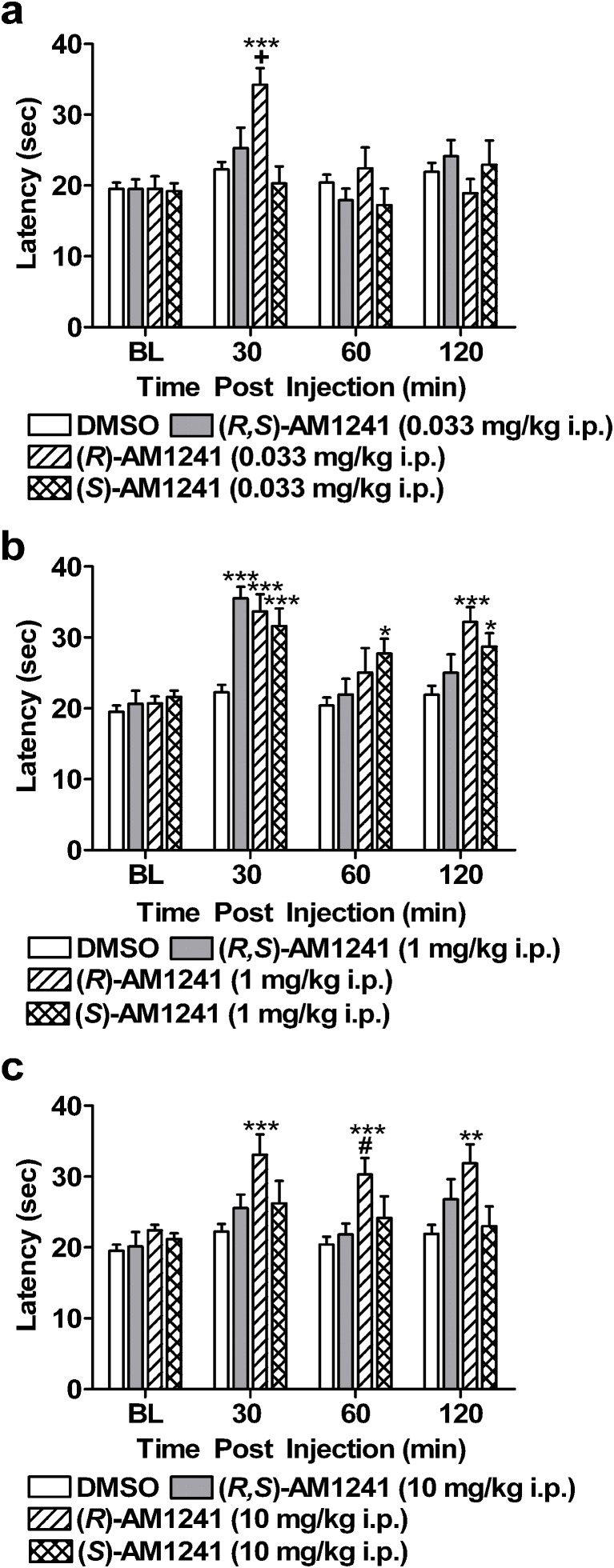
Comparisons were subsequently made between the antinociceptive effects of (R,S)-AM1241, (R)-AM1241, and (S)-AM1241, relative to the DMSO control condition, across the full 120-min time course. The lowest (0.033 mg/kg), middle (1 mg/kg i.p.), and highest (10 mg/kg i.p.) doses were selected for comparison. (R)-AM1241 (0.033 mg/kg i.p.) produced antinociception relative to all other groups tested at 30 min postinjection (F3, 39 = 8.89, P < 0.001; P < 0.05 for each comparison; Fig. 3a). Antinociceptive effects of the lowest dose of (R)-AM1241 were notably absent at subsequent time points (P > 0.25). Racemic AM1241 and (S)-AM1241 (0.033 mg/kg i.p.) failed to produce an antinociceptive effect relative to the DMSO condition at 30 min postinjection (P < 0.29 for each comparison). Both (R,S)-AM1241 (1 mg/kg i.p.) and the enantiomers, (R)-AM1241 (1 mg/kg i.p.) and (S)-AM1241 (1 mg/kg i.p.), produced thermal antinociception in the plantar test at 30 min postinjection relative to the DMSO control condition (F3, 39 = 15.59, P < 0.001; P < 0.001 for each comparison; Fig. 3b). Only (S)-AM1241 (1 mg/kg i.p.), produced an antinociceptive effect at 60 min postinjection (F3, 39 = 2.87, P < 0.05; P < 0.05 for relevant comparison). However, both (R)-AM1241 (1 mg/kg i.p.) and (S)-AM1241 (1 mg/kg i.p.) produced antinociception at 120 min postinjection (F3, 39 = 6.55, P < 0.01; P < 0.05) for each comparison, whereas (R,S)-AM1241 (1 mg/kg i.p.) failed to do so (P > 0.26). The highest dose of (R)-AM1241 (10 mg/kg i.p.) also produced antinociception relative to the vehicle condition at 30 min postinjection (F3, 39 = 5.40, P < 0.01; P < 0.001 for relevant comparison). Antinociceptive effects of (R)-AM1241 (10 mg/kg i.p.) were still present at 120 min postinjection (60 min: F3, 39 = 5.45, P < 0.01; P < 0.05 for relevant comparison; 120 min: F3, 39 = 4.368, P < 0.05; P < 0.05 for relevant comparison; Fig. 3c). Antinociceptive effects of the highest dose of either (R,S)-AM1241 (10 mg/kg i.p.) or (S)-AM1241 (10 mg/kg i.p.) were notably absent at all time points (P > 0.12 for each comparison).
Fig. 3.
Time course of antinociceptive effects of (R,S)-AM1241, (R)-AM1241, and (S)-AM1241. a (R)-AM1241 (0.033 mg/kg i.p.) produced antinociception to thermal stimulation at 30 min postinjection. b (R,S)-AM1241 (1 mg/kg i.p.) and its enantiomers, (R)-AM1241 (1 mg/kg i.p.) and (S)-AM1241 (1 mg/kg i.p.), produced thermal antinociception in the plantar test. c (R)-AM1241 (10 mg/kg i.p.) produced antinociception, whereas (R,S)-AM1241 (10 mg/kg i.p.) and (S)-AM1241 (10 mg/kg i.p.) failed to produce an effect. *P < 0.05, **P < 0.01, ***P < 0.001 vs. DMSO control condition, + P < 0.05 vs. all conditions, # P < 0.05 vs. (R,S)-AM1241 (10 mg/kg i.p.; ANOVA and Dunnett post hoc test). N = 8–19 per group
Pharmacological Specificity
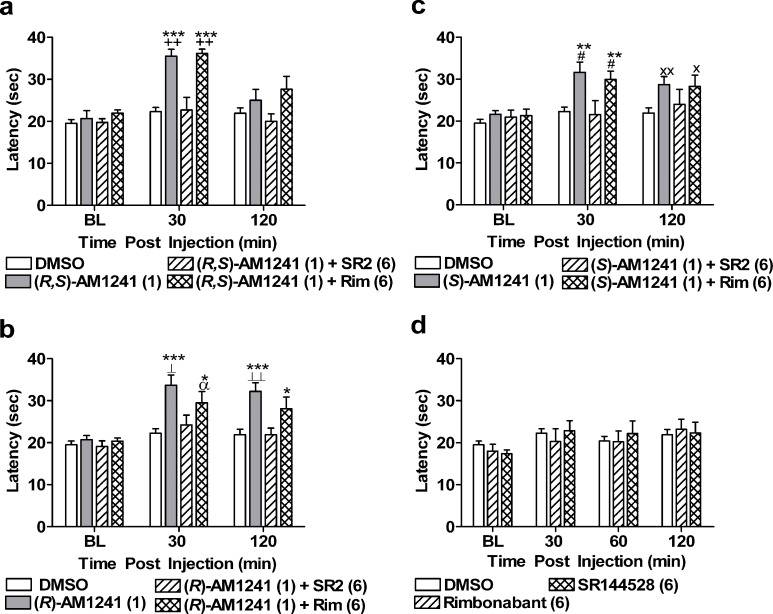
Pharmacological specificity was evaluated using doses of (R,S)-AM1241, (R)-AM1241, and (S)-AM1241 that produced maximal antinociception (1 mg/kg i.p.) for all compounds. (R,S)-AM1241, (R)-AM1241, and (S)-AM1241 produced antinociception to thermal stimulation relative to baseline measurements (P < 0.05). As expected, (R,S)-AM1241 (1 mg/kg i.p.) produced thermal antinociception in the plantar test that was blocked by SR144528 (6 mg/kg i.p.) but not by rimonabant (6 mg/kg i.p.) at 30 min postinjection (F3, 39 = 18.20, P < 0.001; P < 0.01 for each comparison; Fig. 4a). Antinociception produced by either (R)-AM1241 (1 mg/kg i.p.; F3, 39 = 7.88, P < 0.001; P < 0.05 for each comparison; Fig. 4b) or (S)-AM1241 (1 mg/kg i.p.; F3, 39 = 6.56, P < 0.01; P < 0.05 for each comparison; Fig. 4c) was blocked by SR144528 (6 mg/kg i.p.), but not rimonabant (6 mg/kg i.p.), at the same time point. Similar effects were observed for (R)-AM1241 (1 mg/kg i.p.) at 120 min (F3, 39 = 7.10, P < 0.01; P < 0.05 for each comparison; Fig. 4b) postinjection. However, ANOVA failed to reveal a reliable antinociceptive effect of (S)-AM1241 (1 mg/kg i.p.) at 120 min postdrug (P = 0.064). Planned comparisons suggested that (S)-AM1241 (1 mg/kg i.p.), administered either alone or together with rimonabant (6 mg/kg i.p.), produced antinociception at this time point relative to the vehicle condition (P < 0.05 for each planned comparison t test). Rimonabant (6 mg/kg i.p.) and SR144528 (6 mg/kg i.p.) did not alter thermal paw withdrawal latencies relative to vehicle at either 30 (P > 0.66) or 120 (P > 0.88) min postinjection (Fig. 4d).
Fig. 4.
The CB2 antagonist SR144528 (SR2; 6 mg/kg i.p.) but not the CB1 antagonist rimonabant (Rim; 6 mg/kg i.p.) blocked the antinociceptive effects of a (R,S)-AM1241 (1 mg/kg i.p.), b (R)-AM1241 (1 mg/kg i.p.), and c (S)-AM1241 (1 mg/kg i.p.). d Rimonabant (Rim; 6 mg/kg i.p.) and SR144528 (6 mg/kg i.p.) produced no changes in paw withdrawal latencies relative to the vehicle condition. *P < 0.05, **P < 0.01, ***P < 0.001 vs. DMSO control condition, ++ P < 0.01 vs. (R,S)-AM1241 (1) + SR2 (6), ⊥⊥ P < 0.01, ⊥ P < 0.05 vs. (R)-AM1241 (1) + SR2 (6), # P < 0.05 vs. (S)-AM1241 (1) + SR2 (6) (ANOVA; Dunnett and Tukey post hoc tests). XX P < 0.01, X P < 0.05 vs. DMSO control condition, α P < 0.05 vs. (R)-AM1241 (1) + SR2 (6) (Student t test). N = 7–19 per group
Role of Opioid Receptors in Cannabinoid CB2-mediated Antinociception
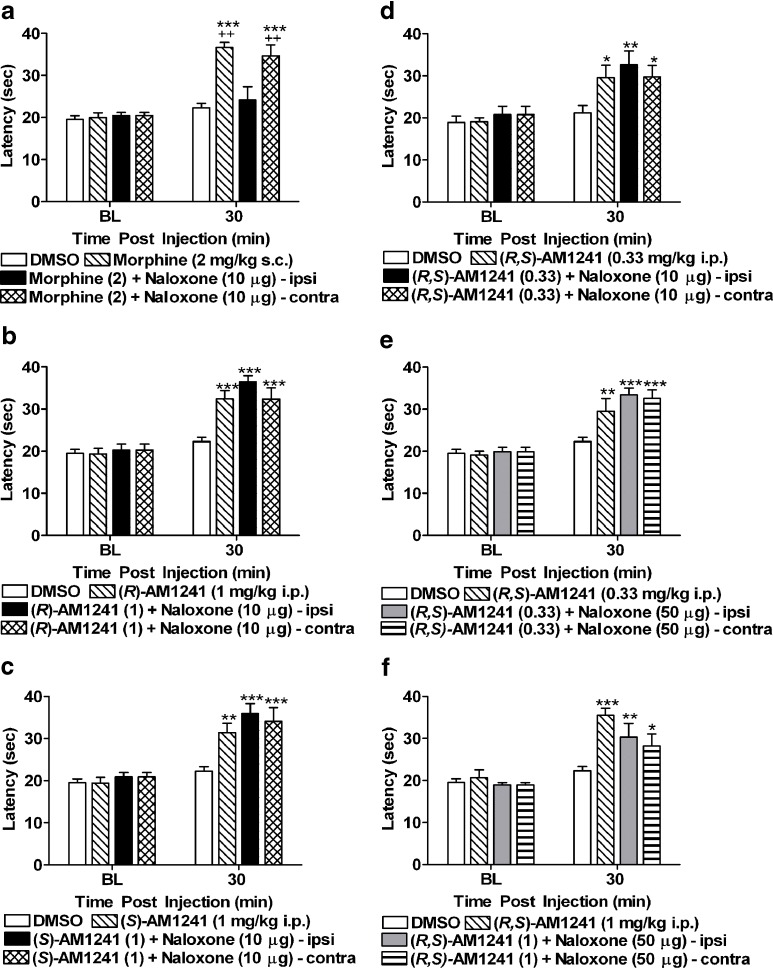
To evaluate the contribution of peripheral opioid receptors to AM1241-induced antinociception, we employed a local dose of naloxone validated previously to block the antinociceptive effects of systemic AM1241 (0.1 mg/kg i.p.) in otherwise naive rats (20). Morphine (2 mg/kg s.c.) produced naloxone-sensitive peripheral antinociception in the plantar test at 30 min postinjection in our study; this effect was completely blocked by local injection of naloxone (10 μg i.paw). A peripheral site of action for this blockade was confirmed by the fact that thermal paw withdrawal latencies remained elevated, relative to baseline (F3, 37 = 13.17, P < 0.001; Fig. 5a) and vehicle treatment (F3, 37 = 17.67, P < 0.001; P < 0.01 for each comparison), in the noninjected paw following systemic morphine administration. Morphine produced antinociception relative to the DMSO condition at 120 min postinjection (F3, 37 = 5.41, P < 0.01; P < 0.05 for relevant comparison; data not shown). However, at this time point, locally injected naloxone was no longer blocking morphine antinociception (P > 0.98). Due to lack of efficacy of naloxone blockade at 120 min, data presented in Fig. 5 are restricted to the 30-min time point. The dose of naloxone (10 μg i.paw) which completely blocked the antinociceptive effects of morphine (2 mg/kg s.c.) failed to block the antinociceptive effects of either (R)-AM1241 (1 mg/kg i.p.; F3, 39 = 17.58, P < 0.001, P < 0.001 for each comparison; Fig. 5b) or (S)-AM1241 (1 mg/kg i.p.; F3, 39 = 12.67, P < 0.001; P < 0.01 for each comparison; Fig. 5c). Moreover, naloxone (10 μg i.paw; F3, 39 = 5.63, P < 0.01; P < 0.05 for each comparison; Fig. 5d) and a fivefold higher dose (50 μg i.paw; F3, 39 = 11.33, P < 0.01; Fig. 5e) failed to block the antinociceptive effects of (R,S)-AM1241 (0.33 mg/kg i.p.) relative to vehicle treatment (P < 0.05 for each comparison). Additionally, naloxone (50 μg i.paw) also failed to block the antinociceptive effects of a higher, more efficacious dose of (R,S)-AM1241 (1 mg/kg i.p.) relative to the vehicle condition (F3, 39 = 9.33, P < 0.001; P < 0.05 for each comparison; Fig. 5f). Under these conditions, naloxone (10 and 50 μg i.paw) did not alter paw withdrawal latencies in either the injected or noninjected paw relative to animals that received local injections of saline (P > 0.72; data not shown).
Fig. 5.
a Naloxone (10 μg i.paw) blocked the antinociceptive effects of morphine (2 mg/kg s.c.). Ipsi denotes the injected paw and contra denotes the noninjected paw. Naloxone (10 μg i.paw) did not block the antinociceptive effects of b (R)-AM1241 (1 mg/kg i.p.), c (S)-AM1241 (1 mg/kg i.p.), or d (R,S)-AM1241 (0.33 mg/kg i.p.). e Naloxone (50 μg i.paw) did not block the effects of (R,S)-AM1241 (0.33 mg/kg i.p.) or f (R,S)-AM1241 (1 mg/kg i.p.). *P < 0.05, **P < 0.01, ***P < 0.001 vs. DMSO control condition, ++ P < 0.01 vs. morphine (2) + naloxone (10 μg)-ipsi (ANOVA; Dunnett and Tukey post hoc tests). N = 6–19 per group
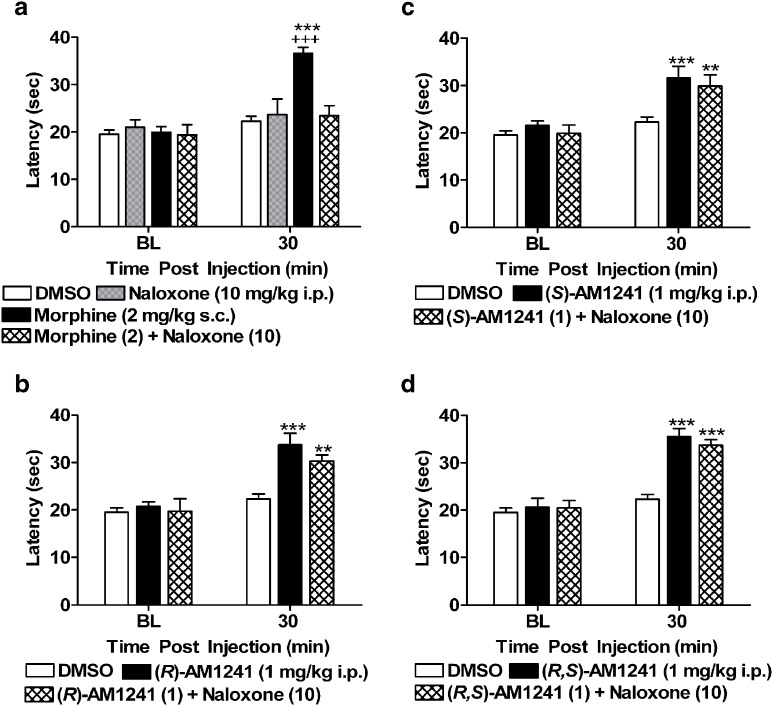
Systemic administration of naloxone (10 mg/kg i.p.) blocked thermal antinociception produced by morphine (2 mg/kg s.c.) at 30 min postinjection (F3, 39 = 12.78, P < 0.001; P < 0.001 for each comparison Fig. 6a), whereas naloxone (10 mg/kg i.p.) alone did not alter paw withdrawal latencies (P > 0.55 for relevant comparison). Morphine (2 mg/kg s.c.) produced an antinociceptive effect at 120 min postinjection relative to both vehicle treatment (F3, 39 = 3.52, P < 0.05; P < 0.05 for relevant comparison) and baseline preinjection thresholds (F3, 39 = 3.47, P < 0.05). However, systemic naloxone (10 mg/kg i.p.) failed to block these observed antinociceptive effects (P > 0.62), suggesting that the duration of action of naloxone blockade was less than 2 h. Data presented in Fig. 6 are consequently restricted to the 30-min time point. Naloxone (10 mg/kg i.p), administered at a dose that completely blocked the antinociceptive effects of morphine (2 mg/kg s.c.) in the same test, failed to block thermal antinociception produced by either (R)-AM1241 (1 mg/kg i.p.; F2, 32 = 17.48, P < 0.001; P < 0.01 for each comparison; Fig. 6b), (S)-AM1241(1 mg/kg i.p.; F2, 32 = 10.00, P < 0.01; P < 0.01 for each comparison; Fig. 6c), or (R,S)-AM1241 (1 mg/kg i.p.; F2, 32 = 36.78, P < 0.001; P < 0.001 for each comparison; Fig. 6d).
Fig. 6.
a Naloxone (10 mg/kg i.p.) blocked the antinociceptive effects of morphine (2 mg/kg s.c.), at a dose that failed to produce an effect when administered alone. Naloxone (10 mg/kg i.p.) did not block the antinociceptive effects of b (R)-AM1241 (1 mg/kg i.p.), c (S)-AM1241 (1 mg/kg i.p.), or d (R,S)-AM1241 (1 mg/kg i.p.). **P < 0.01, ***P < 0.001 vs. DMSO control condition. +++ P < 0.001 vs. all drug groups (ANOVA; Dunnett and Tukey post hoc tests). N = 8–19 per group
DISCUSSION
Racemic AM1241 produces antinociception in the plantar test when administered systemically (16). In our study, (R,S)-AM1241-induced antinociception formed an inverted U-shaped dose–response curve at 30 min postinjection; lower (0.033 and 0.1 mg/kg i.p.) and higher (10 mg/kg i.p.) doses of the drug were less effective at producing antinociception than a dose of 1 mg/kg i.p. Previous reports of (R,S)-AM1241-induced antinociception (16,29) did not test higher doses of (R,S)-AM1241 in the plantar test and therefore did not observe this loss of efficacy. However, the inverted U-shaped dose–response curve could potentially account for conflicting reports of (R,S)-AM1241’s limited antihyperalgesic efficacy (19). Previous work by our lab demonstrated that (R,S)-AM1241 (5 and 10 mg/kg i.p.) was effective at suppressing neuropathic pain induced by administration of the chemotherapeutic agent paclitaxel, whereas a lower dose (1 mg/kg i.p.) failed to produce an effect (30). Thus, it appears that drug efficacy and potency could also be influenced by the receptor state of the animal (i.e., naive vs. neuropathic). As expected, the antinociceptive effects of (R,S)-AM1241 observed in our study were clearly CB2-mediated; these effects were blocked by the CB2 antagonist SR144528 but not by the CB1 antagonist rimonabant. This observation is consistent with previous demonstrations of CB2-mediated antihyperalgesic effects produced by AM1241 in animal models of persistent, inflammatory, and neuropathic pain (30–33).
In contrast to the thermal antinociceptive effects of the CB2 agonists observed here in the plantar test, none of the aminoalkylindoles produced an antinociceptive effect to nonnoxious mechanical stimulation, assessed using a highly sensitive electrovonfrey device. This observation is in marked contrast to the opioid analgesic morphine, which produced reliable, naloxone-sensitive antinociception to mechanical stimulation at the same postinjection time point. Our failure to observe a change in the basal mechanical threshold following administration of either (R,S)-AM1241 or its enantiomers in this test is unlikely to be attributed to selection of an inadequate postinjection time point for evaluation. Malan and colleagues (16) reported robust CB2-mediated antinociception to thermal stimulation following systemic administration of (R,S)-AM1241 at 15 min postinjection. However, our results do not preclude the possibility that antinociception could occur to noxious levels of stimulation (e.g., applied with a Randall Selitto device). Moreover, (R,S)-AM1241 does suppress mechanical hypersensitivity to von Frey stimulation under conditions of injury, during which mechanical thresholds are lowered relative to baseline (3). Coadministration of rimonabant with (R,S)-AM1241 increased mechanical paw withdrawal thresholds. This observation parallels our recent finding of antiallodynia in paclitaxel-treated animals that received rimonabant prior to administration of the CB2 agonist AM1714 (30). Enhanced efficacy of a CB2 agonist following administration of a CB1 antagonist has also been reported in a cerebral ischemic injury model (34). These data suggest that blockade of CB1 receptors with rimonabant may enhance the tone of the endogenous cannabinoid system, thereby increasing the efficacy of the CB2 agonist.
Antinociceptive properties of the enantiomers of (R,S)-AM1241 have not previously been evaluated in naive rats. This characterization is important because of the widespread use of AM1241 as a tool to study functional roles of CB2 receptor activation. Antihyperalgesic effects of (S)-AM1241 were previously reported in a visceral and inflammatory pain model (19). In our study, (S)-AM1241 presented a pharmacological profile which was nearly identical to racemic AM1241. We observed an inverted U-shaped dose–response curve following administration of either (S)-AM1241 or (R,S)-AM1241 at the time point of maximal antinociception (30 min). Our data also illustrate that both the lowest (0.033 and 0.10 mg/kg i.p.) and the highest (10 mg/kg i.p.) doses of (R)-AM1241 produced greater antinociception than comparable doses of either (S)-AM1241 or (R,S)-AM1241. At intermediate doses, the compounds produced similar antinociceptive effects. Previous in vitro work with the enantiomers noted that (R)- and (R,S)-AM1241 are inverse agonists for rat CB2 receptors in the cyclase assay, whereas (S)-AM1241 is a full agonist (19). Thus, it is possible that agonist activity in the cyclase assay predicts the antinociceptive efficacy of (S)-AM1241, thereby reconciling the in vivo observations with results from in vitro receptor binding assays.
Both (R)- and (S)- AM1241 produced thermal antinociception that outlasted that of (R,S)-AM1241 at an identical dose (120-min duration of action as compared to 30 min). This observation may be attributed to the combination of inverse agonist as well as agonist properties of the racemic compound. Differences in metabolic transformation of (R)- and (S)-AM1241 may also contribute to differences in in vivo efficacy of these enantiomers. Although (S)-AM1241 was suggested to be the more active enantiomer in vivo in suppressing acute visceral and inflammatory pain (19), this observation may be dose-dependent. In a chemotherapy model of neuropathic pain, (R)-AM1241, but not (S)-AM1241, was effective in suppressing neuropathic nociception when a high dose of (R)-AM1241 and (S)-AM1241 were evaluated (30). It is important to note that a high dose of (S)-AM1241 (10 mg/kg i.p.) produced seizure-like effects in two of the eight animals tested in our study, effects not observed with either (R,S)-AM1241 or (R)-AM1241. (S)-AM1241 (10 mg/kg i.p.) was previously tested in a chemotherapy model of neuropathic pain and no similar side effects were observed (30). In addition, (S)-AM1241 (10 mg/kg i.p.) was utilized by Bingham and colleagues (19) in visceral (i.e., paraphenyl quinine writhing test) and inflammatory (i.e., carrageenan) pain models, and no similar effects were reported. These latter effects are, therefore, almost certainly due to off-target binding (21).
To our knowledge, this is the first study to examine naloxone sensitivity of (R)- and (S)-AM1241, the enantiomers of (R,S)-AM1241. To accomplish this objective, we employed the opioid antagonist, naloxone, administered both locally and systemically. In our study, local and systemic injections of naloxone completely blocked the antinociceptive effects of morphine. Under these conditions, naloxone, administered alone either intrapaw or intraperitoneally, did not alter paw withdrawal latencies or mechanical withdrawal thresholds relative to comparable controls. We evaluated the contribution of peripheral opioid receptors to the antinociception produced by (R)- and (S)-AM1241 using conditions analogous to those employed by Ibrahim and colleagues (20). Naloxone (10 µg i.paw) was shown previously to block antinociceptive effects of systemic (R,S)-AM1241 (0.1 mg/kg i.p.) in the plantar test (20). However, in our study, this low dose of AM1241 (0.1 mg/kg i.p.) did not produce reliable antinociception relative to vehicle or baseline treatment, so higher doses of racemic and chiral AM1241 (0.33 or 1 mg/kg i.p.) were evaluated for naloxone sensitivity. In our study, locally injected naloxone (10 µg i.paw) completely blocked the antinociceptive effects of systemic morphine in the injected, but not the noninjected paw. However, we were unable to block the antinociceptive effects of either (R)-AM1241, (S)-AM1241, or (R,S)-AM1241 (1 mg/kg i.p.) with locally administered naloxone (10–50 µg i.paw). The lowest dose of (R,S)-AM1241 (0.33 mg/kg i.p.), which produced antinociception, relative to the vehicle condition, in our study was employed as a reference compound in this experiment. However, antinociception produced by (R,S)-AM1241 was not blocked by the local dose of naloxone employed by Ibrahim et al. (20) and was also not blocked by a fivefold higher (50 µg i.paw) dose of naloxone. We observed a similar lack of naloxone-sensitive blockade of (R,S)-AM1241-induced antinociception with both doses of (R,S)-AM1241 (0.33 and 1 mg/kg i.p.), suggesting that dose selection is unlikely to account for these differences. Both our study and that of Ibrahim et al. (20) employed Sprague Dawley rats and a 100% DMSO vehicle for cannabinoid administration. It is possible that the naloxone blockade of (R,S)-AM1241-induced antinociception observed by Ibrahim and colleagues (20) represented a state-dependent or transient phenomenon that was no longer present at 30 min postinjection (the earliest time point at which animals were tested in the plantar test in our study). Differences in animal housing (group housing vs. single housed), animal handling, stress state of the animals tested, or endogenous analgesic tone could contribute to differences in naloxone sensitivity of (R,S)-AM1241-induced antinociception. For example, housing and environmental factors (e.g., objects in the home cage) can decrease nociception in an inflammatory model of pain (35) and may differentially alter endogenous analgesic tone. Thus, under conditions in which endogenous opioid tone is upregulated, a low dose of (R,S)-AM1241 (0.1 mg/kg i.p.) may produce an apparent antinociceptive effect sensitive to blockade by naloxone (19).
We also evaluated whether systemic administration of naloxone (10 mg/kg i.p.) would block the antinociceptive effects of either (R)-AM1241, (S)-AM1241, or (R,S)-AM1241. The ability of systemic naloxone to block the antinociceptive effect of (R,S)-AM1241 has not previously been evaluated in otherwise naive rats. The dose of naloxone employed here was previously shown to block antihyperalgesic effects of (R,S)-AM1241 in a complete Freund’s adjuvant model of chronic inflammatory pain (22) as well as the antiallodynic effects of (R,S)-AM1241 in the spinal nerve ligation model (36). Both of the aforementioned studies employed a high dose of (R,S)-AM1241 (15 mg/kg i.p.). Due to the inverted U-shaped dose–response curve observed for (R,S)-AM1241-induced antinociception, this high dose, in naive rats, might be expected to produce effects comparable to 0.1 or 10 mg/kg i.p. and be less efficacious at inducing antinociception compared to doses of 1 or 5 mg/kg. Moreover, it is also unclear whether this high dose is associated with off-target activity as neither study demonstrated that effects of (R,S)-AM1241 (15 mg/kg i.p.) were CB2-mediated. In our hands, systemic naloxone completely blocked the antinociceptive effects of systemic morphine in the plantar test. However, the same dose of naloxone, administered systemically, failed to block the antinociceptive effects of racemic AM1241 or either of its enantiomers. Our studies suggest that activation of opioid receptors is not sufficient to account for the antinociceptive effects of either (R,S)-AM1241, (R)-AM1241, or (S)-AM1241 in naive animals.
CONCLUSION
The aminoalkylindole (R,S)-AM1241 and its enantiomers (R)-AM1241 and (S)-AM1241 all produce CB2-mediated antinociception that is insensitive to blockade by naloxone and, consequently, is not dependent upon opioid receptor activation. These observations support the hypothesis that the antinociceptive effects of CB2 agonists do not require opioid receptor activation. Our data suggest that the CB2 receptor remains a promising therapeutic target for the treatment of pain.
Electronic supplementary material
(DOC 38.5 KB)
(DOC 37 KB)
ACKNOWLEDGMENTS
The authors are grateful to Ganesh Thakur for helpful discussions. This work was supported R01 DA021644-04, RC1DA028200-01, and R21 DA022478-03 (AGH) and DA9158 and DA3801 (AM). EJR is supported by an APF Graduate Fellowship, a Psi Chi Graduate Research Grant, and an ARCS Foundation Fellowship.
STATEMENT OF CONFLICT OF INTERESTS
AM is on the scientific advisory board of MAK scientific. MAK scientific had no role in this work. There is no conflict of interest.
Abbreviations
- i.p.
intraperitoneal
- i.paw
intrapaw
References
- 1.Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86(8):1946–1947. doi: 10.1021/ja01062a046. [DOI] [Google Scholar]
- 2.Hohmann AG. Spinal and peripheral mechanisms of cannabinoid antinociception: behavioral, neurophysiological and neuroanatomical perspectives. Chem Phys Lipids. 2002;121(1–2):173–190. doi: 10.1016/S0009-3084(02)00154-8. [DOI] [PubMed] [Google Scholar]
- 3.Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol. 2008;153(2):319–334. doi: 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics. 2009;6(4):713–737. doi: 10.1016/j.nurt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96(10):5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onaivi ES. An endocannabinoid hypothesis of drug reward and drug addiction. Ann N Y Acad Sci. 2008;1139:412–421. doi: 10.1196/annals.1432.056. [DOI] [PubMed] [Google Scholar]
- 7.Wiley JL, Martin BR. Cannabinoid pharmacological properties common to other centrally acting drugs. Eur J Pharmacol. 2003;471(3):185–193. doi: 10.1016/S0014-2999(03)01856-9. [DOI] [PubMed] [Google Scholar]
- 8.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310(5746):329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 9.Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- 10.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 11.Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2000;396(2–3):141–149. doi: 10.1016/S0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- 12.Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23(6):1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- 13.Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135(1):235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O'Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17(12):2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- 15.Jhaveri MD, Elmes SJ, Richardson D, Barrett DA, Kendall DA, Mason R, et al. Evidence for a novel functional role of cannabinoid CB receptors in the thalamus of neuropathic rats. Eur J Neurosci. 2008;27(7):1722–1730. doi: 10.1111/j.1460-9568.2008.06162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malan TP, Jr, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, et al. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93(3):239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- 17.Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, et al. HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc Natl Acad Sci USA. 1999;96(25):14228–14233. doi: 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao BB, Mukherjee S, Fan Y, Garrison TR, Daza AV, Grayson GK, et al. In vitro pharmacological characterization of AM1241: a protean agonist at the cannabinoid CB2 receptor? Br J Pharmacol. 2006;149(2):145–154. doi: 10.1038/sj.bjp.0706838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bingham B, Jones PG, Uveges AJ, Kotnis S, Lu P, Smith VA, et al. Species-specific in vitro pharmacological effects of the cannabinoid receptor 2 (CB2) selective ligand AM1241 and its resolved enantiomers. Br J Pharmacol. 2007;151(7):1061–1070. doi: 10.1038/sj.bjp.0707303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci U S A. 2005;102(8):3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao BB, Hsieh G, Daza AV, Fan Y, Grayson GK, Garrison TR, et al. Characterization of a cannabinoid CB2 receptor-selective agonist, A-836339 [2, 2, 3, 3-tetramethyl-cyclopropanecarboxylic acid [3-(2-methoxy-ethyl)-4, 5-dimethyl-3H-thiazol-(2Z)-ylidene]-amide], using in vitro pharmacological assays, in vivo pain models, and pharmacological magnetic resonance imaging. J Pharmacol Exp Ther. 2009;328(1):141–151. doi: 10.1124/jpet.108.145011. [DOI] [PubMed] [Google Scholar]
- 22.Yao BB, Hsieh GC, Frost JM, Fan Y, Garrison TR, Daza AV, et al. In vitro and in vivo characterization of A-796260: a selective cannabinoid CB2 receptor agonist exhibiting analgesic activity in rodent pain models. Br J Pharmacol. 2008;153(2):390–401. doi: 10.1038/sj.bjp.0707568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiteside GT, Gottshall SL, Boulet JM, Chaffer SM, Harrison JE, Pearson MS, et al. A role for cannabinoid receptors, but not endogenous opioids, in the antinociceptive activity of the CB2-selective agonist, GW405833. Eur J Pharmacol. 2005;528(1–3):65–72. doi: 10.1016/j.ejphar.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 24.Thakur GA, Nikas SP, Li C, Makriyannis A. Structural requirements for cannabinoid receptor probes. Handb Exp Pharmacol. 2005;168:209–246. doi: 10.1007/3-540-26573-2_7. [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 26.Nackley AG, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB2 receptors suppresses spinal Fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003;119:747–757. doi: 10.1016/S0306-4522(03)00126-X. [DOI] [PubMed] [Google Scholar]
- 27.Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435(7045):1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 28.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 29.Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, et al. CB2 cannabinoid receptor mediation of antinociception. Pain. 2006;122(1–2):36–42. doi: 10.1016/j.pain.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Rahn EJ, Zvonok AM, Thakur GA, Khanolkar AD, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB2 receptors suppresses neuropathic nociception induced by treatment with the chemotherapeutic agent paclitaxel in rats. J Pharmacol Exp Ther. 2008;327(2):584–591. doi: 10.1124/jpet.108.141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nackley AG, Zvonok AM, Makriyannis A, Hohmann AG. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J Neurophysiol. 2004;92(6):3562–3574. doi: 10.1152/jn.00886.2003. [DOI] [PubMed] [Google Scholar]
- 32.Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutierrez T, Farthing JN, Zvonok AM, Makriyannis A, Hohmann AG. Activation of peripheral cannabinoid CB1 and CB2 receptors suppresses the maintenance of inflammatory nociception: a comparative analysis. Br J Pharmacol. 2007;150(2):153–163. doi: 10.1038/sj.bjp.0706984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M, Martin BR, Adler MW, Razdan RK, Ganea D, Tuma RF. Modulation of the balance between cannabinoid CB(1) and CB(2) receptor activation during cerebral ischemic/reperfusion injury. Neuroscience. 2008;152(3):753–760. doi: 10.1016/j.neuroscience.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tall JM. Housing supplementation decreases the magnitude of inflammation-induced nociception in rats. Behav Brain Res. 2009;197(1):230–233. doi: 10.1016/j.bbr.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Naguib M, Diaz P, Xu JJ, Astruc-Diaz F, Craig S, Vivas-Mejia P, et al. MDA7: a novel selective agonist for CB2 receptors that prevents allodynia in rat neuropathic pain models. Br J Pharmacol. 2008;155(7):1104–1116. doi: 10.1038/bjp.2008.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 38.5 KB)
(DOC 37 KB)